Photo-Durable Molecularly Oriented Liquid Crystalline Copolymer Film based on Photoalignment of N-benzylideneaniline
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Photoreaction
2.3. Hydrolysis of the Oriented Films
2.4. Characterization
3. Results and Discussion
3.1. Thermal and Spectroscopic Properties of the Copolymers
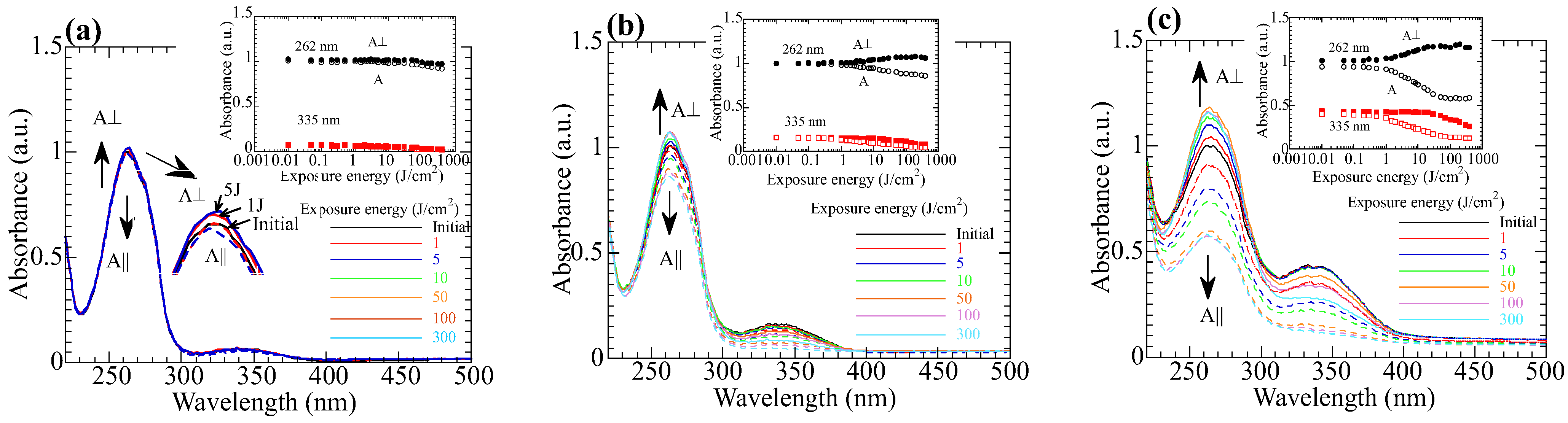
3.2. Axis-Selective Photoreaction of the Copolymer Films
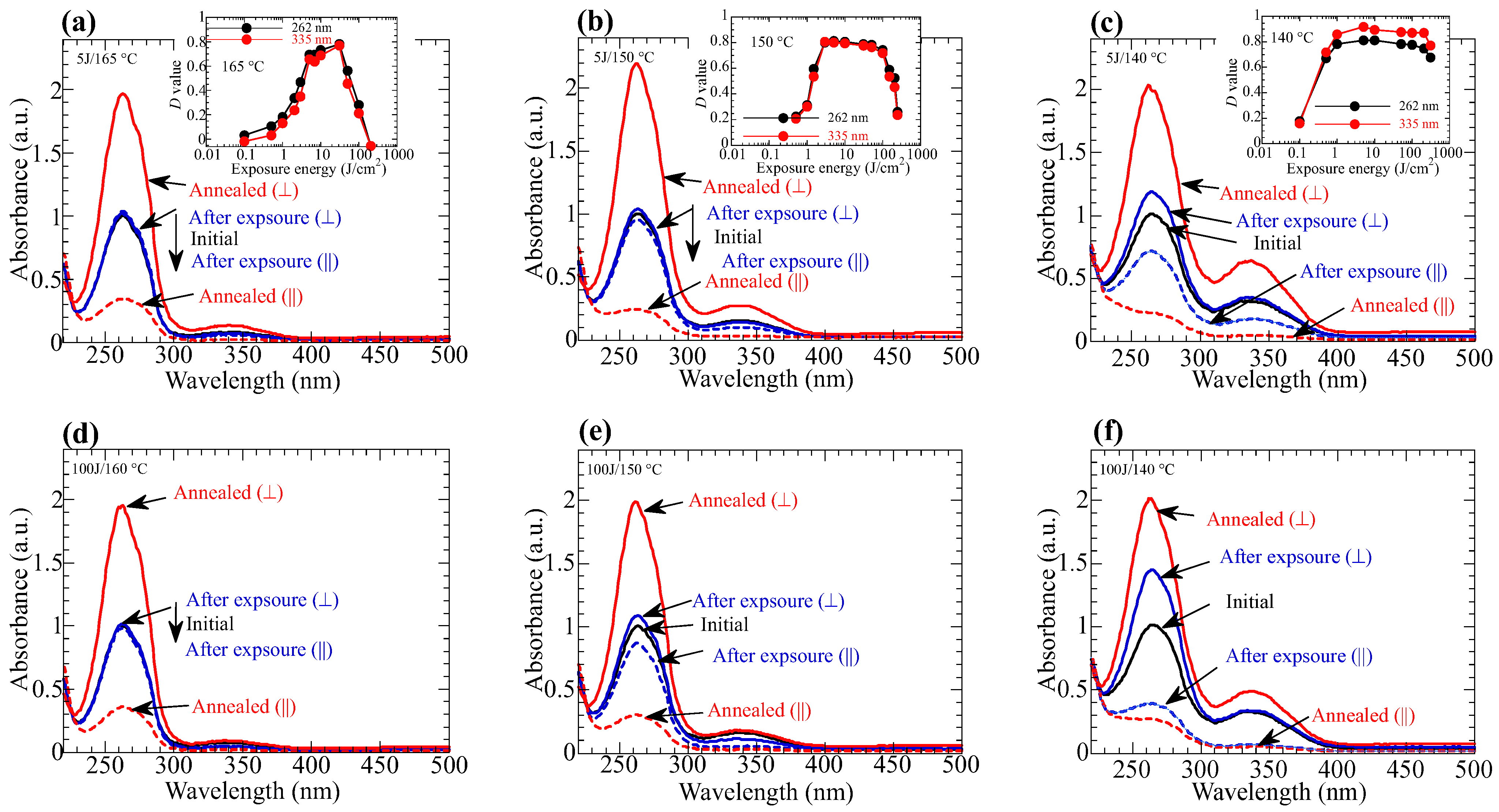
3.3. Thermal Amplification of the Photoinduced Optical Anisotropy
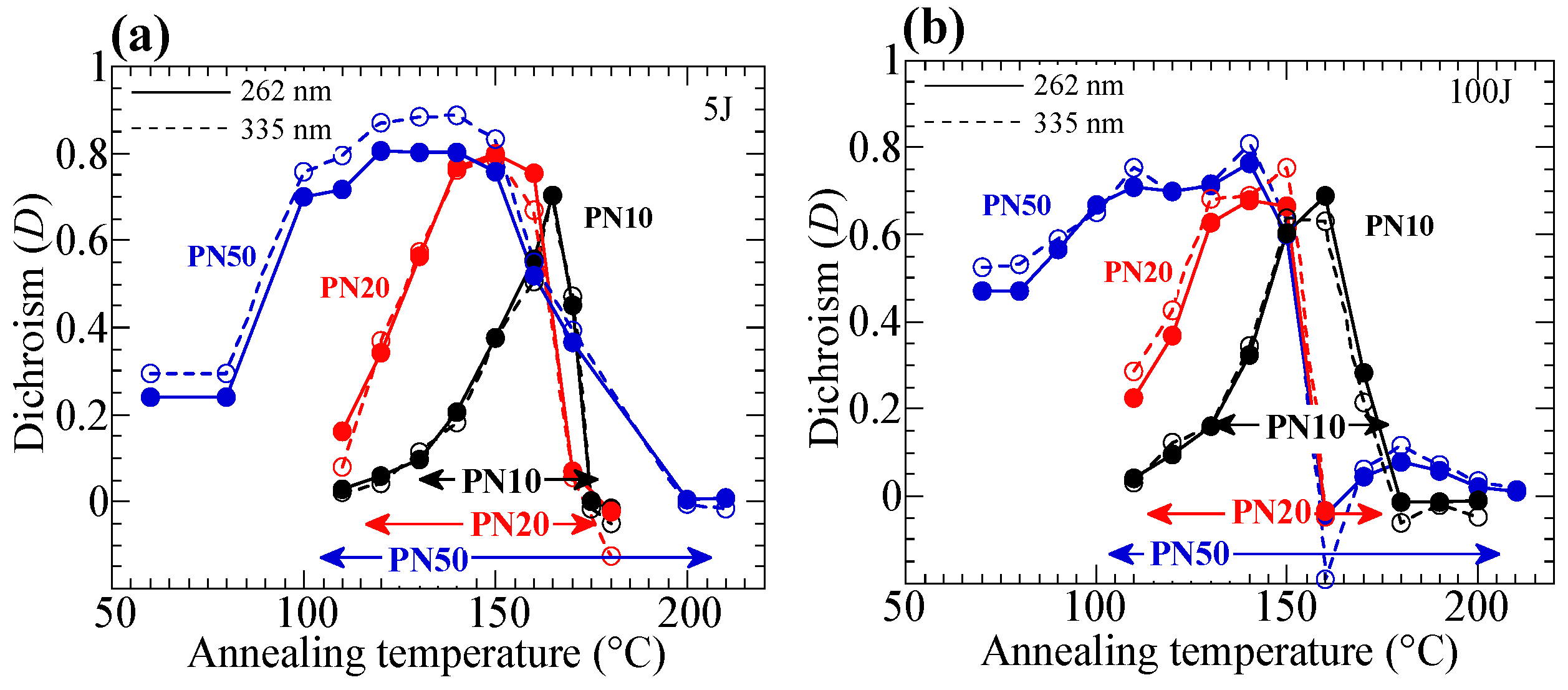
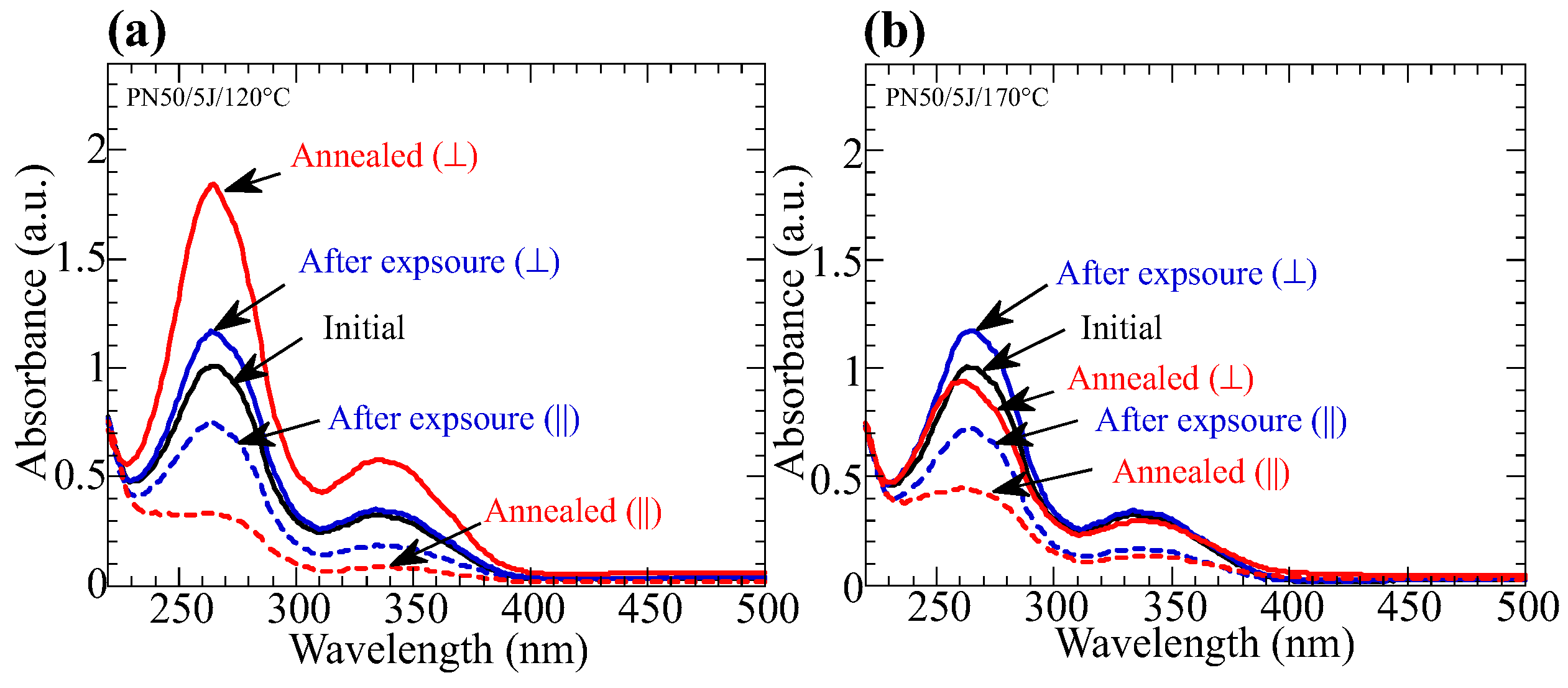
3.4. Influence of the Annelaing Temperature
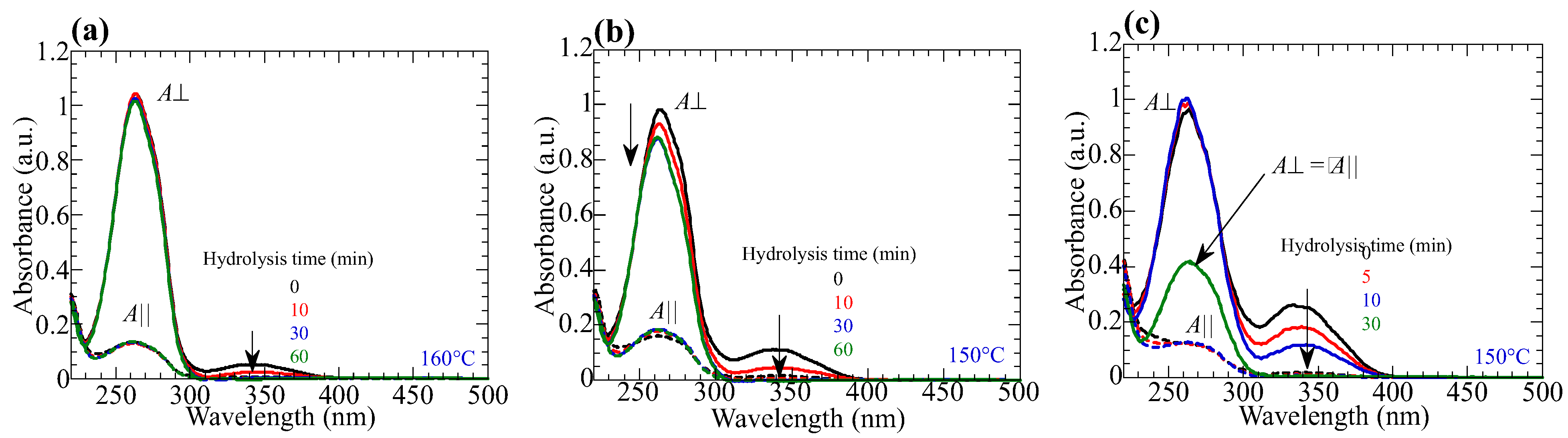
3.5. Hydrolysis of the Reoriented Film
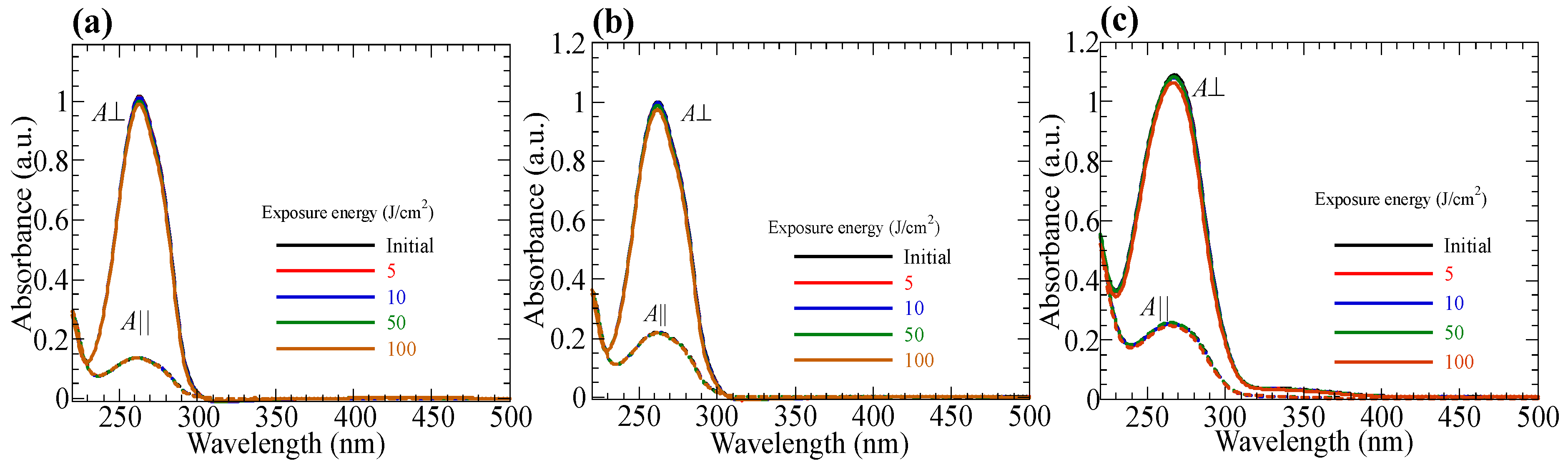
3.6. Photo-Durability of Reoriented Films
3.7. Photo-Durability of Hydrolyzed Oriented Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kawatsuki, N.; Ono, H. Photoinduced Reorientation of Photo-Cross-Linkable Polymer Liquid Crystals and Applications to Highly Functionalized Optical Devices. In Organic Electronics and Photonics; Nalwa, H.S., Ed.; American Sci. Publishers: Stevenson Ranch, CA, USA, 2008; Volume 2, pp. 301–344. [Google Scholar]
- Chigrinov, V.G.; Kozenkov, V.M.; Kwok, H.-S. Photoalignment of Liquid Crystalline Materials; John Wiley & Sons: West Sussex, UK, 2008. [Google Scholar]
- Eich, M.; Wendorff, J.H. Erasable holograms in polymeric liquid crystals. Macromol. Rapid Commun. 1987, 8, 467–471. [Google Scholar] [CrossRef]
