Part A: Biodegradable Bio-Composite Film Reinforced with Cellulose Nanocrystals from Chaetomorpha linum into Thermoplastic Starch Matrices
Abstract
1. Introduction
2. Experimental Work
2.1. Materials
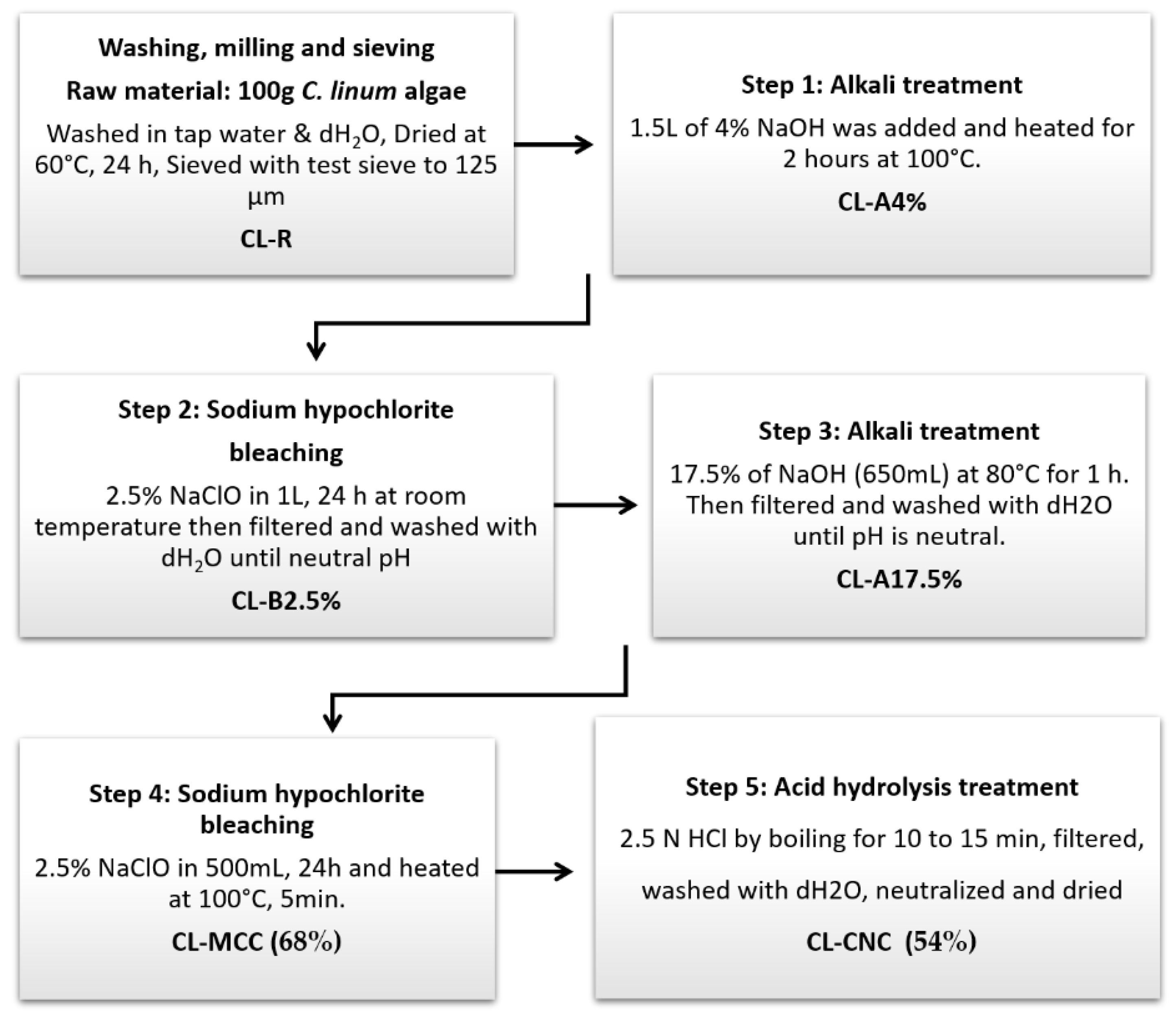
2.1.1. Extract of Chemical Cellulose Nanocrystals
2.1.2. Starch Extraction
2.1.3. Development of Bioplastic Synthesizers from Extracts of Cellulose Nanocrystals and Starch
2.2. Characterization Methods
2.2.1. UV-Visible Analysis
2.2.2. FTIR Analysis
2.2.3. SEM Analysis
2.2.4. Enzymatic Degradation
3. Results and Discussion
3.1. Characterization of Cellulose Nanocrystals Extract
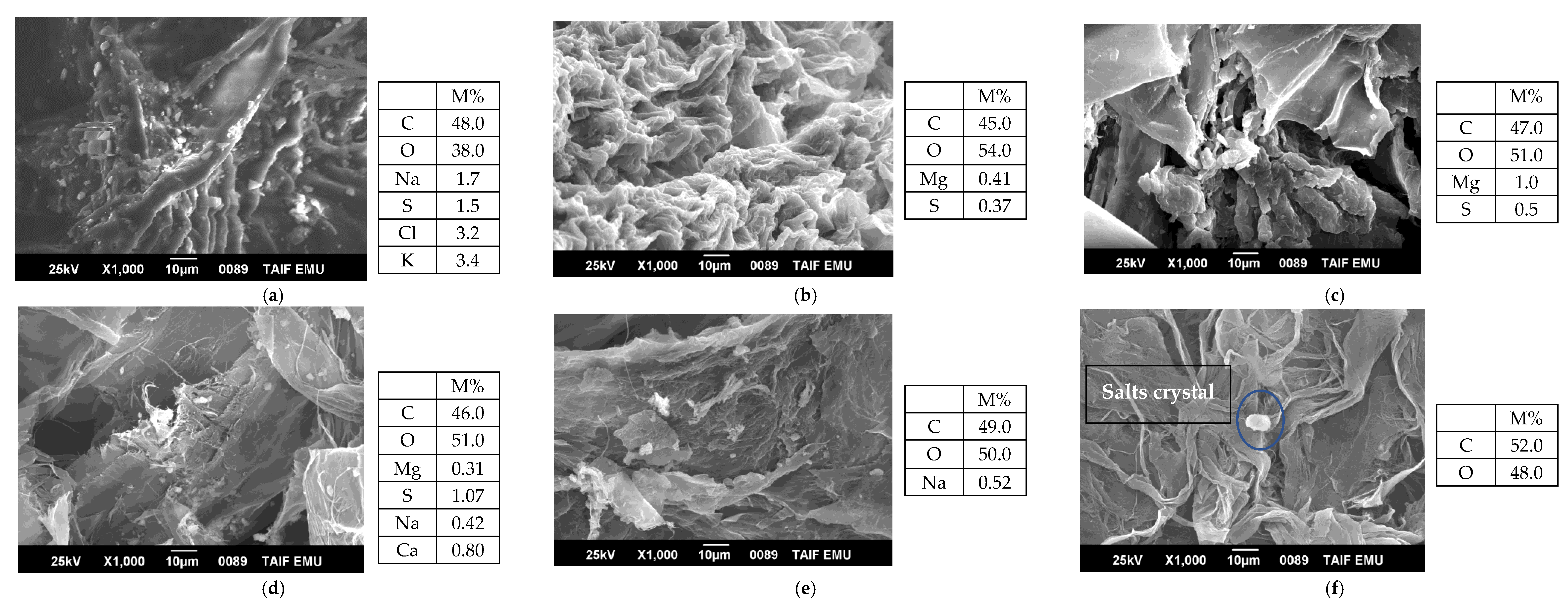
3.1.1. Morphological Analysis of Cellulose Nanocrystals and By-Products during Extraction
3.1.2. Functional Group of Cellulose Nanocrystals and Its By-Products during Extraction
| Main Peak (cm−1) from Isolated CL-CNC and Bio-Composite Films | Main Peak (cm−1) from Pure Glycerol | Main Peak (cm−1) from Cellulose | Main Peak (cm−1) from Corn Starch | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CL-CNC | CS:CL-CNC7:3-50% | CS:CL-CNC 8:2-20% | Reported Glycerol [61] | Reported Cellulose [44] | Reported Corn Starch (Corn-S) [60] | |||||||
| Peak (cm−1) | % T | Peak (cm−1) | % T | Peak (cm−1) | % T | Functional Groups of Glycerol | Λ cm−1 of FT-IR Spectrum | Functional Groups of Cellulose | Λ cm−1 of FT-IR Spectrum | Functional Groups of Corn-S | Λ cm−1 of FT-IR Spectrum | |
| Elongational vibration bands of the O-H link of the primary and secondary alcohol functions | 3363 | 67.60 | 3446 | 64.67 | 3567 3344 | 89.22 56.72 | O–H groups stretching vibration | 3347–3450 | Stretching of hydrogen-bonded hydroxyl groups. | 3410 | ||
| - | - | 3259 | 68.40 | 3169 | 52.14 | Affiliated to O–H bond | 3320 | - | - | - | - | |
| Elongational vibrations of the C-H bond | 2900 | 89.67 | 2928 | 75.91 | - | - | C–H stretching vibration | 2908 | C–H stretching vibration | 2897–2990 | Axial deformation of the CH2 group | 2932 |
| Water adsorbed on cellulose | 1643 | 96.28 | 1677 | 65.01 | 1637 | 69.54 | - | - | H2O absorbed | 1632–1645 | Tightly bound water present in hygroscopic materials | 1651 |
| Crystallinity of cellulosic materials: Vibration of symmetric bending of CH2 | 1430 | 88.52 | 1439 | 40.76 | - | - | - | - | CH2 bending vibration | 1425–1468 | Bending of methyl group | 1452 |
