Synthesis of Chemically Modified Acid-Functionalized Multiwall Carbon Nanotubes with Benzimidazole for Removal of Lead and Cadmium Ions from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
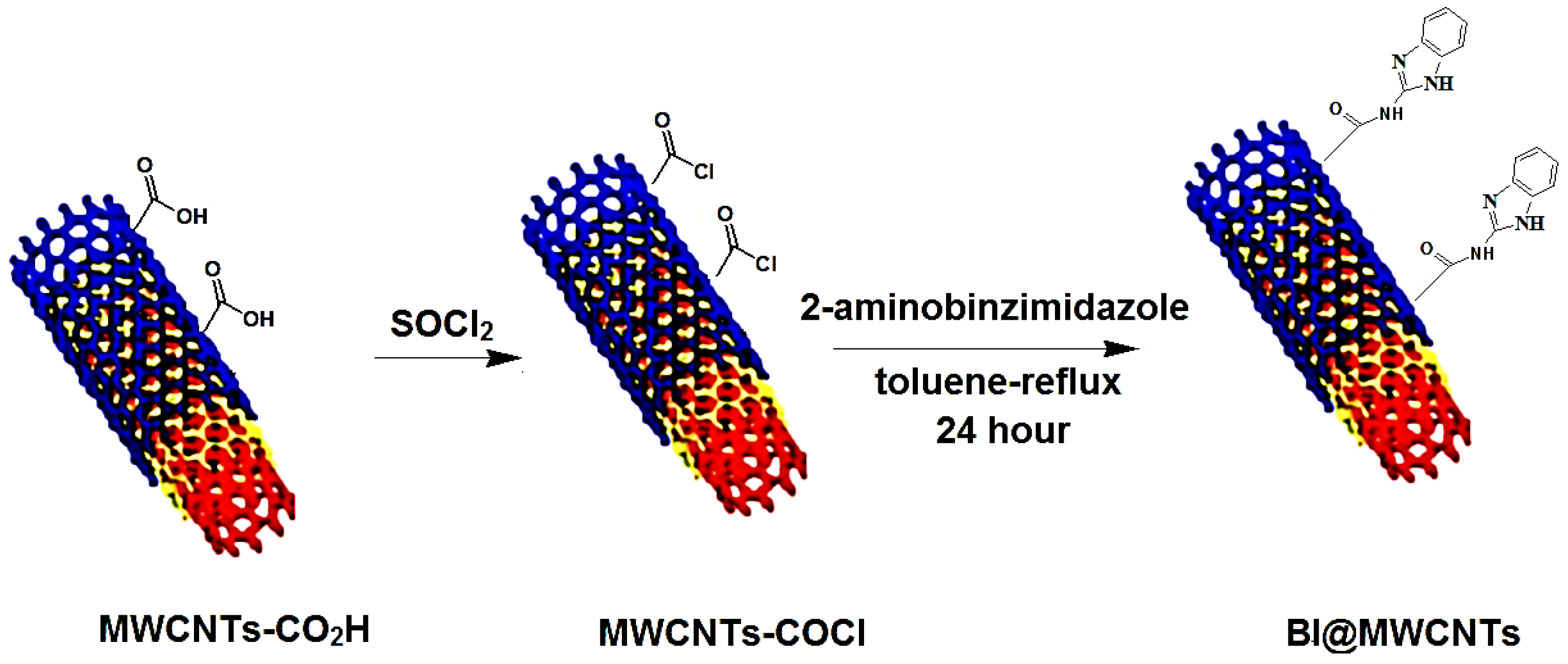
2.2. Preparation of the Functionalized MWCNT Materials
2.3. Characterization of Functionalized MWCNT Materials
2.4. Adsorption Tests
2.5. Adsorption Kinetics Process
2.6. Adsorption Isotherms
2.7. Adsorption Thermodynamics
2.8. Test for Regeneration and Reuse
3. Results and Discussion
3.1. Characterization of BI@MWCNTs
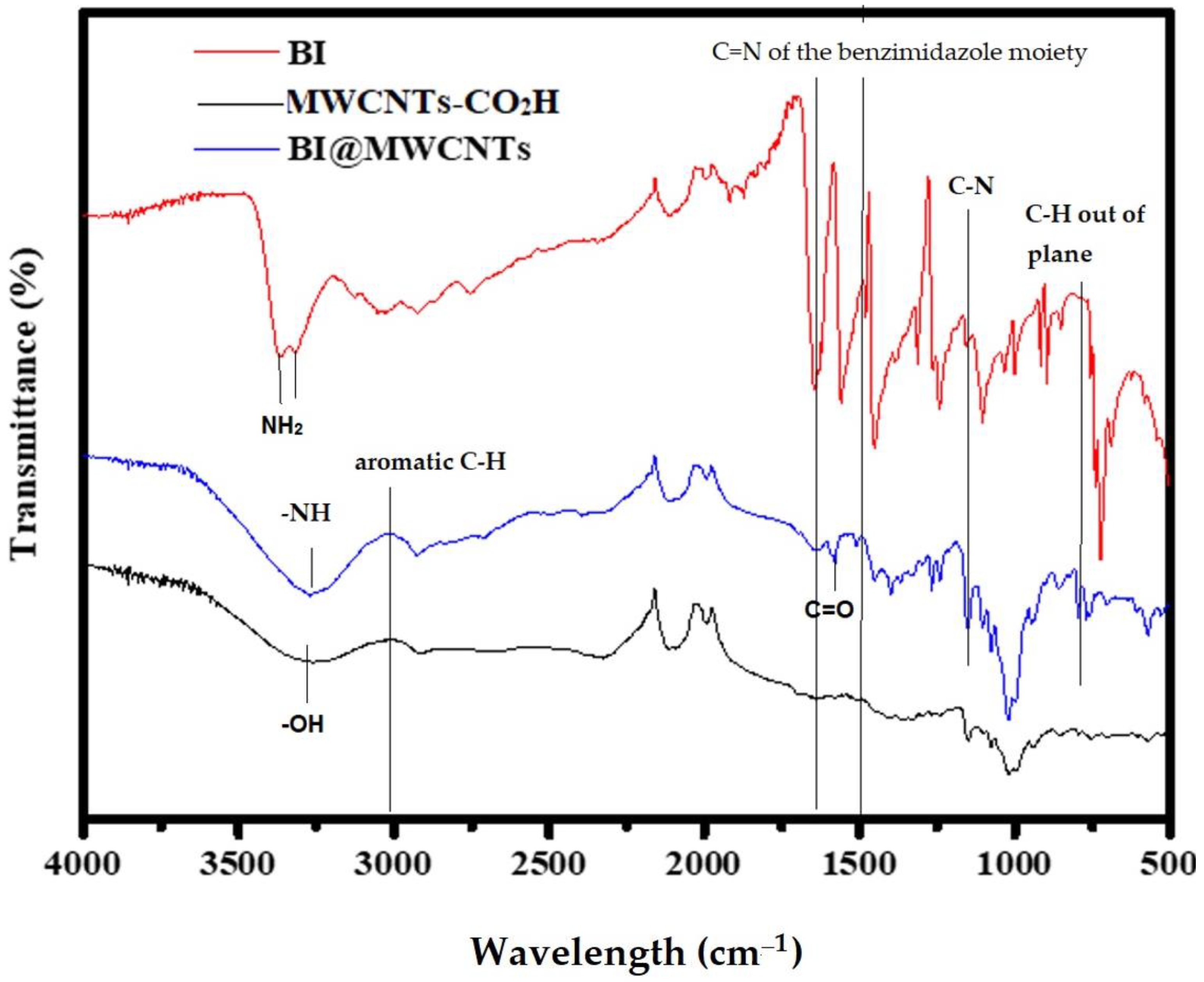
3.1.1. FTIR
3.1.2. SEM
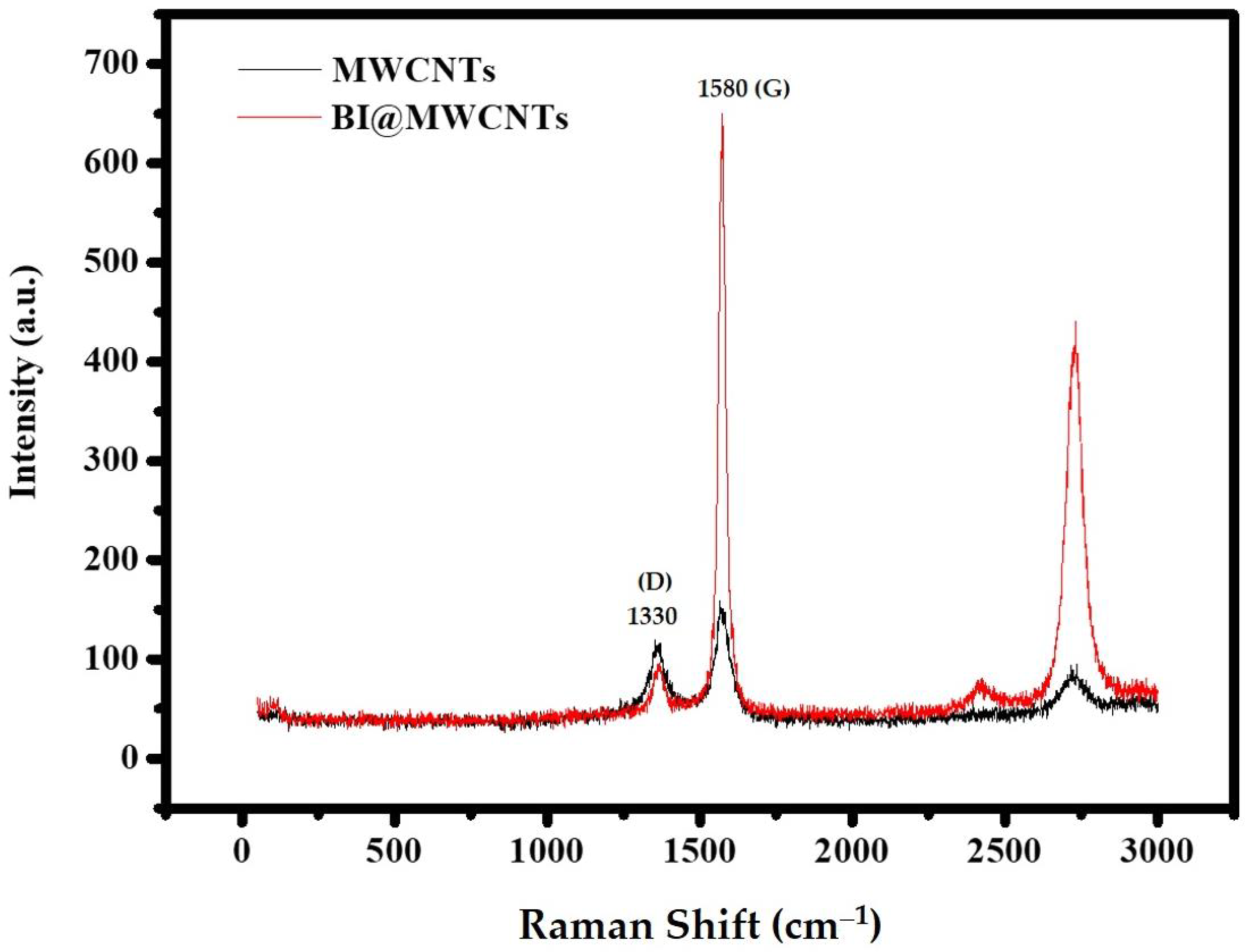
3.1.3. Raman Spectroscopy
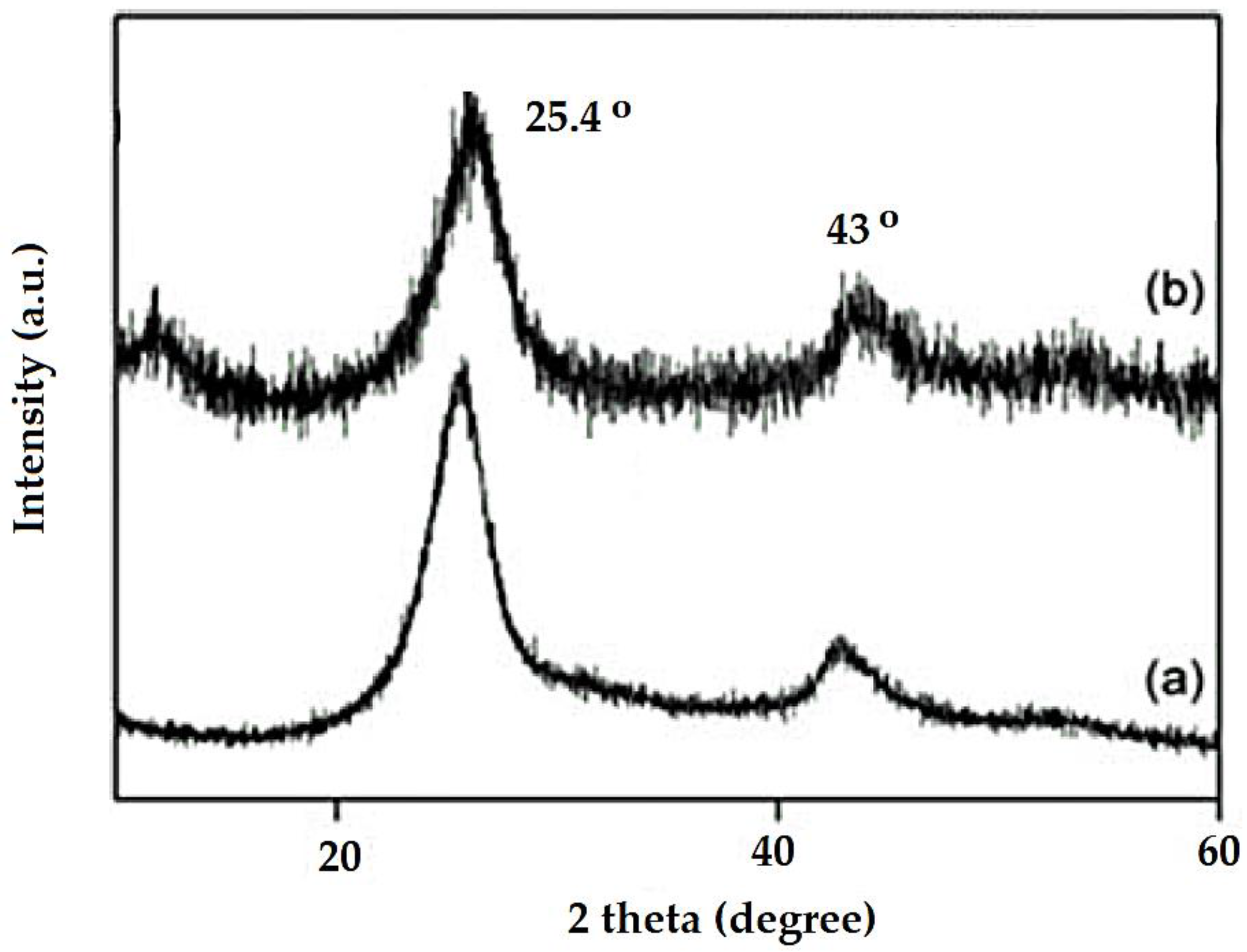
3.1.4. XRD
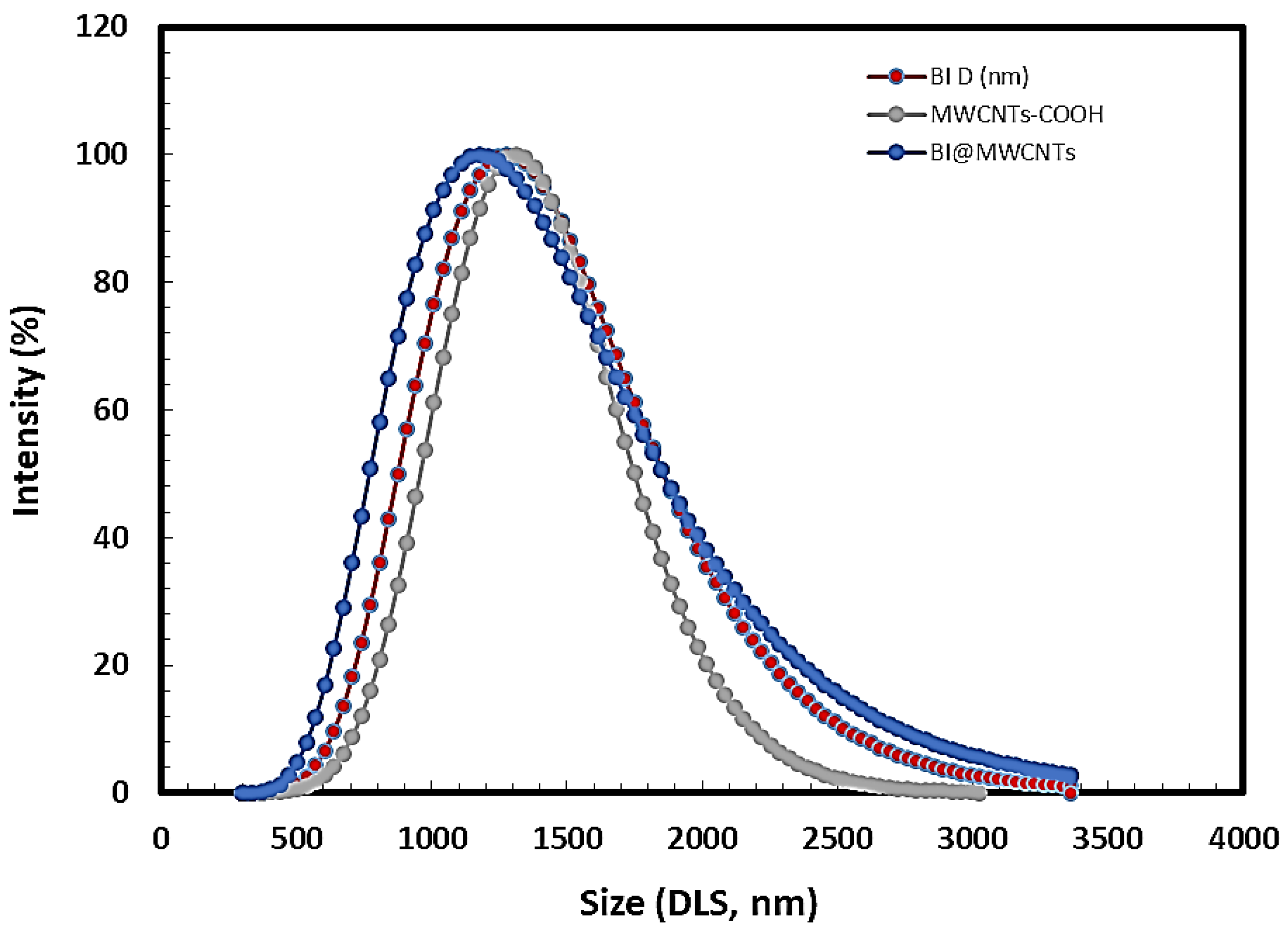
3.1.5. Dynamic Light Scattering (DLS)
3.1.6. BET Analysis
3.2. Adsorption Behaviors
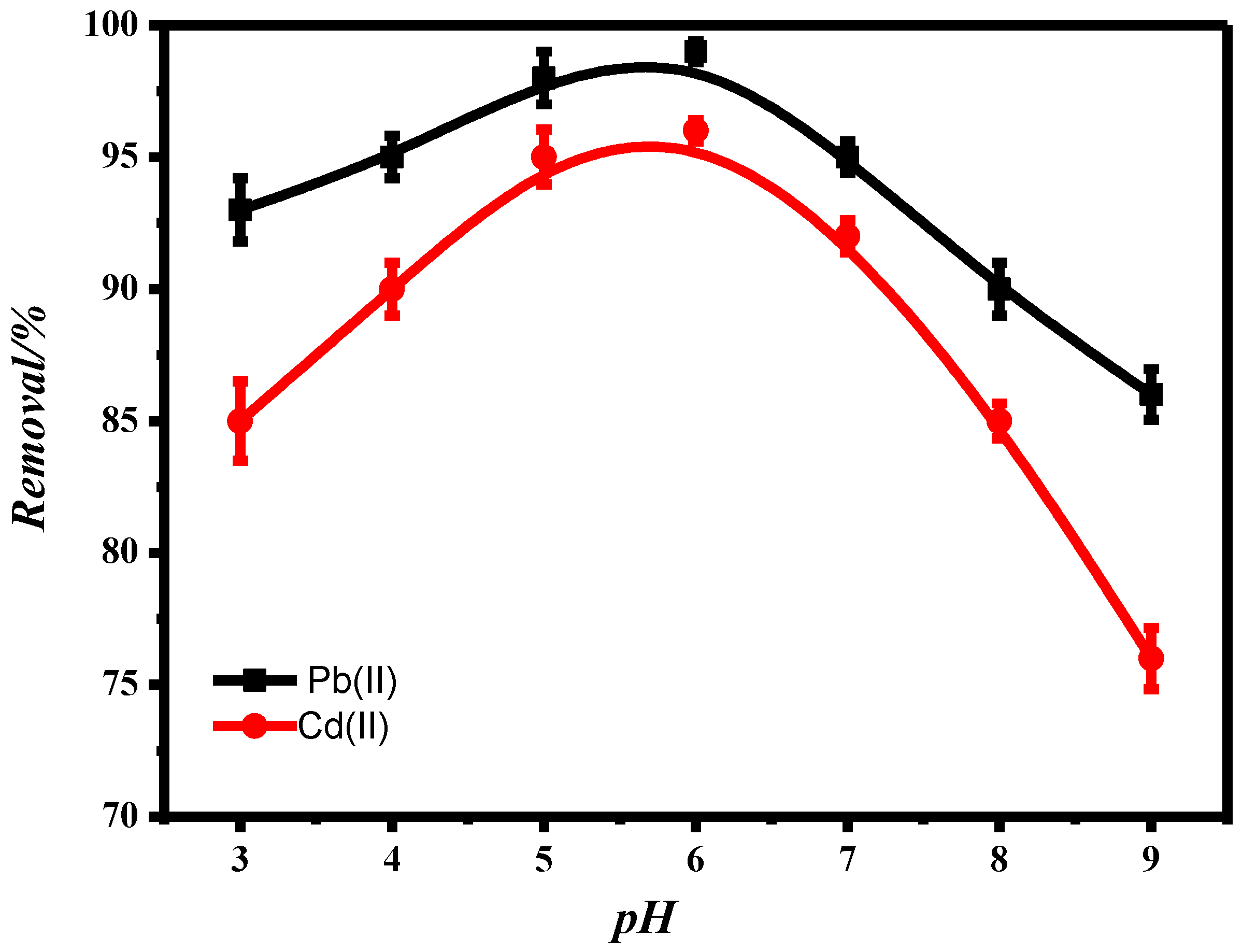
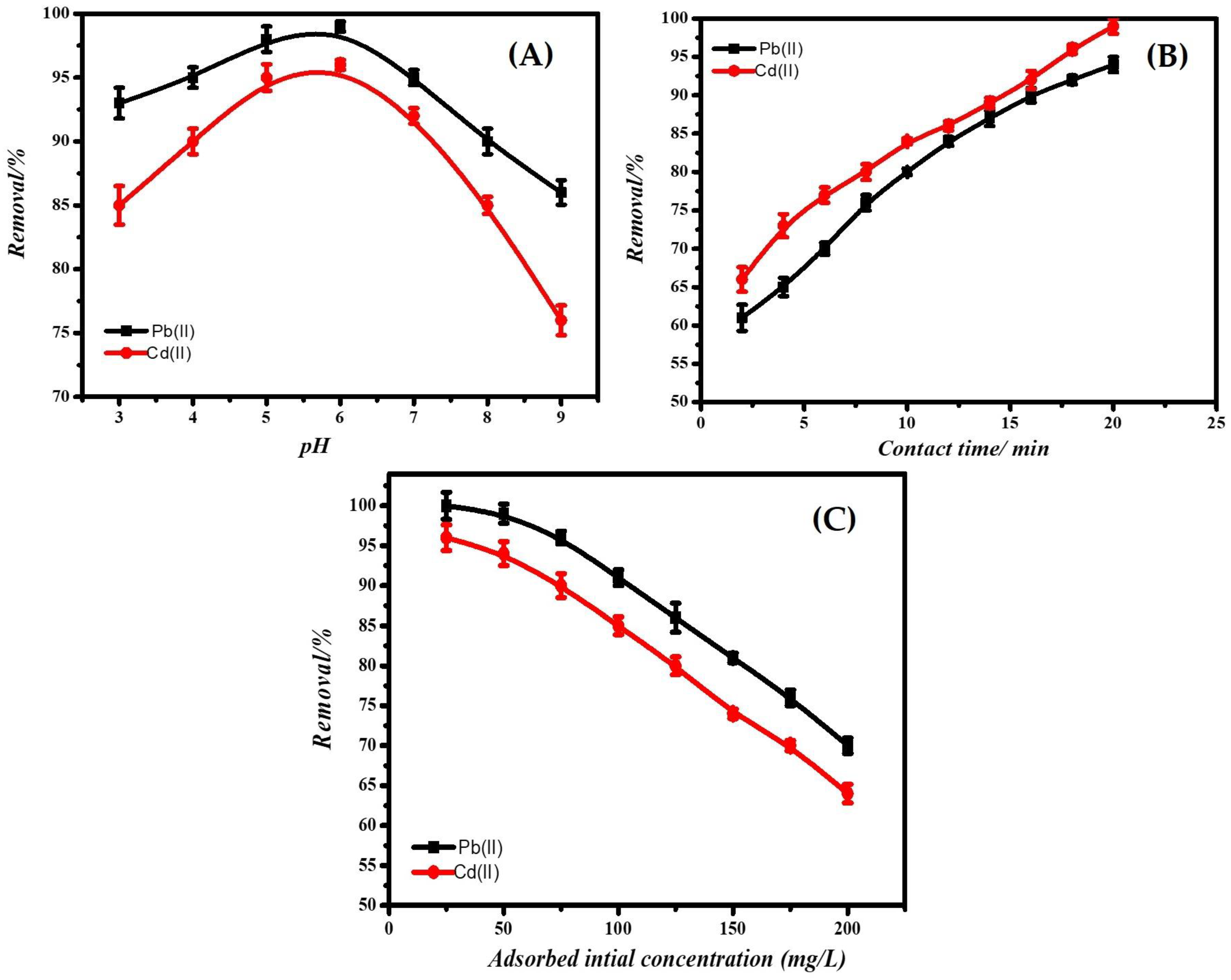
3.2.1. Effect of pH
3.2.2. Adsorption Competition
3.2.3. Pb2+ and Cd2+ Adsorption Optimization
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Adsorption Thermodynamics
3.6. Regeneration Adsorption Tests
3.7. Comparative Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noor-ul-Amin, H.A.; Alamzeb, S.; Begum, S. Accumulation of heavy metals in edible parts of vegetables irrigated with wastewater and their daily intake to adults and children, District Mardan, Pakistan. Food Chem. 2013, 136, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hou, Q.; Yang, Z.; Yu, T.; Zhong, C.; Yang, Y.; Fu, Y. Annual input fluxes of heavy metals in agricultural soil of Hainan Island, China. Environ. Sci. Pollut. 