Facile Fabrication of High-Performance Composite Films Comprising Polyvinyl Alcohol as Matrix and Phenolic Tree Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tannin/Lignin/PVOH (TLP) Film
2.3. Characterizations
2.3.1. SEM Observation
2.3.2. FTIR and ESI-MS
2.3.3. Differential Scanning Calorimetry (DSC) and Thermal Gravimetric Analysis (TGA)
2.3.4. UV-VL Resistance of the Various Films
2.3.5. Mechanical Characterization
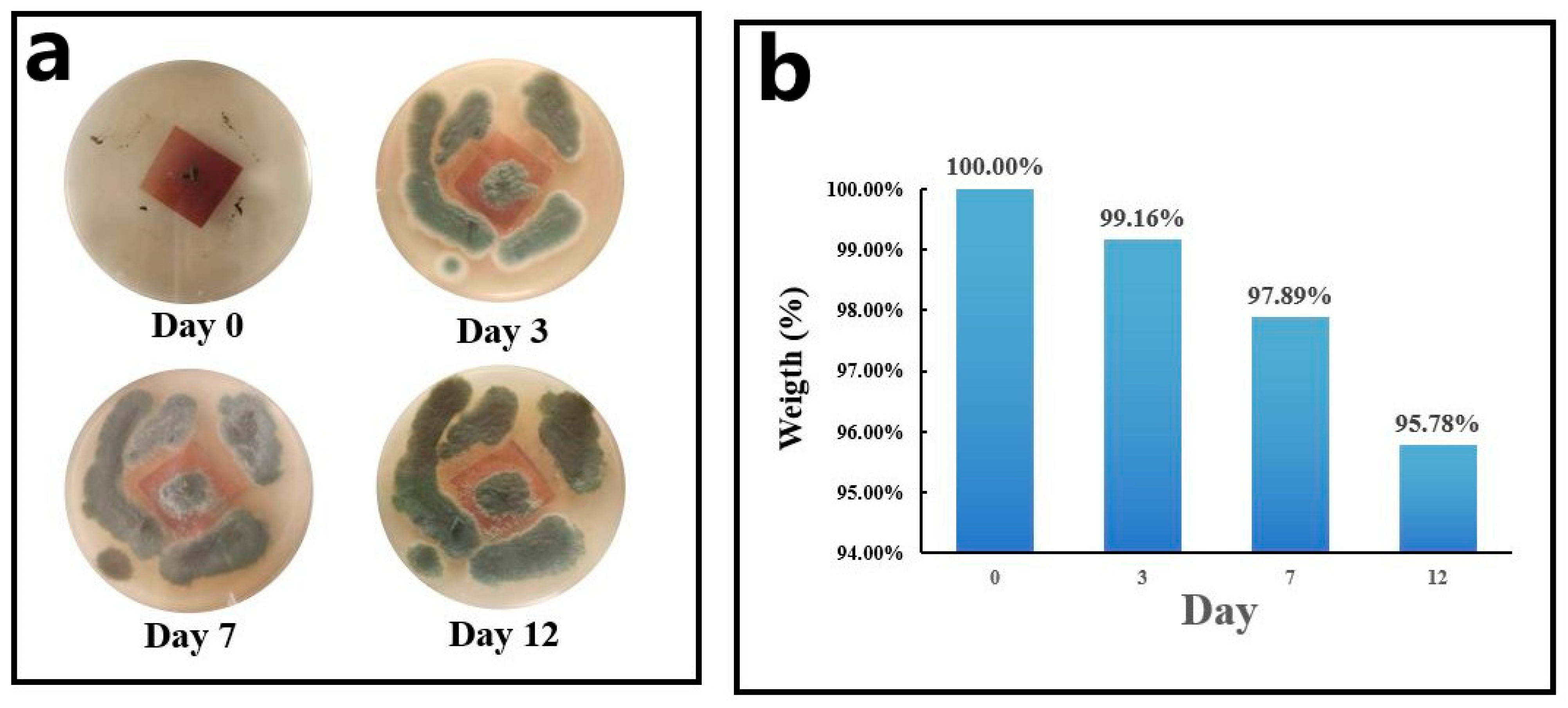
2.3.6. Biodegradability Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Yu, M.; Sun, J.; Zhang, S.; Li, Q.; Teng, L.; Tian, Q.; Xie, R.; Li, G.; Liu, L.; et al. Electrical Failure Mechanism in Stretchable Thin-Film Conductors. ACS Appl. Mater. Interfaces 2022, 2, 14. [Google Scholar] [CrossRef]
- Chate, P.A.; Sathe, D.J. Structural, electrical and thermoelectrical analysis of nickel sulphide thin films. Appl. Phys. A 2016, 122, 600. [Google Scholar] [CrossRef]
- Podgornik, B.; Vižintin, J. Tribology of thin films and their use in the field of machine elements. Vacuum 2002, 68, 39–47. [Google Scholar] [CrossRef]
- Khan, O.; Meyer, J.; Baur, K.; Arafat, S.; Waldschmidt, C. Aperture coupled stacked patch thin film antenna for automotive radar at 77 ghz. Int. J. Microw. Wirel. Technol. 2019, 11, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Dong, T.; Zhong, Z.; Zheng, H.; Xu, W.; Ying, L.; Wang, J.; Peng, J.; Cao, Y. Uniform inkjet-printed films with single solvent. Thin Solid Film. 2018, 667, 21–27. [Google Scholar] [CrossRef]
