A Novel Chitosan/Nano-Hydroxyapatite Composite for the Adsorptive Removal of Cd(II) from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Adsorbent Synthesis
2.2.1. Preparation of Chitosan (CS)
2.2.2. Preparation of Nano-Hydroxyapatite (n-Hap)
2.2.3. Preparation of CS/n-Hap Composite
2.3. Characterization
2.4. Adsorption Studies
2.5. Regeneration Study
3. Results and Discussion
3.1. Characterization
3.1.1. X-ray Diffraction Analysis
3.1.2. Fourier Transform Infra-Red Analysis
3.1.3. Morphological and Elemental Analysis
3.1.4. Thermogravimetric Analysis
3.1.5. N2 Adsorption–Desorption Isotherm Analysis
3.2. Adsorption Studies
3.2.1. pH Effect, Counter Ions, and Organic Compounds
3.2.2. Adsorbent Dose Effect
3.2.3. Contact Time Effect and Kinetic Modeling
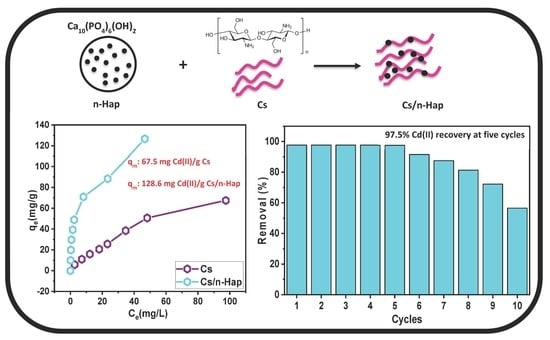
3.2.4. Initial Adsorbate Concentration Effect and Isotherm Modeling
3.2.5. Temperature Effect and Thermodynamic Modeling
3.2.6. Adsorption Mechanisms
3.3. Regeneration Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdellaoui, Y.; El Ibrahimi, B.; Abou Oualid, H.; Kassab, Z.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Gemero-Melo, P. Iron-Zirconium Microwave-Assisted Modification of Small-Pore Zeolite W and Its Alginate Composites for Enhanced Aqueous Removal of As (V) Ions: Experimental and Theoretical Studies. Chem. Eng. J. 2021, 421, 129909. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Bulat, Z.; Đukić-ćosić, D. Cadmium Toxicity Revisited: Focus on Oxidative Stress Induction and Interactions with Zinc Znd Magnesium. Arh. Za Hig. Rada I Toksikol. 2011, 62, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Salah, T.A.; Mohammad, A.M.; Hassan, M.A.; El-Anadouli, B.E. Development of Nano-Hydroxyapatite/Chitosan Composite for Cadmium Ions Removal in Wastewater Treatment. J. Taiwan Inst. Chem. Eng. 2014, 45, 1571–1577. [Google Scholar] [CrossRef]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental Hazards of Cadmium: Past, Present, and Future; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128148655. [Google Scholar]
- Joseph, P. Mechanisms of Cadmium Carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Zamri, M.F.M.A.; Kamaruddin, M.A.; Yusoff, M.S.; Aziz, H.A.; Foo, K.Y. Semi-Aerobic Stabilized Landfill Leachate Treatment by Ion Exchange Resin: Isotherm and Kinetic Study. Appl. Water Sci. 2017, 7, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Abdellaoui, Y.; Gamero-Melo, P.; Díaz-Jiménez, L.; Ponce-Caballero, C.; Giácoman-Vallejos, G. Synthesis and Surface Modification of Small Pore Size Zeolite W for Improving Removal Efficiency of Anionic Contaminants from Water. Bull. Environ. Contam. Toxicol. 2020, 105, 934–940. [Google Scholar] [CrossRef]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A.; Kooh, M.R.R. Efficient Adsorption of Malachite Green Dye Using Artocarpus Odoratissimus Leaves with Artificial Neural Network Modelling. Desalination Water Treat. 2018, 101, 313–324. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.; Morin-crini, N.; Crini, G.; Lichtfouse, E.; Wilson, L.; Conventional, N.M. Conventional and Non-Conventional Adsorbents for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Panić, V.V.; Šešlija, S.I.; Nešić, A.R.; Veličković, S.J. Adsorption of Azo Dyes on Polymer Materials. Hem. Ind. 2013, 67, 881–900. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Bajahzar, A.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Oliveira, M.L.S.; Li, Z. Adsorption of Dyes Brilliant Blue, Sunset Yellow and Tartrazine from Aqueous Solution on Chitosan: Analytical Interpretation via Multilayer Statistical Physics Model. Chem. Eng. J. 2020, 382, 122952. [Google Scholar] [CrossRef]