2014, 21, 7876–7885. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Moosavirad, S.M.; Sarikhani, R.; Shahsavani, E.; Mohammadi, S.Z. Removal of some heavy metals from inorganic industrial wastewaters by ion exchange method. J. Water Chem. Technol. 2015, 37, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Dong, S.; Wang, H.; Cheng, X.; Du, Z. Adsorption performance of low-cost gelatin-montmorillonite nanocomposite for Cr(II) ions. RSC Adv. 2015, 5, 58284–58291. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, C.; Zhao, W.; Zhou, Z.; Yan, W.; Li, X.; Liu, Y.; Sun, Z.; Zhao, G.; Gao, J. Fabrication of chitosan/gelatin foams with ordered porous structures for use in drug release and metal ion adsorption. RSC Adv. 2014, 4, 33840–33847. [Google Scholar] [CrossRef]
- Pan, B.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q.; Zheng, S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009, 151, 19–29. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef]
- Lee, C.-G.; Song, M.-K.; Ryu, J.-C.; Park, C.; Choi, J.-W.; Lee, S.-H. Application of carbon foam for heavy metal removal from industrial plating wastewater and toxicity evaluation of the adsorbent. Chemosphere 2016, 153, 1–9. [Google Scholar] [CrossRef]
- Inagaki, M.; Qiu, J.; Guo, Q. Carbon foam: Preparation and application. Carbon 2015, 87, 128–152. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Yu, G.; Meng, X. Adsorption of heavy metal ions, dyes and proteins by chitosan composites and derivatives—A review. J. Ocean Univ. China 2013, 12, 500–508. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohyd. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Muralidhara, H.B.; Nayaka, Y.A.; Balasubramanyam, J.; Hanumanthappa, H. Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol. 2013, 246, 125–136. [Google Scholar] [CrossRef]
- Hegazy, E.Z.; Abdelmaksod, I.H.; Kosa, S.A. Removal of Heavy Metal Quaternary Cations Systems on Zeolite A and X Mixtures Prepared from Local Kaolin. Clean-Soil Air Water 2014, 42, 775–778. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Lichtfouse, G.E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Yuan, B.; Wood, D.A. Formation Damage during Improved Oil Recovery: Fundamentals and Applications, 1st ed.; Gulf Professional Publishing: Houston, TX, USA; Elsevier: Houston, TX, USA, 2018. [Google Scholar]
- Alkhudhiri, A.; Hakami, M.; Zacharof, M.-P.; Homod, H.A.; Alsadun, A. Mercury, arsenic and lead removal by air gap membrane distillation: Experimental study. Water 2020, 12, 1574. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Al-Faiyz, Y.S.S.; Gouda, M. Multi-Walled Carbon Nanotubes Functionalized with Hydroxamic Acid Derivatives for the Removal of Lead from Wastewater: Kinetics, Isotherm, and Thermodynamic Studies. Polymers 2022, 14, 3870. [Google Scholar] [CrossRef]
- Imran, R.; Chowdhury, S.; Chowdhury, M.; Abu Jafar Mazumder, A.A. Removal of lead ions (Pb2+) from water and wastewater: A review on the low-cost adsorbents. Appl. Water Sci. 2022, 12, 185. [Google Scholar]
