Effect of Hemp Hurd Biochar and Humic Acid on the Flame Retardant and Mechanical Properties of Ethylene Vinyl Acetate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Hemp Hurd Waste Pyrolysis and Composite Preparation
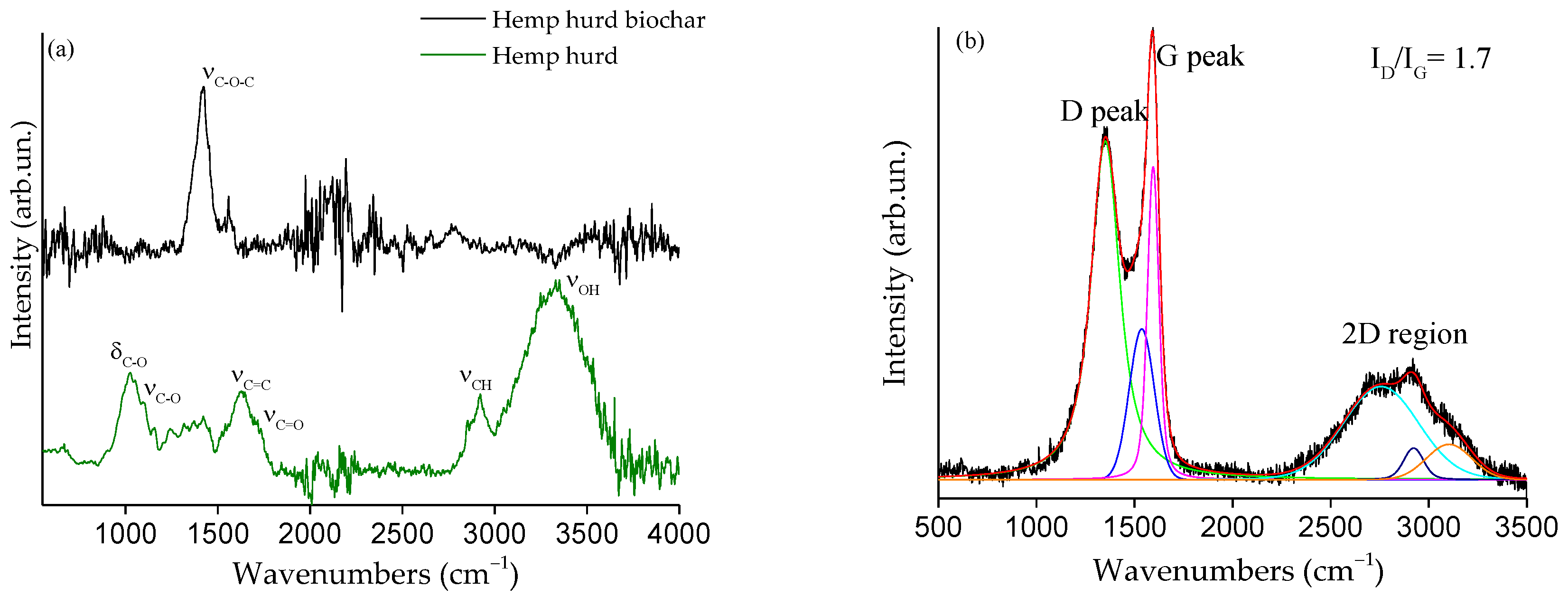
2.2.2. Fourier Transform Infrared (FT-IR) and Raman Analysis
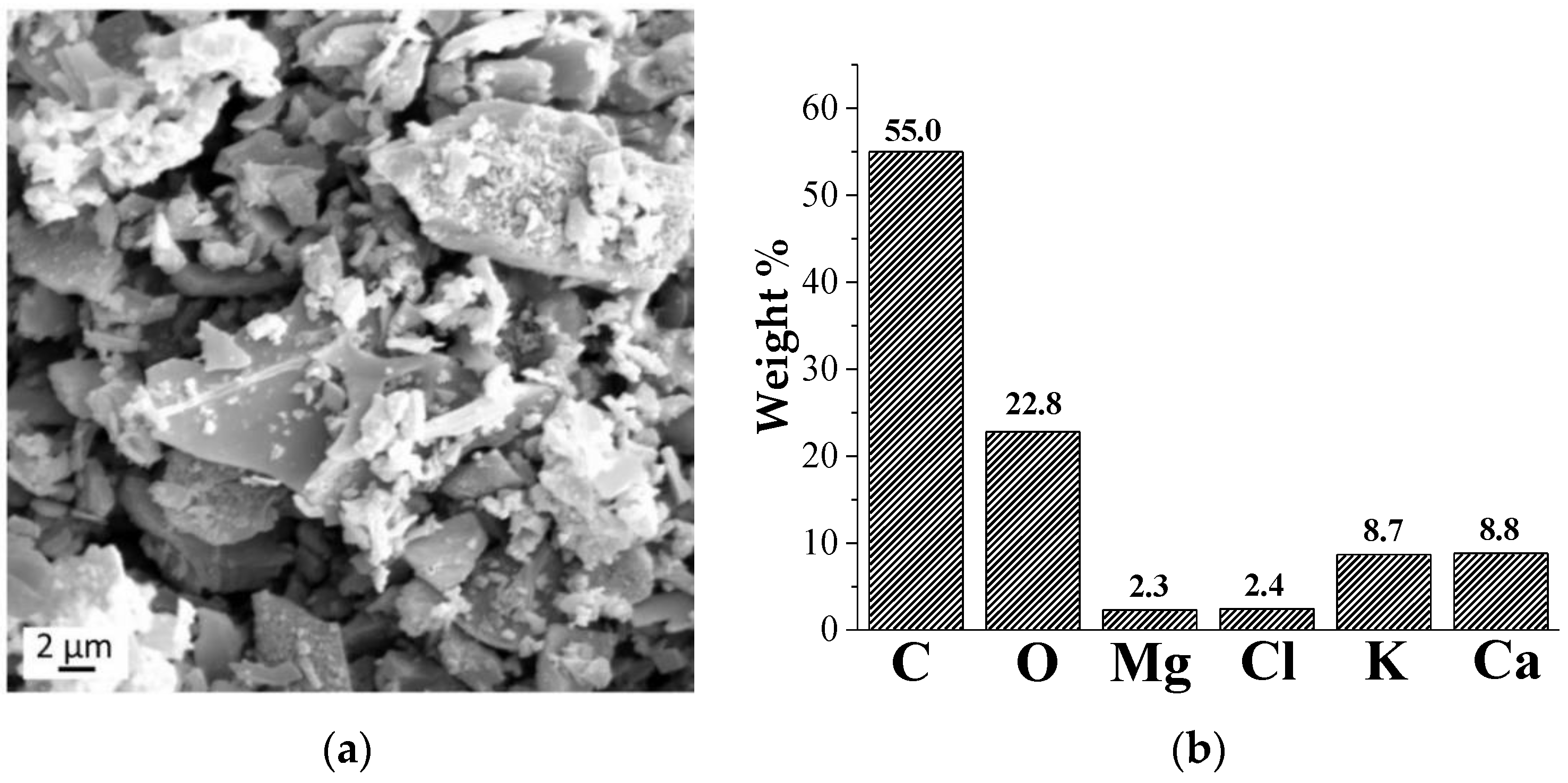
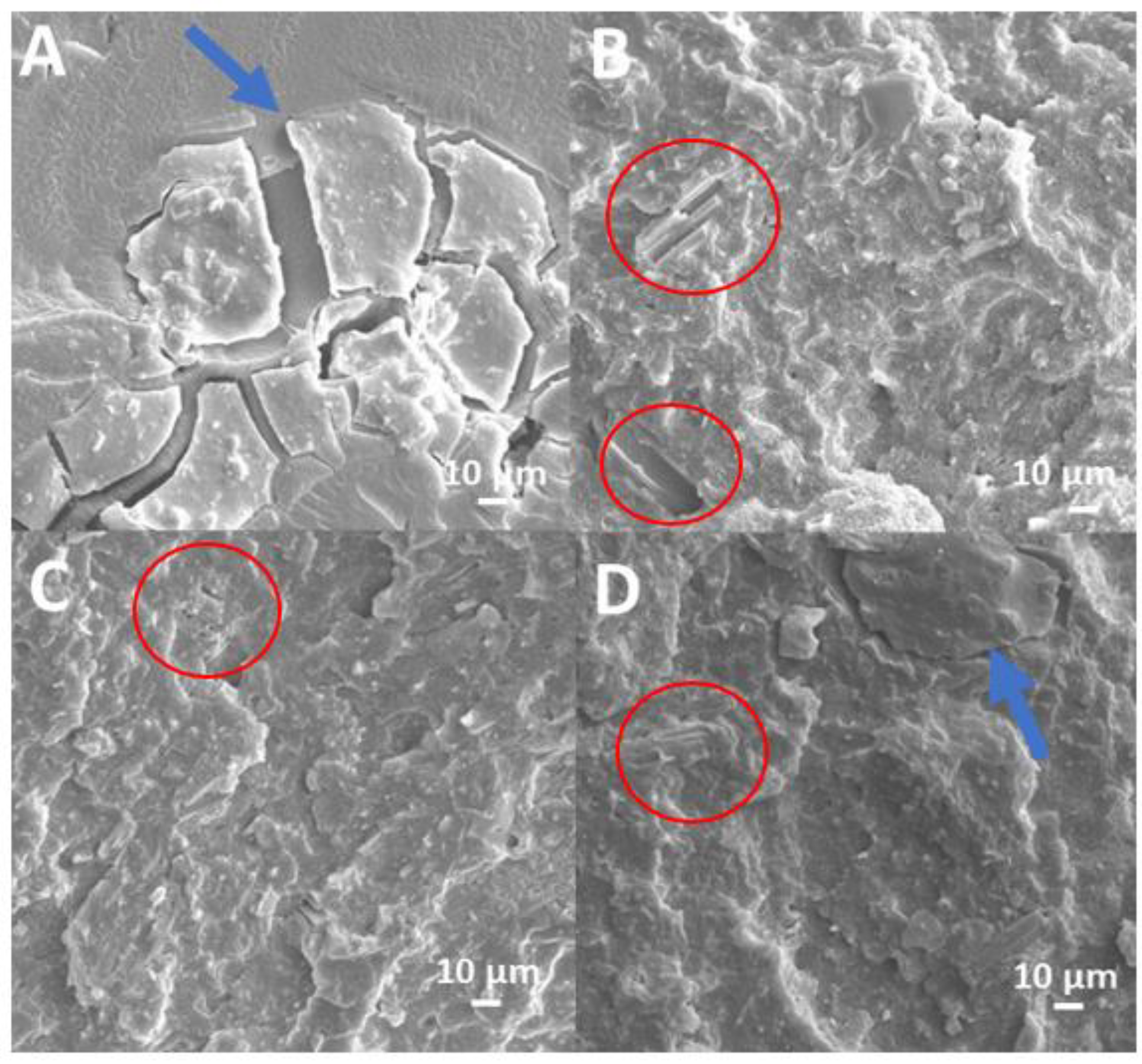
2.2.3. Morphological Analysis (SEM and EDX)
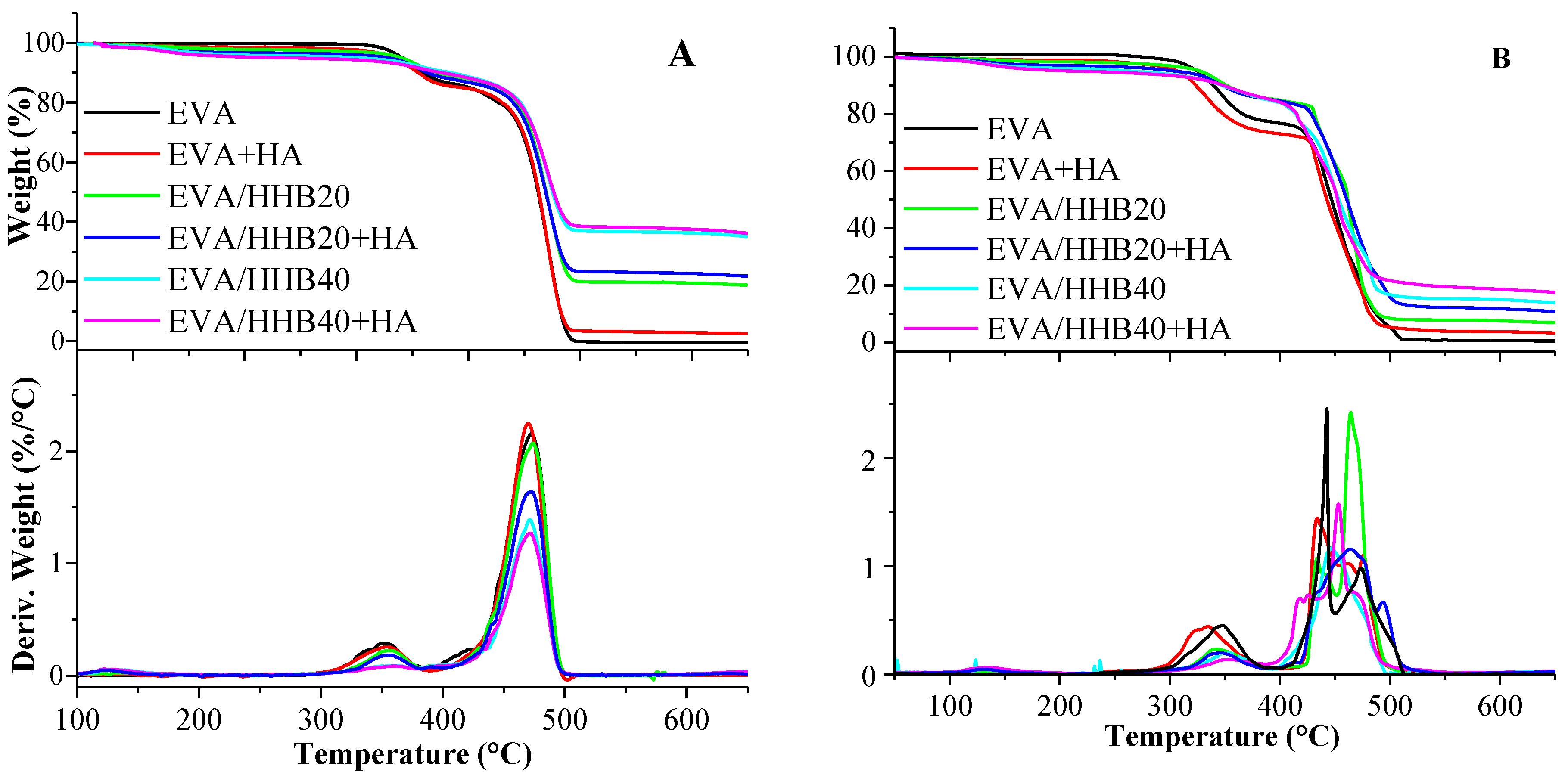
2.2.4. DSC and TGA
2.2.5. Characterization of the Fire Behavior
2.2.6. Mechanical Properties
3. Results and Discussion
3.1. Morphological and Structural (FTIR and Raman) Analysis of Hemp Hurd-Derived Biochar
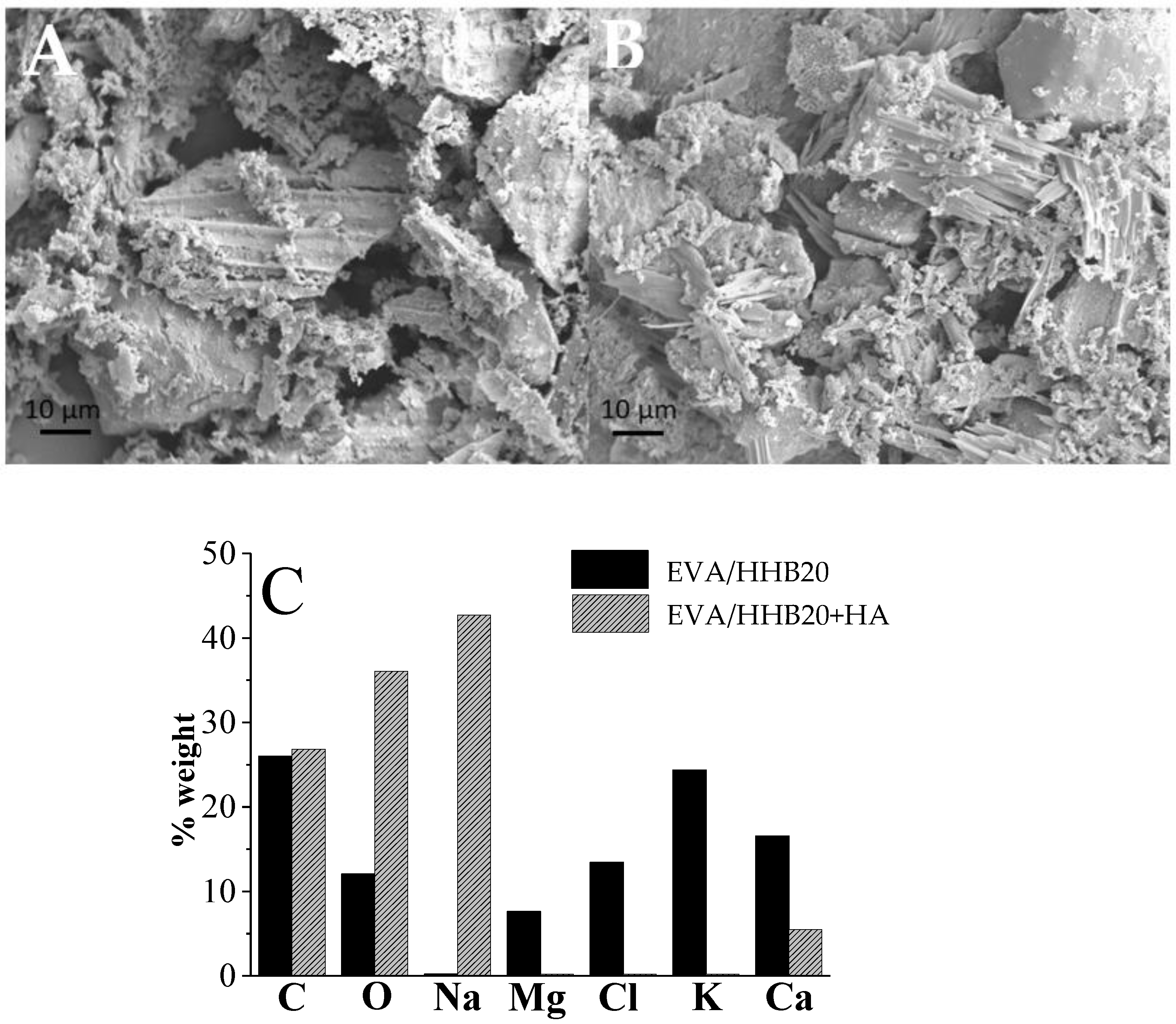
3.2. Morphological Analysis of EVA+HA, EVA/HHB, and EBA/HHB+HA Composites
3.3. Thermal Analysis
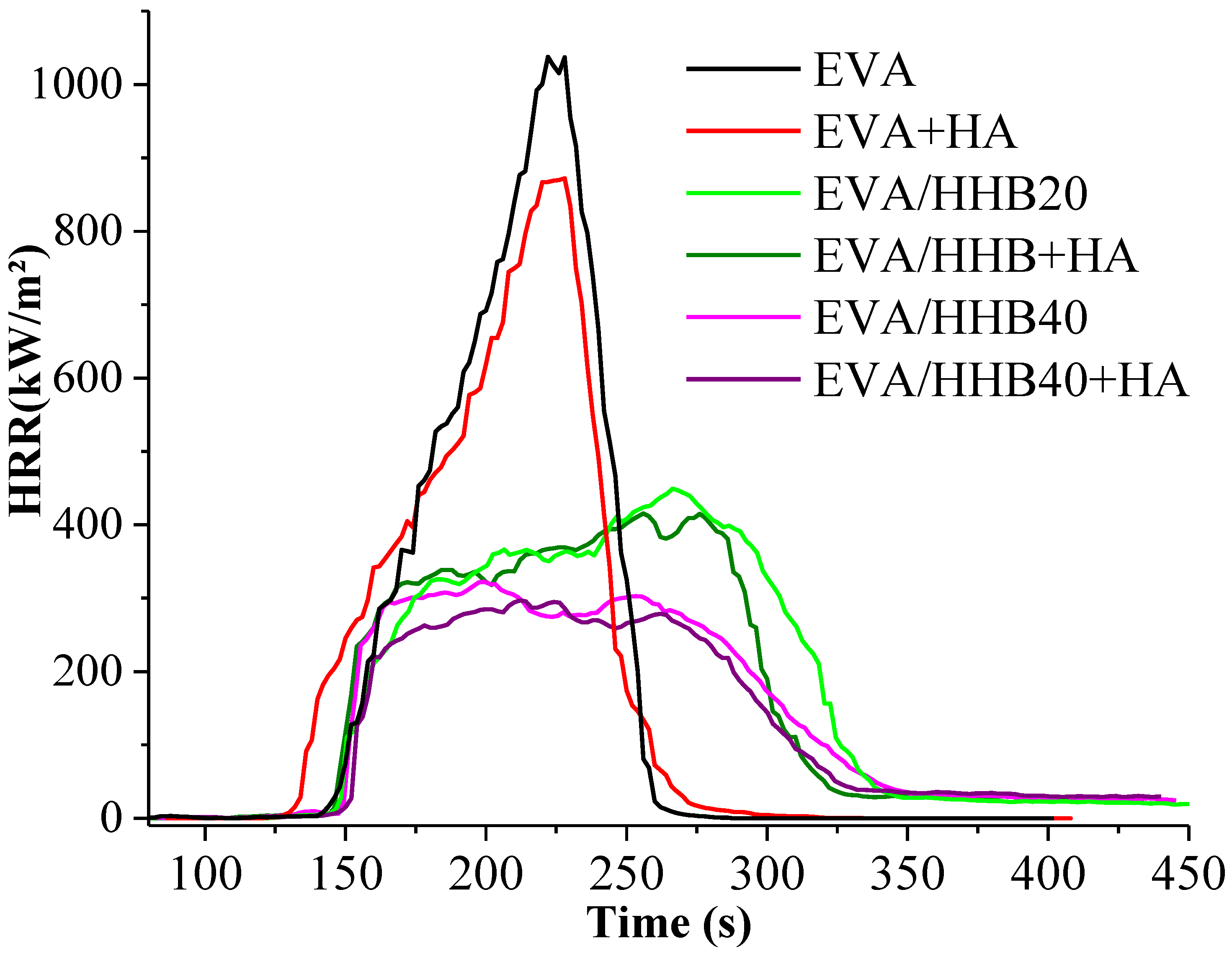
3.4. Fire Behavior
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Kümmerer, K.; Clark, J.H.; Zuin, V.G. Rethinking Chemistry for a Circular Economy. Science 2020, 367, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, I.; Sakai, S.-I. Environmental release and behavior of brominated flame retardants. Environ. Int. 2003, 29, 665–682. [Google Scholar] [CrossRef]
- Hobbs, C.E. Recent Advances in Bio-based Flame Retardant Additives for Synthetic Polymer Materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- Sykam, K.; Försth, M.; Sas, G.; Restás, Á.; Das, O. Phytic acid: A bio-based flame retardant for cotton and wool fabrics. Ind. Crop. Prod. 2021, 164, 113349. [Google Scholar] [CrossRef]