- El Achaby, M.; Ruesgas-Ramón, M.; Fayoud, N.E.H.; Figueroa-Espinoza, M.C.; Trabadelo, V.; Draoui, K.; Ben Youcef, H. Bio-Sourced Porous Cellulose Microfibrils from Coffee Pulp for Wastewater Treatment. Cellulose 2019, 26, 3873–3889. [Google Scholar] [CrossRef]
- Tang, X.; Gan, L.; Duan, Y.; Sun, Y.; Zhang, Y.; Zhang, Z. A Novel Cd2+-Imprinted Chitosan-Based Composite Membrane for Cd2+ Removal from Aqueous Solution. Mater. Lett. 2017, 198, 121–123. [Google Scholar] [CrossRef]
- Ayouch, I.; Barrak, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Impact of the Drying Process on the Efficiency of Alginate Beads for Cadmium Removal from Water: Kinetic, Isotherm and Thermodynamic Study. Environ. Technol. Innov. 2020, 20, 539–547. [Google Scholar] [CrossRef]
- Libio, I.C.; Demori, R.; Ferrão, M.F.; Lionzo, M.I.Z.; da Silveira, N.P. Films Based on Neutralized Chitosan Citrate as Innovative Composition for Cosmetic Application. Mater. Sci. Eng. C 2016, 67, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Shahid-ul-Islam; Butola, B.S. Recent Advances in Chitosan Polysaccharide and Its Derivatives in Antimicrobial Modification of Textile Materials. Int. J. Biol. Macromol. 2019, 121, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current Advancements in Chitosan-Based Film Production for Food Technology; A Review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C. Effect of Gallic Acid Grafted Chitosan Film Packaging on the Postharvest Quality of White Button Mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Smets, G.; Rüdelsheim, P. Biotechnologically Produced Chitosan for Nanoscale Products. A Legal Analysis. New Biotechnol. 2018, 42, 42–47. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, X.; Luo, Y.; Yuan, H.; Ren, T.; Li, X.; Xu, D.; Guo, X.; Wu, Y. Fluorescent Chitosan-Based Hydrogel Incorporating Titanate and Cellulose Nanofibers Modified with Carbon Dots for Adsorption and Detection of Cr (VI). Chem. Eng. J. 2021, 407, 127050. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, P.; Cai, M.; Hu, H.; Fu, Q. Comparative Adsorption of Pb (II), Cu (II) and Cd (II) on Chitosan Saturated Montmorillonite: Kinetic, Thermodynamic and Equilibrium Studies. Appl. Clay Sci. 2017, 143, 320–326. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, K.J.; Wan Ibrahim, W.A.; Jume, B.H. Equilibrium, Kinetic and Mechanism Studies of Cu (II) and Cd (II) Ions Adsorption by Modified Chitosan Beads. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Y.; Li, Y.; Wei, Q.; Hu, L.; Yan, T.; Feng, R.; Yan, L.; Du, B. Phosphorylated Chitosan/CoFe2O4 Composite for the Efficient Removal of Pb (II) and Cd (II) from Aqueous Solution: Adsorption Performance and Mechanism Studies. J. Mol. Liq. 2019, 277, 181–188. [Google Scholar] [CrossRef]
- Ebisike, K.; Okoronkwo, A.E.; Alaneme, K.K. Adsorption of Cd (II) on Chitosan–Silica Hybrid Aerogel from Aqueous Solution. Environ. Technol. Innov. 2019, 14, 100337. [Google Scholar] [CrossRef]
