Fabrication of PbO2/PVDF/CC Composite and Employment for the Removal of Methyl Orange
Abstract
:1. Introduction
2. Experimental Sections
2.1. Materials
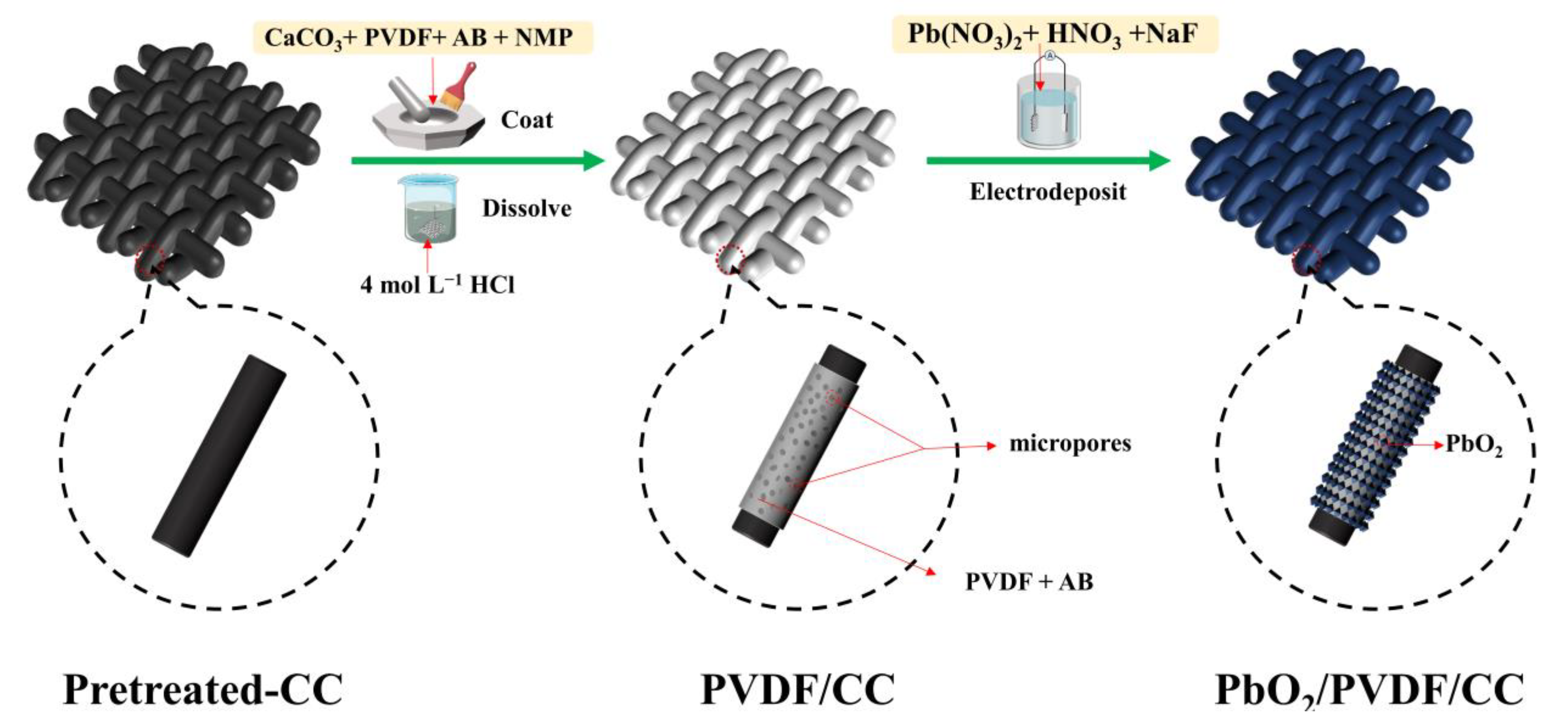
2.2. Fabrication of PbO2/PVDF/CC Composite
2.2.1. Preparation of PVDF/CC Substrate
2.2.2. Preparation of PbO2/PVDF/CC Composite
2.3. Characterization
2.3.1. Conventional Characterization
2.3.2. Electrochemical Characterizations
