Electrochemically Stable Rechargeable Lithium–Sulfur Batteries Equipped with an Electrospun Polyacrylonitrile Nanofiber Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning of Polyacrylonitrile Nanofiber Film
2.2. Material Characterization of Electrospun Polyacrylonitrile Nanofiber Film
2.3. Electrochemical Performance of Electrospun Polyacrylonitrile Nanofiber Film
3. Results and Discussion
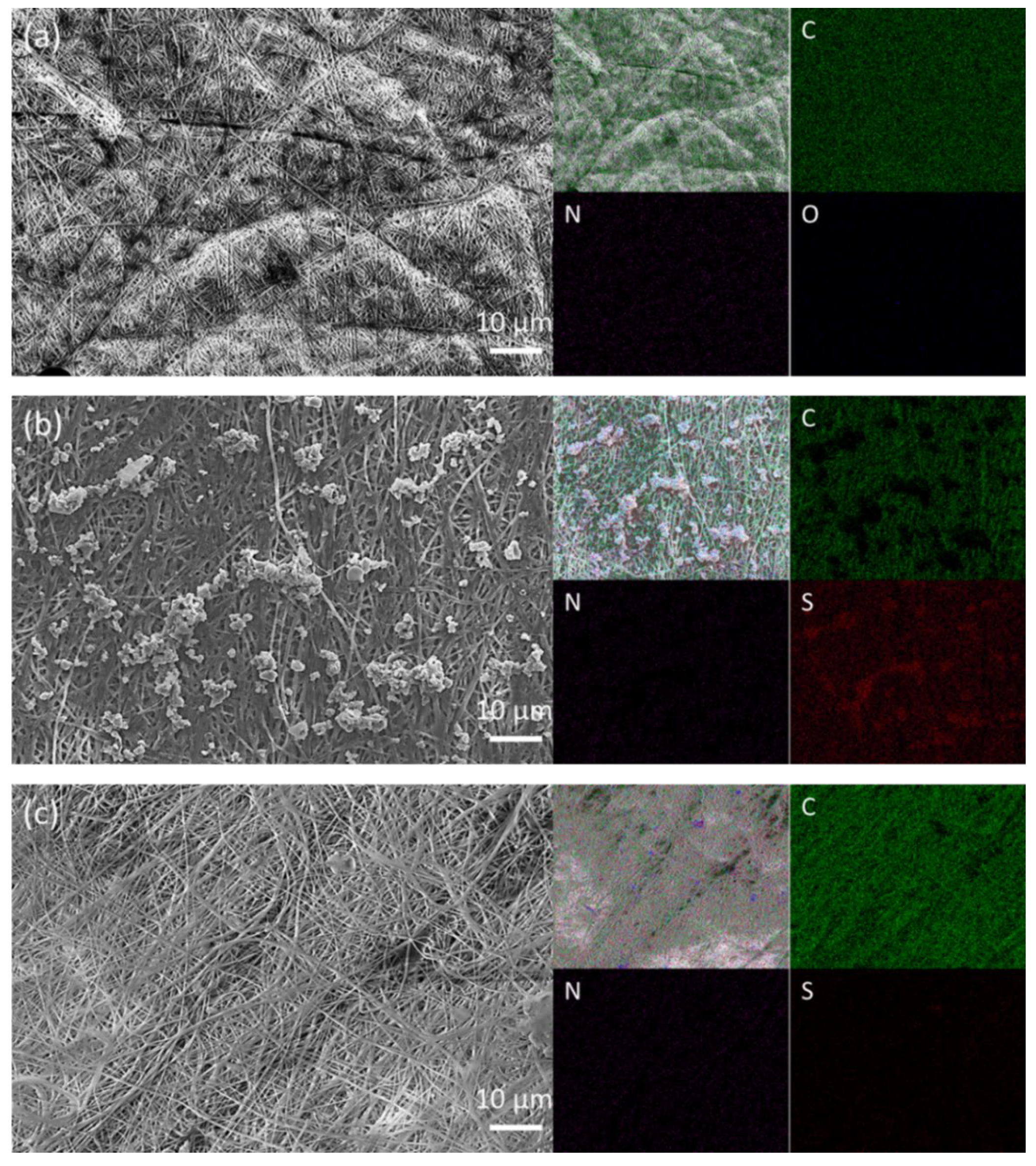
3.1. Material Characterization of Electrospun Polyacrylonitrile Nanofiber Film
3.2. Electrochemical Performance of Electrospun Polyacrylonitrile Nanofiber Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Maier, J.; Yu, Y. Guidelines and Trends for Next-Generation Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.; Román-Ramírez, L.A. Design of Experiments Applied to Lithium-ion Batteries: A Literature Review. Appl. Energy 2022, 320, 119305. [Google Scholar]
- Xiang, J.; Wei, Y.; Zhong, Y.; Yang, Y.; Cheng, H.; Yuan, L.; Xu, H.; Huang, Y. Building Practical High-Voltage Cathode Materials for Lithium-ion Batteries. Adv. Mater. 2022, 34, 2200912. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Fan, Y.; Wang, F.; Lei, T.; Chen, W.; Hu, A.; Xue, L.; Huang, J.; Wu, C.; et al. Mapping Yechniques for the Design of Lithium-Sulfur Batteries. Small 2022, 18, 2106657. [Google Scholar] [CrossRef]
- Feng, S.; Fu, Z.-H.; Chen, X.; Zhang, Q. A Review of the Theoretical Models for Lithium-Sulfur Battery Cathodes. InfoMat 2022, 4, e12304. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A.; Li, S. Encyclopedia of Electrochemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020. [Google Scholar]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium Sulfur Batteries, a Mechanistic Review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.-Q.; Zhang, X.-Q.; Huang, J.-Q.; Zhang, Q. A Perspective toward Practical Lithium–Sulfur Batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Elias, Y.; Meng, J.; Aurbach, D.; Zou, R.; Xia, D.; Pang, Q. Electrolyte Solutions Design for Lithium-Sulfur Batteries. Joule 2021, 5, 2323–2364. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Adair, K.; Zhang, H.; Sun, X. Structural Design of Lithium–Sulfur Batteries: From Fundamental Research to Practical Application. Electrochem. Energ. Rev. 2018, 1, 239–293. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.-W.; Cheng, H.-M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions and Prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium–Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, 2003666. [Google Scholar] [CrossRef]
- Li, S.; Fan, Z. Encapsulation Methods of Sulfur Particles for Lithium-Sulfur Batteries: A Review. Energy Storage Mater. 2021, 34, 107–127. [Google Scholar] [CrossRef]