- Todorov, T.; Nikolova, L.; Tomova, N. Polarization holography. 1: A new high-efficiency organic material with reversible photoinduced birefringence. Appl. Opt. 1984, 23, 4309–4312. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Hasegawa, T.; Ono, H.; Tamoto, T. Formation of Polarization Gratings and Surface Relief Gratings in Photocrosslinkable Polymer Liquid Crystals by Polarization Holography. Adv. Mater. 2003, 15, 991–994. [Google Scholar] [CrossRef]
- Matsunaga, D.; Tamaki, T.; Akiyama, H.; Ichimura, K. Photofabrication of Micro-patterned Polarizing Elements for Stereoscopic Displays. Adv. Mater. 2002, 14, 1477–1480. [Google Scholar] [CrossRef]
- Schadt, M.; Seiberle, H.; Schuster, A.; Kelly, S.M. Photo-Generation of Linearly Polarized Liquid Crystal Aligning Layers Comprising Novel, Integrated Optically Patterned Retarders and Color Filters. Jpn. J. Appl. Phys. 1995, 34, 3240–3249. [Google Scholar] [CrossRef]
- Kang, H.S.; Lee, S.; Park, J. Monolithic, Hierarchical Surface Reliefs by Holographic Photofluidization of Azopolymer Arrays: Direct Visualization of Polymeric Flows. Adv. Funct. Mater. 2011, 21, 4412–4422. [Google Scholar] [CrossRef]
- Hisano, K.; Ota, M.; Aizawa, M.; Akamatsu, N.; Barrett, C.J.; Shishido, A. Single step creation of polarization gratings by scanning wave photopolymerization with unpolarized light. J. Opt. Soc. Am. B 2019, 36, D112–D118. [Google Scholar] [CrossRef]
- Xuan, Y.; Guo, Q.; Zhao, H.; Zhang, H. Full Stokes Polarization Imaging Based on Broadband Liquid Crystal Polarization Gratings. Crystals 2023, 13, 38. [Google Scholar] [CrossRef]
- Oh, C.; Escuti, M.J. Achromatic diffraction from polarization gratings with high efficiency. Opt. Lett. 2008, 33, 2287–2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nersisyan, S.R.; Tabiryan, N.V.; Hoke, L.; Steeves, D.M.; Kimball, B. Polarization insensitive imaging through polarization gtarings. Opt. Lett. 2009, 17, 1817–1830. [Google Scholar]
