Study on the Mechanism of Molecular Weight Reduction of Polyethylene Based on Fe-Montmorillonite and Its Potential Application
Abstract
:1. Introduction
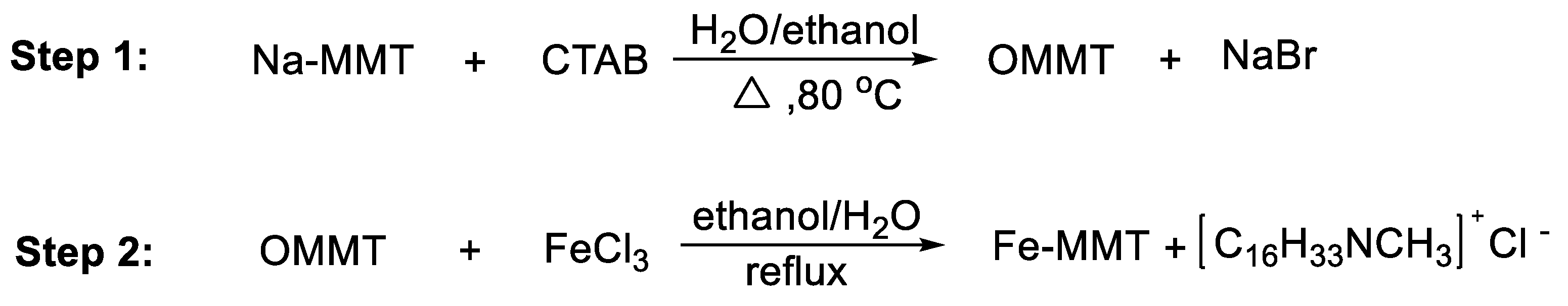
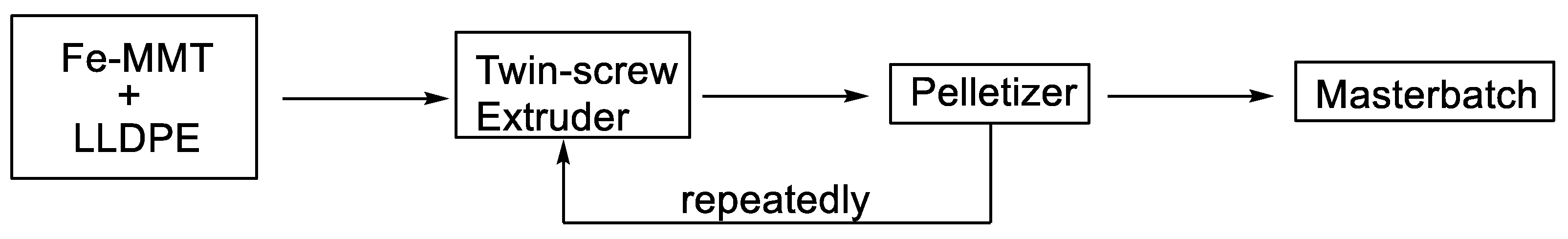
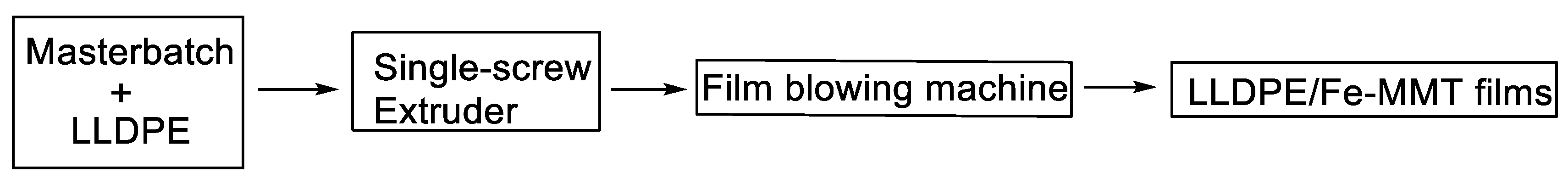
2. Experimental Section
3. Results and Discussion
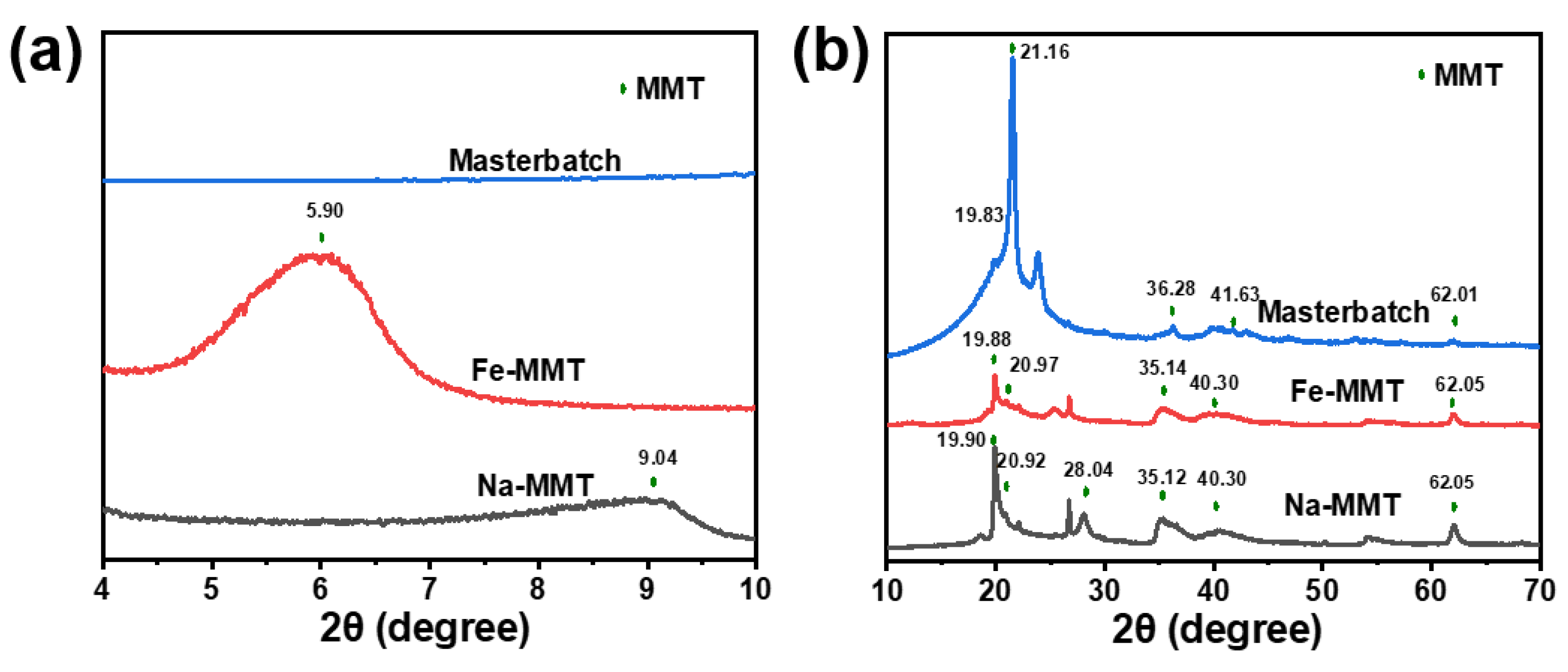
3.1. Structure of Fe-MMT and Its Distribution in Polyethylene Matrix
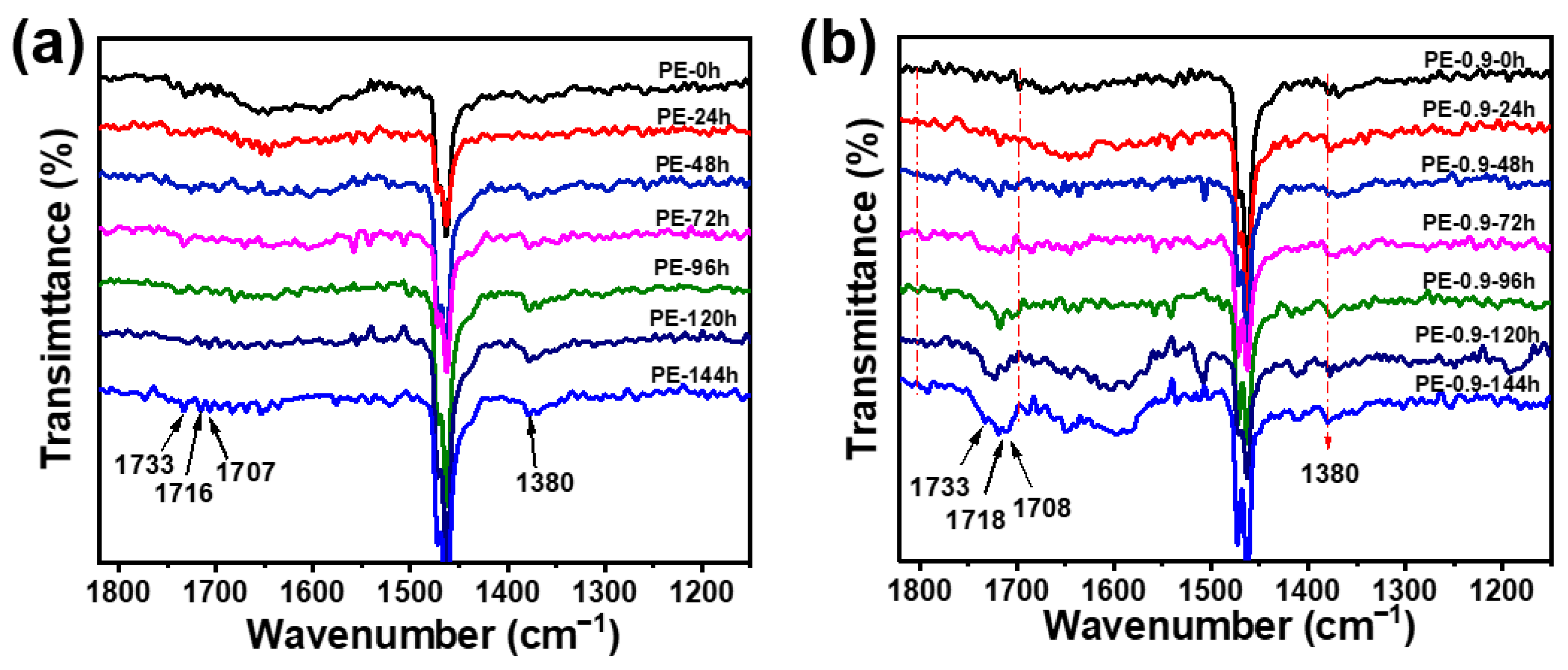
3.2. Analysis of Irradiation Test Results
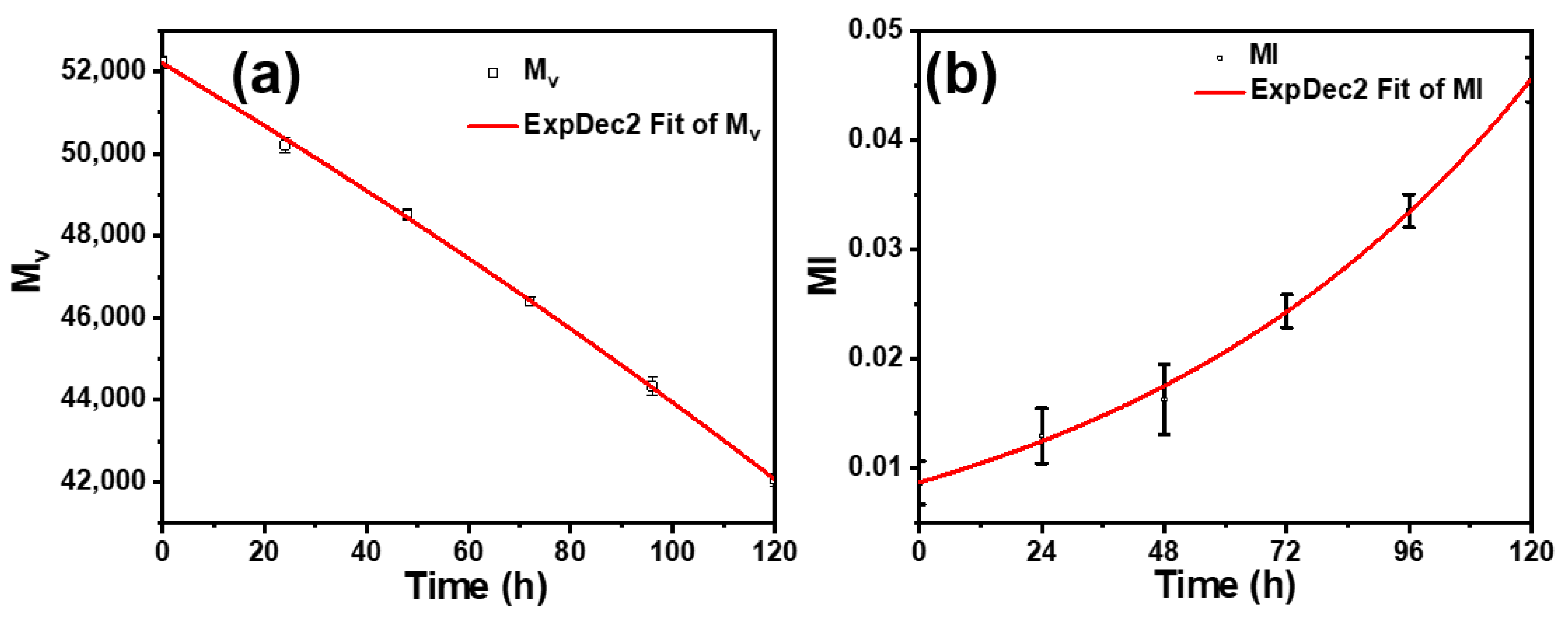
3.3. Analysis of Molecular Weight Changes
3.4. Mechanism of Decreasing PE Molecular Weight under Continuous UV Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Sharma, N. Mechanistic Implications of Plastic Degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Peacock, A.J. Handbook of Polyethylene: Structures, Properties and Applications; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Roy, P.K.; Hakkarainen, M.; Varma, I.K.; Albertsson, A.C. Degradable Polyethylene: Fantasy or Reality. Environ. Sci. Technol. 2011, 45, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of Waste From Polymer Materials: An Overview of the Recent Works. Polym. Degrad Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Wiles, D.; Scott, G. (Polyolefins with Controlled Environmental Degradability. Polym. Degrad. Stab. 2006, 91, 1581–1592. [Google Scholar] [CrossRef]