- Luo, J.; Guan, K.; Lei, W.; Zhang, S.; Jia, Q.; Zhang, H. One Dimensional Carbon-based Composites as Cathodes for Lithium-Sulfur Battery. J. Mater. Sci. Technol. 2022, 122, 101–120. [Google Scholar] [CrossRef]
- Tong, Z.; Huang, L.; Lei, W.; Zhang, H.; Zhang, S. Carbon-containing Electrospun Nanofibers for Lithium–Sulfur Battery: Current Status and Future Directions. J. Energy Chem. 2021, 54, 254–273. [Google Scholar] [CrossRef]
- Quay, Y.-J.; Chung, S.-H. Structural and Surfacial Modification of Carbon Nanofoam as an Interlayer for Electrochemically Stable Lithium-Sulfur Cells. Nanomaterials 2021, 11, 3342. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal–Organic Frameworks for Lithium–Sulfur Batteries. J. Mater. Chem. A 2019, 7, 3469–3491. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Chung, S.-H. Nickel-plated Sulfur Nanocomposites for Electrochemically Stable High-Loading Sulfur Cathodes in a Lean-electrolyte Lithium-Sulfur Cell. Chem. Eng. J. 2022, 429, 132257. [Google Scholar] [CrossRef]
- Zheng, Z.-J.; Ye, H.; Guo, Z.-P. Recent Progress on Pristine Metal/Covalent-Organic Frameworks and their Composites for Lithium–Sulfur Batteries. Energy Environ. Sci. 2021, 14, 1835–1853. [Google Scholar] [CrossRef]
- Wang, P.; Xi, B.; Huang, M.; Chen, W.; Feng, J.; Xiong, S. Emerging Catalysts to Promote Kinetics of Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2002893. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Lim, Y.V.; Wang, Y.; Li, Y.; Zhang, D.; Yang, H.Y. Recent Advances in Heterostructure Engineering for Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2003689. [Google Scholar] [CrossRef]
- Chung, S.-H.; Wu, Y.-H.; Tseng, Y.-H.; Nguyen, T.X.; Ting, J.-M. The High Entropy Oxide (CrMnFeNiMg) 3O4 with Large Compositional Space Shows Long-Term Stability as Cathode in Lithium-Sulfur Batteries. ChemSusChem 2023, e202300135. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Deng, N.; Zhao, H.; Wang, X.; Wei, L.; Wang, M.; Cheng, B.; Kang, W. A Review on Electronically Conducting Polymers for Lithium-Sulfur Battery and Lithium-Selenium Battery: Progress and Prospects. J. Energy Chem. 2021, 58, 523–556. [Google Scholar] [CrossRef]
- Zeng, S.; Li, L.; Xie, L.; Zhao, D.; Wang, N.; Chen, S. Conducting Polymers Crosslinked with Sulfur as Cathode Materials for High-Rate, Ultralong-Life Lithium–Sulfur Batteries. ChemSusChem 2017, 10, 3378–3386. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Q.; Hao, S.-M.; Deng, S.; He, Q.; Lin, Z.; Yang, Y. Polymers in Lithium–Sulfur Batteries. Adv. Sci. 2022, 9, 2103798. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Yang, X.; Zeng, P.; Mao, J.; Dai, K.; Zhang, L.; Sun, X. Recent Progress of Functional Separators with Catalytic Effects for High-performance Lithium-Sulfur Batteries. Nano Energy 2021, 84, 105928. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhang, Y.; Li, M.; Chen, Z.; Lu, J. Revisiting the Role of Polysulfides in Lithium–Sulfur Batteries. Adv. Mater. 2018, 30, 1705590. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Silva, S.R.P.; Ding, B.; Zhang, P.; Shao, G. Lithium–Sulfur Batteries Meet Electrospinning: Recent Advances and the Key Parameters for High Gravimetric and Volume Energy Density. Adv. Sci. 2021, 9, 2103879. [Google Scholar] [CrossRef]
- Liu, M.; Deng, N.; Ju, J.; Fan, L.; Wang, L.; Li, Z.; Zhao, H.; Yang, G.; Kang, W.; Yan, J.; et al. A Review: Electrospun Nanofiber Materials for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1905467. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Wang, Y.; Shen, X.; Wang, F.; Li, H.; Hwang, B.-J.; Zhao, J. Directly Coating a Multifunctional Interlayer on the Cathode via Electrospinning for Advanced Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 29804–29811. [Google Scholar] [CrossRef]
- Shanthi, P.M.; Hanumantha, P.J.; Albuquerque, T.; Gattu, B.; Kumta, P.N. Novel Composite Polymer Electrolytes of PVdF-HFP Derived by Electrospinning with Enhanced Li-Ion Conductivities for Rechargeable Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2018, 1, 483–494. [Google Scholar] [CrossRef]
- Raulo, A.; Bandyopadhyay, S.; Ahamad, S.; Gupta, A.; Srivastava, R.; Formanek, P.; Nandan, B. Bio-inspired Poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate)-Sulfur@polyacrylonitrile Electrospun Nanofibers for Lithium-Sulfur Batteries. J. Power Sources 2019, 431, 250–258. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Chung, S.-H. A Design of the Cathode Substrate for High-loading Polysulfide Cathodes in Lean-electrolyte Lithium-Sulfur Cells. Chem. Eng. J. 2021, 422, 130363. [Google Scholar] [CrossRef]
- Kiai, M.S.; Eroglu, O.; Kizil, H. Electrospun Nanofiber Polyacrylonitrile Coated Separators to Suppress the Shuttle Effect for Long-life Lithium–Sulfur Battery. J. Appl. Polym. Sci. 2019, 137, 48606. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Zhu, P.; Yan, C.; Jia, H.; Kiyak, Y.; Zang, J.; He, J.; Dirican, M.; Zhang, X. Glass Fiber Separator Coated by Porous Carbon Nanofiber Derived from Immiscible PAN/PMMA for High-performance Lithium-Sulfur Batteries. J. Membr. Sci. 2018, 552, 31–42. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, H.R.; Ren, Y.X.; Zhou, D.; Kang, F.Y.; Zhao, T.S. In-situ Fabrication of a Freestanding Acrylate-based Hierarchical Electrolyte for Lithium-Sulfur Batteries. Electrochim. Acta 2016, 213, 871–878. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Hengjing, Z.; Xia, X.; Tu, J. 3D Ultraviolet Polymerized Electrolyte based on PEO Modified PVDF-HFP Electrospun Membrane for High-performance Lithium-Sulfur Batteries. Electrochim. Acta 2020, 329, 135108. [Google Scholar] [CrossRef]