2.4. Electrochemical Oxidation Process
3. Results and Discussion
3.1. Characterizations of PbO2/PVDF/CC Composite
3.2. Electrochemical Characteristics of Fabricated Composites
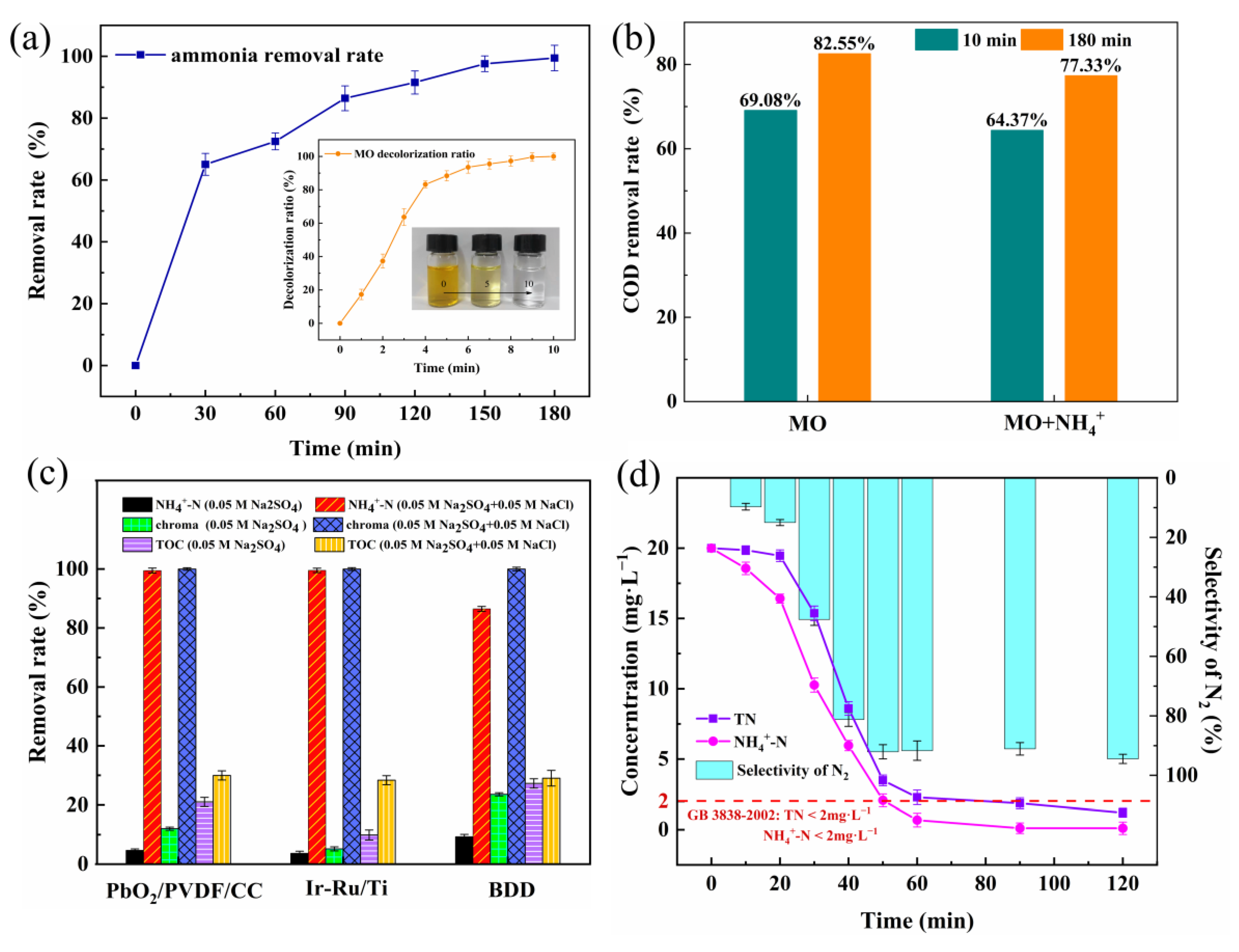
3.3. Electrochemical Oxidation Decolorization of MO
3.3.1. Effect of Conventional Parameters on Decolorization of MO
3.3.2. Free Radical Scavenging Test
3.4. Electrochemical Degradation of MO with the Presence of Ammonium
