Abstract
With the degradation after aging and the destruction of high-intensity exercise, the frequency of tendon injury is also increasing, which will lead to serious pain and disability. Due to the structural specificity of the tendon tissue, the traditional treatment of tendon injury repair has certain limitations. Biodegradable polymer electrospinning technology with good biocompatibility and degradability can effectively repair tendons, and its mechanical properties can be achieved by adjusting the fiber diameter and fiber spacing. Here, this review first briefly introduces the structure and function of the tendon and the repair process after injury. Then, different kinds of biodegradable natural polymers for tendon repair are summarized. Then, the advantages and disadvantages of three-dimensional (3D) electrospun products in tendon repair and regeneration are summarized, as well as the optimization of electrospun fiber scaffolds with different bioactive materials and the latest application in tendon regeneration engineering. Bioactive molecules can optimize the structure of these products and improve their repair performance. Importantly, we discuss the application of the 3D electrospinning scaffold’s superior structure in different stages of tendon repair. Meanwhile, the combination of other advanced technologies has greater potential in tendon repair. Finally, the relevant patents of biodegradable electrospun scaffolds for repairing damaged tendons, as well as their clinical applications, problems in current development, and future directions are summarized. In general, the use of biodegradable electrospun fibers for tendon repair is a promising and exciting research field, but further research is needed to fully understand its potential and optimize its application in tissue engineering.
1. Introduction
Under continuous economic pressure and a global aging environment, the treatment of musculoskeletal diseases is still a major challenge. As a component of the musculoskeletal system, the tendon is the connective tissue connecting muscle and bone, which can store and transfer energy during exercise and play a buffer role. As the tendon bears repeated load strength, it will lead to tearing and pathological changes of the tendon, and then cause pain, swelling, loss of tissue integrity, and functional damage [1,2]. At present, the traditional treatment methods for repairing or replacing damaged tendons, including autotransplantation, allograft transplantation, and prosthetic devices, rarely repair the shape and structure of normal tendons and have some inevitable shortcomings, such as re-rupture of the injured site, adhesion formation, scar formation, and long-term functional defects [3,4]. As tendon tissue repair involves complex coordination between the internal tendon core tissue and the surrounding external synovial tissue, it is necessary to find new and more effective treatment methods to cure tendon injury.
With the maturity of electrospinning technology and the development of technology, electrospinning has a variety of spinnable materials, simple devices, low preparation cost, controllable process, and high production efficiency. The nanofibers prepared using this technology have the advantages of a large specific surface area, high porosity, and adjustable diameter, which can mimic the natural extracellular matrix (ECM) in morphology, so it has great potential in tendon repair [5,6]. Electrospinning equipment mainly consists of a high-voltage power supply, injection pump, spinneret, and receiving plate [7]. In the process of spinning, the high-voltage power supply causes the surface of the droplets at the spinneret to produce a coulomb repulsion force opposite to the surface tension. When the coulomb repulsion force is greater than the surface tension, the droplets spray a thin stream at the conical apex. If the electric field voltage and polymer viscosity are appropriate, the jet stream is stretched and extended, and finally sprayed onto the collection device in a curved and unstable shape to form ultrafine nanofibers [8,9,10,11].
Different preparation methods and raw materials have an impact on the morphology and properties of the final fiber. By selecting the appropriate polymers and process parameters, it is easy to produce fiber structures with different morphologies from tens of nanometers to several microns in diameter, and at the same time, it can produce ECM nanofiber scaffolds with high mechanical strength and bionics [12]. In addition, many studies have also incorporated biomolecules, cells, nanoparticles or drugs into electrostatically spun nanofibre membranes, which can effectively improve their anti-adhesion and repair effects [13,14,15]. The electrospun fibers are structurally controllable, and the electrospun structures of different dimensions will have different effects on cells. A large number of previous studies have proved that, compared with two-dimensional (2D) scaffolds, three-dimensional (3D) nanofiber scaffolds are more conducive to cell morphology change, migration and cell-cell interaction [16]. Traditional 2D biomaterials (e.g., fiber sheets and bio-membranes) are usually stacked very tightly, resulting in a pore size that is too small, which limits cell infiltration and three-dimensional tissue regeneration [17]. The use of a biomimetic 3D nanofiber scaffold can overcome this obstacle and provides the necessary microenvironment for natural cell growth. The terrain and biomechanical clues provided by 3D-oriented nanofibers can guide the tendon differentiation of stem cells and maintain the phenotype of natural tendon cells [18].
The purpose of this review was to summarize the different structures of tendon repair product scaffolds prepared using electrospinning technology and their applications in the biomedical field (Figure 1). Biodegradable polymer electrospinning with different 3D structures is used for tendon repair. Bioactive molecules can optimize the structure of these products and improve their repair performance. The combination of other advanced technologies has greater potential in tendon repair. Biomaterials mixed with electrospinning are reviewed to discuss the influence of different biomaterials on the structure and properties of electrospinning. By discussing the advantages of an electrospun 3D structure in different stages of tendon repair, the current commercial products are listed, highlighting the current development progress. Finally, the opportunities, challenges, and new directions of electrospinning in future development are discussed. It provides a reference for the development of electrospinning technology in tendon repair. Of note, while electrospun fibers have the potential to be an effective tool for tendon repair, there are some potential limitations to consider, and further research is needed to fully understand its potential and optimize its application in tissue-engineering applications.
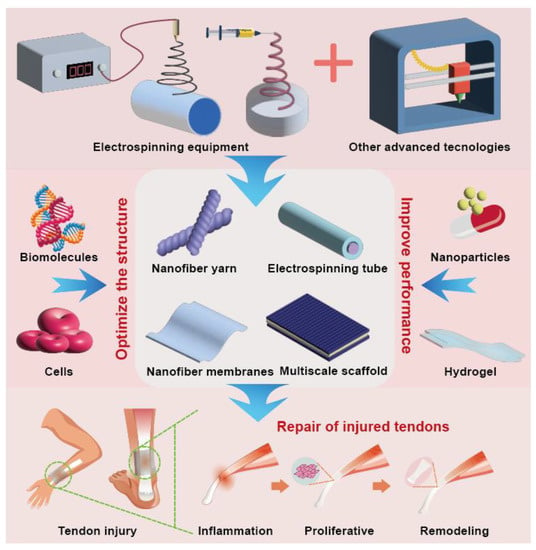
Figure 1.
Diagram of electrospinning technology and its applications in the biomedical field.
2. Repair Process after Tendon Damage
Natural tendon tissue has a layered fiber structure, which is composed of collagen arranged into fibers (type I collagen accounts for 95%) and cells with highly anisotropic mechanical properties. Tendon cells belong to the fibroblast family and are the most common cell type in tendon tissue. They are responsible for forming the structural framework of tendons and can effectively transmit force during muscle contraction [19]. Compared with other musculoskeletal tissues, the repair process is relatively slow due to its inherent connective tissue structure, poor vascular network, and low cell metabolic rate. The process of tendon healing is complex, involving internal and external mechanisms. The intrinsic mechanism is due to the regeneration of the cell group originating from the tendon parenchyma and/or tendon sheath. The external mechanism occurs because the external cells recruit from outside of the injured tendon to the injured site. The extrinsic mechanism involves the migration of cell populations from the blood and surrounding tissues (such as peritendinous tissue and synovial sheath) [20]. In addition, different repair processes will occur after tendon injury in different parts. For example, flexor tendon injury is caused by fibroblasts migrating from the tendon sheath to the tendon and starting to heal, while rotator cuff injury is caused by fibroblasts being exported from the tendon to the bone surface [21].
The first stage of tendon healing is the short cell phase of tendon cells and inflammatory cells invading the wound. After the injury, the initial inflammatory phase begins after a large number of cells die. Subsequently, inflammatory cells, such as neutrophils, monocytes, and macrophages, infiltrate the damaged area. The second stage is the proliferation stage of fibroblasts, which involves the migration of cells to the injured site and the proliferation and deposition of collagen fibers. Finally, in the remodeling stage, which lasts from several months to several years, tendon cells and collagen fibers are arranged along the longitudinal axis of the tendon in the direction of stress, the production of type I collagen increases, and the number of cells decreases [22]. Tendon contains fewer cells and blood vessels than other tissues. Tendon will not regenerate after injury, but will heal through excessive and disordered ECM deposition [23]. During the repair process, the synthetic level of type I collagen is significantly lower than that of healthy tendons. Instead, fibroblasts synthesize randomly oriented type III collagen, which is easy to cause fibrosis of tendon tissue, resulting in poor tissue quality, structural loss, and reduced mechanical strength [24]. In addition, the long healing process of injured tendons usually leads to the formation of fibrous vascular scar tissue, but the shape and mechanical and functional properties of repaired tendons are difficult to return to a normal state, and the risk of re-injury is increased. The adhesion site causes insufficient lubrication between the tendon and surrounding tissues, which can lead to friction and pain [25]. Therefore, it is more beneficial for tendon repair to develop therapies that can regulate different stages and/or different needs to promote regenerative healing.
3. Application of Biodegradable Polymers in Tendon Repair
3.1. Application of Natural Biodegradable Polymers in Tendon Repair
Common biodegradable polymers used in tendon tissue engineering include natural biodegradable polymers and synthetic biodegradable polymers. Compared with synthetic polymers, tendon tissue engineering scaffolds prepared from natural polymers have unique advantages: better cell activity and adhesion, as well as a higher biocompatibility and degradation rate than other materials [26]. Common natural biodegradable polymers used for tendon tissue engineering include collagen, silk protein, gelatin, hyaluronic acid, etc. [27]. In tissue engineering, natural biodegradable polymers are used to prepare tissue scaffolds, which can be combined with growth factors [28] and stem cells [29], or used as drug carriers [30] to reconstruct the natural microenvironment of tendons [31] and help cell adhesion, growth, and differentiation [32]. The scaffold made of natural biodegradable polymer plays a temporary role in supporting the damaged tendon in the human body. During the synthesis and recovery of natural tissue, it can slowly degrade and produce non-toxic metabolites, which are absorbed by surrounding tissues [33]. Here, we briefly discuss the role of some natural biodegradable polymers in tendon repair.