- Vernon, J.P.; Serak, S.V.; Hakobyan, R.S.; Aleksanyan, A.K.; Tondiglia, V.P.; White, T.J.; Bunning, T.J.; Tabiryan, N.V. Recording polarization gratings with a standing spiral wave. Appl. Phys. Lett. 2013, 103, 201101. [Google Scholar] [CrossRef]
- Lin, S.H.; Chen, P.; Chuang, C.; Chao, Y.; Hsu, K.Y. Volume polarization holographic recording in thick phenanthrenequinone-doped poly(methyl methacrylate)photopolymer. Opt. Lett. 2011, 36, 3039–3041. [Google Scholar] [CrossRef] [Green Version]
- Kawatsuki, N. Photoalignment and Photoinduced Molecular Reorientation of Photosensitive Materials. Chem. Lett. 2011, 40, 548–554. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced Motions in Azo-Containing Polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T. Photomodulation of liquid crystal orientations for photonic applications. J. Mater. Chem. 2003, 13, 2037–2057. [Google Scholar] [CrossRef]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Kawanishi, T.; Uchida, E. Photoinduced Cooperative Reorientation in Photoreactive Hydrogen-Bonded Copolymer Films and LC Alignment Using the Resultant Films. Macromolecules 2008, 41, 4642–4650. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Matsushita, H.; Washio, T.; Kozuki, J.; Kondo, M.; Sasaki, T.; Ono, H. Photoinduced Orientation of Photoresponsive Polymers with N-Benzylideneaniline Derivative Side Groups. Macromolecules 2014, 47, 324–332. [Google Scholar] [CrossRef]