| Strain vibrations in the plane of the O-H functions of alcohols | 1326 | 80.91 | - | - | 1365 | 31.63 | - | - | - | - | C-OH bending vibration of starch molecule | 1338 |
| Antisymmetric expansion vibration of the C-O-C glycosidic bond | 1161 | 72.60 | 1159 | 47.78 | - | - | Stretching vibrations of C–O linkages | 1150 | C–O–C glycosidic band stretching vibration | 1162–1172 | Stretching vibrations of C-O bonds in C-O-H of starch molecule | 1157 |
| Vibrations of the C–O bond of carbons 2, 3 and 6 | 1110 | 58.25 | - | - | - | - | Stretching vibrations of C–O in C2 | 1117 | - | - | - | - |
| 1037 | 38.23 | - | - | - | - | Stretching vibrations of the C–O linkage in C1 and C3 | 1045 | - | - | - | - | |
| Crystallinity of cellulosic materials: Vibration of symmetric bending of CH2 | 1016 | 47.56 | 1004 | 34.53 | - | - | Vibration of the skeleton C–C | 995 | - | - | - | - |
| - | - | 918 | 40.82 | - | - | 925 | C–H rock vibration | 896–905 | C-O-C groups in the anhydroglucose ring within the starch structure | 929 | ||
| Attributed to the amorphous region | 908 | 85.94 | - | - | - | - | 850 | |||||
| Associated with the cellulosic β-glycosidic linkages | - | - | 841 | 29.48 | 843 | 54.57 | Vibrations of C–C linkages | 800 | 860 | |||
| - | - | 710 | 54.50 | 731 | 48.42 | - | - | - | - | 763 | ||
| Attributed to δCOH out of plane [26] | 667 612 | 61.58 53.69 | - | - | - | - | - | - | - | - | - | - |
| Attributed to C6-OH torsion [26] | 608 | 53.70 | 559 | 14.46 | 586 | 19.18 | - | - | - | - | - | - |
3.2. Characterization of a Bio-Composite Film
3.2.1. Functional Group of Bio-Composite Film
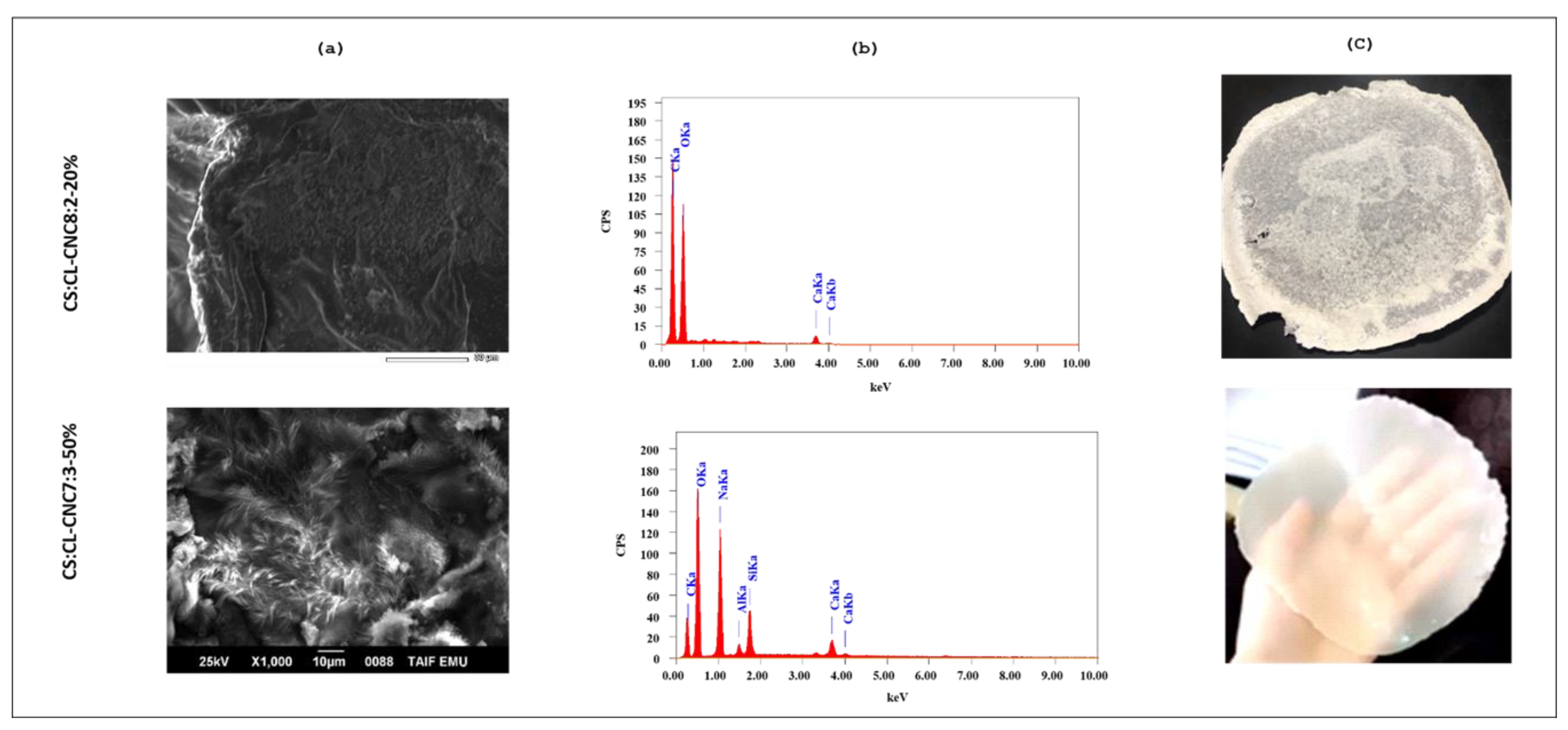
3.2.2. Morphology of Obtained Bio-Composite
3.2.3. Water Content of the Synthesized Bio-Composites Films
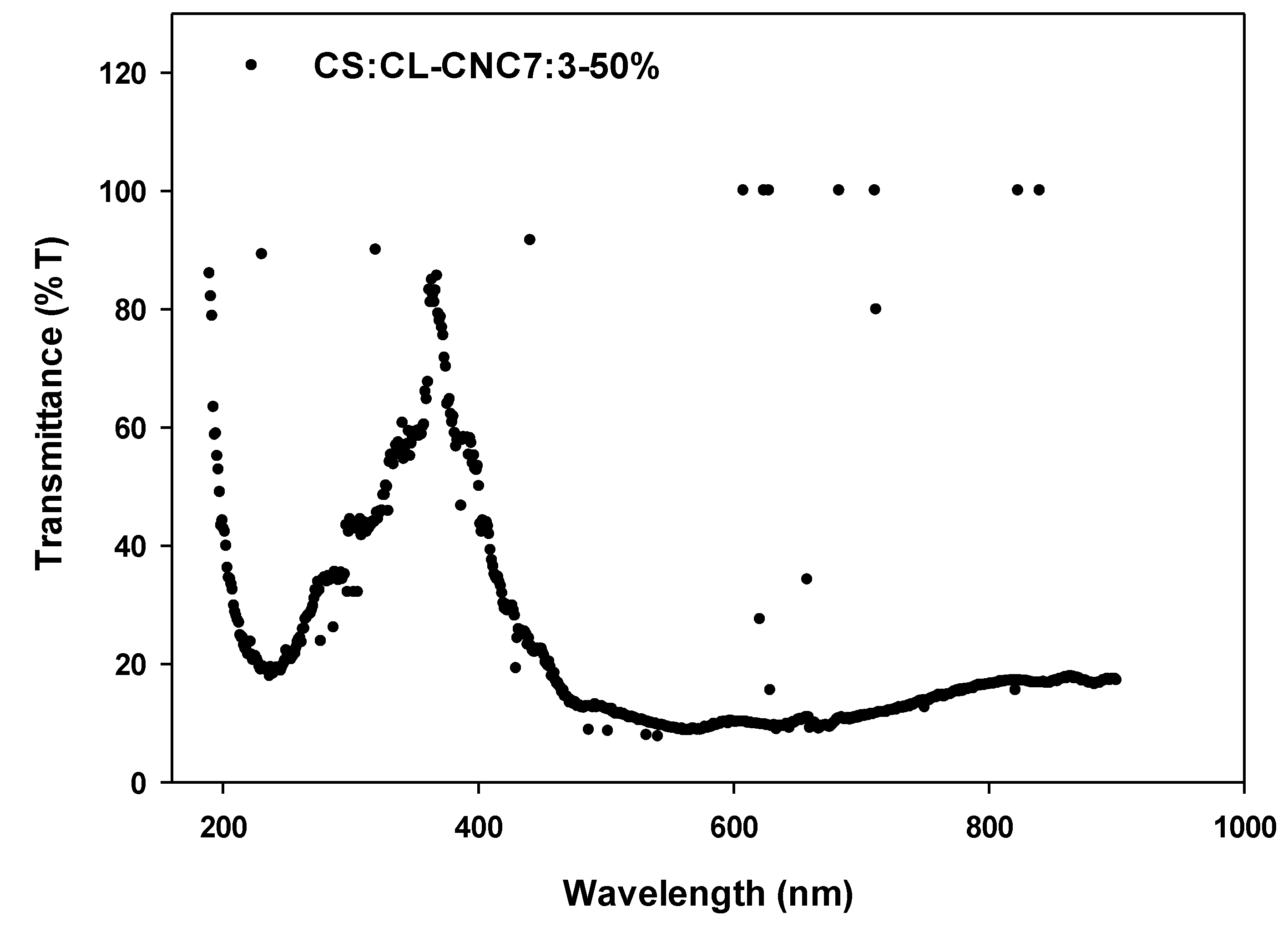
3.2.4. Optical Properties of the Synthesized Bio-Composites Films
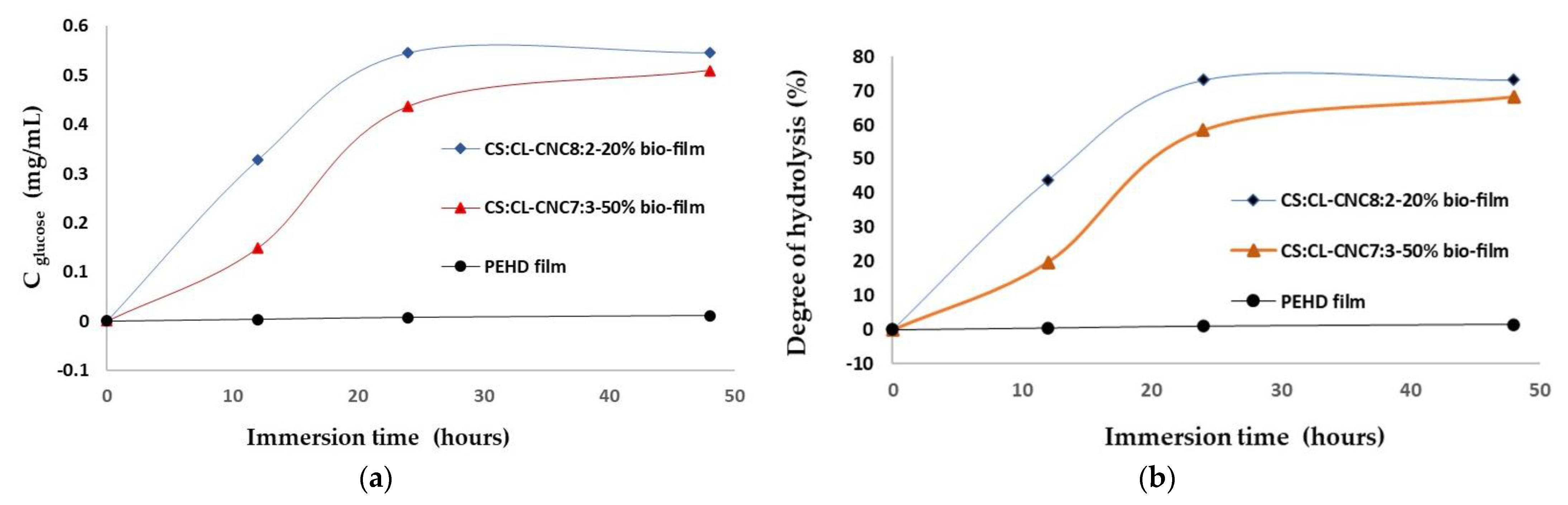
3.3. Biodegradation of Bio-Composite Films with Enzyme Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, P. The green economy transition: The challenges of technological change for sustainability. Sustain. Earth 2020, 3, 6. [Google Scholar] [CrossRef]