- Das, G.; Karak, N. Vegetable oil-based flame retardant epoxy/clay nanocomposites. Polym. Degrad. Stab. 2009, 94, 1948–1954. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Hou, Y.; Hu, Y. Construction of flame retardant coating on polyamide 6.6 via UV grafting of phosphorylated chitosan and sol–gel process of organo-silane. Carbohydr. Polym. 2018, 181, 833. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.S.; Anderson, M.; Xu, C.C. Thermal performance and thermal decomposition kinetics of lignin-based epoxy resins. J. Anal. Appl. Pyrolysis 2016, 119, 124. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, P.; Fang, Z. Synthesis of Phospholipidated β-Cyclodextrin and its Application for Flame-Retardant Poly(Lactic Acid) with Ammonium Polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46054–46064. [Google Scholar] [CrossRef]
- Bosco, F.; Carletto, R.; Alongi, J.; Marmo, L.; Di Blasio, A.; Malucelli, G. Thermal Stability and Flame Resistance of Cotton Fabrics Treated with Whey Proteins. Carbohydr. Polym. 2013, 94, 372–377. [Google Scholar] [CrossRef]
- Alongi, J.; Di Blasio, A.; Milnes, J.; Malucelli, G.; Bourbigot, S.; Kandola, B.; Camino, G. Thermal degradation of DNA, an all-in-one natural intumescent flame retardant. Polym. Degrad. Stab. 2015, 113, 110–118. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, D.Y.; Zhao, J.Q.; Wang, X.L.; Zhu, H.; Wang, C.Y. Meso- and micro-porous composite carbons derived from humic acid for supercapacitors. Electrochim. Acta 2014, 136, 504–512. [Google Scholar] [CrossRef]