- Rathinam, K.; Singh, S.P.; Arnusch, C.J.; Kasher, R. An Environmentally-Friendly Chitosan-Lysozyme Biocomposite for the Effective Removal of Dyes and Heavy Metals from Aqueous Solutions. Carbohydr. Polym. 2018, 199, 506–515. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, L.; Xu, F.; Jiang, H.; Chen, L. Hydroxyapatite Modified Sludge-Based Biochar for the Adsorption of Cu2+ and Cd2+: Adsorption Behavior and Mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Long, Y.; Jiang, J.; Hu, J.; Hu, X.; Yang, Q.; Zhou, S. Removal of Pb (Ⅱ) from Aqueous Solution by Hydroxyapatite/Carbon Composite: Preparation and Adsorption Behavior. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 471–479. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; De La Rosa, G.; Peralta-Videa, J.R. Use of Phytofiltration Technologies in the Removal of Heavy Metals: A Review. Pure Appl. Chem. 2004, 76, 801–813. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, Y.X.; Lu, H.H.; Yang, R.Q.; Yang, S.M. Competitive Adsorption of Pb (II), Cu (II), and Zn (II) Ions onto Hydroxyapatite-Biochar Nanocomposite in Aqueous Solutions. J. Solid State Chem. 2018, 261, 53–61. [Google Scholar] [CrossRef]
- El, R.; Billah, K.; Islam, M.A.; Lgaz, H.; Lima, E.C.; Abdellaoui, Y.; Rakhila, Y.; Goudali, O.; Majdoubi, H.; Alrashdi, A.A.; et al. Shellfish Waste-Derived Mesoporous Chitosan for Impressive Removal of Arsenic (V) from Aqueous Solutions: A Combined Experimental and Computational Approach. Arab. J. Chem. 2022, 15, 104123. [Google Scholar] [CrossRef]
- Abidi, S.S.A.; Murtaza, Q. Synthesis and Characterization of Nano-Hydroxyapatite Powder Using Wet Chemical Precipitation Reaction. J. Mater. Sci. Technol. 2014, 30, 307–310. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Okayama, R.; Machida, M. Adsorption of Aqueous Metal Ions on Cattle-Manure-Compost Based Activated Carbons. J. Hazard. Mater. 2009, 170, 1119–1124. [Google Scholar] [CrossRef]
- Ragab, A.; Ahmed, I.; Bader, D. The Removal of Brilliant Green Dye from Aqueous Solution Using Nano Hydroxyapatite/Chitosan Composite as a Sorbent. Molecules 2019, 24, 847. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-Composite Scaffolds Containing Chitosan/Nano-Hydroxyapatite/Nano-Copper-Zinc for Bone Tissue Engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef]
- Rajiv Gandhi, M.; Kousalya, G.N.; Meenakshi, S. Removal of Copper(II) Using Chitin/Chitosan Nano-Hydroxyapatite Composite. Int. J. Biol. Macromol. 2011, 48, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sairam Sundaram, C.; Viswanathan, N.; Meenakshi, S. Uptake of Fluoride by Nano-Hydroxyapatite/Chitosan, a Bioinorganic Composite. Bioresour. Technol. 2008, 99, 8226–8230. [Google Scholar] [CrossRef]
- Kousalya, G.N.; Rajiv Gandhi, M.; Sairam Sundaram, C.; Meenakshi, S. Synthesis of Nano-Hydroxyapatite Chitin/Chitosan Hybrid Biocomposites for the Removal of Fe (III). Carbohydr. Polym. 2010, 82, 594–599. [Google Scholar] [CrossRef]
- Said, H.A.; Noukrati, H.; Ben Youcef, H.; Bayoussef, A.; Oudadesse, H.; Barroug, A. Mechanical Behavior of Hydroxyapatite-Chitosan Composite: Effect of Processing Parameters. Minerals 2021, 11, 213. [Google Scholar] [CrossRef]
- Nikpour, M.R.; Rabiee, S.M.; Jahanshahi, M. Synthesis and Characterization of Hydroxyapatite/Chitosan Nanocomposite Materials for Medical Engineering Applications. Compos. B Eng. 2012, 43, 1881–1886. [Google Scholar] [CrossRef]
- Attar Nosrati, S.; Alizadeh, R.; Ahmadi, S.J.; Erfani, M. Optimized Precipitation Process for Efficient and Size-Controlled Synthesis of Hydroxyapatite–Chitosan Nanocomposite. J. Korean Ceram. Soc. 2020, 57, 632–644. [Google Scholar] [CrossRef]
