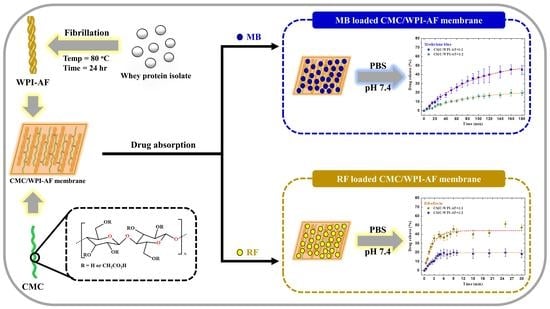
Application of Amyloid-Based Hybrid Membranes in Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Amyloid Fibril Formation of the Whey Protein Isolate (WPI)
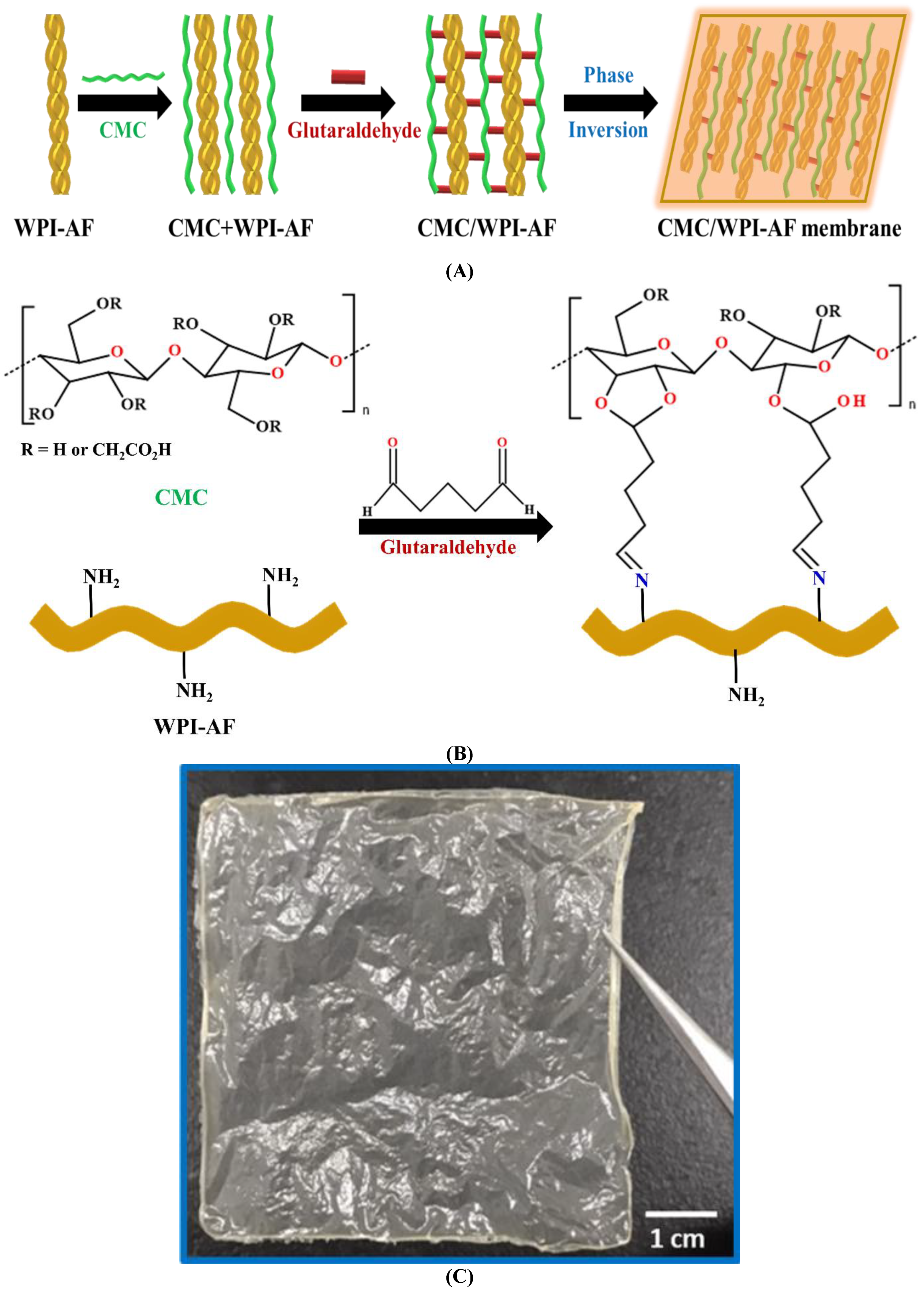
2.3. Fabrication of the Carboxymethyl Cellulose/Whey Protein Isolate Amyloid Fibril (CMC/WPI-AF) Membrane
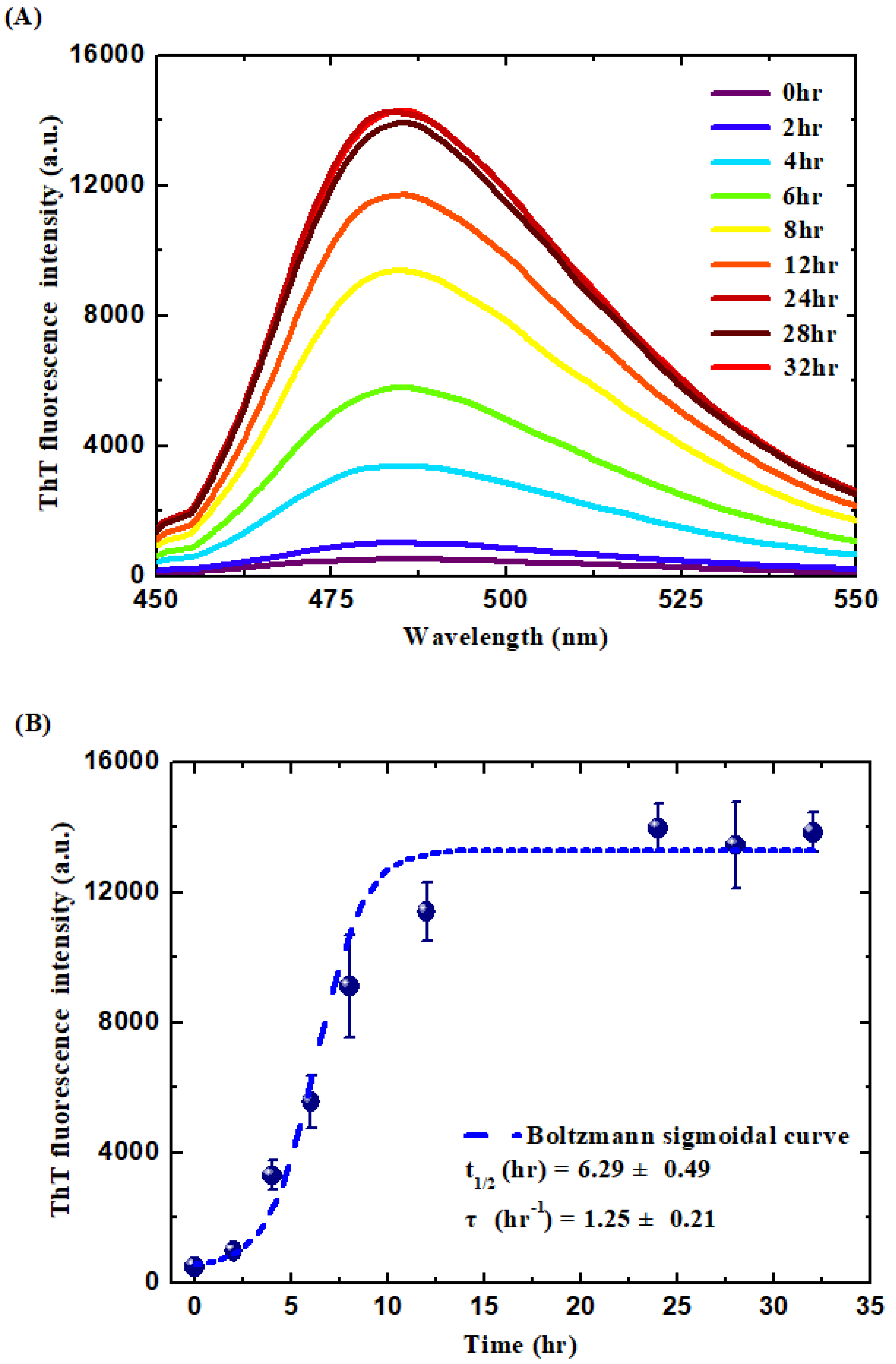
2.4. Thioflavin T (ThT) Binding Assay
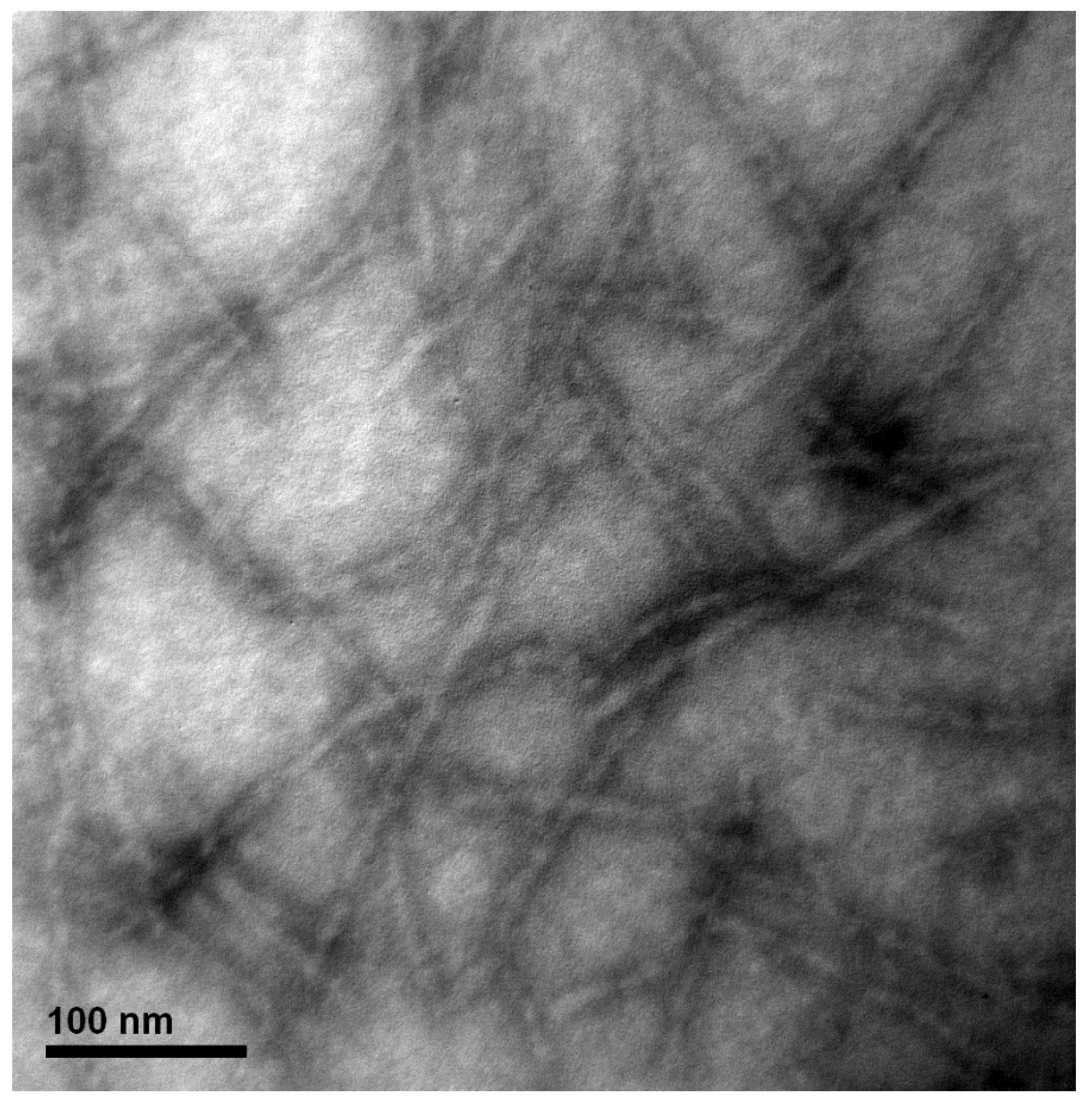
2.5. Transmission Electron Microscopy (TEM)
2.6. Zeta-Potential Measurements
2.7. Fourier Transform Infrared (FT-IR) Spectroscopy
2.8. Scanning Electron Microscopy (SEM)
2.9. Absorption of the Drug in the CMC/WPI-AF Membrane
2.10. In Vitro Passive Drug Delivery Studies
2.11. Statistical Analysis
3. Results and Discussion
3.1. Formation and Characterization of the WPI-AF
3.2. Synthetic Mechanism of the CMC/WPI-AF Membranes
3.3. Zeta Potential Properties of the CMC/WPI-AF Membranes
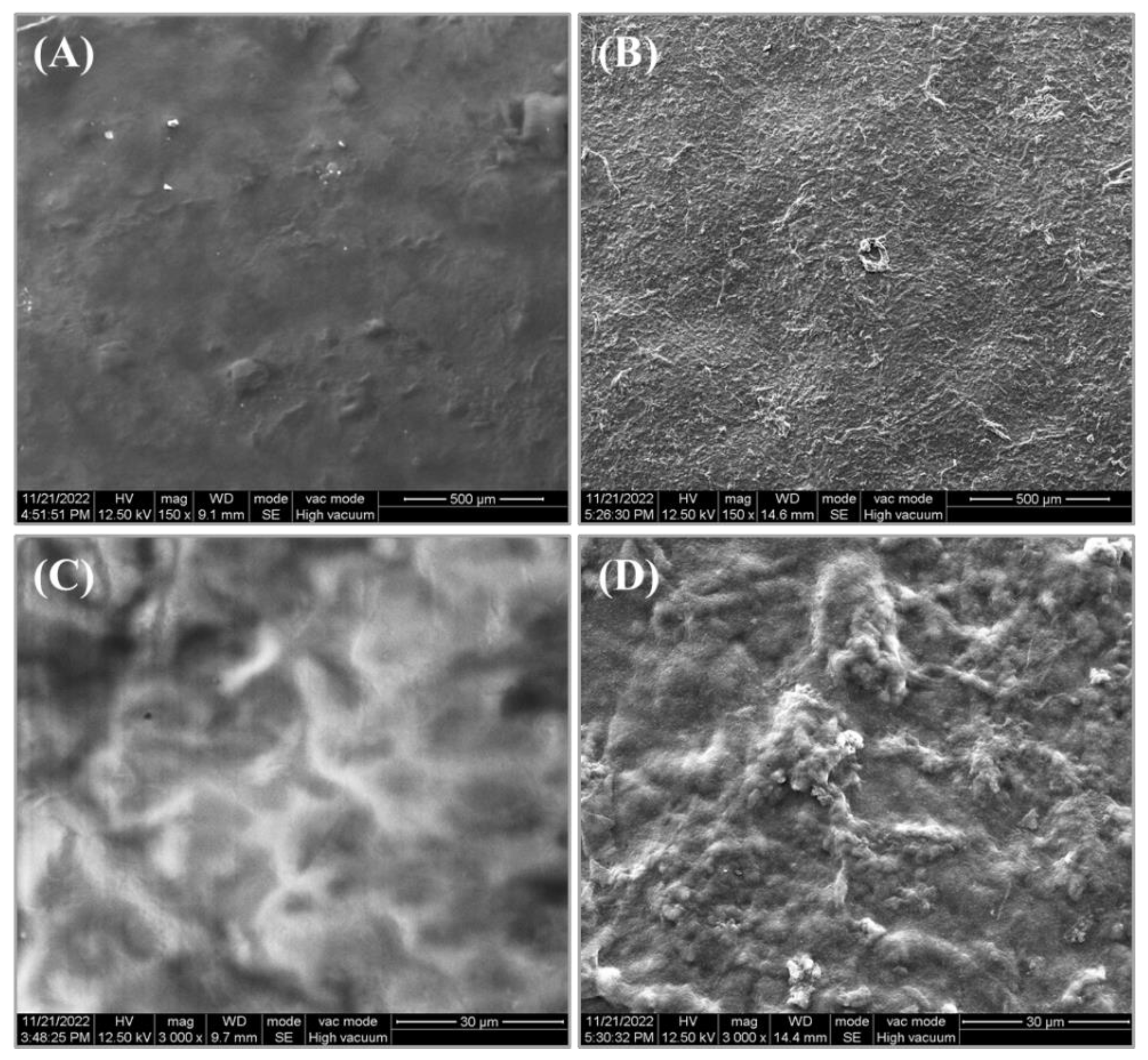
3.4. Surface Microstructure Characterization of the CMC/WPI-AF Membranes
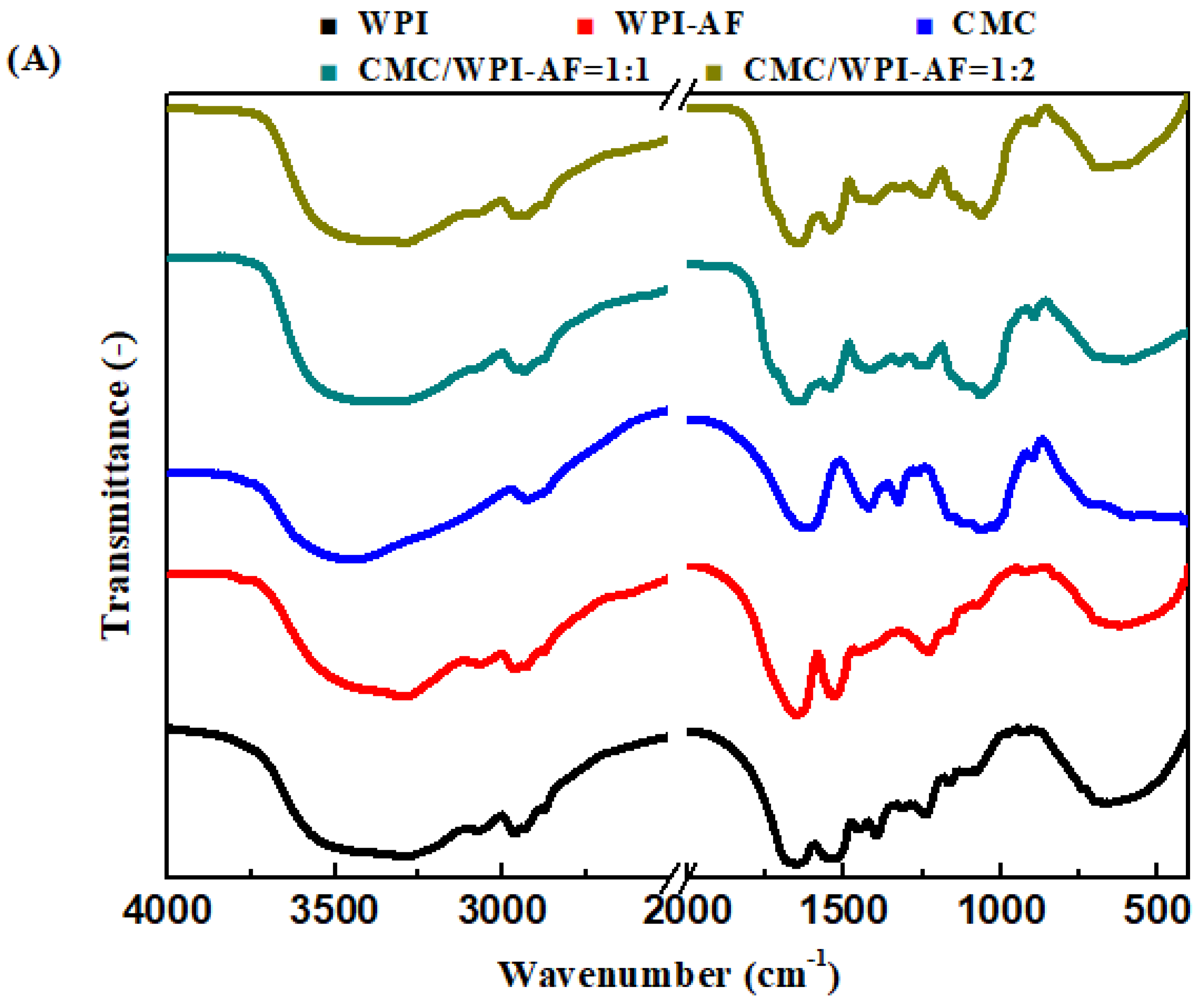
3.5. FT-IR Analysis of the CMC/WPI-AF Membranes
3.6. EE and LC of the Drugs on the CMC/WPI-AF Membranes
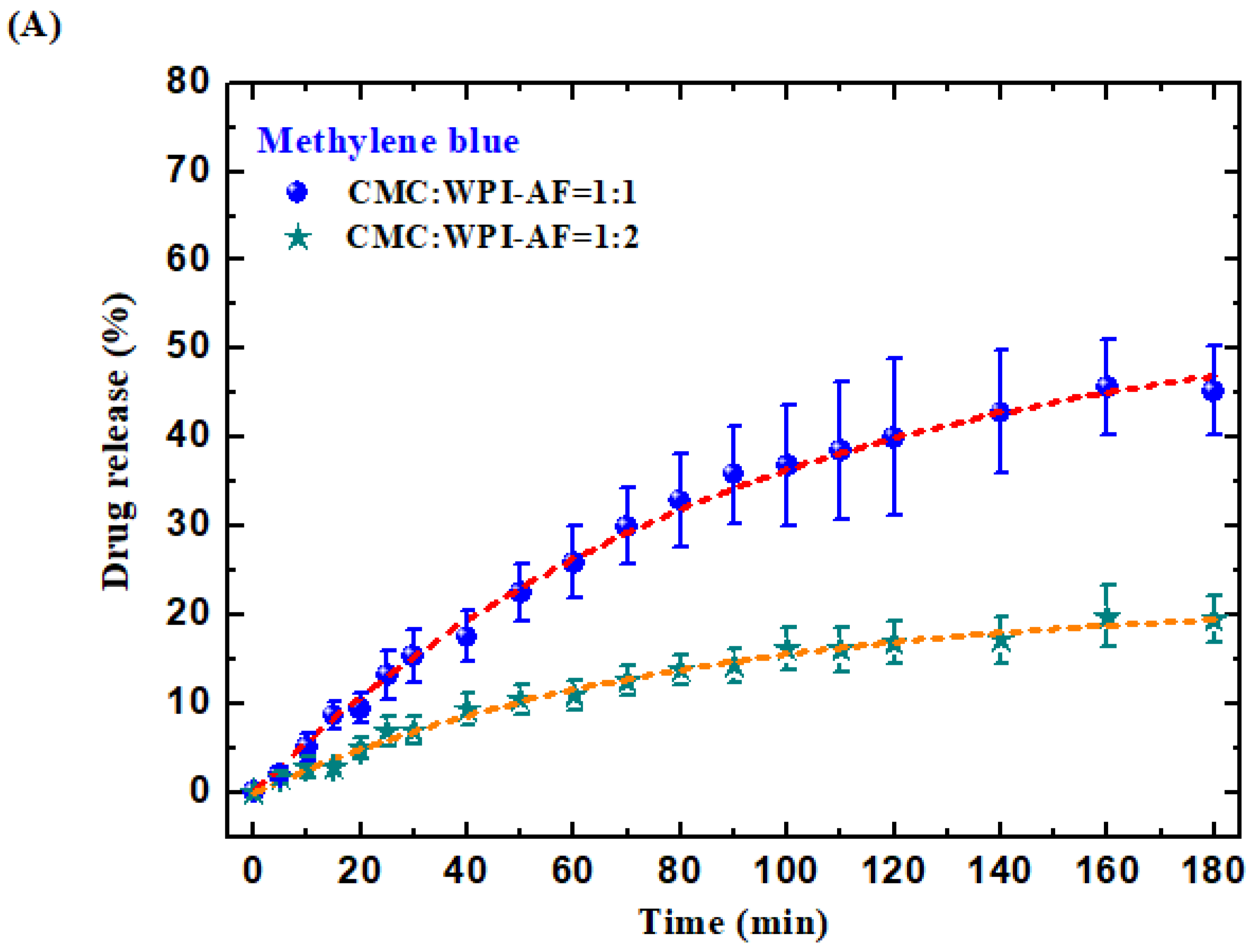
3.7. In Vitro Passive Drug Release of MB and RF from the CMC/WPI-AF Membranes
3.8. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, S.R.; Wu, Y.; Sui, S.F. Study of the correlation of secondary structure of beta-amyloid peptide (Abeta40) with the hydrophobic exposure under different conditions. Gen. Physiol. 2002, 21, 415–427. [Google Scholar]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Dharmadana, D.; Reynolds, N.P.; Conn, C.E.; Valery, C. Molecular interactions of amyloid nanofibrils with biological aggregation modifiers: Implications for cytotoxicity mechanisms and biomaterial design. Interface Focus 2017, 7, 20160160. [Google Scholar] [CrossRef] [Green Version]
- Bellesia, G.; Shea, J.E. Diversity of kinetic pathways in amyloid fibril formation. J. Chem. Phys. 2009, 131, 111102. [Google Scholar] [CrossRef]
- Knowles, T.P.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.S.; Wu, J.W.; Yamamoto, S.; Liu, H.S. Diseases of protein aggregation and the hunt for potential pharmacological agents. Biotechnol. J. Healthc. Nutr. Technol. 2008, 3, 165–192. [Google Scholar] [CrossRef] [PubMed]
- Iadanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.S.S.; Chen, Y.T.; Chen, P.H.; Liu, K.N. A kinetic study on the aggregation behavior of beta-amyloid peptides in different initial solvent environments. Biochem. Eng. J. 2006, 29, 129–138. [Google Scholar] [CrossRef]