3.5. Electrochemical Conversion Pathways of MO and Ammonium
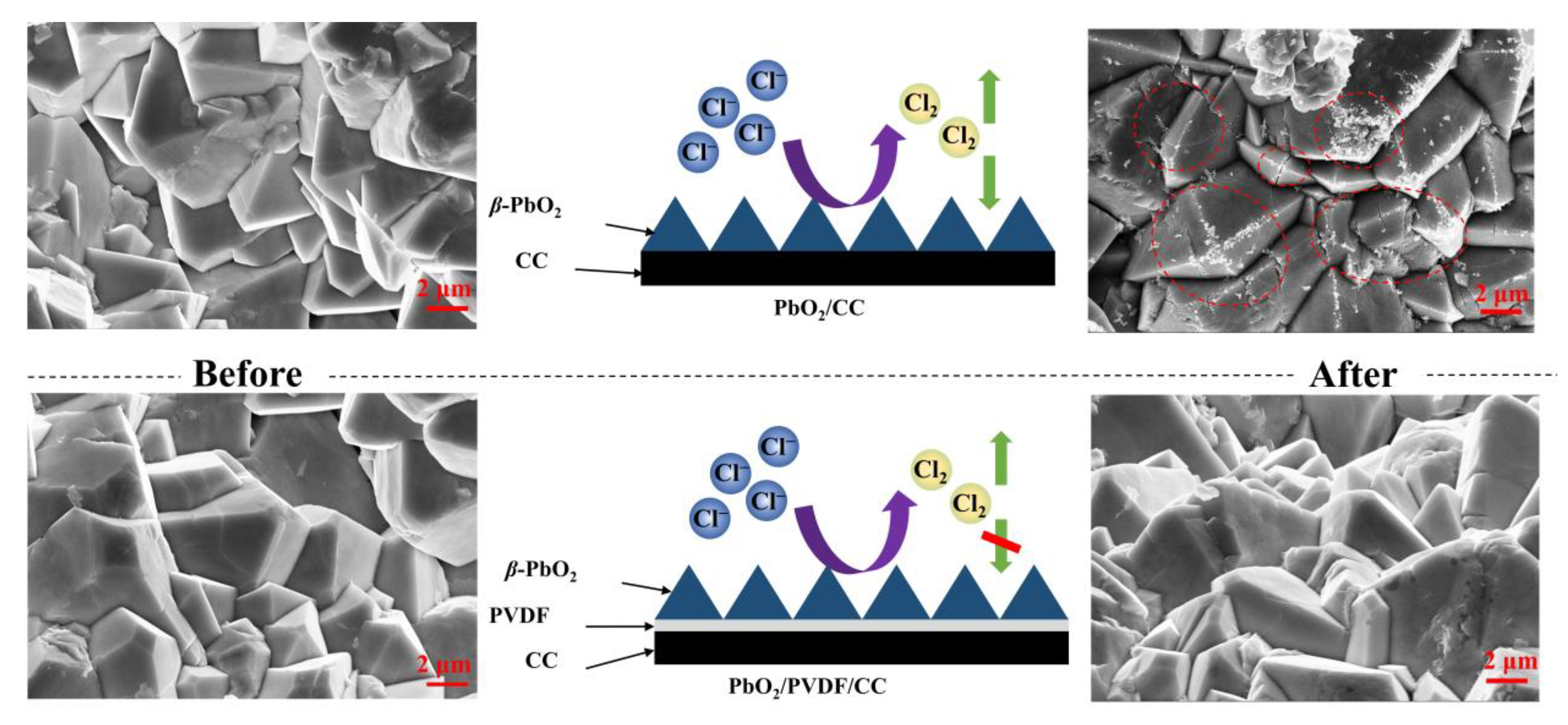
3.6. Stability of PbO2/PVDF/CC Composite
4. Conclusions
- (1)
- The fabricated PbO2/PVDF/CC composite shows a low chlorine evolution potential (1.1 V vs. Ag/AgCl) and a high oxygen evolution potential (1.25 V vs. Ag/AgCl), which is helpful to facilitate the process of chlorine electrochemical oxidation.