- Liu, G.; Shi, H.; Kundu, C.K.; Li, Z.; Li, X.; Zhang, Z. Preparation of novel biomass humate flame retardants and their flame retardancy in epoxy resin. J. Appl. Polym. Sci. 2020, 137, 49601. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Advances in humeomics: Enhanced structural identification of humic molecules after size fractionation of a soil humic acid. Anal. Chim. Acta 2012, 720, 77–90. [Google Scholar] [CrossRef]
- Venezia, V.; Matta, S.; Lehner, S.; Vitiello, G.; Costantini, A.; Gaan, S.; Malucelli, G.; Branda, F.; Luciani, G.; Bifulco, A. Detailed Thermal, Fire, and Mechanical Study of Silicon-Modified Epoxy Resin Containing Humic Acid and Other Additives ACS. Appl. Polym. Mater. 2021, 3, 5969–5981. [Google Scholar] [CrossRef]
- Bartoli, M.; Arrigo, R.; Malucelli, G.; Tagliaferro, A.; Duraccio, D. Recent Advances in Biochar Polymer Composites. Polymers 2022, 14, 2506. [Google Scholar] [CrossRef]
- Faga, M.G.; Duraccio, D.; Di Maro, M.; Pedraza, R.; Bartoli, M.; d’Ayala, G.G.; Torsello, D.; Ghigo, G.; Giulio, M. Ethylene-Vinyl Acetate (EVA) Containing Waste Hemp-Derived Biochar Fibers: Mechanical, Electrical, Thermal and Tribological Behavior. Polymers 2022, 14, 4171. [Google Scholar] [CrossRef]
- Poulose, A.M.; Elnour, A.Y.; Anis, A.; Shaikh, H.; Al-Zahrani, S.; George, J.; Al-Wabel, M.I.; Usman, A.R.; Ok, Y.S.; Tsang, D.C. Date palm biochar-polymer composites: An investigation of electrical, mechanical, thermal and rheological characteristics. Sci. Total Environ. 2018, 619, 311–318. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Hui, D.; Lau, K.-T. Mechanical and flammability characterisations of biochar/polypropylene biocomposites. Compos. Part B Eng. 2016, 106, 120–128. [Google Scholar] [CrossRef]