- Akkermans, C.; Venema, P.; Rogers, S.S.; van der Goot, A.J.; Boom, R.M.; van der Linden, E. Shear pulses nucleate fibril aggregation. Food Biophys. 2006, 1, 144–150. [Google Scholar] [CrossRef]
- Giri, K.; Bhattacharyya, N.P.; Basak, S. pH-dependent self-assembly of polyalanine peptides. Biophys. J. 2007, 92, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Kumar, E.K.; Haque, N.; Prabhu, N.P. Kinetics of protein fibril formation: Methods and mechanisms. Int. J. Biol. Macromol. 2017, 100, 3–10. [Google Scholar] [CrossRef]
- Arosio, P.; Knowles, T.P.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef] [Green Version]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Aso, Y.; Shiraki, K.; Takagi, M. Systematic analysis of aggregates from 38 kinds of non disease-related proteins: Identifying the intrinsic propensity of polypeptides to form amyloid fibrils. Biosci. Biotechnol. Biochem. 2007, 71, 1313–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badtke, M.P.; Hammer, N.D.; Chapman, M.R. Functional amyloids signal their arrival. Sci. Signal. 2009, 2, pe43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGlinchey, R.P.; Shewmaker, F.; Hu, K.-N.; McPhie, P.; Tycko, R.; Wickner, R.B. Repeat domains of melanosome matrix protein Pmel17 orthologs form amyloid fibrils at the acidic melanosomal pH. J. Biol. Chem. 2011, 286, 8385–8393. [Google Scholar] [CrossRef] [Green Version]
- Al-Halifa, S.; Babych, M.; Zottig, X.; Archambault, D.; Bourgault, S. Amyloid self-assembling peptides: Potential applications in nanovaccine engineering and biosensing. Pept. Sci. 2019, 111, e24095. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.; Debelouchina, G.T.; Bayro, M.J.; Clare, D.K.; Caporini, M.A.; Bajaj, V.S.; Jaroniec, C.P.; Wang, L.; Ladizhansky, V.; Müller, S.A. Atomic structure and hierarchical assembly of a cross-β amyloid fibril. Proc. Natl. Acad. Sci. USA 2013, 110, 5468–5473. [Google Scholar] [CrossRef] [Green Version]
- Navarro, S.; Ventura, S. Computational methods to predict protein aggregation. Curr. Opin. Struct. Biol. 2022, 73, 102343. [Google Scholar] [CrossRef]
- Taylor, A.I.; Staniforth, R.A. General Principles Underpinning Amyloid Structure. Front. Neurosci. 2022, 16, 878869. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Madadlou, A. Technological functionality and biological properties of food protein nanofibrils formed by heating at acidic condition. Trends Food Sci. Technol. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Shen, Y.; Levin, A.; Kamada, A.; Toprakcioglu, Z.; Rodriguez-Garcia, M.; Xu, Y.; Knowles, T.P.J. From Protein Building Blocks to Functional Materials. ACS Nano 2021, 15, 5819–5837. [Google Scholar] [CrossRef]