- Arul, P.F.; Thiyagarajan, D. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar]
- Arul, P.F.; Thiyagarajan, D.; Selvam, G.; Venkata, R.P.; Prakhya, B.M.; Sundara, R. Multi-walled carbon nanotube-induced inhalation toxicity: Recognizing nano bis-demethoxy curcumin analog as an ameliorating candidate. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1809–1822. [Google Scholar]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Adebowale, K.O. Synthesis of covalently bonded graphene oxide–iron magnetic nanoparticles and the kinetics of mercury removal. RSC Adv. 2015, 5, 2536–2542. [Google Scholar] [CrossRef]
- El Essawy, N.A.; Ali, S.M.; Farag, H.A.; Konsowa, A.H.; Elnouby, M.; Hamad, H.A. Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol. Environ. Saf. 2017, 145, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Wan, S.; Ding, W.; Wang, Y.; Wu, J.; Gu, Y.; He, F. Manganese oxide nanoparticles impregnated graphene oxide aggregates for cadmium and copper remediation. Chem. Eng. J. 2018, 350, 1135–1143. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Zhou, D.; Han, B. Graphene oxide–tripolyphosphate hybrid used as a potent sorbent for cationic dyes. Carbon 2014, 79, 174–182. [Google Scholar] [CrossRef]
- Rajesh, R.; Hariharasubramanian, A.; Senthilkumar, N.; Ravichandran, Y.D. A biocompatible and load bearing composite of multi-walled carbon nanotubes chitosan and natural hydroxyapatite derived from the chicken bones wasted in the slaughter houses. Int. J. Pharm. Pharm. Sci. 2012, 4, 716–720. [Google Scholar]