- Montero, B.; Rico, M.; Barral, L.; Bouza, R.; López, J.; Schmidt, A.; Bittmann-Hennes, B. Preparation and characterization of bionanocomposite films based on wheat starch and reinforced with cellulose nanocrystals. Cellulose 2021, 28, 7781–7793. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; De Gruyter: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- Agustin, M.B.; Ahmmad, B.; De Leon, E.R.P.; Buenaobra, J.L.; Salazar, J.R.; Hirose, F. Starch-based biocomposite films reinforced with cellulose nanocrystals from garlic stalks. Polym. Compos. 2013, 34, 1325–1332. [Google Scholar] [CrossRef]
- Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Bahrami, B.; Behzad, T.; Salehinik, F.; Zamani, A.; Heidarian, P. Incorporation of Extracted Mucor indicus Fungus Chitin Nanofibers into Starch Biopolymer: Morphological, Physical, and Mechanical Evaluation. Starch-Stärke 2021, 73, 2000218. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Yin, Y.; Hong, Z.; Tian, X.; Zhu, Q.; Jiang, X.; Wang, H.; Gao, W. Cellulose nanocrystals modified with quaternary ammonium salts and its reinforcement of polystyrene. Polym. Bull. 2018, 75, 2151–2166. [Google Scholar] [CrossRef]
- Liu, D.; Sui, G.; Bhattacharyya, D. Synthesis and characterisation of nanocellulose-based polyaniline conducting films. Compos. Sci. Technol. 2014, 99, 31–36. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.S. Comparative Properties Analysis between Microcrystalline Cellulose and Cellulose Nanocrystals Extracted From Rice Straw. Malays. J. Microsc. 2019, 15. [Google Scholar]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Börjesson, M.; Westman, G. Crystalline Nanocellulose—Preparation, Modification, and Properties. In Cellulose: Fundamental Aspects and Current Trends; Books on Demand: Hambourg, Germany, 2015; pp. 159–191. [Google Scholar]
- Ramires, E.C.; Megiatto, J.J.D.; Dufresne, A.; Frollini, E. Cellulose Nanocrystals versus Microcrystalline Cellulose as Reinforcement of Lignopolyurethane Matrix. Fibers 2020, 8, 21. [Google Scholar] [CrossRef]