In tendon tissue engineering, collagen, as the main component of the human tendon, is a natural degradable polymer with a biomimetic tendon ECM structure. Due to its good cell and growth factor binding sites on the surface, it has the function of supporting cell adhesion, migration, growth, and differentiation [34]. Tissue engineering technology has been used to combine a collagen scaffold with growth factors to help repair damaged tendons. Sun et al. [35] used a functional collagen scaffold loaded with recombinant stromal cell-derived factor-1α containing a collagen-binding structural domain (CBD) to recruit mesenchymal stem cells (MSCs) and Achilles tendon fibroblasts, and promote in situ tendon regeneration. In addition, due to its biodegradable properties and good biocompatibility, it achieves both a sustained drug-retentive treatment of the damaged tendon area, promotes the formation of type I collagen, and reduces the inflammatory response due to the implanted scaffold.
Silk proteins have good strength and toughness compared to most natural biodegradable polymers [36] and have a variety of applications in tissue engineering, wound healing, and drug delivery [36]. Silk proteins mimicking the native interface may be more effective in supporting tissue regeneration, maintaining good tensile strength, and initiating tendon regeneration for at least 1 year in the in vivo environment of damaged tendons [29]. Teuschl et al. [37] used silk protein woven into a wire rope shape as an anterior cruciate ligament substitute and combined silk protein fibrous scaffolds with adipose-derived stem cells to promote ligament regeneration in goats over a period of 6 to 12 months, and progressive degradation of silk fibers occurred while regenerating tissue increased.
Gelatin is a unique fibrin, a collagen derivative that mimics the natural connective tissue microenvironment [38]. It significantly improves the infiltration, adhesion, spread, and proliferation of cells on tissue-engineered scaffolds and has low immunogenicity and biodegradability [39]. Liu et al. [27] prepared membranes made from gelatin using the electrostatic spinning technique with micron-sized 3D structures, which significantly promoted the regeneration of type I collagen in a rat chronic rotator cuff tear model. The gelatin scaffolds prepared using electrostatic spinning showed good cytocompatibility and were able to mimic the natural tendon tissue microenvironment to accelerate tendon healing and repair after damage. The gelatin scaffold’s similar structure to the tendon extracellular matrix aided endothelial cell vascularization and transport of active substances in the damaged and healing tendon. The degradation products of gelatin have no side effects on tendon tissue and are slowly enzymatically cleaved as the tendon is stimulated by the gelatin scaffold to return to normal function.
3.2. Limitations and Future Progress Related to the Use of Biodegradable Polymers in Tendon Repair
Although biodegradable polymers have made great strides in the field of regenerative medicine, it is still difficult for any single polymer to meet all the necessary biochemical needs [40]. In order to develop high-performance stents that meet clinical needs, a number of key points need to be considered. Firstly, a rational design of the stent source material is required [41]. Secondly, overcoming problems with mechanical properties and degradation rates requires improved stent-preparation techniques [42]. Natural biodegradable polymers, such as collagen and gelatin, usually share the common disadvantage of lacking mechanical properties and having uncontrolled degradation rates [43]. Collagen, the first naturally occurring biodegradable polymer to be used as a scaffold, is not mechanically strong enough to provide post-operative mechanical support after a tendon tear [34]. To overcome this disadvantage, electrostatic spinning scaffolds have been extensively researched and applied. Electrostatic spinning scaffolds is a technique for spinning polymeric materials into a fibrous form through the means of an electrical charge, which is highly controllable and adjustable. By adjusting the spinning parameters, such as the charge density, flow rate, and distance, fibrous scaffolds of different diameters, morphologies, and porosities can be obtained [44]. The degradation rate and tissue repair of the electrostatic-spun scaffold can also be adjusted by controlling the spinning parameters [45]. For example, Xie et al. [46] designed a collagen/polylactic acid (Col/PLA) hybrid yarn that blended a natural polymer with a synthetic polymer to take advantage of the mechanical strength of the PLA and the biological activity of collagen. The hybrid tendon scaffold combining the biological and synthetic components had the sufficient tensile strength to maintain normal tendon function while promoting host tissue healing. With this in mind, we focus on the prospects for the application of electrostatic spinning technology in tendon repair in the following section.
4. Functional Electrospinning Products of 3D Structures for Tendon Repair
Electrospinning technology occurs when a viscoelastic polymer fluid is extruded from a spinneret under the electrostatic repulsive force of a strong electric field, and the surface tension produces a hanging droplet. After electrification, the electrostatic repulsion between surface charges with the same symbol causes the droplet to be deformed into a Taylor cone, and the charged jet is ejected from the Taylor cone [47]. Different preparation processes will produce multiscale structure repair products. In the application of tendon repair, the main products are nanoyarn, nanotube, spinning film, and a layered multiscale structure electrospinning scaffold (Figure 2A). Different structures have shown an excellent performance in tendon repair.
4.1. Nanofiber Yarn
Tendon is a highly anisotropic tissue, in which the collagen fibers are arranged uniaxially and parallel to each other. Tendon fibroblasts showed highly organized and randomly oriented morphologies in aligned and random parts, respectively [48]. Uniaxially aligned nanofibers can provide contact guidance and alignment for tendon fibroblasts, and can be used for tendon regeneration when their ECMs are organized into highly ordered structural arrays. Tendon fibroblasts showed highly organized and randomly oriented morphologies in aligned and random parts, respectively. Uniaxially aligned nanofibers can provide contact guidance and alignment for tendon fibroblasts, and organize their ECMs into highly ordered structures [49]. Porous engineering electrospun nanofiber materials have highly customized pore traps, topological porous channels, and a high surface area. Compared with oriented electrospun nanofibers, porous nanofibers generally show stronger activity and performance. Macroporous fiber materials usually show a higher loading capacity and stronger accessibility to active sites [50,51]. In addition, compared with linear uniaxially aligned nanofibers, the uniaxially aligned nanofibers with controllable curvature can better simulate the anatomical structure of collagen fibrils in tendon tissue, and better protect tendon fibroblasts under uniaxial strain [52], providing more cell-adhesion sites [53].
Inspired by the multiscale helical fiber structure of natural biological tissue, the helical nanoyarn with a multiscale structure can be prepared using electrospinning and continuous twisting technology. The structure has excellent mechanical properties and ultra-high extensibility. The multiscale periodic topological structure on the material surface can not only change the physical characteristics of cells (survival rate, volume, orientation, growth and shedding), but can also induce the directional differentiation of mesenchymal stem cells into muscle cells by regulating the transport of cell types and specific transcription factors to the nucleus [54]. Electrospun nanofibers coated with polycaprolactone (PCL) or poly (3-hydroxybutyric acid) (P3HB) and twisted silk fibroin (SF) yarn form a nano/micro hybrid structure. PCL or P3HB with good biocompatibility can provide the mechanical strength for the scaffold. A SF yarn contains the unique crimp structure of tendon tissue. The scaffold can promote the adhesion and attachment of fibroblasts and proliferate at a higher speed [55].
When tendons are injured, it is difficult to balance the relationship between exercise and rest. Fortunately, Wang et al. achieved the goal of repairing tissues while exercising properly. They first prepared a kind of electrospun nanofiber composite membrane with a PCL/polyurethane (PU)/PCL sandwich structure, then twisted the fiber membrane into a straight yarn, and then further twisted it to form a spiral yarn support. Due to the non-planar movement and high extensibility of the scaffold, when the rat bone marrow cells cover the whole scaffold to stretch the scaffold, the cells can maintain a high survival rate under the cyclic strain, and gradually proliferate with the passage of time (Figure 2B) [19]. In addition to the twisting technology, the rotating spindle collecting device can also make the aligned fibers curl. The resulting aligned crimped fibers have the same amplitude and wavelength as natural collagen fibers. The crimped fibers can be used to guide the proliferation of fibroblasts in vitro and the synthesis of ECM [56]. The combination of the hot drawing process and electrospinning can enhance the mechanical strength and biological properties of a nanoyarn [57,58].
4.2. Electrospinning Tube
In the surgical treatment of a ruptured tendon, the development of a nanofibrous tube that prevents penetration between the tendon and the surrounding tissues is a possible solution to improve the adhesions that may occur at the injury site in order to cope with the bleeding and infection during the healing process that lead to adhesions. Copolymers of polyhydroxybutyrate and ε-caprolactone (DegraPol®, DP) are biocompatible and biodegradable, and have proven to be ideal tendon materials for electrostatic spinning tubes [59,60]. By using electrostatically spun DP tubing to treat a rabbit model of Achilles tendon rupture, Buschmann et al. found that placing fibrous tubing around a transected Achilles tendon for 12 weeks did not result in overexpression of the inflammatory-associated protein, significantly reduced the formation of adhesions, and underwent partial degradation [61]. In another study that used the strategy of free delivery of growth factors or drugs based on the scaffold, the block copolymer DP was mixed with platelet-derived growth factor BB (PDGF-BB), and a kind of PDGF-BB bilayer bioactive electrospinning tube was developed (Figure 2C). The double-layer structure had a biologically active inner layer and a non-biologically active outer layer, which could continuously deliver PDGF-BB at the tendon rupture and injury site to promote collagen synthesis and increase cell proliferation, while providing a physical barrier for surrounding tissues, thus, significantly reducing peritendinous adhesion [62]. In addition, tubular repair structures were developed in combination with tubular braids in order to enhance the mechanical properties and repair ability of the tubular structures. The electrostatic spinning of a new acrylate capped polyurethane based polymer was able to represent an optimal and controlled environment. Mechanical testing of isolated sheep tendons has shown that the minimum limiting stresses required for flexor tendon repair were fully met. The incorporation of anti-inflammatory and antiadhesive compounds has a minimal inflammatory response and adhesion to surrounding tissues to minimize scar tissue formation and improve the rate of collagenization [63]. An electrospinning tube, as a kind of product to wrap the injured part of tendon, has the application potential of preventing peritendinous adhesion and repairing.