- Azizian, J.; Khoei, D.C.; Tahermansouri, H.; Yadollahzadeh, K. Functionalization of carboxylated multi-walled carbon nanotubes with 1,4-phenylendiamine, phenylisocyanate and phenylisothiocyanate. Fuller. Nanotub. Carbon Nanostruct. 2011, 19, 753–760. [Google Scholar] [CrossRef]
- Nashi, K.A.; Tareq, M.A.; Faisal, A.S.; Hisham, A.M.; Mohamed, G. Carboxymethyl-Cellulose-Containing Ag Nanoparticles as an ElectrochemicalWorking Electrode for Fast Hydroxymethyl-Furfural Sensing in Date Molasses. Polymers 2023, 15, 79. [Google Scholar] [CrossRef]
- Chen, K.; He, J.; Li, Y.; Cai, X.; Zhang, K.; Liu, T.; Hu, Y.; Lin, D.; Kong, L.; Liu, J. Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J. Colloid Interface Sci. 2017, 494, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Naeem, H.; Ajmal, M.; Muntha, S.; Ambreen, J.; Siddiq, M. Synthesis and characterization of graphene oxide sheets integrated with gold nanoparticles and their applications to adsorptive removal and catalytic reduction of water contaminants. RSC Adv. 2018, 8, 3599–3610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: Onepot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Zhang, K.; Liu, T.; Hu, Y.; Chen, X.; Wang, C.; Huang, X.; Kong, L.; Liu, J. Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel. RSC Adv. 2019, 9, 397–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Deng, C.-H.; Yang, H.-C.; Liu, H.-Y.; Huan, S.-Y. Cross-linking to prepare composite graphene oxide-framework membranes with high-flux for dyes and heavy metal ions removal. Chem. Eng. J. 2017, 322, 657–666. [Google Scholar] [CrossRef]