- (2)
- The PbO2/PVDF/CC composite shows a strong decolorization efficiency of methyl orange (~100%), excellent removal of ammonium (>99%), and minimal concentration of lead leaching (0.054 mg L−1), suggesting its predicted engineering application.
- (3)
- In addition, the fabricated PbO2/PVDF/CC composite exhibits desirable stability and environmental safety. After five runs, based on the potential application prospect, the decolorization efficiency of methyl orange, the removal rate of ammonium, and the leached concentration of Pb remain at acceptable levels. The PbO2/PVDF/CC composite deserves to be exploited for wastewater treatment in the textile dyeing and finishing industry.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sutar, S.; Patil, P.; Jadhav, J. Recent advances in biochar technology for textile dyes wastewater remediation: A review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef] [PubMed]
- Ambigadevi, J.; Kumar, P.S.; Vo, D.-V.N.; Haran, S.H.; Raghavan, T.S. Recent developments in photocatalytic remediation of textile effluent using semiconductor based nanostructured catalyst: A review. J. Environ. Chem. Eng. 2021, 9, 104881. [Google Scholar] [CrossRef]
- Singh, A.; Dahiya, S.; Mishra, B.K. Microbial fuel cell coupled hybrid systems for the treatment of dye wastewater: A review on synergistic mechanism and performance. J. Environ. Chem. Eng. 2021, 9, 106765. [Google Scholar] [CrossRef]
- Cifcioglu-Gozuacik, B.; Ergenekon, S.M.; Ozbey-Unal, B.; Balcik, C.; Karagunduz, A.; Dizge, N.; Keskinler, B. Efficient removal of ammoniacal nitrogen from textile printing wastewater by electro-oxidation considering the effects of NaCl and NaOCl addition. Water Sci. Technol. 2021, 84, 752–762. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Chen, H.; Yu, X.; Wang, X.; He, Y.; Zhang, C.; Xue, G.; Liu, Z.; Lao, H.; Song, H.; Chen, W.; et al. Dyeing and finishing wastewater treatment in China: State of the art and perspective. J. Clean. Prod. 2021, 326, 129353. [Google Scholar] [CrossRef]
- Yu, S.; Yu, G.B.; Liu, Y.; Li, G.L.; Feng, S.; Wu, S.C.; Wong, M.H. Urbanization Impairs Surface Water Quality: Eutrophication and Metal Stress in the Grand Canal of China. River Res. Appl. 2012, 28, 1135–1148. [Google Scholar] [CrossRef]
- Zayed, M.A.; Abo-Ayad, Z.A.; Medany, S.S. Catalytic Efficient Electro-oxidation Degradation of DO26 Textile Dye via UV/VIS, COD, and GC/MS Evaluation of By-products. Electrocatalysis 2021, 12, 731–746. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process. Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Swathi, D.; Sabumon, P.C.; Trivedi, A. Simultaneous decolorization and mineralization of high concentrations of methyl orange in an anoxic up-flow packed bed reactor in denitrifying conditions. J. Water Process. Eng. 2021, 40, 101813. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Carolin, F.C.; Jayaraj, T.; Gokulakrishnan, M.; Keerthana, P. Transformation of aqueous methyl orange to green metabolites using bacterial strains isolated from textile industry effluent. Environ. Technol. Innov. 2022, 25, 102126. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Joshiba, G.J. Sustainable approach to decolourize methyl orange dye from aqueous solution using novel bacterial strain and its metabolites characterization. Clean Technol. Environ. Policy 2020, 23, 173–181. [Google Scholar] [CrossRef]