- Ran, J.; Jiang, P.; Sun, G.; Ma, Z.; Hu, J.; Shen, X.; Tong, H. Comparisons among Mg, Zn, Sr, and Si Doped Nano-Hydroxyapatite/Chitosan Composites for Load-Bearing Bone Tissue Engineering Applications. Mater. Chem. Front. 2017, 1, 900–910. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Yilgör, E.; Yilgör, I. Intercalated Chitosan/Hydroxyapatite Nanocomposites: Promising Materials for Bone Tissue Engineering Applications. Carbohydr. Polym. 2017, 175, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, J.; Li, S.; Tong, Y.; Ye, B. Synthesis of Magnetic Microspheres with Sodium Alginate and Activated Carbon for Removal of Methylene Blue. Materials 2017, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Ba Mohammed, B.; Hsini, A.; Abdellaoui, Y.; Abou Oualid, H.; Laabd, M.; El Ouardi, M.; Ait Addi, A.; Yamni, K.; Tijani, N. Fe-ZSM-5 Zeolite for Efficient Removal of Basic Fuchsin Dye from Aqueous Solutions: Synthesis, Characterization and Adsorption Process Optimization Using BBD-RSM Modeling. J. Environ. Chem. Eng. 2020, 8, 104419. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Yakout, A.A.; Abdel-Aal, H.; Osman, M.M. Enhanced Biosorptive Removal of Cadmium from Aqueous Solutions by Silicon Dioxide Nano-Powder, Heat Inactivated and Immobilized Aspergillus Ustus. Desalination 2011, 279, 291–297. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Abou Oualid, H.; Hsini, A.; El Ibrahimi, B.; Laabd, M.; El Ouardi, M.; Giácoman-Vallejos, G.; Gamero-Melo, P. Synthesis of Zirconium-Modified Merlinoite from Fly Ash for Enhanced Removal of Phosphate in Aqueous Medium: Experimental Studies Supported by Monte Carlo/SA Simulations. Chem. Eng. J. 2020, 404, 126600. [Google Scholar] [CrossRef]
- Mittal, H.; Maity, A.; Ray, S.S. Gum Karaya Based Hydrogel Nanocomposites for the Effective Removal of Cationic Dyes from Aqueous Solutions. Appl. Surf. Sci. 2016, 364, 917–930. [Google Scholar] [CrossRef]
- Xia, C.; Jing, Y.; Jia, Y.; Yue, D.; Ma, J.; Yin, X. Adsorption Properties of Congo Red from Aqueous Solution on Modified Hectorite: Kinetic and Thermodynamic Studies. Desalination 2011, 265, 81–87. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Removal of Divalent Cadmium Cations by Means of Synthetic Nano Crystallite Hydroxyapatite. Desalination 2011, 266, 142–148. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Biosorption of Reactive Dyes on the Green Alga Chlorella Vulgaris. Process Biochem. 2005, 40, 1347–1361. [Google Scholar] [CrossRef]
- Ayouch, I.; Barrak, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Improved Recovery of Cadmium from Aqueous Medium by Alginate Composite Beads Filled by Bentonite and Phosphate Washing Sludge. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125305. [Google Scholar] [CrossRef]
- Pelalak, R.; Heidari, Z.; Khatami, S.M.; Kurniawan, T.A.; Marjani, A.; Shirazian, S. Oak wood ash/GO/Fe3O4 adsorption efficiencies for cadmium and lead removal from aqueous solution: Kinetics, equilibrium and thermodynamic evaluation. Arab. J. Chem. 2021, 14, 102991. [Google Scholar] [CrossRef]
- Fu, C.; Zhu, X.; Dong, X.; Zhao, P.; Wang, Z. Study of adsorption property and mechanism of lead(II) and cadmium(II) onto sulfhydryl modified attapulgite. Arab. J. Chem. 2021, 14, 102960. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Mittal, H.; Waanders, F.; Sinha, S. Thermodynamic properties and adsorption behaviour of hydrogel nanocomposites for cadmium removal from mine effluents. J. Ind. Eng. Chem. 2017, 48, 151–161. [Google Scholar] [CrossRef]
- Bagheri, S.; Amini, M.M.; Behbahani, M.; Rabiee, G. Low cost thiol-functionalized mesoporous silica, KIT-6-SH, as a useful adsorbent for cadmium ions removal: A study on the adsorption isotherms and kinetics of KIT-6-SH. Microchem. J. 2019, 145, 460–469. [Google Scholar] [CrossRef]