4.3. Electrospinning Nanofiber Membrane
Compared with random fibers, oriented fibers have better mechanical properties, faster charge transfer, and a more regular spatial structure. The oriented nanofiber structure will change the cell phenotype, thus, affecting the differentiation of stem cells [64]. A hydroxyapatite (HA)/polylactic acid (PLLA)-oriented nanofiber membrane prepared using electrospinning has a rough surface and weak hydrophobicity. The fiber membrane has good cell compatibility, and the cells grow along the fiber arrangement direction, which can effectively promote tendon–bone healing and reduce the rate of rotator cuff repair and tear [65]. When poly-D-L-lactide-glycolide (PLGA) is used as the polymer material of the nanofiber membrane, doxycycline drug elutes the nanofiber membrane. Histological examinations and monitoring of postoperative animal activity levels have shown that the nanofiber membrane can promote the healing of tendon rupture, and can be used as an auxiliary tool for Achilles tendon homotransplantation and reconstruction surgery [66].
Electrospun nanofiber membranes also play an antiadhesive role during tendon repair. However, most electrospun membranes promote cell adhesion due to their relatively hydrophobic surface [67]. The zwitterionic polymer chain is generated in situ from the photopolymerization initiated by the sub-surface of the nanofiber from the inside out, forming a solid network interpenetrating with the polymer chain of the nanofiber matrix, and then the super-lubricated nanofibrous membranes (SLNMs) is grown in situ on each electrospun nanofiber. Research using a rat tendon adhesion model and an abdominal adhesion model showed that a nanofiber membrane with SLNMs can prevent postoperative adhesion. Compared with two antiadhesion products (Interseed® and DK® membrane) used in the clinic, SLNMs can significantly reduce the adhesion around the tendon (Figure 2D). It is not only more effective, but also has the advantage of lower production costs [68]. In addition, a PCL/poly (2-methacryloxyethyl phosphate choline) (PMPC) composite nanofiber membrane with interconnected 3D porous structure, due to the presence of amphoteric ion PMPC, produces a hydrated lubricating surface. When the weight ratio of PMPC to PCL is 0.1, the minimum friction coefficient of the composite nanofiber membrane can reach 0.12. With the increase of mass ratio, the minimum friction coefficient can be less than 0.05. Highly lubricated surfaces effectively inhibit the adhesion of fibroblasts and significantly reduce tissue adhesion during tendon repair in vivo [69].
Compared with the single-layer nanofiber membrane, the double-layer membrane with random and arranged nanofibers in the outer and inner layers provides isotropic mechanical properties, which are more robust and tear resistant after being implanted in the body [70]. The change of pore size distribution in different layers is conducive to cell stratification [71]. Li et al. constructed a PLLA/nHA-PLLA bilayer organic/inorganic flexible bipolar fiber membrane (BFM) using electrospinning. Compared with PLLA’s simple fiber membrane (SFM), BFM significantly increased the glycosaminoglycan staining area at the tendon–bone interface, improved collagen tissue, and promoted the regeneration of attachment points in a rotator cuff tear [72].
4.4. Layered Electrospinning Scaffold
The multiscale structure and polymer type have different effects on the function and mechanical properties of tendon cells. Layered multiscale electrospinning scaffolds can not only highly simulate the hierarchical structure of ECM, but also induce different fibroblast morphological modifications under static and dynamic conditions, thus, changing their shape in the direction of the load application [73]. The layered structure porous scaffold is ingeniously designed and developed. Nanofibers can imitate cell tissue by bonding the biomimetic cell wall of micropores of different sizes (100–1000nm) [74]. In addition, a continuously graded calcium phosphate coating is formed on the non-woven mat of PLGA nanofibers. The mechanical properties and biological activities of the scaffold are significantly affected by the linear gradient of calcium phosphate content [75].
A multilayer nanofiber scaffold is usually made of electrospun high-conductivity solution. The polymer solution mixed with salt or bioactive molecules can adjust the fiber structure and function [76]. One multiscale fiber scaffold was composed of orderly arranged PCL microfibers/collagen bFGF nanofibers (mPCL-nCol-bFGF), and coated with sodium alginate to inhibit the surrounding adhesion. Rabbit tendon cells showed the highest expression of tendinous markers and cell proliferation under dynamic stimulation in vitro. Achilles tendon tissue was regenerated 12 weeks after operation, and collagen was arranged neatly (Figure 2E) [77]. Arranged PCL/Col multiscale fibers can mimic the tendon matrix and enhance porosity by sacrificing collagen nano fibers. Plasma treatment with argon and nitrogen enhances the roughness and hydrophilicity of the fibers, which can significantly enhance cell adhesion and proliferation, and induce tendon differentiation of MSCs [78]. Additionally, Liu et al., after freezing and vacuum drying a fresh amniotic membrane, effectively removed most of the cell components in the amniotic membrane to retain bioactive molecules, and built a growth factor release system that conformed to the tendon healing cycle. Combined with PCL nanofiber membrane, a biomimetic composite membrane with a tendon sheath and putamen structure was constructed, which enhanced the tensile resistance of the amniotic membrane. The 3D porous structure provided sufficient 3D space structure for cell growth. This upregulated the phosphorylation of ERK1/2 and SMAD2/3, promoted the adhesion and proliferation of tendon cells and fibroblasts, and improved collagen synthesis. The rabbit tendon repair model effectively isolated the exogenous tissue adhesion and promoted the endogenous healing of the tendon [79]. The significant advantage of layered and multiscale structures for electrospinning scaffolds is that each layer can have a different function and the different layers can work synergistically with each other to enable a wider range of applications.
The arrangement and topographic characteristics of the multilayer nanofiber scaffold can regulate its mechanical and biological activities [80,81,82]. A study confirmed that the multilayer arrangement scaffold is more conducive to cell proliferation and infiltration, enhanced collagen arrangement, and tendon-related gene expression than the multilayer non-arranged scaffold [80]. In another study, PCL or PLLA nanofiber sheets were stacked and woven together to form a multilayer scaffold aligned with electrospun nanofibers. They had similar nanoscale clues, but different macrostructures. The woven support showed higher tensile strength and suture retention strength, but lower modulus. Due to its larger surface area, the stacked scaffold was better in cell proliferation and migration, and promoted an increase in the total collagen content and total sulfated glycosaminoglycan content. There was no difference between the expression of tendon-related genes and the deposition of extracellular matrix protein [81]. To sum up, it is more beneficial to enhance the effectiveness of tendon repair by comprehensively considering the micro and macro structure of the multiscale scaffold for tendon tissue engineering.
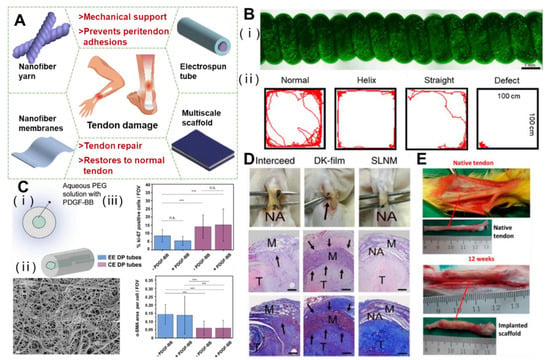
Figure 2.
Functional electrospinning products of 3D structures and the effect on tendon repair. (A) Schematic diagram of electrospinning for various tendon repair. (B) The helical scaffolds are biocompatible and restore motor function in the rat Achilles tendon.Reprinted with permission from Ref. [19]. Copyright 2022 Elsevier Inc. (C) Characterization of coaxial electrospun bilayer tubes, which promote recruitment and proliferation of tendon cells. (*** p < 0.001). Reprinted with permission from Ref. [62]. Copyright 2020 Elsevier Inc. (D) In vivo antitissue adhesion properties of the superlubricated electrospun nanofibrous membranes. The black arrows point to adhesion site. Reprinted with permission from Ref. [68]. Copyright 2022 Springer Nature Limited. (E) The mPCL-nCol-bFGF implantation promoted tendon regeneration after 12 weeks. Reprinted with permission from Ref. [77]. Copyright 2019 American Chemical Society.
5. Electrospinning Composite Scaffold for Tendon Repair
Researchers have recently combined electrospun nanofibers with emerging materials (such as minerals, metals, growth factors, stem cells, drugs, and nanoparticles) to further enhance their physical and chemical properties and biological activities (Figure 3A). The structure and porosity of the scaffold should promote cell activity and the formation of new tissues.
5.1. Parameter Control and Temporary Storage Disadvantages of Electrospinning Materials
Due to the uncontrollable parameters during the preparation of traditional electrospinning technology, the prepared electrospinning products usually have small pore sizes and random fiber diameters, which is not enough for cell infiltration and growth [83]. Therefore, it is still a challenge to manufacture electrospun products with a porosity that can simulate the complex spatial distribution of natural tissue. In order to improve the application performance of electrospinning products in tissue engineering, a variety of improvement strategies have been proposed. Near-field electrospinning (precision electrospinning or direct-writing electrospinning) has proved to be able to accurately control fiber deposition to form patterns. By fixing the spinneret on the motion table, the collector/spinneret can run according to the pre-programmed track [84]. This technology is crucial for the application of classical spinning products in biomedicine.
The shape and diameter of products prepared using electrospinning technology mostly depend on the process parameters. With the update in technology, the fiber diameter, diameter distribution, and orientation of electrospinning products are within the controllable and adjustable range as a result of controlling the conductivity, electric field strength, solution concentration, auxiliary electric field, and other factors [85,86,87,88]. With the development of electrospinning technology, the process parameters that affect the final properties of electrospinning products are more and more accurately controlled, and products with stable physical and chemical properties can be obtained. The electrospinning products prepared can be produced and sold in batches.
However, the weak mechanical properties of electrospun materials are a problem that cannot be ignored, and they are usually not suitable for hard tissue regeneration. It is a feasible method to enhance the tensile strength and other mechanical properties of electrospun products through subsequent extrusion, heating, and other methods [46]. At the same time, the degradation rate of electrospun nano-supports in vivo is not controllable, which can be overcome by mixing other bioactive substances to synthesize new electrospun materials or developing new spinning technologies.
