Fabrication of 3D Printed Polylactic Acid/Polycaprolactone Nanocomposites with Favorable Thermo-Responsive Cyclic Shape Memory Effects, and Crystallization and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modification of PCL Diol
2.2. Synthesis of CNC-OMMT Hybrid Nanofillers
2.3. Preparation of 3D Printing Filaments
2.4. Fabrication of 3D Printed Specimens
2.5. Characterization Methods
3. Results and Discussion
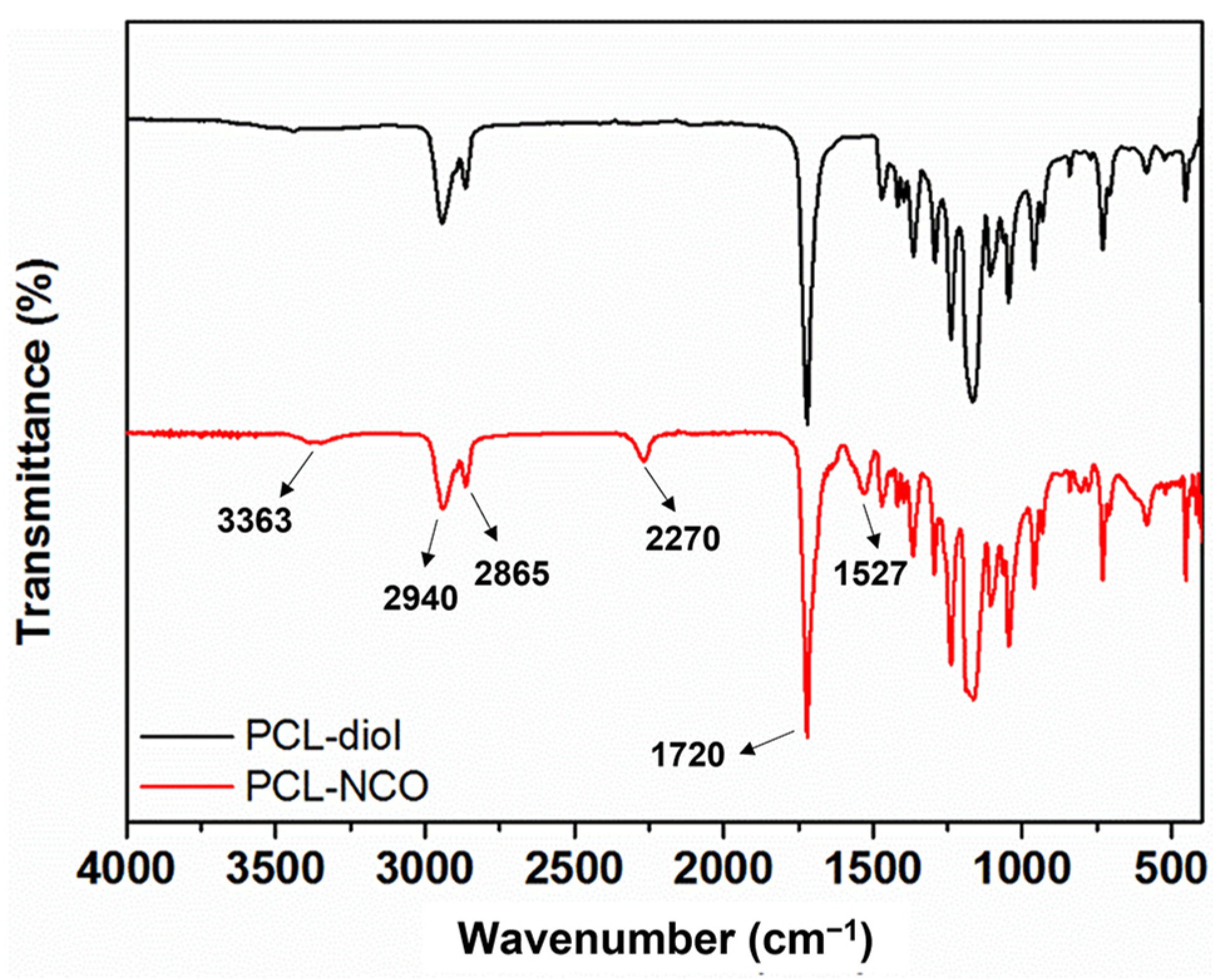
3.1. Characterization of PCL-NCO and Reactive Mechanism
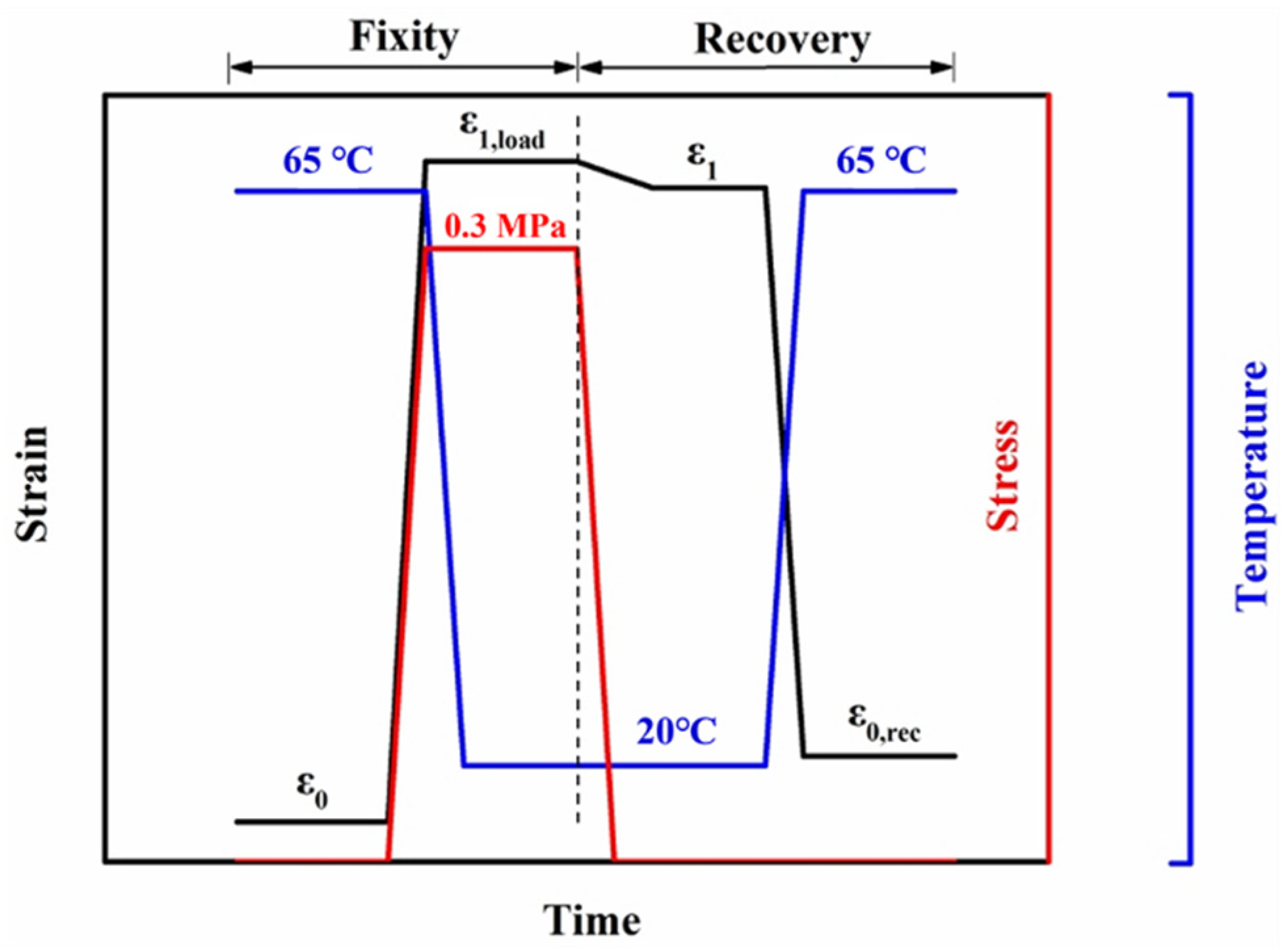
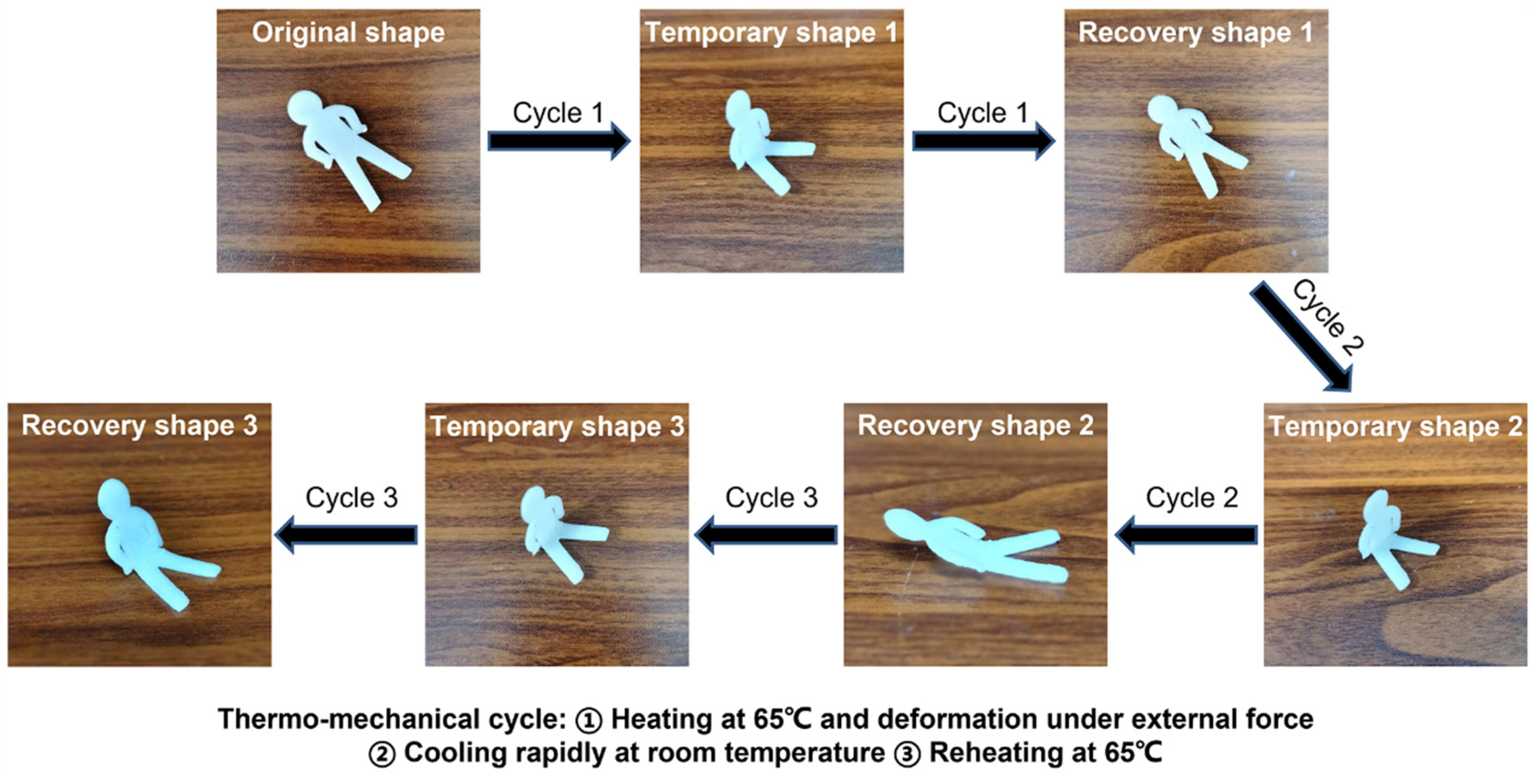
3.2. Cyclic Shape Memory Properties of PLA/PCL Blends Modified by the PCL-NCO
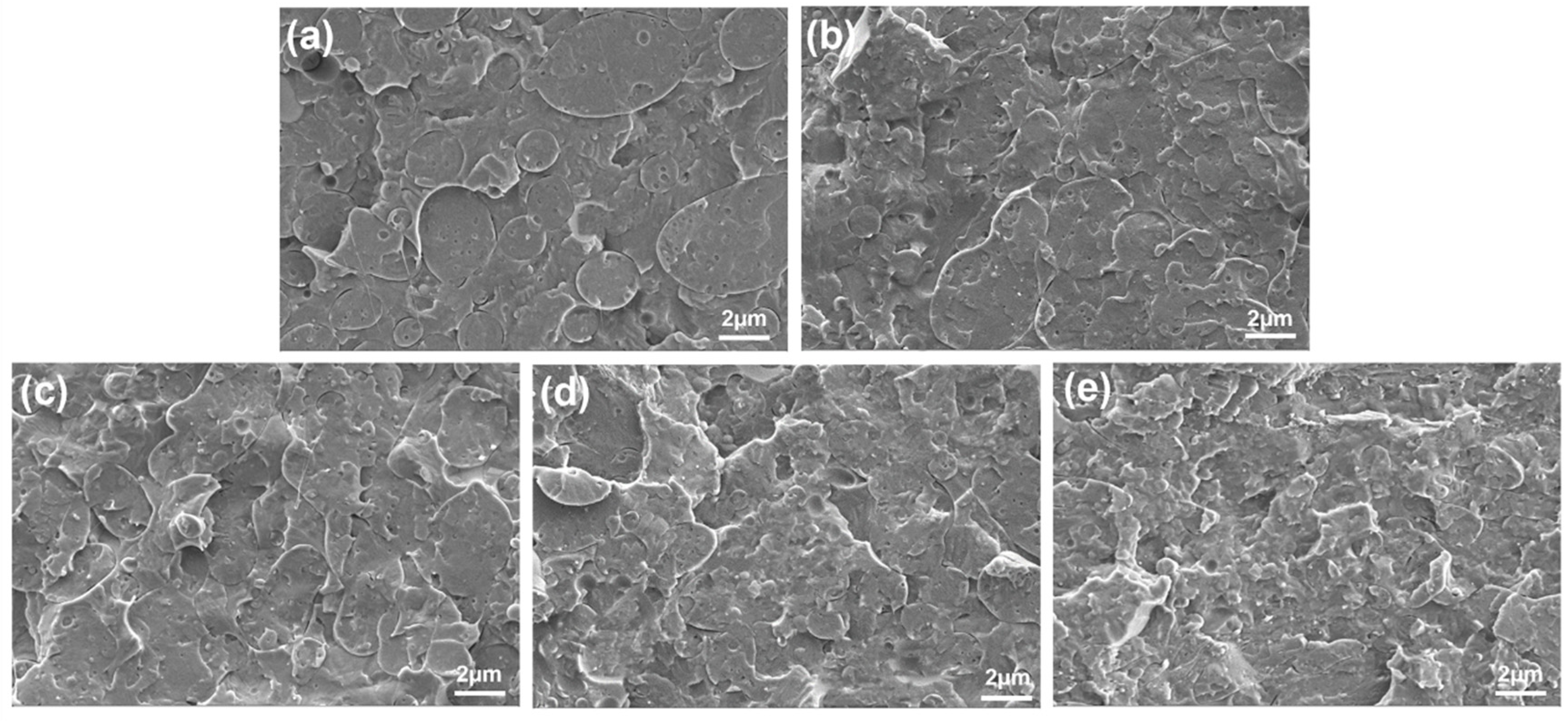
3.3. Morphologies of PLA/PCL Nanocomposites
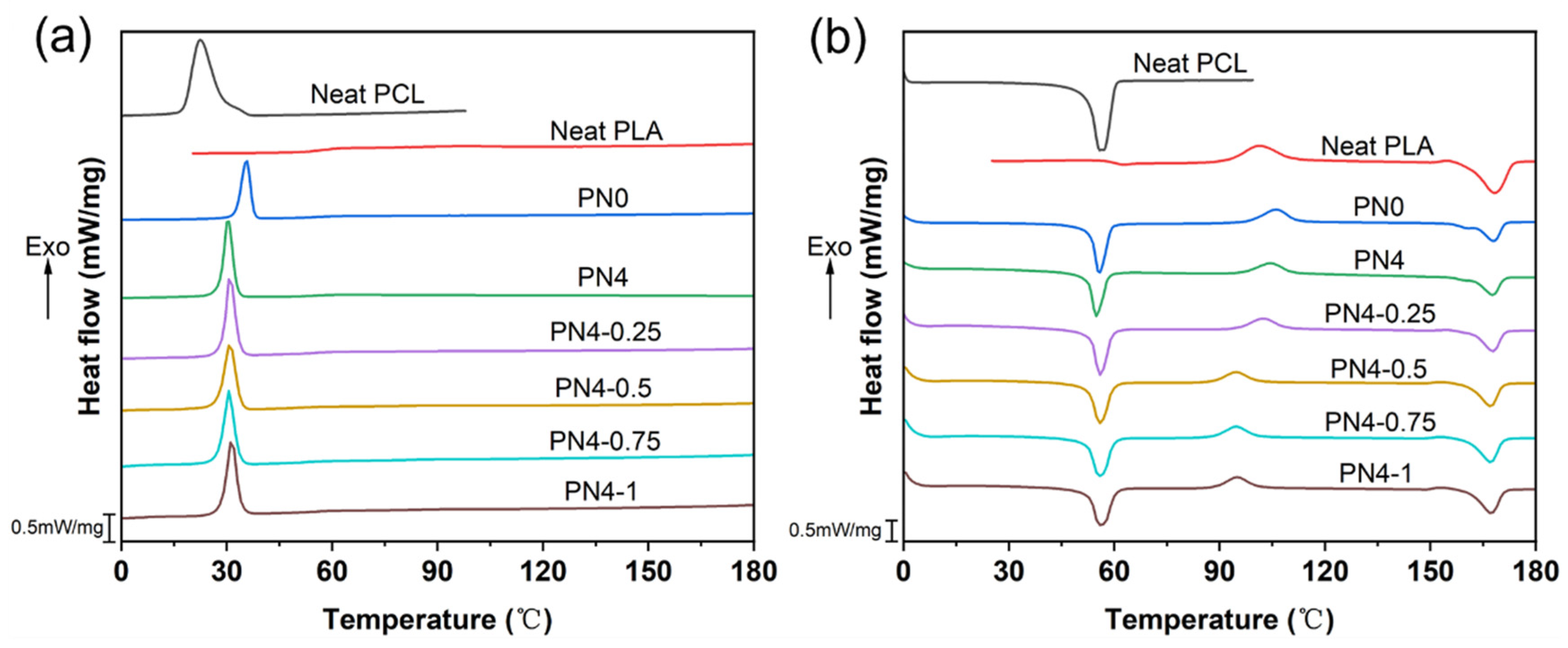
3.4. Thermal Properties of PLA/PCL Nanocomposites
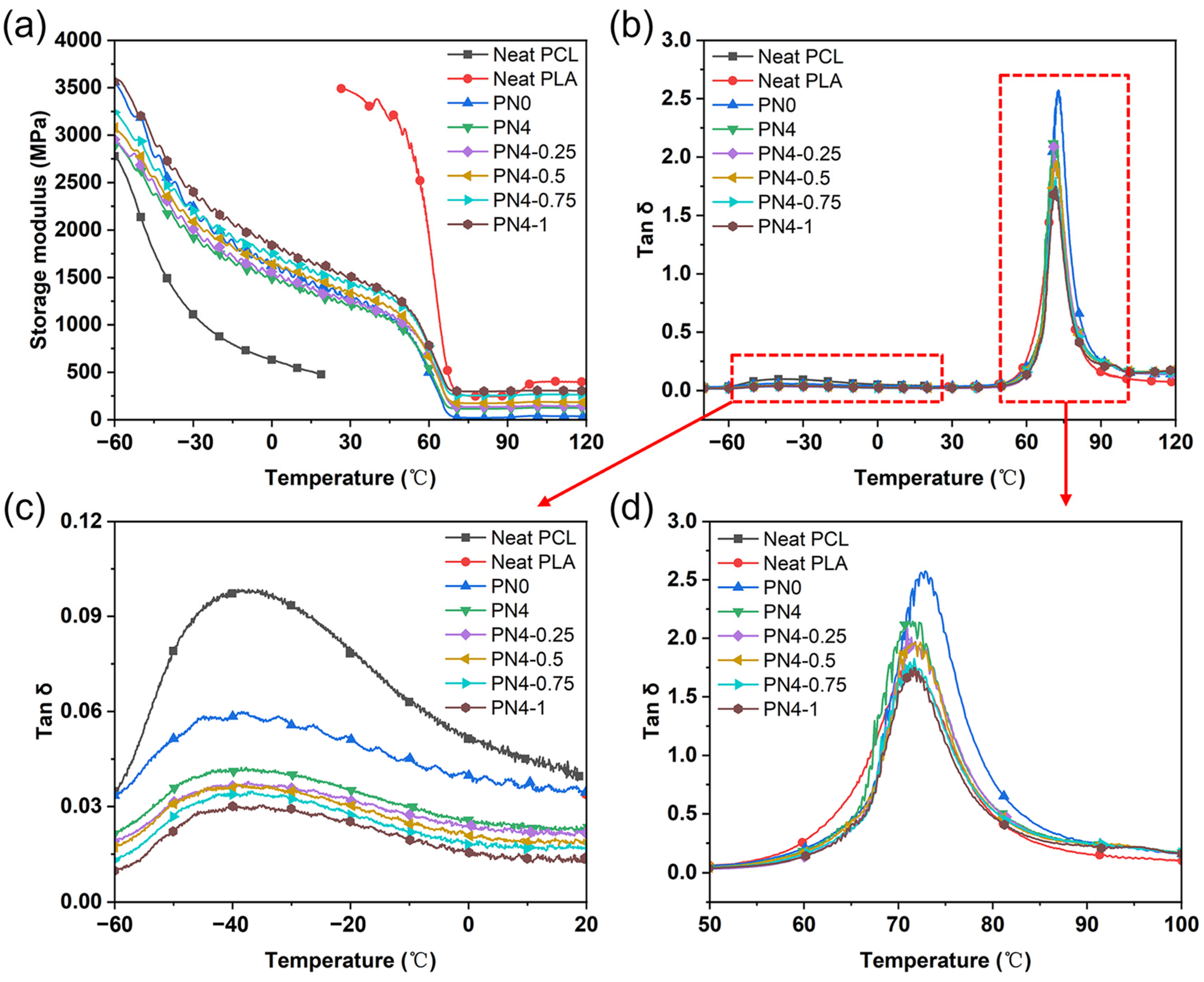
3.5. Dynamic Mechanical Properties of PLA/PCL Nanocomposites
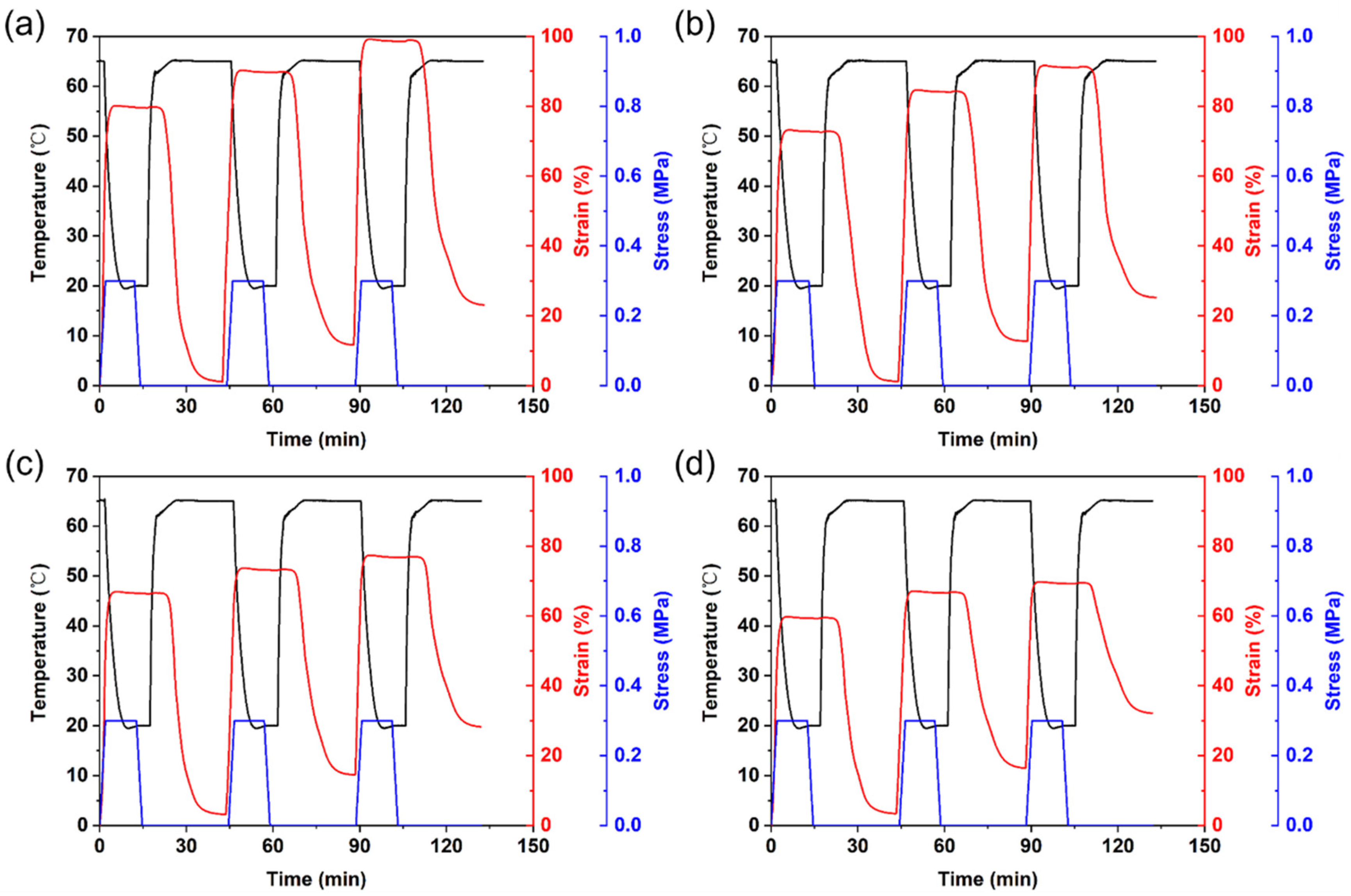
3.6. Cyclic Shape Memory Properties of PLA/PCL Nanocomposites
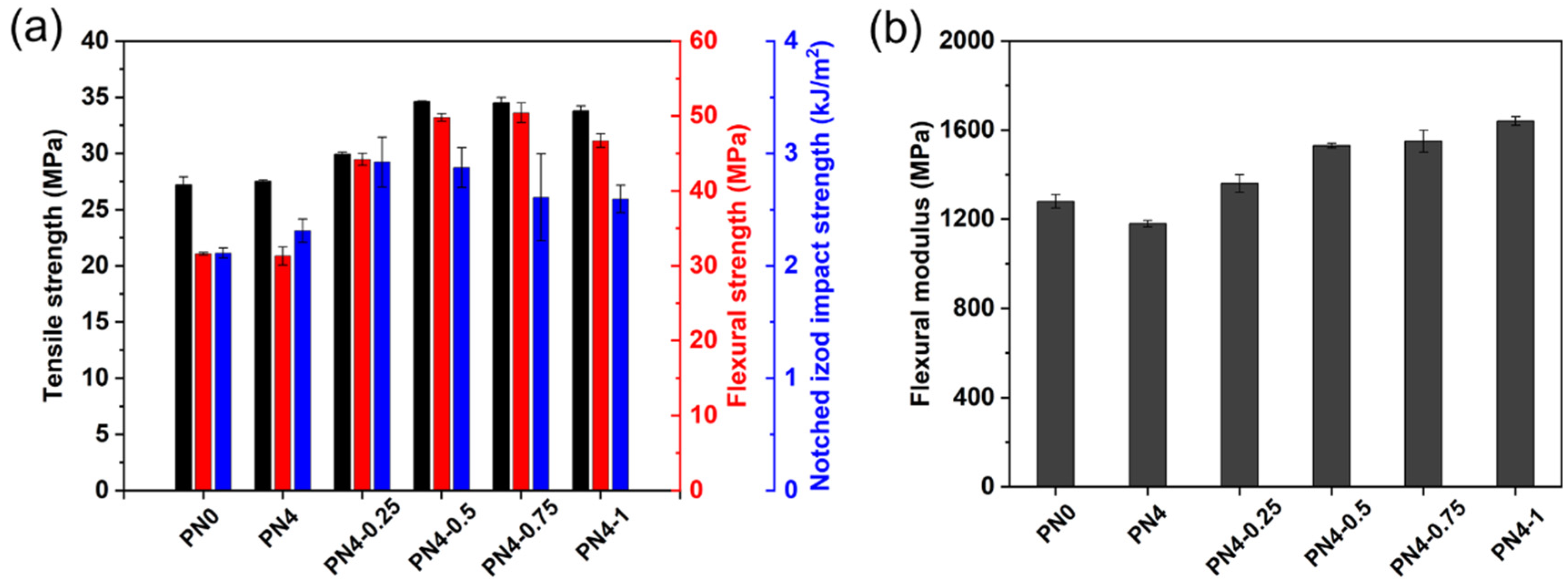
3.7. Mechanical Properties of PLA/PCL Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A review of shape memory polymers and composites: Mechanisms, materials, and applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Arif, Z.U.; Noroozi, R.; Zolfagharian, A.; Bodaghi, M. 4D printing of shape memory polymer composites: A review on fabrication techniques, applications, and future perspectives. J. Manuf. Process. 2022, 81, 759–797. [Google Scholar] [CrossRef]