- Liu, W.; Wang, D.; Soomro, R.A.; Fu, F.; Qiao, N.; Yu, Y.; Wang, R.; Xu, B. Ceramic supported attapulgite-graphene oxide composite membrane for efficient removal of heavy metal contamination. J. Membr. Sci. 2019, 591, 117323. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Chung, T.-S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environ. Sci. Technol. 2015, 49, 10235–10242. [Google Scholar] [CrossRef]
- Samia, A.K.; Ghalia, A.-Z.; Mohamed, A. Removal of heavy metals from aqueous solutions by multi-walled carbon nanotubes modified with 8-hydroxyquinoline. Chem. Eng. J. 2012, 182, 159–168. [Google Scholar]
- Rashi, G.; Neeraj, K.; Elvis, F.-K.; Suprakas, S.R. Efficient Removal of Pb(II) and Cd(II) from Industrial Mine Water by a Hierarchical MoS2/SH-MWCNT Nanocomposite. ACS Omega 2019, 4, 13922–13935. [Google Scholar]
- Atul, A.; D’andrea, A.; Subrata, N.; Kunal, K.; Vida, D.; Shree, R.S.; Don, R.O.; Chris, P.; Robert, D.A.; Michael, E.; et al. Novel covalent approach to bio-conjugate silver coated single walled carbon nanotubes with antimicrobial peptide. J. Nanobiotechnol. 2016, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Bui, H.T.; Nguyen, M.H.L.; Dinh, Q.; Phan, H.K.; Phan, N.M. Application of multi-walled carbon nanotube nanofluid for 450W LED floodlight. J. Nanomater. 2014, 6, 347909. [Google Scholar] [CrossRef] [Green Version]
- Bali, M.; Tlili, H. Removal of heavy metals from wastewater using infiltration-percolation process and adsorption on activated carbon. Int. J. Environ. Sci. Technol. 2019, 16, 249–258. [Google Scholar] [CrossRef]