- Soltani, R.; Pishnamazi, M.; Pelalak, R.; Rezakazemi, M.; Marjani, A.; Dinari, M.; Sarkar, S.M.; Shirazian, S. Preparation of COOH-KCC-1/polyamide 6 composite by in situ ring-opening polymerization: Synthesis, characterization, and Cd(II) adsorption study. J. Environ. Chem. Eng. 2021, 9, 104683. [Google Scholar] [CrossRef]
- Alkan, M.; Çelikçcapa, S.; Demirbaş, Ö.; Dogan, M. Removal of Reactive Blue 221 and Acid Blue 62 Anionic Dyes from Aqueous Solutions by Sepiolite. Dye. Pigment. 2005, 65, 251–259. [Google Scholar] [CrossRef]
- Vilela, P.B.; Matias, C.A.; Dalalibera, A.; Becegato, V.A.; Paulino, A.T. Polyacrylic Acid-Based and Chitosan-Based Hydrogels for Adsorption of Cadmium: Equilibrium Isotherm, Kinetic and Thermodynamic Studies. J. Environ. Chem. Eng. 2019, 7, 103327. [Google Scholar] [CrossRef]
- Shen, X.; Gao, X.; Wei, W.; Zhang, Y.; Zhang, Y.; Ma, L.; Liu, H.; Han, R.; Lin, J. Combined Performance of Hydroxyapatite Adsorption and Magnetic Separation Processes for Cd(II) Removal from Aqueous Solution. J. Dispers. Sci. Technol. 2021, 42, 664–676. [Google Scholar] [CrossRef]
- Xu, Y.; Schwartz, F.W.; Traina, S.J. Sorption of Zn2+ and Cd2+ on Hydroxyapatite Surfaces. Environ. Sci. Technol. 1994, 28, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ghosh, G.; Saha, S. Adsorption Characteristics of Phosphoric Acid Induced Activation of Bio-Carbon: Equilibrium, Kinetics, Thermodynamics and Batch Adsorber Design. Process Saf. Environ. Prot. 2018, 117, 125–142. [Google Scholar] [CrossRef]







| Kinetic Model | Parameters | CS | CS/n-Hap |
|---|---|---|---|
| Pseudo-first order | kf (1/min) | 0.1457 | 0.0167 |
| qe (mg/g) | 11.24 | 8.59 | |
| R² | 0.7221 | 0.6463 | |
| Pseudo-second order | ks (g/mg.min) | 0.0028 | 0.0045 |
| qe (mg/g) | 27.85 | 50.94 | |
| R² | 0.9923 | 0.9981 | |
| Intra-particle diffusion | k1 (mg/min1/2 g) | 3.6242 | 8.0621 |
| C1 | 0.9386 | 1.8667 | |
| R12 | 0.9947 | 0.9886 | |
| k2 (mg/min1/2 g) | 0.0616 | 0.0382 | |
| C2 | 24.394 | 48.557 | |
| R2² | 0.7501 | 0.7589 |
| Adsorbent | Isotherm Models | |||||
|---|---|---|---|---|---|---|
| Langmuir | Freundlich | |||||
| qmax (mg/g) | KL (L/mg) | R2 | KF (mg/g) (L/mg)1/n | n | R2 | |
| CS | 115.07 | 0.0143 | 0.8941 | 2.5804 | 1.3401 | 0.9915 |
| CS/n-Hap | 126.58 | 0.2743 | 0.9637 | 5.818 | 2.7616 | 0.9923 |
| Adsorbent | Thermodynamic Parameters | ||||
|---|---|---|---|---|---|
| ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol K) | |||
| 298 K | 308 K | 318 K | |||
| CS | −28.77 | −30.75 | −32.17 | 124.73 | 460.7 |
| CS/n-Hap | −36.33 | −40.35 | −47.18 | 31.17 | 122.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kaim Billah, R.; Ayouch, I.; Abdellaoui, Y.; Kassab, Z.; Khan, M.A.; Agunaou, M.; Soufiane, A.; Otero, M.; Jeon, B.-H. A Novel Chitosan/Nano-Hydroxyapatite Composite for the Adsorptive Removal of Cd(II) from Aqueous Solution. Polymers 2023, 15, 1524. https://doi.org/10.3390/polym15061524
El Kaim Billah R, Ayouch I, Abdellaoui Y, Kassab Z, Khan MA, Agunaou M, Soufiane A, Otero M, Jeon B-H. A Novel Chitosan/Nano-Hydroxyapatite Composite for the Adsorptive Removal of Cd(II) from Aqueous Solution. Polymers. 2023; 15(6):1524. https://doi.org/10.3390/polym15061524
Chicago/Turabian StyleEl Kaim Billah, Rachid, Ikrame Ayouch, Youness Abdellaoui, Zineb Kassab, Moonis Ali Khan, Mahfoud Agunaou, Abdessadik Soufiane, Marta Otero, and Byong-Hun Jeon. 2023. "A Novel Chitosan/Nano-Hydroxyapatite Composite for the Adsorptive Removal of Cd(II) from Aqueous Solution" Polymers 15, no. 6: 1524. https://doi.org/10.3390/polym15061524