- Ayadi, R.; Koubaa, A.; Braghiroli, F.; Migneault, S.; Wang, H.; Bradai, C. Effect of the pyro-gasification temperature of wood on the physical and mechanical properties of biochar-polymer biocomposites. Materials 2020, 13, 1327. [Google Scholar] [CrossRef] [Green Version]
- Ogunsona, E.O.; Misra, M.; Mohanty, A.K. Impact of interfacial adhesion on the microstructure and property variations of biocarbons reinforced nylon 6 biocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 98, 32–44. [Google Scholar] [CrossRef]
- Das, O.; Kim, N.K.; Kalamkarov, A.L.; Sarmah, A.K.; Bhattacharyya, D. Biochar to the rescue: Balancing the fire performance and mechanical properties of polypropylene composites. Polym. Degrad. Stab. 2017, 144, 485–496. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, H.; Yang, K.; Yi, W. Effect of biochar on mechanical and flame retardant properties of wood–plastic composites. Results Phys. 2017, 7, 2391–2395. [Google Scholar] [CrossRef]
- Zou, D.; Zheng, X.; Ye, Y.; Yan, D.; Xu, H.; Si, S.; Li, X. Effect of different amounts of bamboo charcoal on properties of biodegradable bamboo charcoal/polylactic acid composites. Macromolecules 2022, 216, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.; Bartoli, M.; Frache, A.; Malucelli, G. Investigation of Different Types of Biochar on the Thermal Stability and Fire Retardance of Ethylene-Vinyl Acetate Copolymers. Polymers 2021, 13, 1256. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.; Xu, H.; Lu, W.; Ren, X.; Cai, H.; Lei, H.; Huo, E.; Zhao, Y.; Qian, M.; et al. Biochar filled high-density polyethylene composites with excellent properties: Towards maximizing the utilization of agricultural wastes. Ind. Crop. Prod. 2020, 146, 112185. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, W.; Zhou, L.; Zhang, D.; Cai, H.; Lin, X. Tensile and flammability characterizations of corn straw slagging/high-density polyethylene composites. J. Thermoplast. Compos. Mater. 2020, 33, 1466–1477. [Google Scholar] [CrossRef]
- Park, Y.-J.; Joo, H.-S.; Kim, H.-J.; Lee, Y.-K. Adhesion and rheological properties of EVA-based hot-melt adhesives. Int. J. Adhes. Adhes. 2006, 26, 571–576. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, Y.J.; Jiang, P. Synergistic effects of synthetic phosphonium sulfonates with expandable graphite on flame retardancy for EVA rubber blends. Polym. Degrad. Stab. 2018, 153, 155–164. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Szymczyk, A.; Paszkiewicz, S.; Jędrzejewski, R.; Piesowicz, E.; Siemiński, J. Ethylene vinyl acetate copolymer/halloysite nanotubes nanocomposites with enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2020, 137, 49135. [Google Scholar] [CrossRef]
- Azizi, S.; David, E.; Fréchette, M.F.; Nguyen-Tri, P.; Ouellet-Plamondon, C.M. Electrical and thermal conductivity of ethylene vinyl acetate composite with graphene and carbon black filler. Polym. Test. 2018, 72, 24–31. [Google Scholar] [CrossRef]
