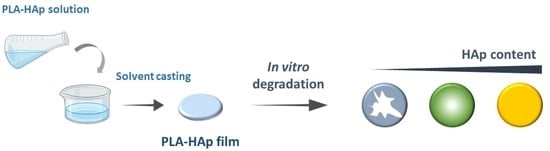
Medical-Grade Poly(Lactic Acid)/Hydroxyapatite Composite Films: Thermal and In Vitro Degradation Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hydroxyapatite
2.2. Preparation of Poly(Lactic Acid)/Hydroxyapatite Composite Films
2.3. In Vitro Degradation Experiment
2.4. Identification and Characterization of Poly(Lactic Acid)/Hydroxyapatite Composite Films
3. Results and Discussion
3.1. Weight Loss
3.2. XRD Analysis
3.3. Thermogravimetric Analysis
3.4. Glass Transition Temperatures and Sample Disintegration
3.5. Sample Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Tg (In)/°C | ||||||
|---|---|---|---|---|---|---|
| Degradation Week | PLA | PLA-10-HAp | PLA-20-HAp | |||
| 0 | 52.8 | ±0.1 | 52.9 | ±0.4 | 53.1 | ±0.4 |
| 2 | 31.3 | ±0.7 | 43.2 | ±3.3 | 51.4 | ±0.7 |
| 5 | 24.4 | ±0.2 | 25.6 | ±0.8 | 51.8 | ±0.3 |
| 8 | 25.8 | ±2.8 | 23.9 | ±1.7 | 52.2 | ±0.4 |
| 10 | - 1 | - | 31.8 | ±0.9 | 36.8 | ±0.5 |
| Tg(Out)/°C | ||||||
|---|---|---|---|---|---|---|
| Degradation Week | PLA | PLA-10-HAp | PLA-20-HAp | |||
| 0 | 52.4 | ±0.6 | 52.3 | ±0.1 | 51.5 | ±1.1 |
| 2 | 50.3 | ±2.3 | 50.8 | ±2.0 | 52.6 | ±0.4 |
| 5 | 45.9 | ±1.1 | 51.5 | ±1.3 | 50.6 | ±1.6 |
| 8 | 44.5 | ±2.5 | 49.8 | ±0.6 | 50.9 | ±0.4 |
| 10 | 43.1 | ±0.7 | 47.7 | ±2.2 | 51.6 | ±0.8 |
Appendix C
References
- Qu, M.; Wang, C.; Zhou, X.; Libanori, A.; Jiang, X.; Xu, W.; Zhu, S.; Chen, Q.; Sun, W.; Khademhosseini, A. Multi-dimensional printing for bone tissue engineering. Adv. Healthc. Mater. 2021, 10, 2001986. [Google Scholar] [CrossRef]
- Chacón, J.M.; Caminero, M.A.; García-Plaza, E.; Núñez, P.J. Additive manufacturing of PLA structures using fused deposition modelling: Effect of process parameters on mechanical properties and their optimal selection. Mater. Des. 2017, 124, 143–157. [Google Scholar] [CrossRef]
- Tang, T.O.; Simon, G.P. Biodegradation of 3D-printed polylactic acid milliprojections under physiological conditions. J. Appl. Polym. Sci. 2020, 137, 49129. [Google Scholar] [CrossRef]
- Distler, T.; Fournier, N.; Grünewald, A.; Polley, C.; Seitz, H.; Detsch, R.; Boccaccini, A.R. Polymer-bioactive glass composite filaments for 3D scaffold manufacturing by fused deposition modeling: Fabrication and characterization. Front. Bioeng. Biotechnol. 2020, 8, 552. [Google Scholar] [CrossRef]
- Alksne, M.; Kalvaityte, M.; Simoliunas, E.; Rinkunaite, I.; Gendviliene, I.; Locs, J.; Rutkunas, V.; Bukelskiene, V. In vitro comparison of 3D printed polylactic acid/hydroxyapatite and polylactic acid/bioglass composite scaffolds: Insights into materials for bone regeneration. J. Mech. Behav. Biomed. Mater. 2020, 104, 103641. [Google Scholar] [CrossRef]
- Amnael Orozco-Díaz, C.; Moorehead, R.; Reilly, G.C.; Gilchrist, F.; Miller, C. Characterization of a composite polylactic acid-hydroxyapatite 3D-printing filament for bone-regeneration. Biomed. Phys. Eng. Express 2020, 7, 25007. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Q.; Shui, H.; Wang, P.; Chen, Q.; Li, Z. Degradation of 3D-printed porous polylactic acid scaffolds under mechanical stimulus. Front. Bioeng. Biotechnol. 2021, 8, 889. [Google Scholar] [CrossRef]
- Deplaine, H.; Acosta-Santamaría, V.A.; Vidaurre, A.; Gómez Ribelles, J.L.; Doblaré, M.; Ochoa, I.; Gallego Ferrer, G. Evolution of the properties of a Poly(L-lactic acid) scaffold with double porosity during in vitro degradation in a phosphate-buffered saline solution. J. Appl. Polym. Sci. 2014, 131, 40956–40966. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Pérez, E. Mechanical performance of in vitro degraded polylactic acid/hydroxyapatite composites. J. Mater. Sci. 2021, 56, 19915–19935. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Berto, F.; Ayatollahi, M.R.; Reinicke, T. Characterization of 3D-printed PLA parts with different raster orientations and printing speeds. Sci. Rep. 2022, 12, 1016. [Google Scholar] [CrossRef]