- Li, A.; Weng, J.; Yan, X.; Li, H.; Shi, H.; Wu, X. Electrochemical oxidation of acid orange 74 using Ru, IrO2, PbO2, and boron doped diamond anodes: Direct and indirect oxidation. J. Electroanal. Chem. 2021, 898, 115622. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process. Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Mandal, P.; Yadav, M.K.; Gupta, A.K.; Dubey, B.K. Chlorine mediated indirect electro-oxidation of ammonia using non-active PbO2 anode: Influencing parameters and mechanism identification. Sep. Purif. Technol. 2020, 247, 116910. [Google Scholar] [CrossRef]
- Aguilar, Z.G.; Brillas, E.; Salazar, M.; Nava, J.L.; Sirés, I. Evidence of Fenton-like reaction with active chlorine during the electrocatalytic oxidation of Acid Yellow 36 azo dye with Ir-Sn-Sb oxide anode in the presence of iron ion. Appl. Catal. B Environ. 2017, 206, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Nasseri, S. Optimization of free chlorine, electric and current efficiency in an electrochemical reactor for water disinfection purposes by RSM. J. Environ. Health Sci. Eng. 2020, 18, 1343–1350. [Google Scholar] [CrossRef]
- Yao, Y.; Teng, G.; Yang, Y.; Huang, C.; Liu, B.; Guo, L. Electrochemical oxidation of acetamiprid using Yb-doped PbO2 electrodes: Electrode characterization, influencing factors and degradation pathways. Sep. Purif. Technol. 2019, 211, 456–466. [Google Scholar] [CrossRef]
- Rodríguez-Narváez, O.M.; Picos, A.R.; Bravo-Yumi, N.; Pacheco-Alvarez, M.; Martínez-Huitle, C.A.; Peralta-Hernández, J.M. Electrochemical oxidation technology to treat textile wastewaters. Curr. Opin. Electrochem. 2021, 29, 100806. [Google Scholar] [CrossRef]
- Rodrigues, A.; Nunes, M.; Lopes, A.; Silva, J.; Ciríaco, L.; Pacheco, M. Electrodegradation of naphthalenic amines: Influence of the relative position of the substituent groups, anode material and electrolyte on the degradation products and kinetics. Chemosphere 2018, 205, 433–442. [Google Scholar] [CrossRef]
- Phetrak, A.; Westerhoff, P.; Garcia-Segura, S. Low energy electrochemical oxidation efficiently oxidizes a common textile dye used in Thailand. J. Electroanal. Chem. 2020, 871, 114301. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S.; Hamid, S.B.A. Recent Advances in Heterogeneous Photocatalytic Decolorization of Synthetic Dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Joorabi, F.T.; Kamali, M.; Sheibani, S. Effect of aqueous inorganic anions on the photocatalytic activity of CuO–Cu2O nanocomposite on MB and MO dyes degradation. Mater. Sci. Semicond. Process. 2022, 139, 106335. [Google Scholar] [CrossRef]
- Zhang, G.; Ruan, J.; Du, T. Recent Advances on Photocatalytic and Electrochemical Oxidation for Ammonia Treatment from Water/Wastewater. ACS Es&T Eng. 2020, 1, 310–325. [Google Scholar] [CrossRef]
- He, Y.; Zhao, D.; Lin, H.; Huang, H.; Li, H.; Guo, Z. Design of diamond anodes in electrochemical degradation of organic pollutants. Curr. Opin. Electrochem. 2022, 32, 100878. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Ye, J.; Lin, Z.; Yang, X. Electrochemical oxidation of methyl orange by a Magnéli phase Ti4O7 anode. Chemosphere 2020, 241, 125084. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Kim, Y.-J.; Kim, I.-T.; Park, G.-I.; Lee, E.-H. Electrochemical conversion characteristics of ammonia to nitrogen. Water Res. 2006, 40, 1431–1441. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Waite, T.D. Active chlorine mediated ammonia oxidation revisited: Reaction mechanism, kinetic modelling and implications. Water Res. 2018, 145, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-J.; Huang, Y.