- Meng, H.; Li, G. A review of stimuli-responsive shape memory polymer composites. Polymer 2013, 54, 2199–2221. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Rodgers, W.R.; Tao, X. Semi-crystalline two-way shape memory elastomer. Polymer 2011, 52, 5320–5325. [Google Scholar] [CrossRef]
- Miaudet, P.; Derre, A.; Maugey, M.; Zakri, C.; Piccione, P.M.; Inoubli, R.; Poulin, P. Shape and temperature memory of nanocomposites with broadened glass transition. Science 2007, 318, 1294–1296. [Google Scholar] [CrossRef]
- Huang, B.; He, H.; Liu, H.; Zhang, Y.; Chen, H.; Ma, Y. Co-precipitated poly(vinyl alcohol)/chitosan composites with excellent mechanical properties and tunable water-induced shape memory. Carbohyd. Polym. 2020, 245, 116445. [Google Scholar] [CrossRef]
- Huang, B.; He, H.; Liu, H.; Wu, W.; Ma, Y.; Zhao, Z. Mechanically strong, heat-resistant, water-induced shape memory poly(vinyl alcohol)/regenerated cellulose biocomposites via a facile co-precipitation method. Biomacromolecules 2019, 20, 3969–3979. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Liu, L.; Leng, J. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 023001. [Google Scholar] [CrossRef]
- Delaey, J.; Dubruel, P.; Van Vlierberghe, S. Shape-memory polymers for biomedical applications. Adv. Funct. Mater. 2020, 30, 1909047. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Zhang, F.; Liu, Y.; Leng, J. The research status and challenges of shape memory polymer-based flexible electronics. Mater. Horiz. 2019, 6, 931–944. [Google Scholar] [CrossRef]
- Jin, B.; Song, H.; Jiang, R.; Song, J.; Zhao, Q.; Xie, T. Programming a crystalline shape memory polymer network with thermo-and photo-reversible bonds toward a single-component soft robot. Sci. Adv. 2018, 4, eaao3865. [Google Scholar] [CrossRef] [Green Version]