- Calvi, S.; Maita, F.; Rapisarda, M.; Fortunato, G.; Valletta, A.; Preziosi, V.; Cassinese, A.; Mariucci, L. Gravure printed organic thin film transistors: Study on the ink printability improvement. Org. Electron. 2018, 61, 104–112. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Y.; Liang, M.; Hou, L.; Li, Y.; Guo, C. Fabrication of stable and corrosion-resisted super-hydrophobic film on mg alloy. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 351–358. [Google Scholar] [CrossRef]
- Yang, C.; Fuat, T.; Sang-Hee, P.; Gyorgy, S. Biobased thin-film composite membranes comprising priamine–genipin selective layer on nanofibrous biodegradable polylactic acid support for oil and solvent-resistant nanofiltration. Green Chem. 2022, 24, 5291–5303. [Google Scholar] [CrossRef]
- Ng, C.; Rao, J.; Nicholls, J. The role of pvd sputtered ptfe and Al2O3 thin films in the development of damage tolerant coating systems. Prog. Artif. Intell. 2020, 9, 675–686. [Google Scholar] [CrossRef]
- Akhavan, A.; Khoylou, F.; Ataeivarjovi, E. Preparation and characterization of gamma irradiated starch/PVA/zno nanocomposite films. Radiat. Phys. Chem. 2017, 138, 49–53. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, Q.; Huang, J.; Zhao, Y.; Li, Q.; Wang, S. Improved Hydrophobic, UV Barrier and Antibacterial Properties of Multifunctional PVA Nanocomposite Films Reinforced with Modified Lignin Contained Cellulose Nanofibers. Polymers 2022, 14, 1705. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Delgado-Regaña, A.; Rodríguez-González, M.A.; Rubio-Alonso, F. Optimization of Tannin Rigid Foam as Adsorbents in Wastewater Treatment. Ind. Crops Prod. 2013, 49, 507–514. [Google Scholar] [CrossRef]
- Pizzi, A. The chemistry and development of tannin/urea–formaldehyde condensates for exterior wood adhesives. J. Appl. Polym. Sci. 1979, 23, 2777–2792. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, W.; Zhang, W.; Li, J. Preparation of tannin–formaldehyde–furfural resin with pretreatment of depolymerization of condensed tannin and ring opening of furfural. J. Adhes. Sci. Technol. 2016, 30, 947–959. [Google Scholar] [CrossRef]
- Esmaeili, N.; Salimi, A.; Zohuriaan-Mehr, M.J.; Vafayan, M.; Meyer, W. Bio-based thermosetting epoxy foam: Tannic acid valorization toward dye-decontaminating and thermo-protecting applications. J. Hazard. Mater. 2018, 357, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, J.; Essawy, H.; Zhang, J.; Zhou, X. Preparation and characterization of novel cellular/nonporous foam structures derived from tannin furanic resin. Ind. Crops Prod. 2021, 162, 113264. [Google Scholar] [CrossRef]