- Knowles, T.P.; Buehler, M.J. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 2011, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lendel, C.; Langton, M.; Olsson, R.T.; Hedenqvist, M.S. Protein nanofibrils: Preparation, properties, and possible applications in industrial nanomaterials. In Industrial Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–63. [Google Scholar]
- Smith, J.F.; Knowles, T.P.; Dobson, C.M.; MacPhee, C.E.; Welland, M.E. Characterization of the nanoscale properties of individual amyloid fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 15806–15811. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, A.W.; Vanacore, G.M.; Zewail, A.H. Nanomechanics and intermolecular forces of amyloid revealed by four-dimensional electron microscopy. Proc. Natl. Acad. Sci. USA 2015, 112, 3380–3385. [Google Scholar] [CrossRef] [Green Version]
- Povilonienė, S.; Časaitė, V.; Bukauskas, V.; Šetkus, A.; Staniulis, J.; Meškys, R. Functionalization of α-synuclein fibrils. Beilstein J. Nanotechnol. 2015, 6, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Knowles, T.P.; Mezzenga, R. Amyloid fibrils as building blocks for natural and artificial functional materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef]
- Li, C.; Alam, M.M.; Bolisetty, S.; Adamcik, J.; Mezzenga, R. New biocompatible thermo-reversible hydrogels from PNiPAM-decorated amyloid fibrils. Chem. Commun. 2011, 47, 2913–2915. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Li, D.; Xi, W.; Luo, F.; Zhang, X.; Zou, M.; Cao, M.; Hu, J.; Wang, W.; Wei, G.; et al. Tunable assembly of amyloid-forming peptides into nanosheets as a retrovirus carrier. Proc. Natl. Acad. Sci. USA 2015, 112, 2996–3001. [Google Scholar] [CrossRef] [Green Version]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Shorter, J.; Lindquist, S. Prions as adaptive conduits of memory and inheritance. Nat. Rev. Genet. 2005, 6, 435–450. [Google Scholar] [CrossRef]
- Jacob, R.S.; Das, S.; Ghosh, S.; Anoop, A.; Jha, N.N.; Khan, T.; Singru, P.; Kumar, A.; Maji, S.K. Amyloid formation of growth hormone in presence of zinc: Relevance to its storage in secretory granules. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.; Su, Z.; Reynolds, N.P.; Arosio, P.; Hamley, I.W.; Gazit, E.; Mezzenga, R. Self-assembling peptide and protein amyloids: From structure to tailored function in nanotechnology. Chem. Soc. Rev. 2017, 46, 4661–4708. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Roberts, S.J.; Meade, S.J.; Gerrard, J.A. Amyloid fibrils as a nanoscaffold for enzyme immobilization. Biotechnol. Prog. 2010, 26, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.S.; Anoop, A.; Maji, S.K. Protein nanofibrils as storage forms of peptide drugs and hormones. Biol. Bio-Inspired Nanomater. 2019, 1174, 265–290. [Google Scholar]