- Yuwawech, K.; Wootthikanokkhan, J.; Tanpichai, S. Enhancement of thermal, mechanical and barrier properties of EVA solar cell encapsulating films by reinforcing with esterified cellulose nanofibers. Polym. Test. 2015, 48, 12–22. [Google Scholar] [CrossRef]
- El Hage, R.; Viretto, A.; Sonnier, R.; Ferry, L.; Lopez-Cuesta, J.-M. Flame retardancy of ethylene vinyl acetate (EVA) using new aluminum-based fillers. Polym. Degrad. Stab. 2014, 108, 56–67. [Google Scholar] [CrossRef]
- Tagliaferro, A.; Rovere, M.; Padovano, E.; Bartoli, M.; Giorcelli, M. Introducing the Novel Mixed Gaussian-Lorentzian Lineshape in the Analysis of the Raman Signal of Biochar. Nanomaterials 2020, 10, 1748. [Google Scholar] [CrossRef] [PubMed]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook; John Wiley and Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Lewin, M.; Weil, E.D. Mechanisms and Modes of Action in Flame Retardancy of Polymers. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2001; Chapter 2; pp. 31–68. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Takidis, G.; Bikiaris, D.; Papageorgiou, G.; Achilias, D.; Sideridou, I. Compatibility of low-density polyethylene/poly(ethylene-co-vinyl acetate) binary blends prepared by melt mixing. J. Appl. Polym. Sci. 2003, 90, 841–852. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez-Siurana, A.; Menargues, S. TG/FTIR study of the thermal pyrolysis of EVA copolymers. J. Anal. Appl. Pyrolysis 2005, 74, 224–230. [Google Scholar] [CrossRef]
- Mohammed, Z.; Jeelani, S.; Rangari, V. Effective reinforcement of engineered sustainable biochar carbon for 3D printed polypropylene biocomposites. Compos. Part C Open Access 2022, 7, 100221. [Google Scholar] [CrossRef]