- Rosa, M.; Medeiros, E.; Malmonge, J.; Gregorski, K.; Wood, D.; Mattoso, L.; Glenn, G.; Orts, W.; Imam, S. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Mueller, S.; Weder, C.; Foster, E.J. Isolation of cellulose nanocrystals from pseudostems of banana plants. RSC Adv. 2013, 4, 907–915. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. Microcrystalline cellulose production from sugarcane bagasse: Sustainable process development and life cycle assessment. J. Clean. Prod. 2020, 249, 119342. [Google Scholar] [CrossRef]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Belali, N.G.; Chaerunisaa, A.Y.; Rusdiana, T. Isolation and Characterization of Microcrystalline Cellulose Derived from Plants as Excipient in Tablet: A Review. Indones. J. Pharm. 2019, 1, 55–61. [Google Scholar] [CrossRef]
- Ilyas, R.; Sapuan, S. The Preparation Methods and Processing of Natural Fibre Bio-polymer Composites. Curr. Org. Synth. 2020, 16, 1068–1070. [Google Scholar] [CrossRef]
- Hassan, I.F.; Ai-Jawhari, H. Polymer Nanocomposite Matrices. 2019. Available online: https://www.researchgate.net/publication/337161132_Polymer_Nanocomposite_Matrices (accessed on 12 February 2023).
- Ilyas, R.A.; Sapuan, S.; Ishak, M.; Zainudin, E.S.; Mahamud, A. Nanocellulose Reinforced Starch Polymer Composites: A Review of Preparation, Properties and Application. In Proceedings of the 5th International Conference on Applied Sciences and Engineering (ICASEA, 2018), Cameron Highlands, Malaysia, 14–15 March 2018. [Google Scholar]
- Lubis, M.; Gana, A.; Maysarah, S.; Ginting, M.H.S.; Harahap, M.B. Production of bioplastic from jackfruit seed starch (Artocarpus heterophyllus) reinforced with microcrystalline cellulose from cocoa pod husk (Theobroma cacao L.) using glycerol as plasticizer. IOP Conf. Series Mater. Sci. Eng. 2018, 309, 012100. [Google Scholar] [CrossRef]
- Potenza, M.; Bergamonti, L.; Lottici, P.P.; Righi, L.; Lazzarini, L.; Graiff, C. Green Extraction of Cellulose Nanocrystals of Polymorph II from Cynara scolymus L.: Challenge for a “Zero Waste” Economy. Crystals 2022, 12, 672. [Google Scholar] [CrossRef]
- Obasi, H.C.; Chaudhry, A.A.; Ijaz, K.; Akhtar, H.; Malik, M.H. Development of biocomposites from coir fibre and poly (caprolactone) by solvent casting technique. Polym. Bull. 2018, 75, 1775–1787. [Google Scholar] [CrossRef]
- Gallardo-Sánchez, M.A.; Diaz-Vidal, T.; Navarro-Hermosillo, A.B.; Figueroa-Ochoa, E.B.; Ramirez Casillas, R.; Anzaldo Hernández, J.; Rosales-Rivera, L.C.; Soltero Martínez, J.F.A.; García Enríquez, S.; Macías-Balleza, E.R. Optimization of the Obtaining of Cellulose Nanocrystals from Agave tequilana Weber Var. Azul Bagasse by Acid Hydrolysis. Nanomaterials 2021, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.T.; Dupré, E.F.; McCall, E.R. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons: Part II: Chemical Modifications. Text. Res. J. 1958, 28, 542–554. [Google Scholar] [CrossRef]