- Fedunova, D.; Antosova, A.; Marek, J.; Vanik, V.; Demjen, E.; Bednarikova, Z.; Gazova, Z. Effect of 1-Ethyl-3-methylimidazolium Tetrafluoroborate and Acetate Ionic Liquids on Stability and Amyloid Aggregation of Lysozyme. Int. J. Mol. Sci. 2022, 23, 783. [Google Scholar] [CrossRef]
- Ziaunys, M.; Sakalauskas, A.; Mikalauskaite, K.; Snieckute, R.; Smirnovas, V. Temperature-dependent structural variability of prion protein amyloid fibrils. Int. J. Mol. Sci. 2021, 22, 5075. [Google Scholar] [CrossRef]
- How, S.-C.; Lin, T.-H.; Chang, C.-C.; Wang, S.S.-S. Examining the effect of bovine serum albumin on the properties and drug release behavior of β-lactoglobulin-derived amyloid fibril-based hydrogels. Int. J. Biol. Macromol. 2021, 184, 79–91. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Jansens, K.J.; Rombouts, I.; Brijs, K.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Conditions governing food protein amyloid fibril formation. part II: Milk and legume proteins. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1277–1291. [Google Scholar] [CrossRef]
- Gade Malmos, K.; Blancas-Mejia, L.M.; Weber, B.; Buchner, J.; Ramirez-Alvarado, M.; Naiki, H.; Otzen, D. ThT 101: A primer on the use of thioflavin T to investigate amyloid formation. Amyloid 2017, 24, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Kumbhakar, M.; Pal, H.; Nath, S. Ultrafast bond twisting dynamics in amyloid fibril sensor. J. Phys. Chem. B 2010, 114, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-R.; Lai, J.-T.; Wang, S.S.-S.; Kuo, Y.-C.; Lin, T.-H. Silver nanoparticle-deposited whey protein isolate amyloid fibrils as catalysts for the reduction of methylene blue. Int. J. Biol. Macromol. 2022, 213, 1098–1114. [Google Scholar] [CrossRef]
- Buhus, G.; Popa, M.; Desbrieres, J. Hydrogels based on carboxymethylcellulose and gelatin for inclusion and release of chloramphenicol. J. Bioact. Compat. Polym. 2009, 24, 525–545. [Google Scholar] [CrossRef]
- Chinyerenwa, A.C.; Wang, H.; Zhang, Q.; Zhuang, Y.; Munna, K.H.; Ying, C.; Yang, H.; Xu, W. Structure and thermal properties of porous polylactic acid membranes prepared via phase inversion induced by hot water droplets. Polymer 2018, 141, 62–69. [Google Scholar] [CrossRef]
