Life Cycle Assessment of Functionalized Bionanocompounds with Ice Nucleation Protein for Freezing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
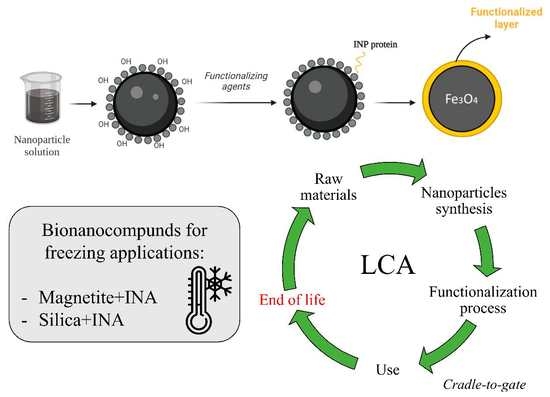
2.2. Synthesis of Fe3O4 Nanoparticles and Surface Modification
2.3. LCA of Bionanocompounds
2.3.1. Goal and Scope Definition
2.3.2. Life Cycle Inventory
2.3.3. Impact Assessment
3. Results
3.1. Results for Energy Consumption
3.2. Results of the Life Cycle Impact Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, R.K.; Prajapati, V.K. Molecular and Immunological Toxic Effects of Nanoparticles. Int. J. Biol. Macromol. 2018, 107 Pt A, 1278–1293. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Sajid, M. Nanomaterials: Types, Properties, Recent Advances, and Toxicity Concerns. Curr. Opin. Environ. Sci. Health 2022, 25, 100319. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured Materials: Basic Concepts and Microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Malakar, A.; Kanel, S.R.; Ray, C.; Snow, D.D.; Nadagouda, M.N. Nanomaterials in the Environment, Human Exposure Pathway, and Health Effects: A Review. Sci. Total Environ. 2021, 759, 143470. [Google Scholar] [CrossRef] [PubMed]
- Long, N.N.; Van Vu, L.; Kiem, C.D.; Doanh, S.C.; Nguyet, C.T.; Hang, P.T.; Thien, N.D.; Quynh, L.M. Synthesis and Optical Properties of Colloidal Gold Nanoparticles. J. Phys. Conf. Ser. 2009, 187, 012026. [Google Scholar] [CrossRef]
- Immanuel, S.; Aparna, T.K.; Sivasubramanian, R. Graphene—Metal Oxide Nanocomposite Modified Electrochemical Sensors. In Graphene-Based Electrochemical Sensors for Biomolecules; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 113–138. [Google Scholar] [CrossRef]
- Muthukumar, H.; Malla, S.; Matheswaran, M. Immobilization of Xylose Reductase Enzyme on Cysteine-Functionalized Murraya Koenigii Mediated Magnetite Nanoparticles. Mater. Lett. 2020, 261, 127125. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Saleh, M.M.; Zaki, M.M.; Nabil, G.M. A Sustainable Nanocomposite for Removal of Heavy Metals from Water Based on Crosslinked Sodium Alginate with Iron Oxide Waste Material from Steel Industry. J. Environ. Chem. Eng. 2020, 8, 104015. [Google Scholar] [CrossRef]
- Pillai, P.; Kakadiya, N.; Timaniya, Z.; Dharaskar, S.; Sillanpaa, M. Removal of Arsenic Using Iron Oxide Amended with Rice Husk Nanoparticles from Aqueous Solution. Mater. Today Proc. 2019, 28, 830–835. [Google Scholar] [CrossRef]
- Shouli, A.; Menati, S.; Sayyahi, S. Comptes Rendus Chimie Copper (II) Chelate-Bonded Magnetite Nanoparticles: A New Magnetically Retrievable Catalyst for the Synthesis of Propargylamines. Comptes Rendus-Chim. 2017, 20, 765–772. [Google Scholar] [CrossRef]
- Rodriguez, A.F.R.; Costa, T.P.; Bini, R.A.; Faria, F.S.E.D.V.; Azevedo, R.B.; Jafelicci, M.; Coaquira, J.A.H.; Martínez, M.A.R.; Mantilla, J.C.; Marques, R.F.C.; et al. Surface Functionalization of Magnetite Nanoparticle: A New Approach Using Condensation of Alkoxysilanes. Phys. B Phys. Condens. Matter 2017, 521, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Pandey, R.; Usui, K.; Livingstone, R.A.; Fischer, S.A.; Pfaendtner, J.; Backus, E.H.G.; Nagata, Y.; Fröhlich-Nowoisky, J.; Schmüser, L.; Mauri, S.; et al. Ice-Nucleating Bacteria Control the Order and Dynamics of Interfacial Water. Sci. Adv. 2016, 