5.2. Scaffold Mixed with Biological Factors
The use of exogenous growth factors has the potential to accelerate cell proliferation, collagen synthesis, and extracellular matrix synthesis, thereby accelerating recovery and enhancing repair. The basic fibroblast growth factor (bFGF) is usually selected as a representative growth factor that affects and enhances tendon/ligament repair and healing, cell differentiation, diffusion, proliferation, and ECM production [89]. Considering the advantages of the above growth factors, Liu et al. doped bFGF with dextran glassy nanoparticles and an electrospun PLLA-glucan composite fiber. Loaded growth factors maintain their biological activity for 30 days, release approximately 48.71 ± 13.53%, and enhance the viability of fibroblasts, collagenase production, capillary endothelial cell proliferation, and tissue regeneration [90]. However, due to the discontinuity, inhomogeneity, and defects introduced into the fiber structure, the mechanical properties of the polymer fiber may be reduced by adding particles into it. In another study, Zhao et al. tested a bFGF-loaded PLGA electrospun fiber membrane with a core–shell structure. Studies have shown that a bFGF-PLGA scaffold can enhance the formation of collagen tissue and fibrocartilage. In addition, compared with the PLGA scaffold, the system shows higher mechanical performance and stiffness. The results showed that the biological activity of bFGF could be preserved for 21 days, significantly accelerating tissue healing and remodeling [91]. Taking advantage bFGF’s ability to enhance the toughness of the product, Zhou et al. uniformly adhered bFGF/vascular endothelial growth factor A (VEGFA) to the surface of the suture through polydopamine. At multiple time points of the chicken model of flexor tendon injury, the final strength of the tendon treated with the bFGF/VEGFA release suture was significantly greater than that of the control suture group (Figure 3B), the tendon slip offset was significantly longer than that of the control naked suture, and the finger flexion work was significantly reduced. In the sixth week, the adhesion score of the bFGF/VEGFA-releasing suture group was significantly lower than that of the control suture group. bFGF/VEGFA has the same effect when applied to the rat Achilles tendon injury model [92]. Extracellular signal-regulated kinase 2 (ERK2) can block tendon adhesion by inhibiting fibroblast proliferation and indirectly reducing the deposition of type III collagen (Col III). The incorporation of ERK2-small interfering RNA (siRNA)/cationic 2,6-pyridyldicarboxylic aldehyde-polyvinimide (PDA) into a PLLA electrospun film can protect the biological activity of ERK2-siRNA and continuously release siRNA to achieve a long-term adhesion prevention effect (Figure 3C) [93].
5.3. Electrospinning Scaffold with Cells
Due to the limited number of stem cells in tendons, the low activity/repair ability severely limits the ability of tendon regeneration. MSCs have a strong self-renewal ability, differentiate into various cell lines, and produce abundant functional paracrine factors. Therefore, MSCs-mediated cell therapy has great potential as a new strategy for tendon tissue regeneration. An SF/methacrylic acid hydrogel (GelMA) bioactive nanofiber scaffold (Figure 3D) designed by Xue et al., silk fibroin, provides support strength and ductility for the scaffold, while methacrylic acid hydrogel promotes cell attachment and growth. The composite scaffold composed of the two is easy to adhere without additional fixation, and can be biodegradable under control. Inoculating mesenchymal stem cells on it greatly improves the efficiency of tendon regeneration [36].
Adipose-derived stem cells (ADSCs) are easy to obtain, have a large storage capacity, and have the potential to differentiate tendon cells. The mesh scaffold composed of a longitudinally arranged PGA fiber inner layer and a braided PGA and PLA fiber outer layer is evenly inoculated with ADSC on the fiber scaffold, and then implanted into the injured Achilles tendon after expansion in vitro. For the treatment in the rabbit Achilles tendon injury model, with the increase of implantation time, the cell seed constructs gradually formed new tendons and became more mature at 45 weeks. A histological examination confirmed the similarity between the new tendon and the natural tissue. Compared with the normal tendon, the tensile strength of the treatment group reached 60% [94].
Skeletal muscle-derived cells (MDCs) are a mixed cell group containing muscle cells, fibroblasts, satellite cells, and muscle-derived mesenchymal stem cells. Compared with mature and fully differentiated tendon cells, MDCs have higher proliferation potential [95]. MDCs from mice were inoculated onto PGA electrospun fibers and sutured to the defect. Compared with tendon cells, they can promote the formation of new tendons and produce stronger tendon tissue. Compared with tendon tissue, thicker collagen fibers show higher maturity. After 12 weeks, MDCs lost the expression of myoD and desmin, and the expression of tendon specific markers increased [96].
5.4. Mixed Scaffold Mixed with Nanoparticles
The performance and function of the original polymer structure can be enhanced by adding nanoparticles into the electrospun fiber. Rinoldi et al. incorporated silica nanoparticles into the beaded electrospun fiber structure, which could improve its wettability, stiffness, and degradation rate, and was conducive to cell proliferation and extracellular matrix deposition [97]. Hydroxyapatite has rich active sites and ion-exchange properties. Using the sol–gel method, HAp nanoparticles were incorporated into PCL/CS nanofiber scaffolds to promote the formation of porous structures in the PCL/CS matrix. A physical and chemical characterization and its biological properties showed that the structure was similar to that of human normal ligaments and tendons, which was conducive to the tendon regeneration reaction of human osteoblasts [98].
Infection after tendon surgery seriously prolongs the repair cycle. Due to the significant antibacterial activity of silver nanoparticles (Ag NPs), many studies have added them to the nanofiber scaffold to effectively reduce the inflammatory reaction and, thus, reduce the adhesion of tissues around the tendon [99,100,101]. Microsol electrospinning can be incorporated to control the release of hydrophilic drugs in hydrophobic polyester fibers. Hydrophilic mitomycin C (MMC) was loaded into HA, then encapsulated in PLLA fiber using electrospinning. The composite fiber membrane can control the stable release of MMC, and a low dose of MMC can inhibit the adhesion, proliferation, and apoptosis of fibroblasts, which is conducive to tendon healing [102]. In addition, zinc oxide nanoparticles (ZnO NPs) inhibit bacterial activity by releasing zinc ions, and participate in various biological processes such as metabolism, cell proliferation and differentiation, and gene expression control. A PCL/HA/ZnO film was constructed using the electrospinning method. After primary mouse fibrochondrocytes and primary mouse tendon cells were cultured on the film, the PCL-5% HA-1% ZnO film (the weight ratio of HA and ZnO was 5% and 1% respectively) showed good antibacterial specificity and excellent cell compatibility and cell adhesion. The PCL-5% HA-1% ZnO film also promoted osteogenesis, cartilage formation, fibrocartilage formation, and tendon healing [103].
5.5. Electrospun Nanofiber/Hydrogel Composite 3D Scaffold
In order to improve the biological reaction of electrospun nanofiber structure, the use of hydrogels with excellent performance is also a feasible strategy. Rinoldi et al. coated a thin layer of GelMA hydrogel containing mesenchymal stem cells on the electrospun nanofiber substrate of PCL/nylon-6 to prepare an optimized 3D multilayer composite scaffold, which can achieve sufficient mechanical and physical properties as well as cell viability and proliferation. Bone morphogenetic protein 12 (BMP-12) was added to promote the differentiation of MSCs, and mechanical stimulation was performed on the scaffold containing cells to imitate the natural function of tendons. The results showed that the synergistic effect of mechanical and biochemical stimulation was conducive to the enhancement of cell viability, proliferation, arrangement and tendon differentiation [104].
The sliding compression of the tendon is prone to rupture of the hydrogel, leading to changes in the polarity of macrophages related to biomaterials and inducing inflammation [105]. The hydrogel coating on the electrospun fiber membrane can enhance the support performance of the scaffold. Self-repairing and deformable HA hydrogel was attached to PCL electrospun nanofibers to form a composite double-layer patch. The electrospun nanofibers of the hydrogel could reduce inflammation and acted as a physical barrier to prevent adhesive tissue from invading the repaired tendon (Figure 3E) [106].
5.6. Electrospinning Combined with Other Advanced Technologies
The combination of electrospinning and other advanced technologies makes up for their respective technical defects and can give full play to the advantages of multitechnology cooperation. For example, electrospinning combined with 3D printing technology shows controlled mechanical properties and improves cell adhesion and proliferation [107]. Touré et al. innovatively electrospun fiber mats of PCL and polyglycerol sebacate (PGS) directly onto 3D-printed PCL–PGS blends containing bioactive glass on one side of the mesh. The scaffold showed good cell growth and penetration of 3D porosity, and excellent mechanical properties and degradation rates [108]. Guner et al. assembled fiber mats produced with rotary jet spinning and wet electrostatic spinning into a biphasic fiber carrier. The outer shell of the duplex support consisted of aligned PCL fibers to ensure better mechanical properties and strength. The support core consisted of PCL or PCL/gelatin fibers which were randomly aligned to provide a bionic structure suitable for cell adhesion. Subsequent in vitro studies have shown that the core fibers were randomly aligned in the aligned PCL fiber shell of the biphasic scaffold, which increased the initial adhesion, proliferation, and differentiation of the mouse fibroblast cell line. The alignment of the PCL fibers promoted elongation of the aligned fibers, thereby improving tendon tissue healing by directing cell proliferation and ECM deposition [109].
Multitechnology cooperation can improve performance in many aspects. Melt deposition modeling, melt electrospinning, and solution electrospinning can provide structural support, promote cell arrangement, and create bionic microenvironment to promote cell functioning. The surface modification of plasma can improve the hydrophilicity of the scaffold and creates a bionic microenvironment to enhance the ability of cell adhesion and proliferation [110]. Furthermore, Hakimi et al. applied both weaving and electrostatic spinning techniques to create strong implantable layered fibrous scaffolds for endogenous tendon repair, both consisting of the same fibrous material, polydioxanone (PDO). Due to the electrostatic spinning of the plates reinforced with woven layers, the scaffold could reach a maximum strength of 65 MPa and a maximum suture strength of 167 N, similar to that of a human rotator cuff tendon. Notably, the nanofibers of the composite scaffold mediated the biological activity and directed the behavior of the tendon cells in vitro and in vivo. Implantation into the rat model matched the mechanical properties of the tendon and progressively embedded it in dense tissue. The scaffold remained well integrated over time with no delamination (Figure 3F) [111]. This solved the problem of weak mechanical performance of electrospinning scaffold in tendon repair.
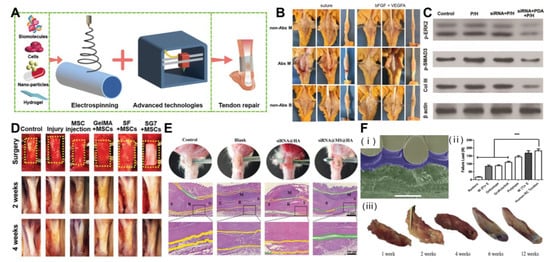
Figure 3.