-H.; Huang, C. Oxidation of ammonia in dilute aqueous solutions over graphite-supported α- and β-lead dioxide electrodes (PbO2@G). Electrochim. Acta 2017, 257, 444–454. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Dai, Q. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: Performance, kinetics and degradation mechanism. Electrochim. Acta 2015, 165, 277–287. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Wang, X.; Wang, X.; Zhang, C.; Zhang, Y. Recent progress in engineering approach towards the design of PbO2-based electrodes for the anodic oxidation of organic pollutants. J. Water Process. Eng. 2021, 42, 102173. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Yan, W. Fabrication and characterization of PbO2 electrode modified with polyvinylidene fluoride (PVDF). Appl. Surf. Sci. 2016, 389, 278–286. [Google Scholar] [CrossRef]
- Tang, K.; Wang, Y.; Zhang, X.; Jamil, S.; Huang, Y.; Cao, S.; Xie, X.; Bai, Y.; Wang, X.; Luo, Z.; et al. High-performance P2-Type Fe/Mn-based oxide cathode materials for sodium-ion batteries. Electrochim. Acta 2019, 312, 45–53. [Google Scholar] [CrossRef]
- Dai, J.; Feng, H.; Shi, K.; Ma, X.; Yan, Y.; Ye, L.; Xia, Y. Electrochemical degradation of antibiotic enoxacin using a novel PbO2 electrode with a graphene nanoplatelets inter-layer: Characteristics, efficiency and mechanism. Chemosphere 2022, 307, 135833. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jiang, L.; Zhang, B.; Jiang, Q.; Su, D.S.; Sun, G. A highly active porous Pt–PbOx/C catalyst toward alcohol electro-oxidation in alkaline electrolyte. Int. J. Hydrogen Energy 2013, 38, 12767–12773. [Google Scholar] [CrossRef]
- Liu, X.; Min, L.; Yu, X.; Zhou, Z.; Sha, L.; Zhang, S. Changes of photoelectrocatalytic, electrocatalytic and pollutant degradation properties during the growth of β-PbO2 into black titanium oxide nanoarrays. Chem. Eng. J. 2021, 417, 127996. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Y.; Wan, J.; Zhu, W.; Jiang, Z. Effect of CeO2 addition on the structure and activity of RuO2/γ-Al2O3 catalyst. Appl. Surf. Sci. 2005, 246, 222–228. [Google Scholar] [CrossRef]
- Sathishkumar, K.; AlSalhi, M.S.; Sanganyado, E.; Devanesan, S.; Arulprakash, A.; Rajasekar, A. Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol. B Biol. 2019, 200, 111655. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Omri, A.; Hamza, W.; Benzina, M. Photo-Fenton oxidation and mineralization of methyl orange using Fe-sand as effective heterogeneous catalyst. J. Photochem. Photobiol. A Chem. 2020, 393, 112444. [Google Scholar] [CrossRef]
- Iovino, P.; Fenti, A.; Galoppo, S.; Najafinejad, M.S.; Chianese, S.; Musmarra, D. Electrochemical Removal of Nitrogen Compounds from a Simulated Saline Wastewater. Molecules 2023, 28, 1306. [Google Scholar] [CrossRef]
- Yang, S.; Jin, R.; He, Z.; Qiao, Y.; Shi, S.; Kong, W.; Wang, Y.; Liu, X. An experimental study on the degradation of methyl orange by combining hydrodynamic cavitation and chlorine dioxide treatments. Chem. Eng. Trans. 2017, 59, 289–294. [Google Scholar] [CrossRef]
- Belal, R.M.; Zayed, M.A.; El-Sherif, R.M.; Ghany, N.A.A. Advanced electrochemical degradation of basic yellow 28 textile dye using IrO2/Ti meshed electrode in different supporting electrolytes. J. Electroanal. Chem. 2021, 882, 114979. [Google Scholar] [CrossRef]
- Bravo-Yumi, N.; Espinoza-Montero, P.; Picos-Benítez, A.; Navarro-Mendoza, R.; Brillas, E.; Peralta-Hernández, J.M. Synthesis and characterization of Sb2O5-doped Ti/SnO2-IrO2 anodes toward efficient degradation tannery dyes by in situ generated oxidizing species. Electrochim. Acta 2020, 358, 136904. [Google Scholar] [CrossRef]