- Lewin, M. Synergism and catalysis in flame retardancy of polymers. Polym. Adv. Technol. 2001, 12, 215–222. [Google Scholar] [CrossRef]


| Samples | Tg (°C) | Tm1,1 (°C) | Tm1,2 (°C) | Tc1 (°C) | Tc2 (°C) | Tm2 (°C) | ΔHm1 (J/g) | χc1 (%) |
|---|---|---|---|---|---|---|---|---|
| EVA | −25.4 | 46.5 | 85.4 | 68.0 | 40.8 | 85.8 | 66.2 | 24 |
| EVA+HA | −25.4 | 46.8 | 86.1 | 67.3 | 40.7 | 85.8 | 58.4 | 23 |
| EVA/HHB20 | −25.7 | 44.6 | 83.7 | 68.8 | 40.7 | 84.2 | 54.6 | 25 |
| EVA/HHB20+HA | −25.8 | 46.0 | 84.7 | 68.1 | 40.6 | 85.3 | 42.8 | 21 |
| EVA/HHB40 | −25.4 | 46.7 | 83.9 | 68.4 | 41.2 | 83.7 | 40.9 | 25 |
| EVA/HHB40+HA | −25.2 | 46.3 | 83.4 | 68.8 | 41.2 | 83.9 | 51.6 | 22 |
| N2 | Air | |||||||
|---|---|---|---|---|---|---|---|---|
| T10% (°C) | Tmax1 (°C) | Tmax2 (°C) | Residue (%) @ 700°C | T10% (°C) | Tmax1 (°C) | T50% (°C) | Residue (%) @ 700°C | |
| EVA | 359 | 352 | 472 | 0.1 | 339 | 350 | 442 | 0 |
| EVA+HA | 355 | 353 | 470 | 2.3 | 324 | 335 | 443 | 2.8 |
| EVA/HHB20 | 370 | 358 | 475 | 18.5 | 352 | 343 | 462 | 6.9 |
| EVA/HHB20+HA | 364 | 358 | 473 | 21.4 | 347 | 342 | 461 | 9.8 |
| EVA/HHB40 | 384 | 362 | 472 | 34.4 | 350 | 354 | 453 | 13.5 |
| EVA/HHB40+HA | 376 | 362 | 473 | 35.4 | 348 | 352 | 453 | 16.2 |
| TTI (s) | pkHRR (kW/m2) | THR (MJ/m2) | SEA (m2/kg) | TSR (m2/m2) | CO/CO2 Ratio | Residue (g) | |
|---|---|---|---|---|---|---|---|
| EVA | 59 ± 1 | 1037 ± 63 | 62 ± 1 | 483 ± 5 | 903 ± 17 | 0.13 ± 0.03 | 0 |
| EVA+HA | 40 ± 5 | 872 ± 62 | 59 ± 2 | 511 ± 5 | 902 ± 22 | 0.03 ± 0.02 | 1.88 ± 0.01 |