Treatment of tendon injury with composite electrospinning technology. (A) Scheme of bioactive materials and/or advanced technology combined with electrospinning for tendon repair. (B) Nanoparticle-coated suture improves the healing strength of injured tendon. Reprinted with permission from Ref. [92]. Copyright 2021 Elsevier Inc. (C) Significant inhibition of adhesion protein expression through PDA/ERK2-siRNA-mediated electrostatic spinning fibers. Reprinted with permission from Ref. [93]. Copyright 2018 Wiley-VCH GmbH. (D) Co-electrospinning cellulose and gelatin methacryloyl tablets with mesenchymal stem cells as seeds for tendon regeneration. The yellow dotted box represents the surgical site. Reprinted with permission from Ref. [36]. Copyright 2022 Wiley-VCH GmbH. (E) Hydrogel composite electrostatically spun nanofibers prevent tendon tissue adhesions and reduce inflammation. (green area: physical surrounding space; yellow area: adhesive tissue; S: su-ture site; R: repair site; M: anti-adhesive material). Reprinted with permission from Ref. [106]. Copyright 2022 Wiley-VCH GmbH. (F) Layered electrostatically spun braided surgical scaffolds for enhanced endogenous tendon repair. (*** p < 0.001). Reprinted with permission from Ref. [111]. Copyright 2021 IOP Publishing Ltd.
6. Advantages of Electrospinning 3D Structure in Tendon Repair at Different Stages
The 3D structure of electrospun fiber is similar to the natural ECM structure of a tendon, which can provide scaffold for tissue growth and support the organization and arrangement of cells in tissue. Meanwhile, it supports the infiltration of cells and nutrients, and promotes the proliferation and regeneration of tendon tissue. Through specific design, the electrospun 3D scaffold has its advantages in the three stages of inflammation, fibroblast proliferation, and remodeling (Figure 4A).
6.1. The Role of Electrospun 3D Structure in the Regulation of Inflammation
Inflammation coexists with the early degeneration of tendon, and the interference between immune cells and tendon fibroblasts will lead to poor tissue healing. The disordered fiber surface may affect the adhesion and gene expression of tendon cells, and make them produce pro-inflammatory signals [112]. In response to this problem, Schoenberger et al. used highly aligned polycaprolactone PCL scaffolds. Compared with randomly oriented nanofiber scaffolds, when primary human tendon fibroblasts were inoculated on PCL aligned nanofiber scaffolds, the expression of key transcription regulators and tendon ECM components collagen I, collagen III, anthocyanins, and disaccharides increased. At the same time, it reduced the expression of the ECM degradation matrix metalloproteinase and reduced the sensitivity of pro-inflammatory signals [113]. In addition, quantitative mechanical load response can promote the polarization of macrophages’ phenotype to an M2-like phenotype, thereby reducing the inflammatory reaction of tendons in vitro and in vivo. The ordered arrangement of PCL scaffolds better promotes tendon healing under mechanical loading (Figure 4B) [114]. In addition, Liu et al. proved that the expression level of the Cluster of Differentiation 68 (CD68) protein gradually decreased with the increase of the SF/PLLA mass ratio, the inflammatory reaction was also significantly inhibited (Figure 4C), and the collagen thickness gradually became thinner with the increase of the SF/PLLA mass ratio [58]. This provided strong support for the design of “immune regulation” characteristics of regenerative biomaterials.
There is an inseparable relationship between the formation of adhesion and the expression of inflammation. It is an effective way to use anti-inflammatory materials to reduce the inflammatory reaction around the tendon. The nanofiber membrane with a polyethylene glycol/polycaprolactone/Ag NPs shell and hyaluronic acid/ibuprofen core prepared with coaxial electrospinning can reduce the adhesion of fibroblasts, and the core–shell structure can reduce pain and increase antibacterial activity by releasing ibuprofen and silver nanoparticles. At the same time, the combined effect of AgNPs and ibuprofen leads to the reduction of macrophages on the tendon tissue to reduce infection and inflammation, so as to meet the needs of an ideal antiadhesion barrier [101].
6.2. The Role of 3D Electrospun Structure in Tendon Proliferation
3D-oriented electrospun fibers exhibit flexible controllability in morphology and fiber density, have excellent biocompatibility in vitro, and are conducive to promoting cell adhesion, proliferation, and differentiation on its microstructure [115,116]. In the process of tendon proliferation, type I collagen (Col I) [117] and tendon regulatory protein (Tenomodulin, Tnmd) [118] are closely related to the tendon repair process and can be used to identify the degree of tendon repair. A bipolar metal flexible electrospun fiber membrane based on the membrane of metal-organic framework (MOF) is used for tendon–bone interface regeneration. Inspired by the biological gradient structure of the tendon–bone interface, the bipolar metal flexible electrospun fiber membrane of MOF is prepared using continuous electrospinning technology, which combines the regulation of osteoblast differentiation and angiogenesis characteristics to achieve synchronous regeneration. As a carrier, MOF can not only realize the sustainable release of metal ions, but also promotes osteogenesis and tendon formation on the scaffold. As a carrier, MOF can realize the sustainable release of metal ions, promote the upregulation of Col I (Figure 4Di) and Tnmd (Figure 4Dii) genes, and mark the proliferation and maturation of tendon cells. After 8 weeks of implantation, the morphology and inflammatory infiltration of the tissue at the interface of the flexible fibrous membrane were significantly improved (Figure 4Diii). This promoted the repair of tendon and bone tissue, as well as the reconstruction of fibrocartilage, and realized the simultaneous regeneration of multiple tissues at the damaged tendon–bone interface [119]. In addition, the pore size and porosity of the 3D electrospun scaffold determined the cell infiltration and regeneration ability. The high porosity of a 3D PCL scaffold can promote the rapid migration of MSCs into the scaffold, and further use of recombinant human connective tissue growth factor to stimulate MSCs can express higher levels of ligament/tendon gene type I collagen and tenomodulin, thus, promoting the proliferation and differentiation of mesenchymal stem cell ligament/tendon lineage [120].
6.3. The Role of 3D Structure of Electrospinning in Hemostasis
During the remodeling stage, the collagen synthesized from the scar at the tendon decreased, and the number of blood vessels and cells produced also decreased sharply. As type III collagen is not completely remolded into type I collagen, the fibrovascular scar produced by adult tendon in the initial healing stage will never be completely replaced [23]. The formation of scar will increase the risk of tendon rupture after repair [121]. In one study, the co-polymer (lactic acid/glycolic acid) electrospun fiber (Him-MFM) supported by the stearyl phosphate ethanolamine layer was connected with the CD11b+/CD68+ scar subpopulation membrane identified in the immune landscape after tendon injury to balance tissue damage. Him-MFM implanted into tendon can change the polarization of type I macrophages, reduce cell apoptosis and metabolic stress, and reduce inflammatory tendon cell response. It is worth noting that the hedging immune strategy increased the secretion of Interleukin-33 (IL-33) in the damaged tendon sheath by 4.36 times, activated the mucus-IL-33-Th2 axis, and directly provided regeneration niche for the proliferation of sheath stem cells [122]. In the mouse flexor tendon injury model, the tendon tissue of Him-MFM group was relatively smooth, no obvious fibrous tissue was found at the junction, and the degree of adhesion was low (Figure 4Ei). Previous studies have shown that the inhibition of the overexpression of myofibroblast markers α-SMA can prevent scar formation after tendon injury [123]. An immunofluorescence assessment of fibroblast infiltration showed that the number of α-SMA positive cells and normalized α-SMA intensity were significantly lower in the Him-MFM group than in the other groups, at 3.11 ± 1.97 and 14.01 ± 3.57, respectively, significantly reducing scar formation during tendon repair (Figure 4Eii), and comparable to mature tendons in terms of structure and function [122]. In addition, the electrospun polydioxanone support had high alignment or a random configuration. The healthy tendon fibroblasts cultured on the scaffold with highly arranged fibers for 7 days showed a unique elongated morphology. The scaffold simulating the alignment of collagen I fibrils with diameter in the process of tissue remodeling will not activate tendon-derived fibroblasts, thus, reducing fiber adhesion and scar formation [124].
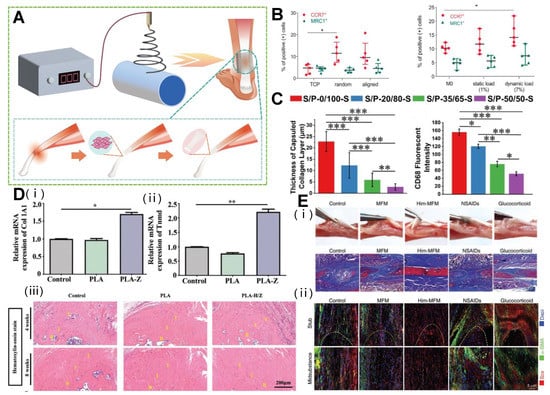
Figure 4.
Electrospinning 3D structure used in different stages of tendon repair. (A) Application of electrospinning products in different stages of tendon repair. (B) Disorganized substrate topography and dynamic mechanical loading drive macrophage polarization toward a pro-inflammatory phenotype in vitro. (* p < 0.05). Reprinted with permission from Ref. [114]. Copyright 2020 Elsevier Inc. (C) Inflammatory response of different SF/PLLA nanoyarn-based woven fabrics 14 days after subcutaneous implantation. (* p < 0.05, ** p < 0.001, *** p < 0.0001). Reprinted with permission from Ref. [58]. Copyright 2022 Elsevier Inc. (D) MOF-based bipolar metal flexible electrospun fiber membrane increases tendon cell proliferation and maturation. (B: bone; I: interface; T: tendon; * p < 0.05, ** p < 0.01). Reprinted with permission from Ref. [119]. Copyright 2022 Wiley-VCH GmbH. (E) The Him-MFM promoted endogenous recovery of tendon and reduced adverse adhesion and scarring. Reprinted with permission from Ref. [122]. Copyright 2022 Wiley-VCH GmbH.