- Duhoranimana, E.; Karangwa, E.; Lai, L.; Xu, X.; Yu, J.; Xia, S.; Zhang, X.; Muhoza, B.; Habinshuti, I. Effect of sodium carboxymethyl cellulose on complex coacervates formation with gelatin: Coacervates characterization, stabilization and formation mechanism. Food Hydrocoll. 2017, 69, 111–120. [Google Scholar] [CrossRef]
- Cai, T.; Yang, Z.; Li, H.; Yang, H.; Li, A.; Cheng, R. Effect of hydrolysis degree of hydrolyzed polyacrylamide grafted carboxymethyl cellulose on dye removal efficiency. Cellulose 2013, 20, 2605–2614. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Saharan, V.; Kukkar, V.; Kataria, M.; Gera, M.; Choudhury, P.K. Dissolution enhancement of drugs. Part I: Technologies and effect of carriers. Int. J. Health Res. 2009, 2, 2. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Hedenqvist, M.S.; Langton, M.; Lendel, C. On the role of peptide hydrolysis for fibrillation kinetics and amyloid fibril morphology. RSC Adv. 2018, 8, 6915–6924. [Google Scholar] [CrossRef] [Green Version]
- Habibi, N. Preparation of biocompatible magnetite-carboxymethyl cellulose nanocomposite: Characterization of nanocomposite by FTIR, XRD, FESEM and TEM. Spectrochim. Acta A Mol. 2014, 131, 55–58. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Musale, D.A.; Kumar, A. Effects of surface crosslinking on sieving characteristics of chitosan/poly (acrylonitrile) composite nanofiltration membranes. Sep. Purif. Technol. 2000, 21, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, S.; Robles, I.; Godínez, L.A.; Acelas, N.; Flórez, E. Experimental and theoretical insights on methylene blue removal from wastewater using an adsorbent obtained from the residues of the orange industry. Molecules 2021, 26, 4555. [Google Scholar] [CrossRef]
- Liu, F.; Zou, H.; Hu, J.; Liu, H.; Peng, J.; Chen, Y.; Lu, F.; Huo, Y. Fast removal of methylene blue from aqueous solution using porous soy protein isolate based composite beads. Chem. Eng. J. 2016, 287, 410–418. [Google Scholar] [CrossRef]
- Chakraborty, P.; Bairi, P.; Roy, B.; Nandi, A.K. Rheological and fluorescent properties of riboflavin-poly (N-isopropylacrylamide) hybrid hydrogel with a potentiality of forming Ag nanoparticle. RSC Adv. 2014, 4, 54684–54693. [Google Scholar] [CrossRef]
- Ge, X.; Sun, Y.; Kong, J.; Mao, M.; Yu, H.; Arora, A.; Suppavorasatit, I.; Wang, Y. The thermal resistance and targeting release of zein-sodium alginate binary complexes as a vehicle for the oral delivery of riboflavin. J. Food Sci. Technol. 2022, 60, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta-Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, G.; Jing, P. Polyphenol binding disassembles glycation-modified bovine serum albumin amyloid fibrils. Spectrochim. Acta A Mol. 2021, 246, 119001. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bolisetty, S.; Mohammadi, T.; Mezzenga, R. Assessing the binding performance of amyloid-carbon membranes toward heavy metal ions. Langmuir 2019, 35, 4161–4170. [Google Scholar] [CrossRef]