- Ehrmann, G.; Ehrmann, A. 3D printing of shape memory polymers. J. Appl. Polym. Sci. 2021, 138, 50847. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Feng, J. 3D printing of tunable shape memory polymer blends. J. Mater. Chem. C 2017, 5, 8361–8365. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Peng, X.; Huang, B.; Li, J. Three-dimensional printing of poly (lactic acid) bio-based composites with sugarcane bagasse fiber: Effect of printing orientation on tensile performance. Polym. Adv. Technol. 2019, 30, 910–922. [Google Scholar] [CrossRef]
- Geng, Y.; He, H.; Liu, H.; Jing, H. Preparation of polycarbonate/poly (lactic acid) with improved printability and processability for fused deposition modeling. Polym. Adv. Technol. 2020, 31, 2848–2862. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Huang, B. Favorable thermoresponsive shape memory effects of 3D printed poly(lactic acid)/poly(ε-caprolactone) blends fabricated by fused deposition modeling. Macromol. Mater. Eng. 2020, 305, 2000295. [Google Scholar] [CrossRef]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-memory polymers and their composites: Stimulus methods and applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Kalita, H.; Karak, N. Bio-based hyperbranched polyurethane/multi-walled carbon nanotube nanocomposites as shape memory materials. Polym. Compos. 2013, 35, 636–643. [Google Scholar] [CrossRef]
- Gardella, L.; Calabrese, M.; Monticelli, O. PLA maleation: An easy and effective method to modify the properties of PLA/PCL immiscible blends. Colloid Polym. Sci. 2014, 292, 2391–2398. [Google Scholar] [CrossRef]