- Meier, J.G.; Ruhmann, R.; Stumpe, J. Planar and Homeotropic Alignment of LC polymers by the Combination of Photoorientation and Self-Organization. Macromolecules 2000, 33, 843–850. [Google Scholar] [CrossRef]
- Barachevsky, V.A. Photoanisotropic polymeric media and their application in optical devices. Proc. SPIE 1991, 1559, 184–193. [Google Scholar]
- Ichimura, K.; Akita, Y.; Akiyama, H.; Kudo, K.; Hayashi, Y. Photoreactivity of Polymers with Regioisomeric Cinnamate Side Chains and Their Ability to Regulate Liquid Crystal Alignment. Macromolecules 1997, 30, 903–911. [Google Scholar] [CrossRef]
- Yaroshchuk, O.; Pelzl, G.; Pirwitz, G.; Reznikov, Y.; Zaschke, H.; Kim, J.; Kwon, S.B. Photosensitive Materials on a Base of Polysiloxane for the Alignment of Nematic Liquid Crystals. Jpn. J. Appl. Phys. 1997, 36, 5693–5695. [Google Scholar] [CrossRef]
- Obi, M.; Morino, S.; Ichimura, K. Reversion of Photoalignment Direction of Liquid Crystals Induced by Cinnamate Polymer Films. Jpn. J. Appl. Phys. 1999, 38, L145–L147. [Google Scholar] [CrossRef]
- Ito, A.; Norisada, Y.; Inada, S.; Kondo, M.; Sasaki, T.; Sakamoto, M.; Ono, H.; Kawatsuki, N. Photoinduced Reorientation and Photofunctional Control of Liquid Crystalline Copolymers with In Situ-Formed N-Benzylideneaniline Derivative Side Groups. Langmuir 2021, 37, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Nishizono, T.; Kondo, M.; Kawatsuki, N. Photoinduced Molecular Reorientation of a Liquid Crystalline Polymer with a High Birefringence. Chem. Lett. 2021, 50, 912–915. [Google Scholar] [CrossRef]
- Sakai, A.; Nishizono, T.; Kondo, M.; Sasaki, T.; Sakamoto, M.; Ono, H.; Kawatsuki, N. Birefringence Control of Photoalignable Liquid Crystalline Polymers Based on an In Situ Exchange of Oriented Mesogenic Side Groups. Chem. Lett. 2022, 51, 91–93. [Google Scholar] [CrossRef]