- Khundamri, N.; Aouf, C.; Fulcrand, H.; Dubreucq, E.; Tanrattanakul, V. Bio-based flexible epoxy foam synthesized from epoxidized soybean oil and epoxidized mangosteen tannin. Ind. Crops Prod. 2019, 128, 556–565. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Iswanto, A.H. Recent developments in lignin-and tannin-based non-isocyanate polyurethane resins for wood adhesives—A review. Appl. Sci. 2021, 11, 4242. [Google Scholar] [CrossRef]
- Chen, M.S.; Luo, J.; Shi, R.Q.; Zhang, J.Z.; Gao, Q.; Li, J. Improved adhesion performance of soy protein-based adhesives with a larch tannin-based resin. Polymers 2017, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Ghahri, S.; Chen, X.; Pizzi, A.; Hajihassani, R.; Papadopoulos, A.N. Natural tannins as new cross-linking materials for soy-based adhesives. Polymers 2021, 13, 595. [Google Scholar] [CrossRef]
- Lagel, M.C.; Pizzi, A.; Basso, M.C.; Abdalla, S. Development and characterization of abrasive grinding wheels with a tannin-furanic resins matrix. Ind. Crops Prod. 2015, 65, 343–348. [Google Scholar] [CrossRef]
- Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S.; Zhou, X. Polypropylene blend with polyphenols through dynamic vulcanization: Mechanical, rheological, crystalline, thermal, and uv protective property. Polymers 2019, 11, 1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Li, J.; Yang, F.; Zhu, Y.; Wang, H.; Du, G.; Essawy, H.; Zhou, X. Assisted