- Kruer-Zerhusen, N.; Cantero-Tubilla, B.; Wilson, D.B. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2017, 25, 37–48. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ioelovich, M. Optimal Conditions for Isolation of Nanocrystalline Cellulose Particles. Nanosci. Nanotechnol. 2012, 2, 9–13. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Cellulose Fibers: Bio- and Nano-Polymer Composites: Green Chemistry and Technology; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Bodros, E.; Baley, C. Study of the tensile properties of stinging nettle fibres (Urtica dioica). Mater. Lett. 2008, 62, 2143–2145. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.D. Recent developments in chemical modification and characterization of natural fiber-reinforced composites. Polym. Compos. 2007, 29, 187–207. [Google Scholar] [CrossRef]
- Teixeira, E.D.M.; Corrêa, A.C.; Manzoli, A.; Leite, F.; De Oliveira, C.R.; Mattoso, L.H.C. Cellulose nanofibers from white and naturally colored cotton fibers. Cellulose 2010, 17, 595–606. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Zhou, J.; Zhang, L. Structure and physical properties of methylcellulose synthesized in NaOH/urea solution. Polym. Bull. 2006, 56, 349–357. [Google Scholar] [CrossRef]
- Qi, H.; Liebert, T.; Meister, F.; Heinze, T. Homogenous carboxymethylation of cellulose in the NaOH/urea aqueous solution. React. Funct. Polym. 2009, 69, 779–784. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.; Misra, M. Characterization of natural fiber surfaces and natural fiber composites. Compos. Part A: Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Isogai, A.; Atalla, R.H. Dissolution of Cellulose in Aqueous NaOH Solutions. Cellulose 1998, 5, 309–319. [Google Scholar] [CrossRef]