- Shishido, A. Rewritable holograms based on azobenzene-containing liquid-crystalline polymers. Polym. J. 2010, 42, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Rochon, P.; Gosselin, J. Optically induced and erased birefringence and dichroism in azoaromatic polymers. Appl. Phys. Lett. 1992, 60, 4–6. [Google Scholar] [CrossRef]
- Eich, M.; Wendroff, J. Laser-induced gratings and spectroscopy in monodomains of liquid-crystalline polymers. J. Opt. Soc. Am. B 1990, 7, 1428–1436. [Google Scholar] [CrossRef]
- Seki, T. New strategies and implications for the photoalignment of liquid crystalline polymers. Polym, J. 2014, 46, 751–768. [Google Scholar] [CrossRef] [Green Version]
- Seki, T.; Nagano, S.; Hara, M. Versatility of photoalignment techniques: From nematics to a wide range of functional materials. Polymer 2013, 54, 6053–6072. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, M.; Sato, D.; Shishido, A.; Ikeda, T. Bragg-Type Polarization Gratings Formed in Thick Polymer Films Containing Azobenzene and Tolane Moieties. Langmuir 2007, 23, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Light-directed alignment, surface morphing and related processes: Recent trends. J. Mater. Chem. C 2016, 4, 7895–7910. [Google Scholar] [CrossRef]
- Shibaev, V.P.; Kostromin, S.G.; Ivanov, S.A. Polymers as Electroactive and Photooptical Media; Shibaev, V., Ed.; Springer: Berlin, Germany, 1996; pp. 37–110. [Google Scholar]
- Zettsu, N.; Ubukata, T.; Seki, T.; Ichimura, K. Soft Crosslinkable Azo Polymer for Rapid Surface Relief Formation and Persistent Fixation. Adv. Mater. 2001, 13, 1693–1697. [Google Scholar] [CrossRef]