- Molavi, F.K.; Ghasemi, I.; Messori, M.; Esfandeh, M. Nanocomposites based on poly (L-lactide)/poly (ε-caprolactone) blends with triple-shape memory behavior: Effect of the incorporation of graphene nanoplatelets (GNps). Compos. Sci. Technol. 2017, 151, 219–227. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, Z.; Dai, B.; Liu, J.; Chen, Y.; Zeng, G.; He, Y.; Liu, Y.; Liu, R. Preparation and characterization of reinforced starch-based composites with compatibilizer by simple extrusion. Carbohyd. Polym. 2019, 223, 115122. [Google Scholar] [CrossRef]
- Lee, J.C.; Choi, M.-C.; Choi, D.-H.; Ha, C.-S. Toughness enhancement of poly (lactic acid) through hybridisation with epoxide-functionalised silane via reactive extrusion. Polym. Degrad. Stabil. 2019, 160, 195–202. [Google Scholar] [CrossRef]
- Nocita, D.; Forte, G.; Drakopoulos, S.X.; Visco, A.; Gianporcaro, A.; Ronca, S. Processing and characterization of bio-polyester reactive blends: From thermoplastic blends to cross-linked networks. Polymer 2017, 132, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Visco, A.; Nocita, D.; Giamporcaro, A.; Ronca, S.; Forte, G.; Pistone, A.; Espro, C. Effect of Ethyl Ester L-Lysine Triisocyanate addition to produce reactive PLA/PCL bio-polyester blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 68, 308–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufresne, A. Cellulose nanomaterial reinforced polymer nanocomposites. Curr. Opin. Colloid. Inter. Sci. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Kenny, J.M.; Peponi, L. Thermally-activated shape memory behaviour of bionanocomposites reinforced with cellulose nanocrystals. Cellulose 2014, 21, 4231–4246. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; He, H.; Huang, B. Cellulose nanocrystals-organic montmorillonite nanohybrid material by electrostatic self-assembly. J. Appl. Polym. Sci. 2020, 137, 49263. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, B.; Zhou, L.; Li, J.; Zhang, J.; Chen, X.; Xu, S.; He, H. Synergistic effects of cellulose nanocrystals-organic montmorillonite as hybrid nanofillers for enhancing mechanical, crystallization, and heat-resistant properties of three-dimensional printed poly (lactic acid) nanocomposites. Polym. Eng. Sci. 2021, 61, 2985–3000. [Google Scholar] [CrossRef]
- Bai, H.; Huang, C.; Xiu, H.; Gao, Y.; Zhang, Q.; Fu, Q. Toughening of poly(L-lactide) with poly(ε-caprolactone): Combined effects of matrix crystallization and impact modifier particle size. Polymer 2013, 54, 5257–5266. [Google Scholar] [CrossRef]
- Bai, H.; Xiu, H.; Gao, J.; Deng, H.; Zhang, Q.; Yang, M.; Fu, Q. Tailoring impact toughness of poly(L-lactide)/poly(ε-caprolactone)/(PLLA/PCL) blends by controlling crystallization of PLLA matrix. ACS Appl. Mater. Interfaces 2012, 4, 897–905. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Sonseca, A.; Gimenez, E.; Marcos-Fernandez, A.; Kenny, J.M. Synthesis and characterization of PCL-PLLA polyurethane with shape memory behavior. Eur. Polym. J. 2013, 49, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Taipina, M.O.; Ferrarezi, M.M.F.; Yoshida, I.V.P.; Goncalves, M.C. Surface modification of cotton nanocrystals with a silane agent. Cellulose 2013, 20, 217–226. [Google Scholar] [CrossRef]