7. Patents Related to Electrospinning of Biodegradable Polymers
At present, many researchers are working on patented tendon repair technology using biodegradable polymers as materials. These techniques can increase the health of tendon tissue, which can improve joint mobility and function. We conducted a comprehensive search on Google patents using the keywords “biodegradable polymers” and “tendon repair” and yielded 155 results. In a careful review, 30 techniques related to tendon repair were listed, as shown in Table 1. Here, several biodegradable polymer products for tendon repair with different structures and different uses are presented. For example, Chinese Patent No. CN-103071185-A provides a bilayer-structured bionic-tendon-sheath membrane comprising a fibrous layer and a synovial layer, wherein the synovial layer of the bionic tendon sheath membrane contains HA or its metal salts, which enables the controlled release of HA or its metal salts, mimics the function of HA secretion by the inner layer of biological tendon sheaths, provides an environment with long-lasting lubricating effect for tendons, promotes endogenous healing of tendons, improves tendon healing quality, and improves postoperative tendon function. The fibrous layer mainly acts as a physical barrier to reduce peritendinous tissue adhesions and prevent exogenous healing. The biodegradable polymer material is also self-degrading and biocompatible, improving efficacy and reducing side effects [125]. US Patent No. US-2015190222-A1 comprises biodegradable/polymer fiber-based three-dimensional woven scaffolds with a woven structure comprising three regions, two ends designated for attachment to the structure, and an intermediate region used as a replacement ligament or tendon. The graft material can be surgically implanted into a patient’s ligament or tendon injury site to promote healing and repair of the injured ligament or tendon [126]. In addition, Canada Patent No. CA-2917427-C is a biodegradable polymer technology for tendon repair that addresses the integrity of the tendon by uniting biodegradable polymers through the suturing of the tendon tissue [127]. It is important to note that these patented technologies are only some of the current biodegradable polymer technologies that can be used for tendon repair, and many others are being researched and developed. In addition, different patented technologies may have different limitations and scope of application and should be selected and applied on a case-by-case basis.

Table 1.
Patents for biodegradable polymers for tendon repair.
8. Status and Commercialization of Clinical Trials of Biodegradable Polymer Electrospinning in Tendon Repair
The effective application of biodegradable polymers in complex in vivo environments, and in particular the eventual clinical translation of the materials, still faces a number of key issues and challenges. Therefore, clinical trials of biodegradable polymers in tendon repair are summarized in Table 2. In order to adapt to medical applications in different in vivo environments, biodegradable polymers are made into different types of materials or products, such as micro- and nanoparticles, gels, and implantable devices, by means of self-assembly, chemical reactions, and molding processes. Methods of application of biodegradable polymers in tendon repair include use alone or in combination with other materials. For example, Park et al. randomized 40 patients to either a braided absorbable polylactide suture group or a braided non-absorbable polyethylene terephthalate suture group. After completing 12 months of follow-up, the absorbable sutures were found to be no worse than the non-absorbable sutures in repairing acute Achilles tendon ruptures [155]. PXL01 is a synthetic peptide derived serially from human lactoferrin with antibacterial and anti-inflammatory properties. Wiig et al. used a prospective, double-blind trial to verify that HA-loaded lactoferrin peptide (PXL01) improved hand recovery after flexor tendon repair [156]. Despite the potential advantages of biodegradable polymers in tendon repair, more research and experimental validation are needed before clinical application. Mentzel et al. [157] and Liew et al. [158] designed clinical protocols to validate the role of ADCON®-T/N in zone II flexor tendon repair, respectively, and did not obtain consistent results. Therefore, when performing tendon repair, the physician needs to consider the advantages and disadvantages of using biodegradable polymers to make the best treatment decision, depending on the specific condition and the patient’s circumstances.

Table 2.
Clinical trials of biodegradable polymers in tendon repair.
Biological scaffolds for biomedical applications are not only available for research but also have real commercial potential. Products of animal origin or products prepared from natural biomaterials of animal origin as raw materials have been approved by the FDA 510(k) for the strengthening of soft tissues. Most commercial bio-scaffolds are prepared from natural biomaterials, such as collagen [167], or collagen cross-linked with synthetic biomaterials, such as polylactic acid [168], PLGA [169], and PLCL [170], which are synthetic polymers that have been approved by the Food and Drug Administration (FDA) for use in humans. The materials for commercially approved scaffolds are mainly type I collagen obtained from mammalian (bovine, porcine, equine) tissues, which have the natural structure and bioactivity to promote cell proliferation and tissue growth. Scaffolds that have been registered and used in the clinic for tendon reconstruction include TAPESTRY® [171], GTR® [172] and TenoMedTM [173]. The electrostatic spinning bio-scaffold has an ECM-like structure that facilitates cell adhesion and tissue regeneration, and offers structural advantages and biocompatibility in tendon repair. The electrostatic spinning bio-scaffold could be easily implanted into defective areas of injured tendon, for example, the TAPESTRY® [171] Bio-integrative Implant developed by Embody, Inc. has been specifically designed for the management and protection of tendon injuries. TAPESTRY® is designed for type I collagen and Poly (D, L-lactide) to provide a layer between the tendon and the surrounding tissue. Its constituent ingredients are currently approved by the FDA for clinical use. The medical collagen membrane with a bilayer structure developed by GTR Bio-Tech.Co., Ltd. is purified from bovine Achilles tendon tissue and has a bilayer structure, which matches the rate of degradation to the rate of tissue regeneration after implantation and is now in clinical use [172]. In addition, the composition and application of FDA registered biological scaffolds, as well as the manufacturer’s information, are summarized in Table 3. As the limitations of biomaterials in human applications are overcome, new commercial tendon repair products are being developed.

Table 3.
FDA-registered commercial products for tendon repair.
9. Conclusions
Electrospun nanofibers with controllable diameter, arrangement, high porosity, and a large surface area produced using electrospinning are usually easily able to meet the requirements of tendon repair tissue engineering, have the characteristics of low production costs and high efficiency, and are an ideal treatment choice for tendon injury [5,6,13,14,15]. Its mode of action involves providing a microenvironment for cell survival in vitro and expanding target cells, reducing the occurrence of inflammation at the injury site after implantation in vivo, and promoting the proliferation of tendon cells and the arrangement of collagen fibers.
A 3D electrospun-nanofiber scaffold based on the controllability of the electrospinning process can better simulate the structure and shape of ECM. A 3D electrospun-nanofiber scaffold with high porosity can be manufactured using direct electrospinning, post-processing technology, and by adjusting the fiber collection technology or combining these technologies [180]. In addition, researchers are exploring the use of new polymer materials, and mixing cells, bioactive molecules, and biomaterials into electrospun fibers to improve their performance and biological activity [181,182]. Natural biodegradable polymers play an integral role in tendon repair due to their tendon-tissue-mimicking structure and excellent biocompatibility. Currently, in the field of regenerative medicine, biological scaffolds with both biocompatibility and great mechanical properties have been prepared by combining natural biodegradable polymers with other polymers or with other biotechnologies such as 3D printing and freeze-drying.
The 3D structure of electrospun fiber provides several advantages for tendon repair, including the ability to provide a scaffold for tissue growth, support the infiltration of cells and nutrients, and be customized to match the characteristics of natural tissue. However, more research is needed to fully understand the potential mechanism of the positive effect of electrospun fibers on tissue repair. By optimizing its design and manufacturing methods, electrospun 3D scaffolds can display their capabilities in three stages of inflammation, proliferation, and remodeling after tendon injury.
In the past, the use of 3D electrostatic spun wire scaffolds for tendon repair still posed many challenges. The weak mechanical properties of electrospun products prevented them from being used in hard tissue repair. The uncontrollable degradation rate of electrospun products made it a limitation in the application of regenerative medicine. However, as conventional electrospun technology is updated, there is increasingly great control over the diameter distribution of the membrane through operations such as the running track of the electrospun nozzle. At the same time, the solution concentration, electric/magnetic field, and conductivity of the solution are adjusted during the production process so that the properties of the fiber membrane are stable and controllable. In short, with the development and progress of electrospinning technology, the scalability and cost-effectiveness of electrospinning fibers used in tendon repair tissue engineering applications can be improved.