- Rajakaruna, R.A.D.N.V.; Subeshan, B.; Asmatulu, E. Fabrication of hydrophobic PLA filaments for additive manufacturing. J. Mater. Sci. 2022, 57, 8987–9001. [Google Scholar] [CrossRef]
- Vacaras, S.; Baciut, M.; Lucaciu, O.; Dinu, C.; Baciut, G.; Crisan, L.; Hedesiu, M.; Crisan, B.; Onisor, F.; Armencea, G.; et al. Understanding the basis of medical use of Poly-lactide-based resorbable polymers and composites—A review of the clinical and metabolic impact. Drug Metab. Rev. 2019, 51, 570–588. [Google Scholar] [CrossRef]
- Ahlinder, A.; Fuoco, T.; Finne-Wistrand, A. Medical grade polylactide, copolyesters and polydioxanone: Rheological properties and melt stability. Polym. Test. 2018, 72, 214–222. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Ivankovic, H.; Gallego Ferrer, G.; Tkalcec, E.; Orlic, S.; Ivankovic, M. Preparation of highly porous hydroxyapatite from cuttlefish bone. J. Mater. Sci. Mater. Med. 2009, 20, 1039–1046. [Google Scholar] [CrossRef]
- Johnsson, M.S.A.; Nancollas, G.H. The role of brushite and octacalcium phosphate in apatite formation. Crit. Rev. Oral Biol. Med. 2016, 3, 61–82. [Google Scholar] [CrossRef]
- Tas, A.C.; Bhaduri, S.B. Chemical processing of CaHPO4 2H2O: Its conversion to hydroxyapatite. J. Am. Ceram. Soc. 2004, 87, 2195–2200. [Google Scholar] [CrossRef]
- Bauer, L.; Antunović, M.; Rogina, A.; Ivanković, M.; Ivanković, H. Bone-mimetic porous hydroxyapatite/whitlockite scaffolds: Preparation, characterization and interactions with human mesenchymal stem cells. J. Mater. Sci. 2021, 56, 3947–3969. [Google Scholar] [CrossRef]
- Carrasco, F.; Pérez, O.S.; Maspoch, M.L. Kinetics of the thermal degradation of Poly(lactic acid) and polyamide bioblends. Polymers 2021, 13, 3996. [Google Scholar] [CrossRef]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates—2. Polylactide: Degradation under isothermal conditions, thermal degradation mechanism and photolysis of the polymer. Polym. Degrad. Stab. 1985, 11, 309–326. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of Poly(L-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight Poly(L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomater. Silver Jubil. Compend. 1996, 17, 117–128. [Google Scholar] [CrossRef]
- Vasanthan, N.; Ly, O. Effect of microstructure on hydrolytic degradation studies of Poly(L-lactic acid) by FTIR spectroscopy and differential scanning calorimetry. Polym. Degrad. Stab. 2009, 94, 1364–1372. [Google Scholar] [CrossRef]
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar] [CrossRef]
- Blaker, J.J.; Nazhat, S.N.; Maquet, V.; Boccaccini, A.R. Long-term in vitro degradation of PDLLA/Bioglass® bone scaffolds in acellular simulated body fluid. Acta Biomater. 2011, 7, 829–840. [Google Scholar] [CrossRef]
- Kang, Y.; Yao, Y.; Yin, G.; Huang, Z.; Liao, X.; Xu, X.; Zhao, G. A study on the in vitro degradation properties of Poly(L-lactic acid)/β-tricalcuim phosphate(PLLA/β-TCP) scaffold under dynamic loading. Med. Eng. Phys. 2009, 31, 589–594. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, Y.; Tian, J. Study on the biodegradability of modified starch/polylactic acid (PLA) composite materials. RSC Adv. 2020, 10, 26298–26307. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2020, 6, 490–502. [Google Scholar] [CrossRef]
- Albano, C.; González, G.; Palacios, J.; Karam, A.; Castillo, R.V.; Covis, M. Characterization of poly L-Lactide/Hydroxyapatite composite: Chemical, thermal and thermomechanical properties. Rev. Fac. Ing. Univ. Cent. Venez. 2013, 28, 97–107. [Google Scholar]