- Sessini, V.; Navarro-Baena, I.; Arrieta, M.P.; Dominici, F.; Lopez, D.; Torre, L.; Kenny, J.M.; Dubois, P.; Raquez, J.-M.; Peponi, L. Effect of the addition of polyester-grafted-cellulose nanocrystals on the shape memory properties of biodegradable PLA/PCL nanocomposites. Polym. Degrad. Stabil. 2018, 152, 126–138. [Google Scholar] [CrossRef]
- Jérémy, O.; Raquez, J.-M.; Thomassin, J.-M.; Gloaguen, J.-M.; Lauro, F.; Jérôme, C.; Lefebvre, J.-M.; Dubois, P. Mechanistic insights on nanosilica self-networking inducing ultra-toughness of rubber-modified polylactide-based materials. Nanocomposites 2015, 1, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Sakai, F.; Nishikawa, K.; Inoue, Y.; Yazawa, K. Nucleation enhancement effect in poly(L-lactide) (PLLA)-poly(ɛ-caprolactone) (PCL) blend induced by locally activated chain mobility resulting from limited miscibility. Macromolecules 2009, 42, 8335–8342. [Google Scholar] [CrossRef]
- Wu, W.; Ye, W.; Wu, Z.; Geng, P.; Wang, Y.; Zhao, J. Influence of layer thickness, raster angle, deformation temperature and recovery temperature on the shape-memory effect of 3D-printed polylactic acid samples. Materials 2017, 10, 970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; He, H.; Li, M.-C.; Huang, S.; Mei, C.; Wu, Q. Enhancing mechanical properties of poly(lactic acid) through its in-situ crosslinking with maleic anhydride-modified cellulose nanocrystals from cottonseed hulls. Ind. Crop. Prod. 2018, 112, 449–459. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Peng, X.; He, H.; Jia, Y.; Liu, H.; Geng, Y.; Huang, B.; Luo, C. Shape memory effect of three-dimensional printed products based on polypropylene/nylon 6 alloy. J. Mater. Sci. 2019, 54, 9235–9246. [Google Scholar] [CrossRef]