Author Contributions
Conceptualization, Y.Z. and Y.X.; validation, Y.X.; writing—original draft preparation, Y.Z. and Y.X.; writing—review and editing, Y.L.; visualization, Y.R. and X.L.; project administration, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China of Ministry of Science and Technology of the People’s Republic of China (2022YFC2409700 and 2022YFC2401804), the Key-Area Research and Development Program of Guangdong Province (2020B0101020001), and the National Natural Science Foundation of China (31971318, 81973001 and 22027810).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Glossary
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ECM | Extracellular matrix |
| CBD | Collagen-binding structural domain |
| MSCs | Mesenchymal stem cells |
| Col | Collagen |
| PLA | Polylactic acid |
| PCL | Polycaprolactone |
| P3HB | Poly (3-hydroxybutyric acid) |
| SF | Silk fibroin |
| PU | Polyurethane |
| DP | Degrapol® |
| PDGF-BB | Platelet-derived growth factor BB |
| HA | Hydroxyapatite |
| PLLA | Polylactic acid |
| PLGA | Poly-D-L-lactide-glycolide |
| SLNM | Super-lubricated nanofibrous membranes |
| PMPC | Poly (2-methacryloxyethyl phosphate choline) |
| BFM | Bipolar fiber membrane |
| SFM | Simple fiber membrane |
| mPCL-nCol-bFGF | PCL microfibers/collagen bfgf |
| bFGF | Basic fibroblast growth factor |
| VEGFA | Vascular endothelial growth factor A |
| ERK2 | Extracellular signal-regulated kinase 2 |
| siRNA | Small interfering RNA |
| Col III | Type III collagen |
| PDA | 2,6-pyridyldicarboxylic aldehyde-polyvinimide |
| GelMA | Methacrylic acid hydrogel |
| ADSCs | Adipose derived stem cells |
| MDCs | Muscle derived cells |
| Ag NPs | Silver nanoparticles |
| MMC | Mitomycin C |
| ZnO NPs | Zinc oxide nanoparticles |
| BMP-12 | Bone morphogenetic protein 12 |
| PGS | Polyglycerol sebacate |
| PDO | Polydioxanone |
| CD68 | Cluster of Differentiation 68 |
| Col I | Type I collagen |
| Tnmd | Tenomodulin |
| MOF | Metal-organic framework |
| Him-MFM | Co-polymer (lactic acid/glycolic acid) electrospun fiber |
| IL-33 | Interleukin-33 |
| PXL01 | Lactoferrin peptide |
References
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal. Interact. 2006, 6, 181–190. [Google Scholar]
- Sakabe, T.; Sakai, T. Musculoskeletal diseases—Tendon. British. Medical. Bulletin. 2011, 99, 211–225. [Google Scholar] [CrossRef]
- Coons, D.A.; Alan Barber, F. Tendon Graft Substitutes—Rotator Cuff Patches. Sports. Med. Arthrosc. Rev. 2006, 14, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta. Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, R.; Lakshminarayanan, R.; Madhaiyan, K.; Amutha Barathi, V.; Lim, K.H.; Ramakrishna, S. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: Applications in tissue regeneration, drug delivery and pharmaceuticals. Chem. Soc. Rev. 2015, 44, 790–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, W.; Gu, J.; Yan, X.; Chen, Y.; Guo, M.; Zhou, G.; Tong, S.; Ge, M.; Liu, Y.; et al. MOF-based fibrous membranes adsorb PM efficiently and capture toxic gases selectively. Nanoscale 2019, 11, 17782–17790. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, N.; Li, J.; Wang, H.; Wang, C.; Zhang, Z.; Gu, J.g.; Wang, M.; Han, C.C.; Xu, S.; et al. Dual-Functional Esophageal Stent Coating Composed of Paclitaxel-Loaded Electrospun Membrane and Protective Film. J. Biomed. Nanotechnol. 2019, 15, 2108–2120. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y.; Liu, W.; Li, S.; Wang, X.; Zhou, W.; Zhang, G.; Liu, K.; Zhang, H.; Zhao, Y.; et al. Untethered artificial muscles powered by wearable sweat-based energy generator. Nano Today 2023, 49, 101765. [Google Scholar] [CrossRef]
- Rinoldi, C.; Kijenska-Gawronska, E.; Khademhosseini, A.; Tamayol, A.; Swieszkowski, W. Fibrous Systems as Potential Solutions for Tendon and Ligament Repair, Healing, and Regeneration. Adv. Healthc. Mater. 2021, 10, e2001305. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S.H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, C.; Mao, R.; Liang, X.; Wang, H.; Lin, Z.; Li, J.; Li, S.; Jiang, J.; Zhang, T.; et al. Surface structure change properties: Auto-soft bionic fibrous membrane in reducing postoperative adhesion. Bioact. Mater. 2022, 12, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release. 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.R.; He, R.Y.; Sha, B.Y.; Li, W.F.; Qing, H.B.; Teng, R.; Xu, F. Electrospun three-dimensional aligned nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 92, 995–1005. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Laranjeira, M.; Domingues, R.M.A.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. 3D Mimicry of Native-Tissue-Fiber Architecture Guides Tendon-Derived Cells and Adipose Stem Cells into Artificial Tendon Constructs. Small 2017, 13, 1700689. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Wang, N.; Yue, G.; Wang, X.; Cai, B.; Hao, Y.; Li, Y.; Guo, F.; Zhang, Z.; et al. Bioinspired stretchable helical nanofiber yarn scaffold for locomotive tissue dynamic regeneration. Matter 2022, 5, 4480–4501. [Google Scholar] [CrossRef]
- Cook, J.L.; Purdam, C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Brit. J. Sport. Med. 2008, 43, 409–416. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Galatz, L.M.; Gerstenfeld, L.; Heber-Katz, E.; Rodeo, S.A. Tendon regeneration and scar formation: The concept of scarless healing. J. Orthop. Res. 2015, 33, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Kim, J.E.; Yoon, K.S.; Shin, S. Platelet-Rich Plasma Stimulates Cell Proliferation and Enhances Matrix Gene Expression and Synthesis in Tenocytes from Human Rotator Cuff Tendons with Degenerative Tears. Am. J. Sport. Med. 2012, 40, 1035–1045. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, S.J.; Wang, H.-Y.; Tang, S.C.; Yang, S.-W. Evaluation of the ability of xanthan gum/gellan gum/hyaluronan hydrogel membranes to prevent the adhesion of postrepaired tendons. Carbohyd. Polym. 2014, 114, 230–237. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural polymeric scaffolds in bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, S.; Wu, Y.; Liu, L.; Su, S.; Liang, T.; He, R.; Guo, Z.; Zhang, Y.; Lin, Z.; et al. The Regenerative Role of Gelatin in PLLA Electrospun Membranes for the Treatment of Chronic Massive Rotator Cuff Injuries. Macromol. Biosci. 2022, 22, 2100281. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Y.; Zhu, W.; Zhang, G.; Yang, Y.P.; Zhao, C. The role of MicroRNAs in tendon injury, repair, and related tissue engineering. Biomaterials 2021, 277, 121083. [Google Scholar] [CrossRef]
- Ruan, D.; Zhu, T.; Huang, J.; Le, H.; Hu, Y.; Zheng, Z.; Tang, C.; Chen, Y.; Ran, J.; Chen, X.; et al. Knitted Silk-Collagen Scaffold Incorporated with Ligament Stem/Progenitor Cells Sheet for Anterior Cruciate Ligament Reconstruction and Osteoarthritis Prevention. ACS. Biomater. Sci. Eng. 2019, 5, 5412–5421. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharmaceut. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.-F.; Liu, R.-C.; Shen, W.-L.; Yin, Z.; Chen, X. Physical Microenvironment-Based Inducible Scaffold for Stem Cell Differentiation and Tendon Regeneration. Tissue. Eng. Part. B. Re. 2018, 24, 443–453. [Google Scholar] [CrossRef]
- Tu, T.; Shen, Y.; Wang, X.; Zhang, W.; Zhou, G.; Zhang, Y.; Wang, W.; Liu, W. Tendon ECM modified bioactive electrospun fibers promote MSC tenogenic differentiation and tendon regeneration. Appl. Mater. Today. 2020, 18, 100495. [Google Scholar] [CrossRef]
- Micheli, L.; Parisio, C.; Lucarini, E.; Carrino, D.; Ciampi, C.; Toti, A.; Ferrara, V.; Pacini, A.; Ghelardini, C.; Di Cesare Mannelli, L. Restorative and pain-relieving effects of fibroin in preclinical models of tendinopathy. Biomed. Pharmacother. 2022, 148, 112693. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.; Lake, S.P.; Zellers, J.A. Effect of diabetes on tendon structure and function: Not limited to collagen crosslinking. J. Diabetes. Sci. Technol. 2023, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mou, C.; Shi, Q.; Chen, B.; Hou, X.; Zhang, W.; Li, X.; Zhuang, Y.; Shi, J.; Chen, Y.; et al. Controlled release of collagen-binding SDF-1α from the collagen scaffold promoted tendon regeneration in a rat Achilles tendon defect model. Biomaterials 2018, 162, 22–33. [Google Scholar] [CrossRef]
- Xue, Y.; Kim, H.-J.; Lee, J.; Liu, Y.; Hoffman, T.; Chen, Y.; Zhou, X.; Sun, W.; Zhang, S.; Cho, H.-J.; et al. Co-Electrospun Silk Fibroin and Gelatin Methacryloyl Sheet Seeded with Mesenchymal Stem Cells for Tendon Regeneration. Small 2022, 18, 2107714. [Google Scholar] [CrossRef]
- Teuschl, A.; Heimel, P.; Nürnberger, S.; van Griensven, M.; Redl, H.; Nau, T. A Novel Silk Fiber–Based Scaffold for Regeneration of the Anterior Cruciate Ligament. Am. J. Sport. Med. 2016, 44, 1547–1557. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharmaceut. 2017, 523, 441–453. [Google Scholar] [CrossRef]
- Francois, E.; Dorcemus, D.; Nukavarapu, S. Biomaterials and scaffolds for musculoskeletal tissue engineering. In Interfaces Regenerative Engineering of Musculoskeletal Tissues and Interfaces; Nukavarapu, S., Freeman, J., Laurencin, C., Eds.; Woodhead Publishing: Cambridge, UK, 2015; ISBN 9781782423010. [Google Scholar]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.u.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, R.; Deng, N.; Zhao, X.; Li, X.; Guo, C. Natural polymer-based scaffolds for soft tissue repair. Front. Bioeng. Biotech. 2022, 10, 1280. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Qian, Y.; Jin, Y.; Wang, S.; Li, J.; Yuan, W.E.; Fan, C. Biomimetic multilayer polycaprolactone/sodium alginate hydrogel scaffolds loaded with melatonin facilitate tendon regeneration. Carbohydr. Polym. 2022, 277, 118865. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.-E.; Inai, R.; Ramakrishna, S. Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mat. 2019, 12, 013002. [Google Scholar] [CrossRef]