- Mitsui, S.; Nagano, S.; Hara, M.; Seki, T. SRG Inscription in Supramolecular Liquid Crystalline Polymer Film: Replacement of Mesogens. Crystals 2017, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, Y.; Sasaki, Y.; Igawa, K.; Tsuji, H. Hydrogen bonding liquid crystalline benzoic acids with alkylthio groups: Phase transition behavior and insights into the cybotactic nematic phase. New J. Chem. 2017, 41, 6514–6522. [Google Scholar] [CrossRef]
- Kato, T.; Fréchet, M.J. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Praefcke, K.; Kohne, B.; Gündogan, B. 2,3,4-Trihexyloxy Cinnamic Acid–The First Example of a Novel Series of Biaxial Nematic Liquid Crystals. Mol. Cryst. Liq. Cryst. Lett. 1990, 7, 27–32. [Google Scholar]
- Gray, G.W.; Jones, B. Mesomorphism and Chemical Constitution. Part I. The n-Alkoxynaphtoic Acids. J. Chem. Soc. 1954, 683–686. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M.J. Stabilization of a liquid-crystalline phase through noncovalent interaction with a polymer side chain. Macromolecules 1989, 22, 3818–3819. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional Liquid-Crystalline Assemblies: Self-Organized Soft Materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef]
- Kumar, U.; Kato, T.; Fréchet, J.M.J. Use of Intermolecular Hydrogen Bonding for the Induction od Liquid Crystallinity on the Side Chain of Polysiloxanes. J. Am. Chem. Soc. 1992, 114, 6630–6639. [Google Scholar] [CrossRef]
- Kan, R.O.; Furey, R.L. Photochemicsal Formation of 1,3-Diazetidines. J. Am. Chem. Soc. 1968, 90, 1666–1667. [Google Scholar] [CrossRef]