| Parameter | Nozzle Diameter | Nozzle Temperature | Printing Speed | Layer Thickness | Raster Angle | Infill Density | Platform Temperature |
|---|---|---|---|---|---|---|---|
| Value | 0.6 mm | 190 °C | 40 mm/s | 0.05 mm | 45°/−45° | 100% | 30 °C |
| Sample | Rf1 (%) | Rr1 (%) | Rf2 (%) | Rr2 (%) | Rf3 (%) | Rr3 (%) |
|---|---|---|---|---|---|---|
| PN0 | 98.25 | 92.66 | 97.72 | 65.48 | 96.55 | 58.72 |
| PN2 | 98.65 | 94.89 | 98.76 | 82.31 | 98.49 | 81.79 |
| PN4 | 98.87 | 97.14 | 99.08 | 85.97 | 99.01 | 84.30 |
| PN6 | 99.23 | 92.59 | 99.26 | 81.15 | 99.14 | 78.72 |
| PN8 | 99.04 | 91.50 | 99.09 | 78.67 | 98.84 | 75.35 |
| Sample | Tmc a (°C) | ∆Hmc b (J/g) | Tm c (°C) | ∆Hm d (J/g) | Xc e (%) |
|---|---|---|---|---|---|
| Neat PCL | 22.5 | 62.2 | 56.7 | 61.0 | 44.9 |
| PN0 | 35.8 | 29.3 | 55.9 | 29.2 | 42.9 |
| PN4 | 30.4 | 29.1 | 55.2 | 27.1 | 39.9 |
| PN4-0.25 | 30.6 | 31.2 | 55.9 | 30.9 | 45.4 |
| PN4-0.5 | 30.6 | 31.0 | 55.8 | 30.8 | 45.3 |
| PN4-0.75 | 30.5 | 31.4 | 55.9 | 31.3 | 46.0 |
| PN4-1 | 31.0 | 32.0 | 56.0 | 32.0 | 47.1 |
| Sample | Tcc a (°C) | Tm (°C) | ∆Hcc b (J/g) | ∆Hm (J/g) | Xc (%) |
|---|---|---|---|---|---|
| Neat PLA | 101.3 | 168.1 | 30.7 | 35.6 | 5.2 |
| PN0 | 106.1 | 168.0 | 16.5 | 17.5 | 2.1 |
| PN4 | 104.4 | 167.7 | 13.8 | 15.8 | 4.3 |
| PN4-0.25 | 101.5 | 167.9 | 13.9 | 17.7 | 8.1 |
| PN4-0.5 | 93.6 | 167.2 | 12.4 | 21.7 | 19.9 |
| PN4-0.75 | 93.8 | 167.2 | 12.8 | 23.1 | 22.0 |
| PN4-1 | 94.0 | 167.4 | 12.8 | 23.6 | 23.1 |
| Sample | Storage Modulus E’ at 25 °C (MPa) | Tan δ Peak Temperature (°C) | Tan δ Peak Value |
|---|---|---|---|
| Neat PCL | 478 | −39.2 | 0.10 |