- Naduparambath, S.; Purushothaman, E. Sago seed shell: Determination of the composition and isolation of microcrystalline cellulose (MCC). Cellulose 2016, 23, 1803–1812. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Zhong, L.; Fu, S.; Peng, X.; Zhan, H.; Sun, R. Colloidal stability of negatively charged cellulose nanocrystalline in aqueous systems. Carbohydr. Polym. 2012, 90, 644–649. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; Malladi, R.; Afrin, S.; Ansari, M.; Asad, M.; Karim, Z. Cellulose Nanocrystals-Based Nanocomposites: Preparation, Processing, Properties & Performance. In Bio-Based Polymers and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 49–65. [Google Scholar]
- Sain, M.; Panthapulakkal, S. Bioprocess preparation of wheat straw fibers and their characterization. Ind. Crop. Prod. 2006, 23, 1–8. [Google Scholar] [CrossRef]
- Carrillo, I.; Mendonça, R.T.; Ago, M.; Rojas, O.J. Comparative study of cellulosic components isolated from different Eucalyptus species. Cellulose 2018, 25, 1011–1029. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Zhou, D. Cellulose Nanocrystals (CNCs) from Corn Stalk: Activation Energy Analysis. Materials 2017, 10, 80. [Google Scholar] [CrossRef]
- Mihály, J.; Deák, R.; Szigyártó, I.C.; Bóta, A.; Beke-Somfai, T.; Varga, Z. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and C H stretching vibrations. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 459–466. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Fernández Pérez, A.; Reyes, G.; Oviedo, C.; Gacitúa, W.; Gonzalez, R.; Uyarte, O. Isolation and Characterization of Cellulose Nanocrystals from Rejected Fibers Originated in the Kraft Pulping Process. Polymers 2018, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, X.; Zhang, J.; Guo, D.; Kong, F.; Zhang, N. Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr. Polym. 2015, 125, 360–366. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Trabelsi, L.; M’Sakni, N.H.; Ouada, H.B.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol. Bioprocess Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the green macroalgae Ulva lactuca: Isolation, characterization, optotracing, and production of cellulose nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Effect of Alkali Treatment on Crystalline Structure of Cellulose Fiber from Mendong (Fimbristylis globulosa) Straw. Key Eng. Mater. 2014, 594–595, 720–724. [Google Scholar] [CrossRef]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in I β cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. IR Study on Cellulose with the Varied Moisture Contents: Insight into the Supramolecular Structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Júnior; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocoll. 2015, 46, 93–102. [Google Scholar] [CrossRef]