- Padwa, A.; Bergmark, W.; Pashayan, D. On the Mechanism of the Photoreduction of Aryl N-Alkylimines. J. Am. Chem. Soc. 1969, 91, 2653–2660. [Google Scholar] [CrossRef]
- Balgoh, G.; Schryver, F. The photochemistry of N-substituted benzaldimines. Tetrahedron Lett. 1969, 17, 1371–1372. [Google Scholar] [CrossRef]
- Wu, Y.; Demachi, Y.; Tsutsumi, O.; Kanazawa, A.; Shiono, T.; Ikeda, T. Photoinduced Alignment of Polymer Liquid Crystals Containing Azobenzene Moieties in the Side Chain. 2. Effect of Spacer Length of the Azobenzene Unit on the Alignment Behavior. Macromolecules 1998, 31, 1104–1108. [Google Scholar] [CrossRef]
- Wu, Y.; Demachi, Y.; Tsutsumi, O.; Kanazawa, A.; Shiono, T.; Ikeda, T. Photoinduced Alignment of Polymer Liquid Crystals Containing Azobenzene Moieties in the Side Chain. 3. Effect of Structure of Photochromic Moieties on the Alignment Behavior. Macromolecules 1998, 31, 4457–4463. [Google Scholar] [CrossRef]
- Date, R.W.; Fawcett, A.H.; Geue, T.; Haferkorn, J.; Malcolm, R.K.; Stumpe, J. Self-Ordering within Thin Films of Poly(olefin sulfone)s. Macromolecules 1998, 31, 4935–4949. [Google Scholar] [CrossRef] [PubMed]
- Charette, J.J.; Hoffmann, E.D. Physicochemical properties of Schiff bases. 4. Tautomeric equilibrium and kinetics of hydrolysis of N-benzylideneaniline derivatives. J. Org. Chem. 1979, 44, 2256–2262. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, B.; Behera, G.S. Hydrolysis of Schiff bases, 1. Kinetics and mechanism of spontaneous, acid, and base hydrolysis of N-(2/4-hydroxybenzylidene)-2-aminobenzothiazoles. Int. J. Chem. Kinet. 1991, 23, 639–654. [Google Scholar] [CrossRef]