- Yang, C.J.; Gong, C.Q.; Peng, T.Y.; Deng, K.J.; Zan, L. High photocatalytic degradation activity of the polyvinyl chloride (PVC)–vitamin C (VC)–TiO2 nano-composite film. J. Hazard. Mater. 2010, 178, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Weon, J. Effects of thermal ageing on mechanical and thermal behaviors of linear low density polyethylene pipe. Polym. Degrad. Stab. 2010, 95, 14–20. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Gregersen, Ø.W.; Männle, F. Thermal Oxidation of Polyolefins by Mild Pro-Oxidant Additives Based on Iron Carboxylates and Lipophilic Amines: Degradability in the Absence of Light and Effect on the Adhesion to Paperboard. Polymers 2015, 7, 1522–1540. [Google Scholar] [CrossRef]
- Costa, L.; Bracco, P. Mechanisms of Cross-Linking, Oxidative Degradation, and Stabilization of UHMWPE. In UHMWPE Biomateials Handbook Ultra High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices, 3rd ed.; Elsever Inc.: Amsterdam, The Netherlands, 2015; pp. 467–487. [Google Scholar]
- Bracco, P.; Costa, L.; Luda, M.P.; Billingham, N.A. Review of Experimental Studies of the Role of Free-Radicals in Polyethylene Oxidation. Polym. Degrad. Stab. 2018, 155, 67–83. [Google Scholar] [CrossRef]