- Hate, S.S.; Reutzel-Edens, S.M.; Taylor, L.S. Influence of Drug-Silica Electrostatic Interactions on Drug Release from Mesoporous Silica-Based Oral Delivery Systems. Mol. Pharm. 2020, 17, 3435–3446. [Google Scholar] [CrossRef]
- Feng, Z.; Zheng, Y.; Zhao, L.; Zhang, Z.; Sun, Y.; Qiao, K.; Xie, Y.; Wang, Y.; He, W. An ultrasound-controllable release system based on waterborne polyurethane/chitosan membrane for implantable enhanced anticancer therapy. Mater. Sci. Eng. C 2019, 104, 109944. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.; Molina, E.; Chiavacci, L.; Santilli, C.V.; Briois, V.; Pulcinelli, S.H. Drug-matrix interaction of sodium diclofenac incorporated into ureasil-poly (ethylene oxide) hybrid materials. RSC Adv. 2012, 2, 5629–5636. [Google Scholar] [CrossRef]
- Zandi, M.; Mohebbi, M.; Varidi, M.; Ramezanian, N. Evaluation of diacetyl encapsulated alginate-whey protein microspheres release kinetics and mechanism at simulated mouth conditions. Food Res. Int. 2014, 56, 211–217. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-C.; Hu, C.-C.; Li, C.-Y.; Li, S.-C.; Cai, Z.-W.; Wei, Y.; Don, T.-M. Theophylline-Loaded Pectin/Chitosan Hydrochloride Submicron Particles Prepared by Spray Drying with a Continuous Feeding Ultrasonic Atomizer. Polymers 2022, 14, 4538. [Google Scholar] [CrossRef]
- Rac, V.; Lević, S.; Balanč, B.; Graells, B.O.; Bijelić, G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids Surf. B 2019, 180, 441–448. [Google Scholar] [CrossRef]
- Janićijević, Ž.; Radovanović, F. Polyethersulfone/poly (acrylic acid) composite hydrogel membrane reservoirs for controlled delivery of cationic drug formulations. Polymer 2018, 147, 56–66. [Google Scholar] [CrossRef]
- Im, J.S.; Bai, B.C.; Lee, Y.-S. The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery system. Biomaterials 2010, 31, 1414–1419. [Google Scholar] [CrossRef]
- Sheng, S.; Yin, X.; Chen, F.; Lv, Y.; Zhang, L.; Cao, M.; Sun, Y. Preparation and characterization of PVA-co-PE drug-loaded nanofiber membrane by electrospinning technology. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Qiao, Z.; Tran, L.; Parks, J.; Zhao, Y.; Hai, N.; Zhong, Y.; Ji, H.F. Highly stretchable gelatin-polyacrylamide hydrogel for potential transdermal drug release. Nano Select 2021, 2, 107–115. [Google Scholar] [CrossRef]