compatibility, and balanced regulation of the mechanical, thermal, and antioxidant activity of polyvinyl alcohol-chinese bayberry tannin extract films using different di-aldehydes as cross-linkers. J. Renew. Mater. 2022, 10, 359–372. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Sanjivkasbe, P.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and characterization of lignin-polyurethane based wood adhesive. Int. J. Adhes. Adhes. 2019, 95, 102427. [Google Scholar] [CrossRef]
- Sathawong, S.; Sridach, W.; Techato, K.A. Lignin: Isolation and preparing the lignin based hydrogel. J. Environ. Chem. Eng. 2018, 6, 5879–5888. [Google Scholar] [CrossRef]
- Li, N.; An, X.; Xiao, X.; An, W.; Zhang, Q. Recent advances in the treatment of lignin in papermaking wastewater. World J. Microbiol. Biotechnol. 2022, 38, 116. [Google Scholar] [CrossRef]
- Zhu, W.; Westman, G.; Theliander, H. Lignin separation from kraft black liquor by combined ultrafiltration and precipitation: A study of solubility of lignin with different molecular properties. Nord. Pulp Pap. Res. J. 2016, 31, 270–278. [Google Scholar] [CrossRef]
- Gong, X.; Meng, Y.; Lu, J.; Tao, Y.; Cheng, Y.; Wang, H. A review on lignin-based phenolic resin adhesive. Macromol. Chem. Phys. 2022, 223, 2100434. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Du, G.; Gerardin, C.; Amirou, S. Oxidized demethylated lignin as a bio-based adhesive for wood bonding. J. Adhes. 2021, 97, 873–890. [Google Scholar] [CrossRef]