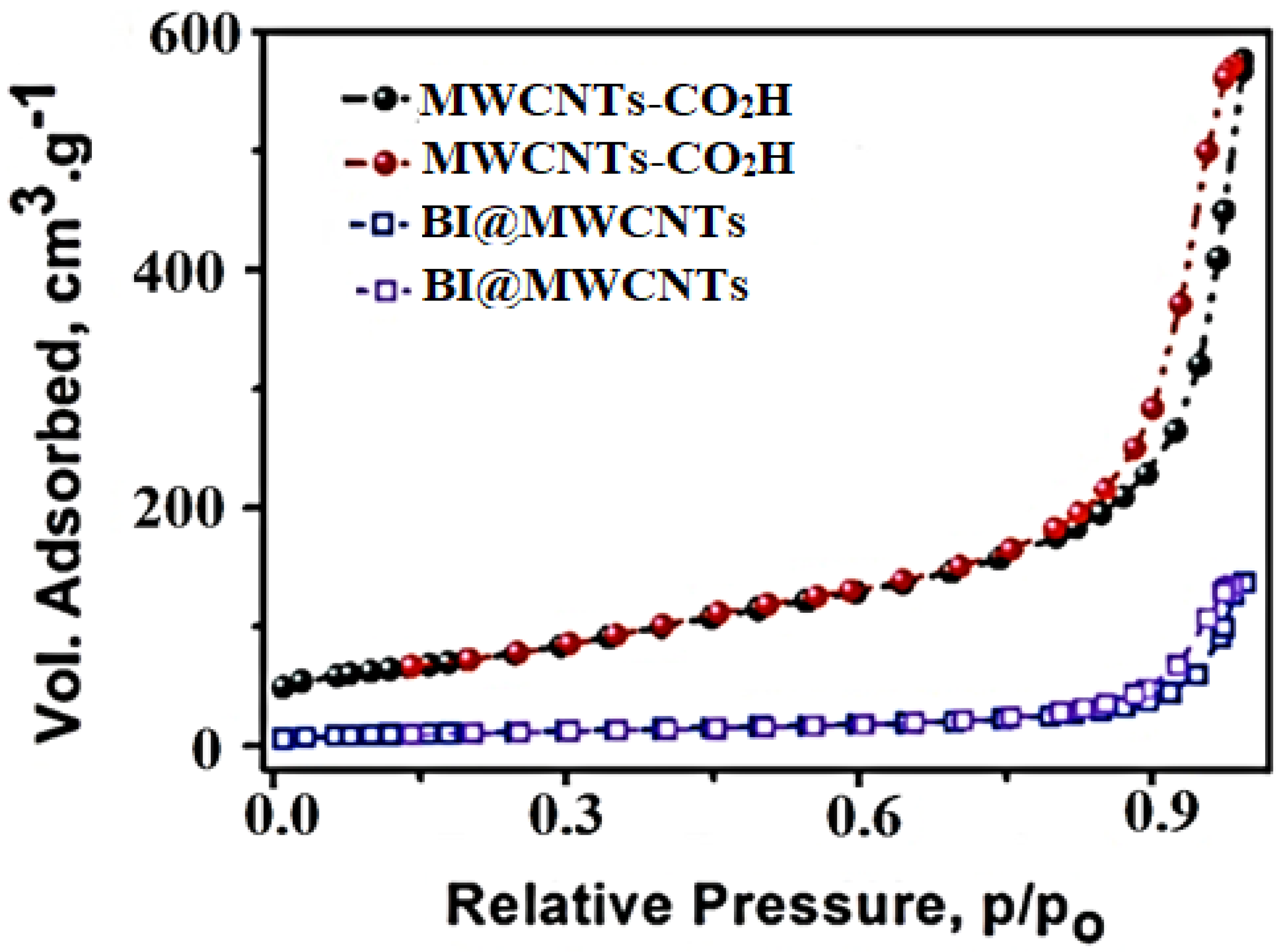



| Type | BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (m3/g) |
|---|---|---|---|
| MWCNTs–CO2H | 252.6 | 14 | 0.7999 |
| BI@MWCNTs | 45.3 | 20 | 0.3041 |
| Maximum Adsorption Capacity (mg/g) | Maximum (%) Removal | Distribution Coefficient (L/g) | ||||
|---|---|---|---|---|---|---|
| Pb2+ | Cd2+ | Pb2+ | Cd2+ | Pb2+ | Cd2+ | |
| BI@MWCNTs | 485 | 470 | 97 | 94 | 1000 | 110 |
| Cd(II) Initial Concentrations (mg/L) Adsorbed on BI@MWCNTs | Pb(II) Initial Concentrations (mg/L) Adsorbed on BI@MWCNTs | |||||
|---|---|---|---|---|---|---|
| 50 | 150 | 200 | 50 | 150 | 200 | |
| qe,exp (mg/g) a | 350 | 490 | 970 | 380 | 500 | 1017 |
| Pseudo-first order | ||||||
| qe,cal (mg/g) | 32 | 57 | 850 | 0.36 | 4.2 | 35 |
| k1 (min−1) | 0.16 | 0.17 | 0.21 | 0.05 | 0.08 | 0.1 |
| R2 | 0.7 | 0.88 | 0.96 | 0.92 | 0.89 | 0.80 |
| Pseudo-second order | ||||||
| qe,cal (mg/g) | 356 | 499 | 990 | 380 | 500 | 1016 |
| K2 (min−1) | 0.003 | 0.0005 | 0.0006 | 0.98 | 0.07 | 0.01 |
| R2 | 0.999 | 1 | 1 | 0.999 | 1 | 1 |
| Cd(II) Adsorption on BI@MWCNTs | Pb(II) Adsorption on BI@MWCNTs | |||
|---|---|---|---|---|
| 25 °C | 30 °C | 25 °C | 30 °C | |
| Langmuir isotherm | ||||
| qm (mg/g) | 840 | 916 | 930 | 931 |
| KL (L/mg) | 0.45 | 0.73 | 0.8 | 0.81 |
| R2 | 0.997 | 0.998 | 0.999 | 0.999 |
| RL | 0.008 | 0.008 | 0.0069 | 0.0069 |
| Freundlich isotherm | ||||
| KF (mg/g) | 220 | 216 | 228 | 232 |
| 1/nF | 0.22 | 0.21 | 0.24 | 0.21 |
| R2 | 0.998 | 0.998 | 0.997 | 0.995 |
| T (K) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | |||