- Lin, Y.; Pitcheri, R.; Zhu, J.; Jiao, C.; Guo, Y.; Li, J.; Qiu, Y. Electrospun PVDF/PSSLi Ionomer Films as a Functional Separator for Lithium-Sulfur Batteries. J. Alloy. Compd. 2019, 785, 627–633. [Google Scholar] [CrossRef]
- Rana, M.; Ahad, S.A.; Li, M.; Luo, B.; Wang, L.; Gentle, I.; Knibbe, R. Review on Areal Capacities and Long-term Cycling Performances of Lithium Sulfur Battery at High Sulfur Loading. Energy Storage Mater. 2019, 18, 289–310. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Peng, H.-J.; Zhao, M.; Huang, J.-Q. Heterogeneous/Homogeneous Mediators for High-Energy-Density Lithium–Sulfur Batteries: Progress and Prospects. Adv. Funct. Mater. 2018, 28, 1707536. [Google Scholar] [CrossRef]
- Groves, P. Diffusion Ordered Spectroscopy (DOSY) as Applied to Polymers. Polym. Chem. 2017, 8, 6700–6708. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, J.; Zhu, X.; Chen, K.; Ouyang, Y.; Wu, Y.; Miao, Y.-E.; Liu, T. A dual-functional Poly(vinyl alcohol)/poly(lithium acrylate) Composite Nanofiber Separator for Ionic Shielding of Polysulfides Enables High-rate and Ultra-stable Li-S Batteries. Nano Res. 2021, 14, 1541–1550. [Google Scholar] [CrossRef]
- Chiu, L.-L.; Chung, S.-H. Composite gel-polymer electrolyte for high-loading polysulfide cathodes. J. Mater. Chem. A 2022, 10, 13719–13726. [Google Scholar] [CrossRef]
- Judez, X.; Zhang, H.; Li, C.; Eshetu, G.G.; González-Marcos, J.A.; Armand, M.; Rodriguez-Martinez, L.M. Review—Solid Electrolytes for Safe and High Energy Density Lithium-Sulfur Batteries: Promises and Challenges. J. Electrochem. Soc. 2018, 165, A6008. [Google Scholar] [CrossRef]
- Carbone, L.; Coneglian, T.; Gobet, M.; Munoz, S.; Devany, M.; Greenbaum, S.; Hassoun, J. A Simple Approach for Making a Viable, Safe, and High-performances Lithium-Sulfur Battery. J. Power Sources 2018, 377, 26–35. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical Impedance Spectroscopy Study of a Lithium/Sulfur Battery: Modeling and Analysis of Capacity Fading. J. Electrochem. Soc. 2013, 160, A553. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Hung, C.-C.; Chung, S.-H. High-loading Polysulfide/Cement Cathode for the Manufacture of Dynamically and Statically Stable Lean-electrolyte Lithium–Sulfur Batteries. Ceram. Int. 2023, 49, 11846–11853. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, S.; Fu, Y. Recent Advances of Functional Electrolyte Additives for Lithium-Sulfur Batteries. J. Electrochem. 2022, 2217005. [Google Scholar] [CrossRef]
- Zhu, X.; Ouyang, Y.; Chen, J.; Zhu, X.; Luo, X.; Lai, F.; Zhang, H.; Miao, Y.-E.; Liu, T. In Situ Extracted Poly(acrylic acid) Contributing to Electrospun Nanofiber Separators with Precisely Tuned Pore Structures for Ultra-stable Lithium–Sulfur Batteries. J. Mater. Chem. A 2019, 7, 3253–3263. [Google Scholar] [CrossRef]