- Da Cruz, J.A.; Da Silva, A.B.; Ramin, B.B.; Souza, P.R.; Popat, K.C.; Zola, R.S.; Martins, A.F. Poly (vinyl alcohol)/cationic tannin blend films with antioxidant and antimicrobial activities. Mater. Sci. Eng. C 2020, 107, 110357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Essawy, H.; Liu, A.; Yang, C.; Hou, D.; Zhou, X.; Zhang, J. Preparation of Film Based on Polyvinyl Alcohol Modified by Alkaline Starch and Lignin Fiber. J. Renew. Mater. 2023, 11, 837–852. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Du, G.; Chen, J. Isolation and culture characterization of a new polyvinyl alcohol-degrading strain: Penicillium sp. WSH02-21. World J. Microbiol. Biotechnol. 2004, 20, 587–591. [Google Scholar] [CrossRef]
- Leitão, A.L. Potential of Penicillium Species in the Bioremediation Field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef] [Green Version]
- Muntoni, A.P.; Braunstein, A.; Pagnani, A.; Martino, D.D.; Martino, A.D. Relationship between fitness and heterogeneity in exponentially growing microbial populations. Biophys. J. 2021, 121, 1919–1930. [Google Scholar] [CrossRef]
- Abou-Zeid, D.M.; Müller, R.J.; Deckwer, W.D. Degradation of natural and synthetic polyesters under anaerobic conditions. J. Biotechnol. 2001, 86, 113–126. [Google Scholar] [CrossRef]