- Jiang, Y.-Y.; Chen, Z.-W.; Li, M.-M.; Xiang, Q.-H.; Wang, X.-X.; Miao, H.-F.; Ruan, W.-Q. Degradation of diclofenac sodium using Fenton-like technology based on nano-calcium peroxide. Sci. Total. Environ. 2021, 773, 144801. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Yuan, D.; Zhang, Y.; Qi, J.; Rao, Y.; Lu, G.; Li, B.; Wang, K.; Yin, K. Enhanced photocatalytic performance of BiVO4 for degradation of methylene blue under LED visible light irradiation assisted by peroxymonosulfate. Int. J. Electrochem. Sci. 2020, 15, 2470–2480. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, C.; Tang, S.; Wang, Z.; Sun, Q.; Zhang, X.; Jiao, T.; Zhang, Q. Ferric ion-ascorbic acid complex catalyzed calcium peroxide for organic wastewater treatment: Optimized by response surface method. Chin. Chem. Lett. 2021, 32, 3387–3392. [Google Scholar] [CrossRef]
- Lei, J.; Xu, Z.; Xu, H.; Qiao, D.; Liao, Z.; Yan, W.; Wang, Y. Pulsed electrochemical oxidation of acid Red G and crystal violet by PbO2 anode. J. Environ. Chem. Eng. 2020, 8, 103773. [Google Scholar] [CrossRef]
- Isarain-Chávez, E.; Baró, M.D.; Rossinyol, E.; Morales-Ortiz, U.; Sort, J.; Brillas, E.; Pellicer, E. Comparative electrochemical oxidation of methyl orange azo dye using Ti/Ir-Pb, Ti/Ir-Sn, Ti/Ru-Pb, Ti/Pt-Pd and Ti/RuO2 anodes. Electrochim. Acta 2017, 244, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, H.; Xue, G.; Liu, Y.; Chen, S.; Jia, C. A critical review of the aniline transformation fate in azo dye wastewater treatment. J. Clean. Prod. 2021, 321, 128971. [Google Scholar] [CrossRef]
- Doumbi, R.T.; Noumi, G.B. Dip coating deposition of manganese oxide nanoparticles on graphite by sol gel technique for the indirect electrochemical oxidation of methyl orange dye: Parameter’s optimization using box-behnken design. Case Stud. Chem. Environ. Eng. 2021, 3, 100068. [Google Scholar] [CrossRef]
- Valica, M.; Hostin, S. Electrochemical Treatment of Water Contaminated with Methylorange. Nova Biotechnol. Chim. 2016, 15, 55–64. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chen, Z.L.; Guo, P.F.; Fei, B.; Wu, R.B. B-doping-induced amorphization of LDH for large-current-density hydrogen evolution reaction. Appl. Catal. B Environ. 2020, 261, 118240. [Google Scholar] [CrossRef]
- Zuo, S.J.; Zhang, Y.Q.; Guo, R.X.; Chen, J.Q. Efficient removal of ammonia nitrogen by an electrochemical process for spent caustic wastewater treatment. Catalysts 2022, 12, 1357. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Liu, C.; Liang, L.; Ma, Y.; Wang, X.; Ma, J.; Li, Z.; Yang, S. Fabrication of PbO2/PVDF/CC Composite and Employment for the Removal of Methyl Orange. Polymers 2023, 15, 1462. https://doi.org/10.3390/polym15061462
Song L, Liu C, Liang L, Ma Y, Wang X, Ma J, Li Z, Yang S. Fabrication of PbO2/PVDF/CC Composite and Employment for the Removal of Methyl Orange. Polymers. 2023; 15(6):1462. https://doi.org/10.3390/polym15061462
Chicago/Turabian StyleSong, Laizhou, Cuicui Liu, Lifen Liang, Yalong Ma, Xiuli Wang, Jizhong Ma, Zeya Li, and Shuqin Yang. 2023. "Fabrication of PbO2/PVDF/CC Composite and Employment for the Removal of Methyl Orange" Polymers 15, no. 6: 1462. https://doi.org/10.3390/polym15061462
APA StyleSong, L., Liu, C., Liang, L., Ma, Y., Wang, X., Ma, J., Li, Z., & Yang, S. (2023). Fabrication of PbO2/PVDF/CC Composite and Employment for the Removal of Methyl Orange. Polymers, 15(6), 1462. https://doi.org/10.3390/polym15061462