- Zhou, C.; He, Q.; Li, Z.; Meng, J.; Hong, X.; Li, Y.; Zhao, Y.; Xu, X.; Mai, L. A Robust Electrospun Separator Modified with In Situ Grown Metal-organic Frameworks for Lithium-Sulfur Batteries. Chem. Eng. J. 2020, 395, 124979. [Google Scholar] [CrossRef]
- Hu, M.; Ma, Q.; Yuan, Y.; Pan, Y.; Chen, M.; Zhang, Y.; Long, D. Grafting Polyethyleneimine on Electrospun Nanofiber Separator to Stabilize Lithium Metal Anode for Lithium Sulfur Batteries. Chem. Eng. J. 2020, 388, 124258. [Google Scholar] [CrossRef]
- Deng, N.; Wang, L.; Feng, Y.; Liu, M.; Li, Q.; Wang, G.; Zhang, L.; Kang, W.; Cheng, B.; Liu, Y. Co-based and Cu-based MOFs Modified Separators to Strengthen the Kinetics of Redox Reaction and inhibit Lithium-dendrite for Long-life Lithium-Sulfur Batteries. Chem. Eng. J. 2020, 388, 124241. [Google Scholar] [CrossRef]
- Zheng, S.; Zhu, X.; Ouyang, Y.; Chen, K.; Chen, A.-L.; Fan, X.; Miao, Y.-E.; Liu, T.; Xie, Y. Metal–Organic Framework Decorated Polymer Nanofiber Composite Separator for Physiochemically Shielding Polysulfides in Stable Lithium–Sulfur Batteries. Energy Fuels 2021, 35, 19154–19163. [Google Scholar] [CrossRef]
- Lan, F.; Zhang, H.; Fan, J.; Xu, Q.; Li, H.; Min, Y. Electrospun Polymer Nanofibers with TiO2@NiCo-LDH as Efficient Polysulfide Barriers for Wide-Temperature-Range Li–S Batteries. ACS Appl. Mater. Interfaces 2021, 13, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, G.; Kang, W.; Deng, N.; Cheng, B. Taming Polysulfides and Facilitating Lithium-ion Migration: Novel Electrospinning MOFs@PVDF-based Composite Separator with Spiderweb-like Structure for Li-S Batteries. Electrochim. Acta 2021, 365, 137344. [Google Scholar] [CrossRef]
- Liang, X.; Wang, L.; Wang, Y.; Liu, Y.; Sun, Y.; Xiang, H. Constructing Multi-functional Composite Separator of PVDF-HFP/h-BN Supported Co-CNF Membrane for Lithium–Sulfur Batteries. Sustain. Energy Fuels 2022, 6, 440–448. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, L.-L.; Chung, S.-H. Electrochemically Stable Rechargeable Lithium–Sulfur Batteries Equipped with an Electrospun Polyacrylonitrile Nanofiber Film. Polymers 2023, 15, 1460. https://doi.org/10.3390/polym15061460
Chiu L-L, Chung S-H. Electrochemically Stable Rechargeable Lithium–Sulfur Batteries Equipped with an Electrospun Polyacrylonitrile Nanofiber Film. Polymers. 2023; 15(6):1460. https://doi.org/10.3390/polym15061460
Chicago/Turabian StyleChiu, Li-Ling, and Sheng-Heng Chung. 2023. "Electrochemically Stable Rechargeable Lithium–Sulfur Batteries Equipped with an Electrospun Polyacrylonitrile Nanofiber Film" Polymers 15, no. 6: 1460. https://doi.org/10.3390/polym15061460