- Lazar, M.; Rychly, J.; Klimo, V.; Pelikan, P.; Valko, L. Free Radicals in Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Reddy, M.M.; Deighton, M.; Gupta, R.K.; Bhattacharya, S.N.; Parthasarathy, R. Biodegradation of oxo-biodegradable polyethylene. J. Appl. Polym. Sci. 2009, 111, 1426–1432. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Medrano, D.E.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- North, E.J.; Halden, R.U. Plastics and Environmental Health: The Road Ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Seymour, R.B. Polymer Science Before and After 1899: Notable Developments During the Lifetime of Maurits Dekker. J. Macromol. Sci. Chem. 1989, 26, 1023–1032. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and Release of Chemicals From Plastics to the Environment and to Wildlife. Philos. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [Green Version]
- Rochman, C.M. Microplastics research-from sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H. Polymer Handbook; Wiley: Toronto, ON, Canada, 1986; Volume II. [Google Scholar]
- Eskandarabadi, S.M.; Mahmoudian, M.; Farah, K.R.; Abdali, A.; Nozad, E.; Enayati, M. Active Intelligent Packaging Film Based on Ethylene Vinyl Acetate Nanocomposite Containing Extracted Anthocyanin, Rosemary Extract and ZnO/Fe-MMT Nanoparticles. Food Packag. Shelf Life 2019, 22, 100389. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.; Yang, H.; Ouyang, J. Perovskite LaFeO3/Montmorillonite Nanocomposites: Synthesis, Interface Characteristics and Enhanced Photocatalytic Activity. Sci. Rep. 2016, 6, 19723. [Google Scholar] [CrossRef] [Green Version]
- Ávila-Torres, Y.; Huerta, L.; Barba-Behrens, N. XPS-Characterization of Heterometallic Coordination Compounds with Optically Active Ligands. J. Chem. 2013, 2013, 370637–370645. [Google Scholar] [CrossRef] [Green Version]
- Khabbaz, F.; Albertsson, A.C.; Karlsson, S. Chemical and Morphological Changes of Environmentally Degradable Polyethylene Films Exposed to Thermo-Oxidation. Polym. Degrad. Stab. 1999, 63, 127–138. [Google Scholar] [CrossRef]
- Setnescu, R.; Jipa, S.; Osawa, Z. Chemiluminescence Study on the Oxidation of Several Polyolefins—I. Thermal-Induced Degradation of Additive-Free Polyolefins. Polym. Degrad. Stab. 1998, 60, 377–383. [Google Scholar] [CrossRef]
- Valadez-Gonzalez, A.; Cervantes-Uc, J.M.; Veleva, L. Mineral Filler Influence on the Photo-Oxidation of High Density Polyethylene: I. Accelerated UV Chamber Exposure Test. Polym. Degrad. Stab. 1999, 63, 253–260. [Google Scholar] [CrossRef]
- Lomakin, S.M.; Novokshonova, L.A.; Brevnov, P.N.; Shchegolikhin, A.N. Thermal Properties of Polyethylene/Montmorillonite Nanocomposites Prepared by Intercalative Polymerization. J. Mater. Sci. 2008, 43, 1340–1353. [Google Scholar] [CrossRef]