- Stimson, V.R. Energy of Activation for the Acid-catalysed Hydrolysis of Esters. Nature 1955, 47, 175. [Google Scholar] [CrossRef]

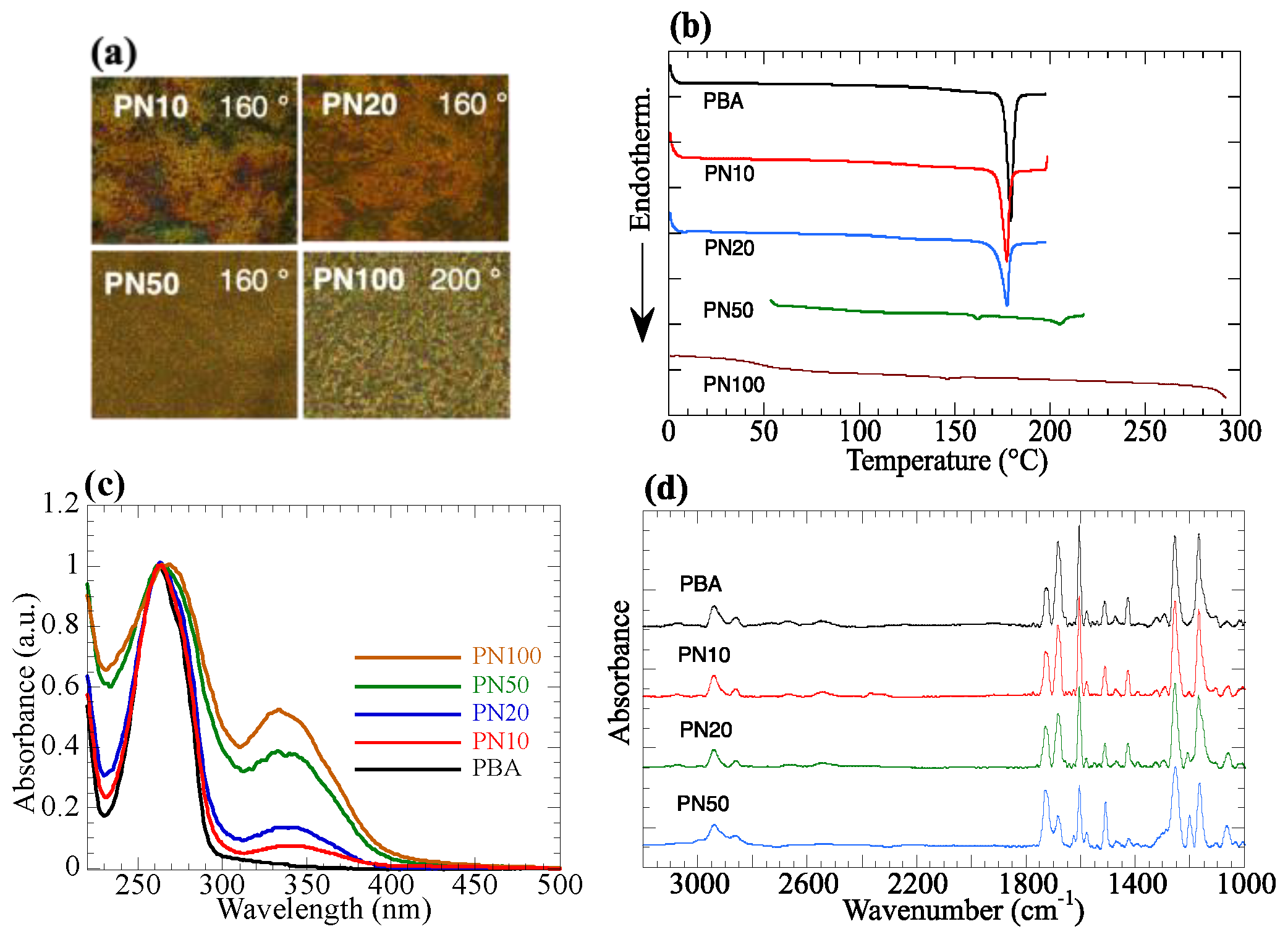




| (Co)Polymers | X (a) | Molecular Weight (b) Mn (Mw/Mn) | Thermal Property (°C) (c) (Transition Enthalpy, J/g) |
|---|---|---|---|
| PBA | 0 | 22,000 (1.5) | G 137 N 179 (29.3) I |
| PN10 | 9 | 37,000 (2.6) | G 121 N 177 (24.6) I |
| PN20 | 18 | 28,000 (2.5) | G 112 N 174 (19.0) I |
| PN50 | 48 | 29,000 (2.1) | G 105 N1 163 (0.63) N2 205 (2.3) I |
| PN100 | 100 | 49,000 (1.9) | G 75 N 295 I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, G.; Kondo, M.; Sakamoto, M.; Sasaki, T.; Ono, H.; Kawatsuki, N. Photo-Durable Molecularly Oriented Liquid Crystalline Copolymer Film based on Photoalignment of N-benzylideneaniline. Polymers 2023, 15, 1408. https://doi.org/10.3390/polym15061408
Nakajima G, Kondo M, Sakamoto M, Sasaki T, Ono H, Kawatsuki N. Photo-Durable Molecularly Oriented Liquid Crystalline Copolymer Film based on Photoalignment of N-benzylideneaniline. Polymers. 2023; 15(6):1408. https://doi.org/10.3390/polym15061408
Chicago/Turabian StyleNakajima, Gento, Mizuho Kondo, Moritsugu Sakamoto, Tomoyuki Sasaki, Hiroshi Ono, and Nobuhiro Kawatsuki. 2023. "Photo-Durable Molecularly Oriented Liquid Crystalline Copolymer Film based on Photoalignment of N-benzylideneaniline" Polymers 15, no. 6: 1408. https://doi.org/10.3390/polym15061408
APA StyleNakajima, G., Kondo, M., Sakamoto, M., Sasaki, T., Ono, H., & Kawatsuki, N. (2023). Photo-Durable Molecularly Oriented Liquid Crystalline Copolymer Film based on Photoalignment of N-benzylideneaniline. Polymers, 15(6), 1408. https://doi.org/10.3390/polym15061408