- Langer, R. Invited review polymeric delivery systems for controlled drug release. Chem. Eng. Commun. 1980, 6, 1–48. [Google Scholar] [CrossRef]
- Lassé, M.; Ulluwishewa, D.; Healy, J.; Thompson, D.; Miller, A.; Roy, N.; Chitcholtan, K.; Gerrard, J.A. Evaluation of protease resistance and toxicity of amyloid-like food fibrils from whey, soy, kidney bean, and egg white. Food Chem. 2016, 192, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, F.D.; Rodero, C.F.; Lutz-Bueno, V.; Chorilli, M.; Mezzenga, R. Amyloid Fibrils Enhance the Topical Bio-Adhesivity of Liquid Crystalline Mesophase-Based Drug Formulations. Adv. Healthc. Mater. 2023, 2202720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, P. Amyloid-Mediated Fabrication of Organic—Inorganic Hybrid Materials and Their Biomedical Applications. Adv. Mater. Interfaces 2020, 7, 2001060. [Google Scholar] [CrossRef]
- Bolisetty, S.; Boddupalli, C.S.; Handschin, S.; Chaitanya, K.; Adamcik, J.; Saito, Y.; Manz, M.G.; Mezzenga, R. Amyloid fibrils enhance transport of metal nanoparticles in living cells and induced cytotoxicity. Biomacromolecules 2014, 15, 2793–2799. [Google Scholar] [CrossRef]
- Shen, Y.; Posavec, L.; Bolisetty, S.; Hilty, F.M.; Nyström, G.; Kohlbrecher, J.; Hilbe, M.; Rossi, A.; Baumgartner, J.; Zimmermann, M.B. Amyloid fibril systems reduce, stabilize and deliver bioavailable nanosized iron. Nat. Nanotechnol. 2017, 12, 642–647. [Google Scholar] [CrossRef]
- Yue, J.; Shu, M.; Yao, X.; Chen, X.; Li, D.; Yang, D.; Liu, N.; Nishinari, K.; Jiang, F. Fibrillar assembly of whey protein isolate and gum Arabic as iron carrier for food fortification. Food Hydrocoll. 2022, 128, 107608. [Google Scholar] [CrossRef]
- Shimanovich, U.; Efimov, I.; Mason, T.O.; Flagmeier, P.; Buell, A.K.; Gedanken, A.; Linse, S.; Åkerfeldt, K.S.; Dobson, C.M.; Weitz, D.A. Protein microgels from amyloid fibril networks. ACS Nano 2015, 9, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mains, J.; Lamprou, D.A.; McIntosh, L.; Oswald, I.D.; Urquhart, A.J. Beta-adrenoceptor antagonists affect amyloid nanostructure; amyloid hydrogels as drug delivery vehicles. Chem. Commun. 2013, 49, 5082–5084. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Kwon, Y.; Nam, E.-J.; Park, H.; Paik, S.R. Disulfide-mediated elongation of amyloid fibrils of α-synuclein for use in producing self-healing hydrogel and dye-absorbing aerogel. Acta Biomater. 2022, 145, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, H.; Yao, L.; Yu, Y.; Ma, G. Amyloid-based injectable hydrogel derived from hydrolyzed hen egg white lysozyme. Acs Omega 2019, 4, 8071–8080. [Google Scholar] [CrossRef] [Green Version]