| EVA/HHB20 | 62 ± 4 | 450 ± 12 | 62 ± 1 | 467 ± 2 | 877 ± 29 | 0.08 ± 0.01 | 2.13 ± 0.11 |
| EVA/HHB20+HA | 61 ± 4 | 415 ± 25 | 58 ± 2 | 531 ± 30 | 919 ± 25 | 0.06 ± 0.01 | 2.98 ± 0.03 |
| EVA/HHB40 | 56 ± 4 | 322 ± 24 | 50 ± 3 | 506 ± 33 | 774 ± 90 | 0.06 ± 0.02 | 4.77 ± 0.02 |
| EVA/HHB40+HA | 54 ± 7 | 294 ± 9 | 44 ± 1 | 383 ± 67 | 590 ± 50 | 0.05 ± 0.01 | 5.75 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Maro, M.; Faga, M.G.; Pedraza, R.; Malucelli, G.; Bartoli, M.; Gomez d’Ayala, G.; Duraccio, D. Effect of Hemp Hurd Biochar and Humic Acid on the Flame Retardant and Mechanical Properties of Ethylene Vinyl Acetate. Polymers 2023, 15, 1411. https://doi.org/10.3390/polym15061411
Di Maro M, Faga MG, Pedraza R, Malucelli G, Bartoli M, Gomez d’Ayala G, Duraccio D. Effect of Hemp Hurd Biochar and Humic Acid on the Flame Retardant and Mechanical Properties of Ethylene Vinyl Acetate. Polymers. 2023; 15(6):1411. https://doi.org/10.3390/polym15061411
Chicago/Turabian StyleDi Maro, Mattia, Maria Giulia Faga, Riccardo Pedraza, Giulio Malucelli, Mattia Bartoli, Giovanna Gomez d’Ayala, and Donatella Duraccio. 2023. "Effect of Hemp Hurd Biochar and Humic Acid on the Flame Retardant and Mechanical Properties of Ethylene Vinyl Acetate" Polymers 15, no. 6: 1411. https://doi.org/10.3390/polym15061411
APA StyleDi Maro, M., Faga, M. G., Pedraza, R., Malucelli, G., Bartoli, M., Gomez d’Ayala, G., & Duraccio, D. (2023). Effect of Hemp Hurd Biochar and Humic Acid on the Flame Retardant and Mechanical Properties of Ethylene Vinyl Acetate. Polymers, 15(6), 1411. https://doi.org/10.3390/polym15061411