- Tazibt, N.; Kaci, M.; Dehouche, N.; Ragoubi, M.; Atanase, L.I. Effect of filler content on the morphology and physical properties of Poly(Lactic Acid)-hydroxyapatite composites. Materials 2023, 16, 809. [Google Scholar] [CrossRef] [PubMed]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Remmler, M.; Mackenzie, K.; Möder, M.; Wachsen, O. Thermal decomposition of biodegradable polyesters—II. Poly(lactic acid). Polym. Degrad. Stab. 1996, 53, 329–342. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for Poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Schmitz, J.P.; Agrawal, C.M. The effects of porosity on in vitro degradation of Polylactic Acid–Polyglycolic acid implants used in repair of articular cartilage. Tissue Eng. 1998, 4, 53–63. [Google Scholar] [CrossRef]
- Pitt, G.G.; Gratzl, M.M.; Kimmel, G.L.; Surles, J.; Sohindler, A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (ε-caprolactone), and their copolymers in vivo. Biomaterials 1981, 2, 215–220. [Google Scholar] [CrossRef]
- Li, S.M.; Garreau, H.; Vert, M. Structure-property relationships in the case of the degradation of massive aliphatic poly-(α-hydroxy acids) in aqueous media—Part 1: Poly(dl-lactic acid). J. Mater. Sci. Mater. Med. 1990, 1, 123–130. [Google Scholar] [CrossRef]
- Szczepanska, P.; Szymanowski, H.; Suwalska, M.; Rosinska, K.; Skrodzki, M.; Uznanski, P.; Bociaga, D.; Bubko, I.; Drozd, E.; Gruber-Bzura, B.; et al. Influence of ceramic phosphate powders on the physicochemical and biological properties of Poly(L-lactide). Ceram. Int. 2023, 49, 7692–7709. [Google Scholar] [CrossRef]
- Behera, K.; Sivanjineyulu, V.; Chang, Y.H.; Chiu, F.C. Thermal properties, phase morphology and stability of biodegradable PLA/PBSL/HAp composites. Polym. Degrad. Stab. 2018, 154, 248–260. [Google Scholar] [CrossRef]
- Kaavessina, M.; Chafidz, A.; Ali, I.; Al-Zahrani, S.M. Characterization of Poly(Lactic acid)/hydroxyapatite prepared by a solvent-blending technique: Viscoelasticity and in vitro hydrolytic degradation. J. Elastomers Plast. 2015, 47, 753–768. [Google Scholar] [CrossRef]
- Weng, W.; Song, S.; Cao, L.; Chen, X.; Cai, Y.; Li, H.; Zhou, Q.; Zhang, J.; Su, J. A comparative study of bioartificial bone tissue Poly-L-lactic Acid/Polycaprolactone and PLLA scaffolds applied in bone regeneration. J. Nanomater. 2015, 2014, 935149. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Liu, A.; Hong, Z.; Jing, X. Electrospun Poly(L-lactide)-grafted hydroxyapatite/Poly(L-lactide) nanocomposite fibers. Eur. Polym. J. 2007, 43, 3187–3196. [Google Scholar] [CrossRef]
- Alex, A.; Ilango, N.K.; Ghosh, P. Comparative role of chain scission and solvation in the biodegradation of Polylactic Acid (PLA). J. Phys. Chem. B 2018, 122, 9516–9526. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, L.; Rogina, A.; Ivanković, M.; Ivanković, H. Medical-Grade Poly(Lactic Acid)/Hydroxyapatite Composite Films: Thermal and In Vitro Degradation Properties. Polymers 2023, 15, 1512. https://doi.org/10.3390/polym15061512
Bauer L, Rogina A, Ivanković M, Ivanković H. Medical-Grade Poly(Lactic Acid)/Hydroxyapatite Composite Films: Thermal and In Vitro Degradation Properties. Polymers. 2023; 15(6):1512. https://doi.org/10.3390/polym15061512
Chicago/Turabian StyleBauer, Leonard, Anamarija Rogina, Marica Ivanković, and Hrvoje Ivanković. 2023. "Medical-Grade Poly(Lactic Acid)/Hydroxyapatite Composite Films: Thermal and In Vitro Degradation Properties" Polymers 15, no. 6: 1512. https://doi.org/10.3390/polym15061512
APA StyleBauer, L., Rogina, A., Ivanković, M., & Ivanković, H. (2023). Medical-Grade Poly(Lactic Acid)/Hydroxyapatite Composite Films: Thermal and In Vitro Degradation Properties. Polymers, 15(6), 1512. https://doi.org/10.3390/polym15061512