- Altunbas, A.; Lee, S.J.; Rajasekaran, S.A.; Schneider, J.P.; Pochan, D.J. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 2011, 32, 5906–5914. [Google Scholar] [CrossRef] [Green Version]
- Kabay, G.; Meydan, A.E.; Can, G.K.; Demirci, C.; Mutlu, M. Controlled release of a hydrophilic drug from electrospun amyloid-like protein blend nanofibers. Mater. Sci. Eng. C 2017, 81, 271–279. [Google Scholar] [CrossRef]



| CMC/WPI-AF Membranes | Methylene Blue (MB) | |
|---|---|---|
| Encapsulation Efficiency (%) | Loading Capacity (%) | |
| CMC:WPI-AF = 1:1 | 18.31 ± 3.14 | 106.71 ± 7.97 |
| CMC:WPI-AF = 1:2 | 46.80 ± 2.88 | 197.38 ± 9.43 |
| CMC/WPI-AF membranes | Riboflavin (RF) | |
| Encapsulation efficiency (%) | Loading capacity (%) | |
| CMC:WPI-AF = 1:1 | 10.74 ± 1.63 | 21.89 ± 1.21 |
| CMC:WPI-AF = 1:2 | 16.49 ± 3.48 | 24.80 ± 1.49 |
| CMC/WPI-AF Membranes | Methylene Blue (MB) | ||
|---|---|---|---|
| M∞ | kf (min−1) | R2 | |
| CMC:WPI-AF = 1:1 | 54.35 ± 1.41 | 0.0111 ± 0.0005 | 0.996 |
| CMC:WPI-AF = 1:2 | 21.66 ± 0.67 | 0.0127 ± 0.0008 | 0.993 |
| CMC/WPI-AF membranes | Riboflavin (RF) | ||
| M∞ | kf (min−1) | R2 | |
| CMC:WPI-AF = 1:1 | 43.92 ± 1.19 | 0.4294 ± 0.0439 | 0.951 |
| CMC:WPI-AF = 1:2 | 19.86 ± 0.53 | 0.3442 ± 0.0293 | 0.961 |
| CMC/WPI-AF Membranes | Methylene Blue (MB) | ||
|---|---|---|---|
| kp (min−n) | N | R2 | |
| CMC:WPI-AF = 1:1 | 0.015 ± 0.002 | 0.852 ± 0.025 | 0.996 |
| CMC:WPI-AF = 1:2 | 0.024 ± 0.005 | 0.755 ± 0.055 | 0.976 |
| CMC/WPI-AF membranes | Riboflavin (RF) | ||
| kp (min−n) | N | R2 | |
| CMC:WPI-AF = 1:1 | 0.326 ± 0.014 | 0.821 ± 0.057 | 0.992 |
| CMC:WPI-AF = 1:2 | 0.249 ± 0.020 | 0.750 ± 0.090 | 0.973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-R.; Wang, S.S.-S.; Hsu, T.-L.; Chou, S.-H.; How, S.-C.; Lin, T.-H. Application of Amyloid-Based Hybrid Membranes in Drug Delivery. Polymers 2023, 15, 1444. https://doi.org/10.3390/polym15061444
Lai Y-R, Wang SS-S, Hsu T-L, Chou S-H, How S-C, Lin T-H. Application of Amyloid-Based Hybrid Membranes in Drug Delivery. Polymers. 2023; 15(6):1444. https://doi.org/10.3390/polym15061444
Chicago/Turabian StyleLai, You-Ren, Steven S.-S. Wang, Ti-Lun Hsu, Szu-Hui Chou, Su-Chun How, and Ta-Hsien Lin. 2023. "Application of Amyloid-Based Hybrid Membranes in Drug Delivery" Polymers 15, no. 6: 1444. https://doi.org/10.3390/polym15061444
APA StyleLai, Y.-R., Wang, S. S.-S., Hsu, T.-L., Chou, S.-H., How, S.-C., & Lin, T.-H. (2023). Application of Amyloid-Based Hybrid Membranes in Drug Delivery. Polymers, 15(6), 1444. https://doi.org/10.3390/polym15061444