2, e1501630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yan, Q.; Chen, J.; He, Y.; Wang, J.; Zhang, H.; Yu, Z.; Li, L. Molecular Characterization of an Ice Nucleation Protein Variant (InaQ) from Pseudomonas Syringae and the Analysis of Its Transmembrane Transport Activity in Escherichia Coli. Int. J. Biol. Sci. 2012, 8, 1097–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monfared, B.; Furberg, R.; Palm, B. Magnetic vs. Vapor-Compression Household Refrigerators: A Preliminary Comparative Life Cycle Assessment. Int. J. Refrig. 2014, 42, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Luglietti, R.; Rosa, P.; Pastore, A.; Terzi, S.; Taisch, M. Life Cycle Assessment Tool Implemented in Household Refrigeration Industry: A Magnetic Cooling Prototype Development. In Procedia Manufacturing; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 8, pp. 231–238. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organisation for Stardardization: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 10 October 2022).
- Sotelo, D.C.; Ornelas-Soto, N.; Osma, J.F. Novel Magnetic Polymeric Filters with Laccase-Based Nanoparticles for Improving Congo Red Decolorization in Bioreactors. Polymers 2022, 14, 2328. [Google Scholar] [CrossRef]
- Pulido, I.; Camacho, D.; Calderón, L.; Angulo, J.; Osma, J.; Danies, G.; Obregón, C. Bio-Nanocompound as an Agent for Nucleating Aqueous-Based Compounds and Production Method Thereof. WO2021/224833, 11.11.2021. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2021224833&_cid=P21-LB43QP-23865-1 (accessed on 30 November 2022).
- Feijoo, S.; González-García, S.; Moldes-Diz, Y.; Vazquez-Vazquez, C.; Feijoo, G.; Moreira, M.T. Comparative Life Cycle Assessment of Different Synthesis Routes of Magnetic Nanoparticles. J. Clean. Prod. 2017, 143, 528–538. [Google Scholar] [CrossRef]
- Azouz, R.A.; Korany, R.M.S. Toxic Impacts of Amorphous Silica Nanoparticles on Liver and Kidney of Male Adult Rats: An In Vivo Study. Biol. Trace Elem. Res. 2021, 199, 2653–2662. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Chang, Z.; Li, L.; Xing, H.; Dong, W.F. Combined Toxicity of Silica Nanoparticles and Cadmium Chloride on the Cardiovascular System of Zebrafish (Danio Rerio) Larvae. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2021, 239, 108895. [Google Scholar] [CrossRef]
- Hozayen, W.G.; Mahmoud, A.M.; Desouky, E.M.; El-Nahass, E.S.; Soliman, H.A.; Farghali, A.A. Cardiac and Pulmonary Toxicity of Mesoporous Silica Nanoparticles Is Associated with Excessive ROS Production and Redox Imbalance in Wistar Rats. Biomed. Pharmacother. 2019, 109, 2527–2538. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and Green Synthesis of Nanoparticles from Plant Extracts with Antiviral and Antimicrobial Properties: A Critical Review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Sharma, R.; Tripathi, A. Green Synthesis of Nanoparticles and Its Key Applications in Various Sectors. Mater. Today Proc. 2021, 48, 1626–1632. [Google Scholar] [CrossRef]
- Marimón-Bolívar, W.; González, E.E. Green Synthesis with Enhanced Magnetization and Life Cycle Assessment of Fe3O4 Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 9, 58–66. [Google Scholar] [CrossRef]
- Fuentes, O.P.; Noguera, M.J.; Peñaranda, P.A.; Flores, S.L.; Cruz, J.C.; Osma, J.F. Micromixers for Wastewater Treatment and Their Life Cycle Assessment (LCA). In Advances in Microfluidics and Nanofluids; Sohel Murshed, S.M., Ed.; IntechOpen: London, UK, 2021; pp. 1–15. [Google Scholar] [CrossRef]