| Neat PLA | 3489 | 71.5 | 1.72 |
| PN0 | 1322 | 72.9 | 2.57 |
| PN4 | 1234 | 70.6 | 2.11 |
| PN4-0.25 | 1283 | 70.9 | 2.08 |
| PN4-0.5 | 1392 | 71.7 | 1.96 |
| PN4-0.75 | 1485 | 71.2 | 1.79 |
| PN4-1 | 1550 | 71.6 | 1.75 |
| Sample | Rf1 (%) | Rr1 (%) | Rf2 (%) | Rr2 (%) | Rf3 (%) | Rr3 (%) |
|---|---|---|---|---|---|---|
| PN4-0.25 | 99.15 | 98.50 | 99.24 | 88.07 | 99.24 | 86.81 |
| PN4-0.5 | 99.17 | 97.98 | 99.19 | 85.80 | 99.26 | 85.23 |
| PN4-0.75 | 99.26 | 94.75 | 99.17 | 84.05 | 98.98 | 78.14 |
| PN4-1 | 99.06 | 94.02 | 99.16 | 80.42 | 98.81 | 70.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, C.; Chen, S.; Chen, P.; Li, J.; Jian, H.; Guo, G.; Chen, X.; Zhu, X.; Wu, J. Fabrication of 3D Printed Polylactic Acid/Polycaprolactone Nanocomposites with Favorable Thermo-Responsive Cyclic Shape Memory Effects, and Crystallization and Mechanical Properties. Polymers 2023, 15, 1533. https://doi.org/10.3390/polym15061533
Liu H, Li C, Chen S, Chen P, Li J, Jian H, Guo G, Chen X, Zhu X, Wu J. Fabrication of 3D Printed Polylactic Acid/Polycaprolactone Nanocomposites with Favorable Thermo-Responsive Cyclic Shape Memory Effects, and Crystallization and Mechanical Properties. Polymers. 2023; 15(6):1533. https://doi.org/10.3390/polym15061533
Chicago/Turabian StyleLiu, Hao, Chengdi Li, Simin Chen, Ping Chen, Jinbo Li, Huihua Jian, Guoyi Guo, Xiao Chen, Xiaofeng Zhu, and Jun Wu. 2023. "Fabrication of 3D Printed Polylactic Acid/Polycaprolactone Nanocomposites with Favorable Thermo-Responsive Cyclic Shape Memory Effects, and Crystallization and Mechanical Properties" Polymers 15, no. 6: 1533. https://doi.org/10.3390/polym15061533