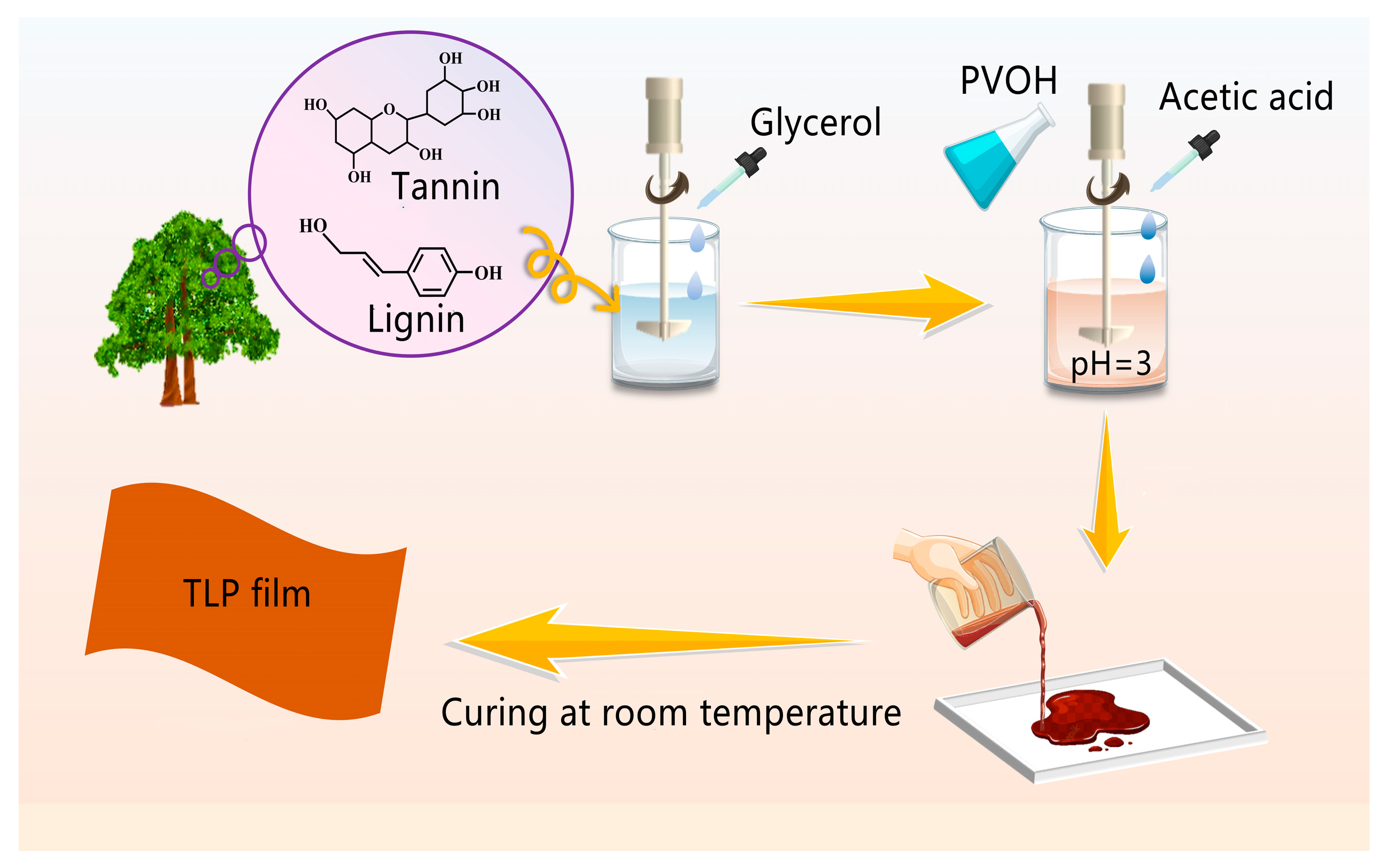
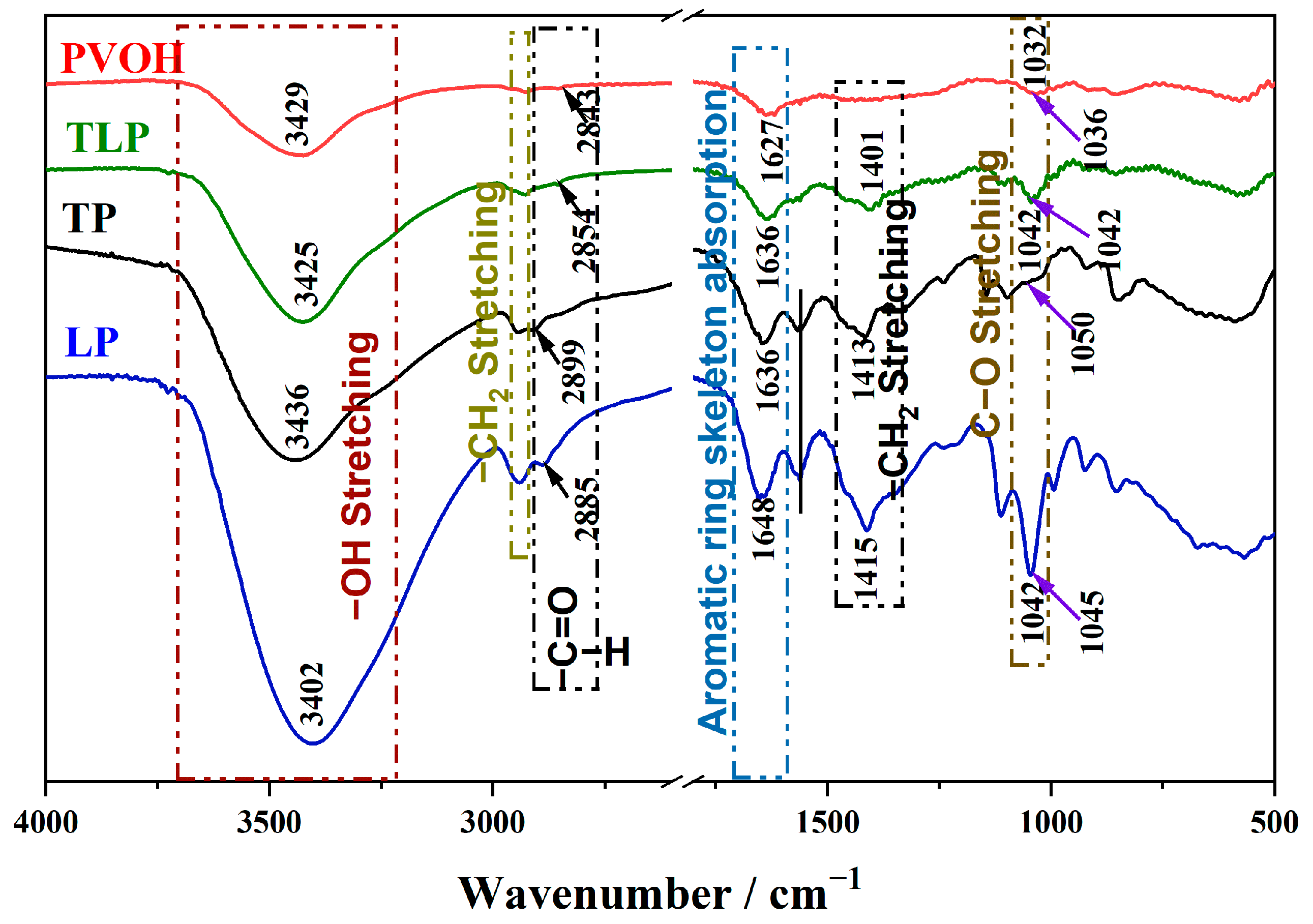



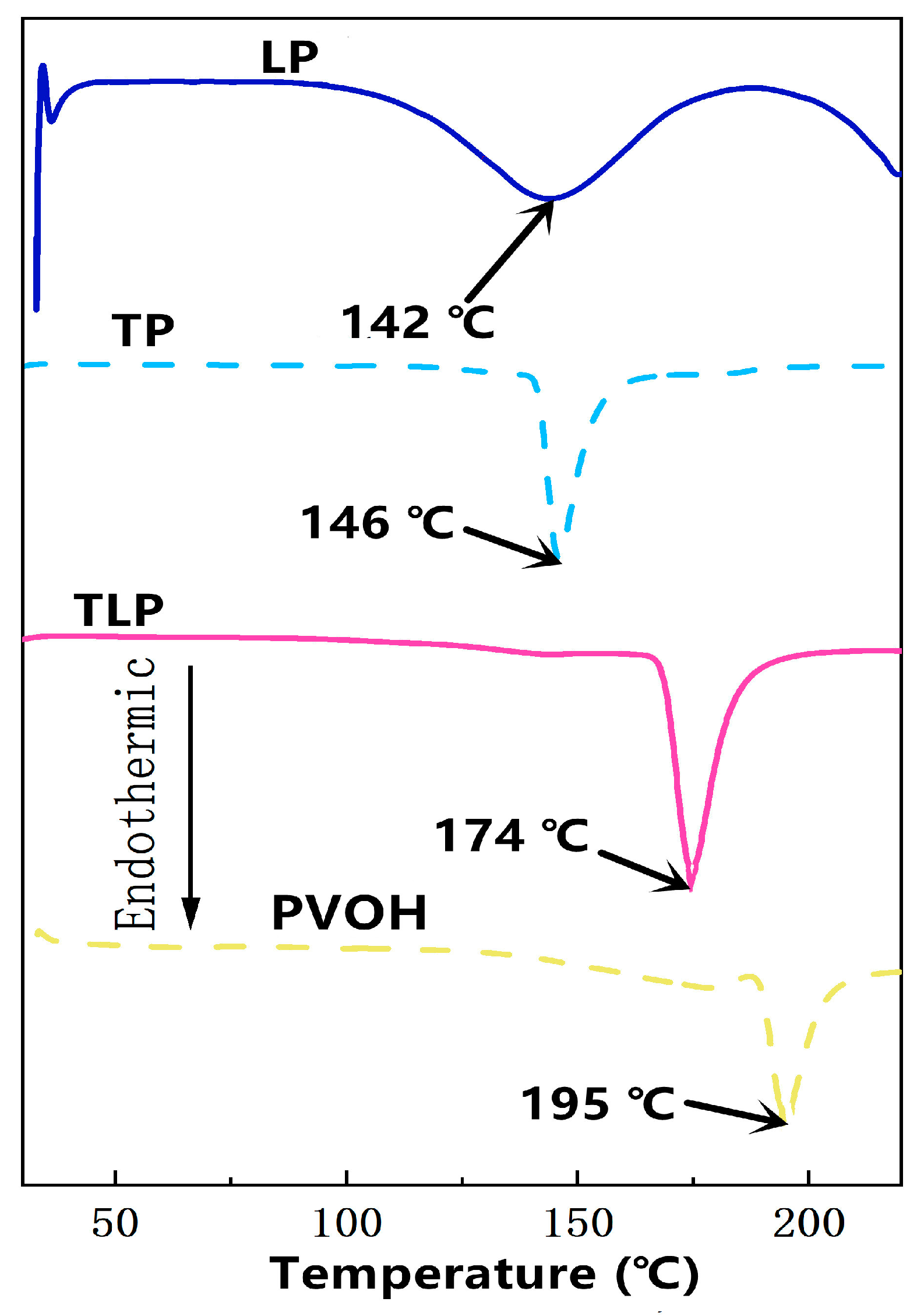

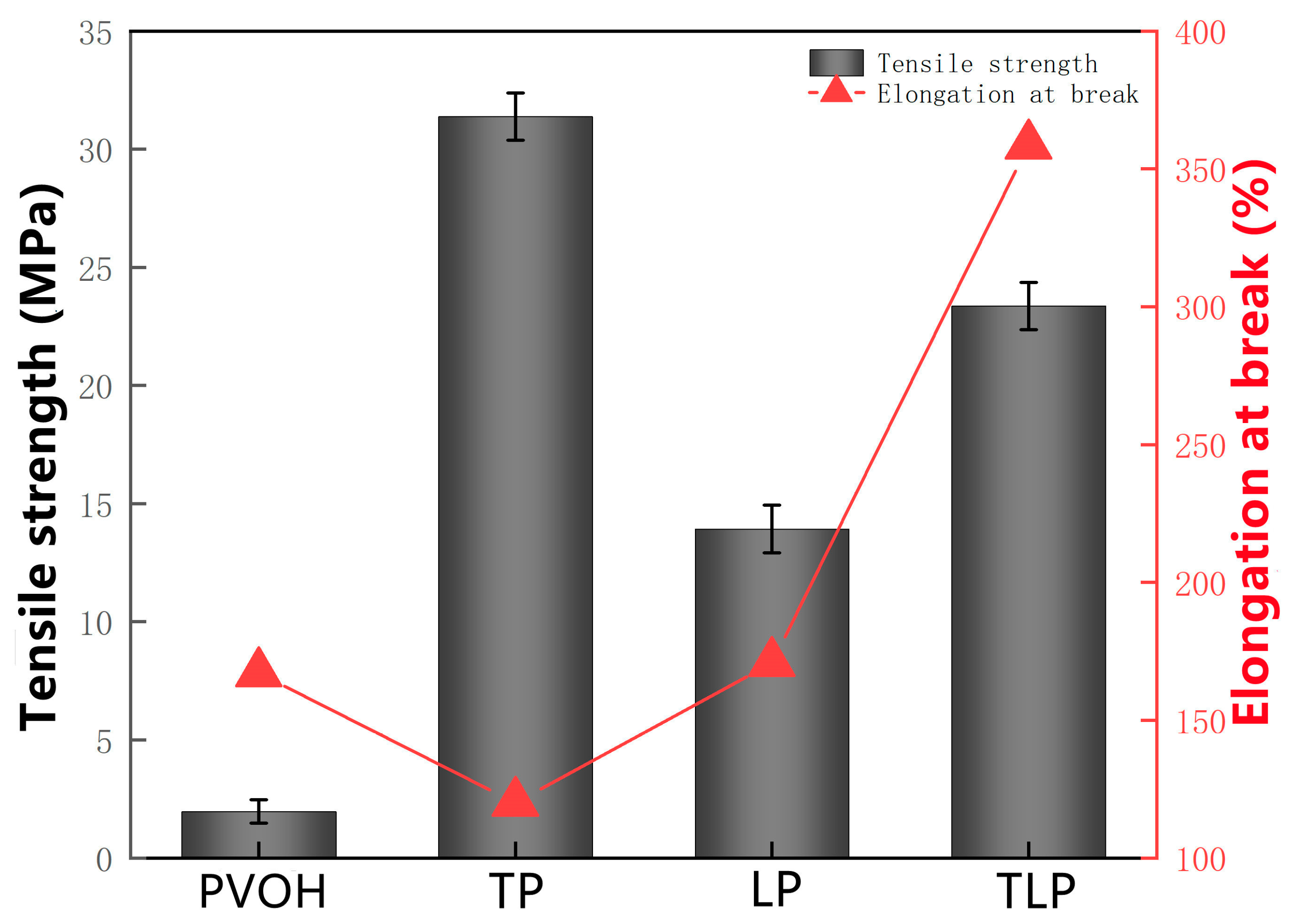

| Type | Tannin/g | Lignin/g | PVOH/g | DW/mL | Sodium Hydroxide/mL | Glacial Acetic Acid/mL | Glycerin/g |
|---|---|---|---|---|---|---|---|
| PVOH | / | / | 30 | 52 | / | 5 | 3 |
| TP | 0.5 | / | 30 | 52 | / | 5 | 3 |
| LP | / | 0.5 | 30 | 52 | 0.2 | 5 | 3 |
| TLP | 0.5 | 0.5 | 30 | 52 | 0.2 | 5 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liu, B.; Zheng, L.; Zhou, Y.; Essawy, H.; Chen, X.; Zhou, X.; Zhang, J. Facile Fabrication of High-Performance Composite Films Comprising Polyvinyl Alcohol as Matrix and Phenolic Tree Extracts. Polymers 2023, 15, 1424. https://doi.org/10.3390/polym15061424
Xu Y, Liu B, Zheng L, Zhou Y, Essawy H, Chen X, Zhou X, Zhang J. Facile Fabrication of High-Performance Composite Films Comprising Polyvinyl Alcohol as Matrix and Phenolic Tree Extracts. Polymers. 2023; 15(6):1424. https://doi.org/10.3390/polym15061424
Chicago/Turabian StyleXu, Ying, Bowen Liu, Lulu Zheng, Yunxia Zhou, Hisham Essawy, Xinyi Chen, Xiaojian Zhou, and Jun Zhang. 2023. "Facile Fabrication of High-Performance Composite Films Comprising Polyvinyl Alcohol as Matrix and Phenolic Tree Extracts" Polymers 15, no. 6: 1424. https://doi.org/10.3390/polym15061424