|---|---|---|---|---|---|---|
| Cd(II) | Pb(II) | Cd(II) | Pb(II) | Cd(II) | Pb(II) | |
| 298 | 40.38760 | 53.2325 | −10,971 | −16,325.7 | 2.97768 | 2.91339 |
| Carbon-Based Materials | % Removal | Reference | |
|---|---|---|---|
| Pb2+ | Cd2+ | ||
| Graphene oxide (GO)/isophorone diisocyanate | 70 | 40 | [38] |
| Ceramic supported GO/attapulgite composite | 99 | 77 | [39] |
| GO framework/ethylenediamine | 89 | 80 | [40] |
| MWCNTs–hydroxyquinoline | 95 | 89 | [41] |
| MWCNT–CO2H | 88 | - | [22] |
| MoS2/SH–MWCNTs | 95 | 80 | [42] |
| Ag-coated SWCNTs | 93 | 15 | [43] |
| Al2O3–MWCNTs | 90 | - | [44] |
| Commercial activated carbon | 74 | 74 | [45] |
| BI@MWCNTs | 100 | 98 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elghamry, I.; Gouda, M.; Al-Fayiz, Y.S.S. Synthesis of Chemically Modified Acid-Functionalized Multiwall Carbon Nanotubes with Benzimidazole for Removal of Lead and Cadmium Ions from Wastewater. Polymers 2023, 15, 1421. https://doi.org/10.3390/polym15061421
Elghamry I, Gouda M, Al-Fayiz YSS. Synthesis of Chemically Modified Acid-Functionalized Multiwall Carbon Nanotubes with Benzimidazole for Removal of Lead and Cadmium Ions from Wastewater. Polymers. 2023; 15(6):1421. https://doi.org/10.3390/polym15061421
Chicago/Turabian StyleElghamry, Ibrahim, Mohamed Gouda, and Yasair S. S. Al-Fayiz. 2023. "Synthesis of Chemically Modified Acid-Functionalized Multiwall Carbon Nanotubes with Benzimidazole for Removal of Lead and Cadmium Ions from Wastewater" Polymers 15, no. 6: 1421. https://doi.org/10.3390/polym15061421