- Orue, A.; Corcuera, M.A.; Peña, C.; Eceiza, A.; Arbelaiz, A. Bionanocomposites based on thermoplastic starch and cellulose nanofibers. J. Thermoplast. Compos. Mater. 2014, 29, 817–832. [Google Scholar] [CrossRef]
- van Soest, J.J.; Tournois, H.; de Wit, D.; Vliegenthart, J.F. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Liu, P.; Xie, F.; Li, M.; Liu, X.; Yu, L.; Halley, P.J.; Chen, L. Phase transitions of maize starches with different amylose contents in glycerol–water systems. Carbohydr. Polym. 2011, 85, 180–187. [Google Scholar] [CrossRef]
- Ai, B.; Zheng, L.; Li, W.; Zheng, X.; Yang, Y.; Xiao, D.; Shi, J.; Sheng, Z. Biodegradable Cellulose Film Prepared From Banana Pseudo-Stem Using an Ionic Liquid for Mango Preservation. Front. Plant Sci. 2021, 12, 625878. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Z.; Chen, C.; Xia, Q.; Zhou, Y.; Jing, S.; Wang, R.; Ray, U.; Gan, W.; Li, C.; et al. All-Natural, Degradable, Rolled-Up Straws Based on Cellulose Micro- and Nano-Hybrid Fibers. Adv. Funct. Mater. 2020, 30, 1910417. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. Int. J. Food Prop. 2019, 22, 925–941. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Metzger, C.; Briesen, H. Thermoplastic Starch Nanocomposites Reinforced with Cellulose Nanocrystal Suspensions Containing Residual Salt from Neutralization. Macromol. Mater. Eng. 2021, 306, 2100161. [Google Scholar] [CrossRef]
- Bahrami, M.; Abenojar, J.; Martínez, M. Recent Progress in Hybrid Biocomposites: Mechanical Properties, Water Absorption, and Flame Retardancy. Materials 2020, 13, 5145. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Zhang, Z.; Richardson, M. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Thomason, J.L. The interface region in glass fibre-reinforced epoxy resin composites: 2. Water absorption, voids and the interface. Composites 1995, 26, 477–485. [Google Scholar] [CrossRef]