- Andrady, A.L. Wavelength Sensitivity in Polymer Photodegradation. Adv. Polym. Sci. 1997, 128, 47–94. [Google Scholar]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Degradation Profile of Polyethylene After Artificial Accelerated Weathering. Polym. Degrad. Stab. 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M.; Wang, Y. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef] [PubMed]



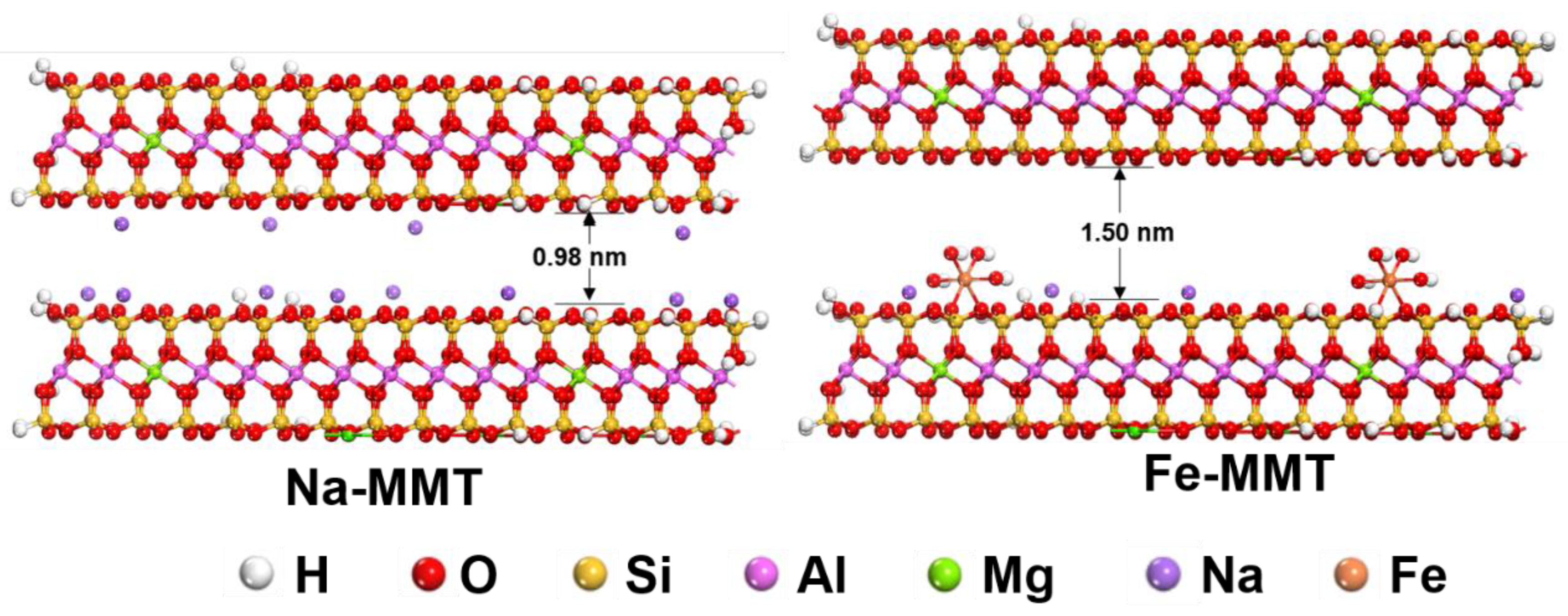





| Sample Name | LLDPE (wt%) | Masterbatch (wt%) | Fe-MMT (wt%) |
|---|---|---|---|
| PE | 100 | 0 | 0 |
| PE-0.1 | 99 | 1 | 0.1 |
| PE-0.3 | 97 | 3 | 0.3 |
| PE-0.5 | 95 | 5 | 0.5 |
| PE-0.7 | 93 | 7 | 0.7 |
| PE-0.9 | 91 | 9 | 0.9 |
| Sample | Binding Energy (ev) | |||
|---|---|---|---|---|
| Fe 2p | O 1s | Si 2p | Al 2p | |
| Na-MMT | -- | -- | 103.80 102.80 | 74.89 |
| Fe-MMT | 726.61 (2p1/2) 713.01 (2p3/2) | 533.33 (H2O) 532.12 (-OH) 530.80 (Lattice O) | 103.63 102.56 | 74.85 |
| Sample | PE | PE-0.1 | PE-0.3 | PE-0.5 | PE-0.7 | PE-0.9 |
|---|---|---|---|---|---|---|
| X (%) | 328 ± 26 | 319 ± 30 | 318 ± 6 | 306 ± 6 | 327 ± 12 | 342 ± 15 |
| Wavenumber (cm−1) | Attribution |
|---|---|
| 1780 | γ-lactones |
| 1733 | aldehydes or esters |
| 1716 | ketones |
| 1707 | carboxylic acids |
| 1380 | methyl |
| Sample | Time (h) a |
|---|---|
| PE | 72 |
| PE-0.1 | 72 |
| PE-0.3 | 48 |
| PE-0.5 | 48 |