- Jin, J.; Yurkow, E.J.; Adler, D.; Lee, T.C. Improved Freeze Drying Efficiency by Ice Nucleation Proteins with Ice Morphology Modification. Food Res. Int. 2018, 106, 90–97. [Google Scholar] [CrossRef] [PubMed]




| Stage | Process | Inventory | Amount | Unit |
|---|---|---|---|---|
| Manufacturing | Raw materials | Magnetite nanoparticles (Fe3O4) | 0.1 | g |
| Silica nanoparticles | 0.1 | g | ||
| Energy (precision scale) | 0.0033 | kWh | ||
| Water consumption | 0.005 | L | ||
| Re-suspension | Tetramethylammonium hydroxide (TMAH) | 0.02 | L | |
| Energy (ultrasonic bath) | 0.08 | kWh | ||
| Water consumption | 0.1 | L | ||
| Silanization | (3-Aminopropyl) triethoxysilane (APTES) | 0.001 | L | |
| Energy (ultrasonic bath) | 0.04 | kWh | ||
| Water consumption | 0.1 | L | ||
| Crosslinker | Glutaraldehyde (GLU) | 0.002 | L | |
| Energy (vortex) | 0.0006 | kWh | ||
| Water consumption | 0.1 | L | ||
| Energy (refrigerator) | 0.144 | kWh | ||
| Second layer | INA protein (Snowmax) | 0.01 | g | |
| Energy (vortex) | 0.0006 | kWh | ||
| Water consumption | 0.001 | L | ||
| Energy (refrigerator) | 3.456 | kWh | ||
| Washing and re-dispersion | Energy (ultrasonic bath) | 0.3 | kWh | |
| Energy (centrifuge) | 3.5 | kWh | ||
| Energy (vortex) | 0.044 | kWh | ||
| Water consumption | 0.15 | L | ||
| Operation | Operation | Magnetite + INA bionanocompound | 40 | mL |
| Energy (refrigerator) | 3.943 | kWh | ||
| Silica + INA bionanocompund | 40 | mL | ||
| Energy (refrigerator) | 7.487 | kWh | ||
| Water consumption | 40 | mL | ||
| Energy (freezer) | 0.267 | kWh |
| Impact Categories | Unit | Magnetite + INA | Silica + INA | Water |
|---|---|---|---|---|
| Human toxicity, non-carcinogenic effects | CTU | 5.86 × 10−5 | 1.04 × 10−4 | 1.12 × 10−4 |
| Human toxicity, carcinogenic effects | CTU | 7.79 × 10−9 | 1.38 × 10−8 | 1.49 × 10−8 |
| Ecotoxicity of freshwater | CTU | 0.55 | 0.98 | 1.06 |
| Climate change | kg CO2-Eq | 1.45 | 2.57 | 2.77 |
| Resource depletion, minerals and metals | kg Sb-Eq | 1.86 × 10−6 | 3.35 × 10−6 | 3.55 × 10−6 |
| Resource depletion, dissipated water | m3 water-Eq | 0.70 | 1.23 | 1.33 |
| Photochemical ozone formation | kg NMVOC-Eq | 4.34 × 10−3 | 7.69 × 10−3 | 8.29 × 10−3 |
| Freshwater and terrestrial acidification | mol H+-Eq | 1.13 × 10−2 | 2.00 × 10−2 | 2.15 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, O.P.; Osma, J.F. Life Cycle Assessment of Functionalized Bionanocompounds with Ice Nucleation Protein for Freezing Applications. Polymers 2023, 15, 1457. https://doi.org/10.3390/polym15061457
Fuentes OP, Osma JF. Life Cycle Assessment of Functionalized Bionanocompounds with Ice Nucleation Protein for Freezing Applications. Polymers. 2023; 15(6):1457. https://doi.org/10.3390/polym15061457
Chicago/Turabian StyleFuentes, Olga P., and Johann F. Osma. 2023. "Life Cycle Assessment of Functionalized Bionanocompounds with Ice Nucleation Protein for Freezing Applications" Polymers 15, no. 6: 1457. https://doi.org/10.3390/polym15061457
APA StyleFuentes, O. P., & Osma, J. F. (2023). Life Cycle Assessment of Functionalized Bionanocompounds with Ice Nucleation Protein for Freezing Applications. Polymers, 15(6), 1457. https://doi.org/10.3390/polym15061457