- Herrera-Gómez, A.; Velázquez-Cruz, G.; Martín-Polo, M. Analysis of the water bound to a polymer matrix by infrared spectroscopy. J. Appl. Phys. 2001, 89, 5431–5437. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Biria, D. The synergetic effect of starch and alpha amylase on the biodegradation of n-alkanes. Chemosphere 2016, 152, 166–172. [Google Scholar] [CrossRef]
- Yahia, R.; Owda, M.E.; Abou-Zeid, R.E.; Abdelhai, F.; El-Gamil, H.Y.; Abdo, A.M.; Ali, A.A. Biodegradable, UV absorber and thermal stable bioplastic films from waxy corn starch/polyvinyl alcohol blends. Biomass-Convers. Biorefinery 2023, 1–8. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.; Amaraweera, S.M.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Kulatunga, A.K.; Rajapaksha, S.M.; Dassanayake, R.S.; Fernando, C.A.N.; et al. Compatibilization of Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications: A Review. J. Compos. Sci. 2021, 5, 300. [Google Scholar] [CrossRef]
| Sample Code | CS (g) | NaOH (mL) | CL-CNC (g) | CS (g) | Distilled Water (mL) | Glycerol (mL) |
|---|---|---|---|---|---|---|
| CS:CL-CNC 7:3-50% | 7.00 | 40 | 3.00 | 7.00 | 140 | 90.0 |
| CS:CL-CNC 8:2-20% | 8.00 | 40 | 2.00 | 8.00 | 160 | 40.0 |
| LOI A1430/A908 | TCI A1326/A2903 | HBI A3350/A1326 | |
|---|---|---|---|
| CL-MCC | 0.994 ± 0.005 | 1.011 ± 0.006 | 1.014 ± 0.002 |
| CL-CNC | 1.003 ± 0.005 | 1.023 ± 0.006 | 1.035 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsufyani, T.; M’sakni, N.H. Part A: Biodegradable Bio-Composite Film Reinforced with Cellulose Nanocrystals from Chaetomorpha linum into Thermoplastic Starch Matrices. Polymers 2023, 15, 1542. https://doi.org/10.3390/polym15061542
Alsufyani T, M’sakni NH. Part A: Biodegradable Bio-Composite Film Reinforced with Cellulose Nanocrystals from Chaetomorpha linum into Thermoplastic Starch Matrices. Polymers. 2023; 15(6):1542. https://doi.org/10.3390/polym15061542
Chicago/Turabian StyleAlsufyani, Taghreed, and Nour Houda M’sakni. 2023. "Part A: Biodegradable Bio-Composite Film Reinforced with Cellulose Nanocrystals from Chaetomorpha linum into Thermoplastic Starch Matrices" Polymers 15, no. 6: 1542. https://doi.org/10.3390/polym15061542
APA StyleAlsufyani, T., & M’sakni, N. H. (2023). Part A: Biodegradable Bio-Composite Film Reinforced with Cellulose Nanocrystals from Chaetomorpha linum into Thermoplastic Starch Matrices. Polymers, 15(6), 1542. https://doi.org/10.3390/polym15061542