| PE-0.7 | 24 |
| PE-0.9 | 24 |
| Sample | Mv at UV Irradiation Time | ||||||
|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | 144 h | |
| PE | 52,231 | 50,212 | 48,517 | 46,401 | 44,334 | 42,066 | 39,584 |
| PE-0.1 | 49,455 | 48,324 | 45,704 | 43,076 | 41,562 | 39,912 | 36,568 |
| PE-0.3 | 49,329 | 47,102 | 43,514 | 41,035 | 38,755 | 36,301 | 33,515 |
| PE-0.5 | 49,237 | 46,027 | 42,506 | 39,689 | 36,469 | 33,294 | 28,933 |
| PE-0.7 | 49,150 | 43,642 | 33,455 | 24,413 | 20,160 | 15,660 | 12,888 |
| PE-0.9 | 49,090 | 29,159 | 17,600 | 13,511 | 8587 | 5600 | 4344 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, H.; Zhang, Y.; Wang, Q. Study on the Mechanism of Molecular Weight Reduction of Polyethylene Based on Fe-Montmorillonite and Its Potential Application. Polymers 2023, 15, 1429. https://doi.org/10.3390/polym15061429
Wang Z, Chen H, Zhang Y, Wang Q. Study on the Mechanism of Molecular Weight Reduction of Polyethylene Based on Fe-Montmorillonite and Its Potential Application. Polymers. 2023; 15(6):1429. https://doi.org/10.3390/polym15061429
Chicago/Turabian StyleWang, Zhiming, Huimin Chen, Yunpeng Zhang, and Qingzhao Wang. 2023. "Study on the Mechanism of Molecular Weight Reduction of Polyethylene Based on Fe-Montmorillonite and Its Potential Application" Polymers 15, no. 6: 1429. https://doi.org/10.3390/polym15061429