- Si, Y.; Shi, S.; Hu, J. Applications of electrospinning in human health: From detection, protection, regulation to reconstruction. Nano Today 2023, 48, 101723. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, F.; Akkus, O.; King, M.W. A collagen/PLA hybrid scaffold supports tendon-derived cell growth for tendon repair and regeneration. J. Biomed. Mater. Res. B. 2022, 110, 2624–2635. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Z.; Xiang, L.; Zhao, Z.; Cui, W. Functional biomaterials for tendon/ligament repair and regeneration. Regen. Biomater. 2022, 9, rbac062. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, X.; Chen, J.L.; Shen, W.L.; Hieu Nguyen, T.M.; Gao, L.; Ouyang, H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 2010, 31, 2163–2175. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Lipner, J.; Manning, C.N.; Schwartz, A.G.; Thomopoulos, S.; Xia, Y. “Aligned-to-random” nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale 2010, 2, 923–926. [Google Scholar] [CrossRef]
- Zhang, F.; Si, Y.; Yu, J.; Ding, B. Electrospun porous engineered nanofiber materials: A versatile medium for energy and environmental applications. Chem. Eng. J. 2023, 456, 140989. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Wang, N.; Yu, J.; Liu, Y.T.; Ding, B. From 1D Nanofibers to 3D Nanofibrous Aerogels: A Marvellous Evolution of Electrospun SiO(2) Nanofibers for Emerging Applications. Nanomicro. Lett. 2022, 14, 194. [Google Scholar] [CrossRef]
- Liu, W.; Lipner, J.; Moran, C.H.; Feng, L.; Li, X.; Thomopoulos, S.; Xia, Y. Generation of electrospun nanofibers with controllable degrees of crimping through a simple, plasticizer-based treatment. Adv. Mater. 2015, 27, 2583–2588. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhai, H.; Sun, Y.; Wu, S.; Chen, S. Developing high strength poly(L-lactic acid) nanofiber yarns for biomedical textile materials: A comparative study of novel nanofiber yarns and traditional microfiber yarns. Mater. Lett. 2021, 300, 130229. [Google Scholar] [CrossRef]
- Li, Y.; Guo, F.; Hao, Y.; Gupta, S.K.; Hu, J.; Wang, Y.; Wang, N.; Zhao, Y.; Guo, M. Helical nanofiber yarn enabling highly stretchable engineered microtissue. Proc. Natl. Acad. Sci. USA 2019, 116, 9245–9250. [Google Scholar] [CrossRef] [PubMed]
- Naghashzargar, E.; Farè, S.; Catto, V.; Bertoldi, S.; Semnani, D.; Karbasi, S.; Tanzi, M.C. Nano/Micro Hybrid Scaffold of PCL or P3HB Nanofibers Combined with Silk Fibroin for Tendon and Ligament Tissue Engineering. J. Appl. Biomater. Func. 2015, 13, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Surrao, D.C.; Hayami, J.W.S.; Waldman, S.D.; Amsden, B.G. Self-Crimping, Biodegradable, Electrospun Polymer Microfibers. Biomacromolecules 2010, 11, 3624–3629. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Cai, J.; Zhao, J.; Duan, B.; Chen, S. Combining electrospinning with hot drawing process to fabricate high performance poly (L-lactic acid) nanofiber yarns for advanced nanostructured bio-textiles. Biofabrication 2021, 13, 045018. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Zhang, H.; Zhao, W.; Qu, L.; Chen, S.; Wu, S. Electrospun strong, bioactive, and bioabsorbable silk fibroin/poly (L-lactic-acid) nanoyarns for constructing advanced nanotextile tissue scaffolds. Mater. Today. Bio. 2022, 14, 100243. [Google Scholar] [CrossRef]
- Buschmann, J.; Calcagni, M.; Bürgisser, G.M.; Bonavoglia, E.; Neuenschwander, P.; Milleret, V.; Giovanoli, P. Synthesis, characterization and histomorphometric analysis of cellular response to a new elastic DegraPol® polymer for rabbit Achilles tendon rupture repair. J. Tissue. Eng. Regen. M. 2012, 9, 584–594. [Google Scholar] [CrossRef]
- Meier Bürgisser, G.; Calcagni, M.; Müller, A.; Bonavoglia, E.; Fessel, G.; Snedeker, J.G.; Giovanoli, P.; Buschmann, J. Prevention of Peritendinous Adhesions Using an Electrospun DegraPol Polymer Tube: A Histological, Ultrasonographic, and Biomechanical Study in Rabbits. Biomed. Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Bürgisser, G.M.; Evrova, O.; Heuberger, D.M.; Wolint, P.; Rieber, J.; Miescher, I.; Schüpbach, R.A.; Giovanoli, P.; Calcagni, M.; Buschmann, J. Electrospun tube reduces adhesion in rabbit Achilles tendon 12 weeks post-surgery without PAR-2 overexpression. Sci. Rep. 2021, 11, 23293. [Google Scholar] [CrossRef]
- Evrova, O.; Burgisser, G.M.; Ebnother, C.; Adathala, A.; Calcagni, M.; Bachmann, E.; Snedeker, J.G.; Scalera, C.; Giovanoli, P.; Vogel, V.; et al. Elastic and surgeon friendly electrospun tubes delivering PDGF-BB positively impact tendon rupture healing in a rabbit Achilles tendon model. Biomaterials 2020, 232, 119722. [Google Scholar] [CrossRef]
- Pien, N.; Van de Maele, Y.; Parmentier, L.; Meeremans, M.; Mignon, A.; De Schauwer, C.; Peeters, I.; De Wilde, L.; Martens, A.; Mantovani, D.; et al. Design of an electrospun tubular construct combining a mechanical and biological approach to improve tendon repair. J. Mater. Sci. Mater. M 2022, 33, 51. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shi, X.; Li, X.; Wang, J.; Wang, Y.; Luo, Y. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration. Biomaterials 2019, 207, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Sang, X.; Tian, Z.; Jiang, S.; Li, C.; Guo, Q.; Wang, C.; Hu, P.; Liu, Y. Electrospun hydroxyapatite loaded L-polylactic acid aligned nanofibrous membrane patch for rotator cuff repair. Int. J. Biol. Macromol. 2022, 217, 180–187. [Google Scholar] [CrossRef]
- Weng, C.J.; Wu, Y.C.; Hsu, M.Y.; Chang, F.P.; Liu, S.J. Electrospun, Resorbable, Drug-Eluting, Nanofibrous Membranes Promote Healing of Allograft Tendons. Membranes 2022, 12, 529. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chen, S.-H.; Shalumon, K.T.; Chen, J.-P. Prevention of peritendinous adhesions with electrospun polyethylene glycol/polycaprolactone nanofibrous membranes. Colloid. Surface. B. 2015, 133, 221–230. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.H.; Zhai, W.J.; Zhang, Z.N.; Liu, Y.H.; Cheng, S.J.; Zhang, H.Y. In-situ growth of robust superlubricated nano-skin on electrospun nanofibers for post-operative adhesion prevention. Nat. Commun. 2022, 13, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.; Wen, S.; Zhou, S.; Wang, Z.; Deng, L.; Mao, H.-Q.; Cui, W.; Zhang, H. Ice-Inspired Superlubricated Electrospun Nanofibrous Membrane for Preventing Tissue Adhesion. Nano. Letters. 2020, 20, 6420–6428. [Google Scholar] [CrossRef]
- Xie, J.; MacEwan, M.R.; Liu, W.; Jesuraj, N.; Li, X.; Hunter, D.; Xia, Y. Nerve Guidance Conduits Based on Double-Layered Scaffolds of Electrospun Nanofibers for Repairing the Peripheral Nervous System. ACS. Appl. Mater. Inter. 2014, 6, 9472–9480. [Google Scholar] [CrossRef]
- Pal, P.; Dadhich, P.; Srivas, P.K.; Das, B.; Maulik, D.; Dhara, S. Bilayered nanofibrous 3D hierarchy as skin rudiment by emulsion electrospinning for burn wound management. Biomater. Sci. 2017, 5, 1786–1799. [Google Scholar] [CrossRef]
- Li, X.; Cheng, R.; Sun, Z.; Su, W.; Pan, G.; Zhao, S.; Zhao, J.; Cui, W. Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing. Acta Biomater. 2017, 61, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Shin, M.; Kim, S.-H.; Lee, H.; Jang, J.-H. SpONGE: Spontaneous Organization of Numerous-Layer Generation by Electrospray. Angew. Chem. Int. Edit. 2015, 54, 7587–7591. [Google Scholar] [CrossRef]
- Si, Y.; Wang, X.; Dou, L.; Yu, J.; Ding, B. Ultralight and fire-resistant ceramic nanofibrous aerogels with temperature-invariant superelasticity. Sci. Adv. 2018, 4, eaas8925. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Lipner, J.; Yuan, X.; Thomopoulos, S.; Xia, Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano. Lett. 2009, 9, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Shen, S.J.; Hsu, Y.H.; Chou, Y.C.; Yu, P.C.; Liu, S.J. Tri-Layered Doxycycline-, Collagen- and Bupivacaine-Loaded Poly(lactic-co-glycolic acid) Nanofibrous Scaffolds for Tendon Rupture Repair. Polymers 2022, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Jayasree, A.; Kottappally Thankappan, S.; Ramachandran, R.; Sundaram, M.N.; Chen, C.H.; Mony, U.; Chen, J.P.; Jayakumar, R. Bioengineered Braided Micro-Nano (Multiscale) Fibrous Scaffolds for Tendon Reconstruction. ACS. Biomater. Sci. Eng. 2019, 5, 1476–1486. [Google Scholar] [CrossRef]
- Sankar, D.; Mony, U.; Rangasamy, J. Combinatorial effect of plasma treatment, fiber alignment and fiber scale of poly (ε-caprolactone)/collagen multiscale fibers in inducing tenogenesis in non-tenogenic media. Mat. Sci. Eng. C. Mater. 2021, 127, 112206. [Google Scholar] [CrossRef]
- Liu, C.; Tian, S.; Bai, J.; Yu, K.; Liu, L.; Liu, G.; Dong, R.; Tian, D. Regulation of ERK1/2 and SMAD2/3 Pathways by Using Multi-Layered Electrospun PCL-Amnion Nanofibrous Membranes for the Prevention of Post-Surgical Tendon Adhesion. Int. J. Nanomedicine. 2020, 15, 927–942. [Google Scholar] [CrossRef]
- Orr, S.B.; Chainani, A.; Hippensteel, K.J.; Kishan, A.; Gilchrist, C.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta. Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef]
- Rothrauff, B.B.; Lauro, B.B.; Yang, G.; Debski, R.E.; Musahl, V.; Tuan, R.S. Braided and Stacked Electrospun Nanofibrous Scaffolds for Tendon and Ligament Tissue Engineering. Tissue. Eng. Part A 2017, 23, 378–389. [Google Scholar] [CrossRef]
- Nowotny, J.; Aibibu, D.; Farack, J.; Nimtschke, U.; Hild, M.; Gelinsky, M.; Kasten, P.; Cherif, C. Novel fiber-based pure chitosan scaffold for tendon augmentation: Biomechanical and cell biological evaluation. J. Biomater. Sci.-Polym. Ed. 2016, 27, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable polymer electrospun nanofibers: History, shapes and application for tissue engineering. Chinese. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Oh, H.; Jeong, Y.H.; Cho, D.-W. Fabrication of a three-dimensional nanofibrous scaffold with lattice pores using direct-write electrospinning. Mater. Lett. 2013, 93, 397–400. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly (vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Yarin, A.L.; Zussman, E. Upward needleless electrospinning of nanofibers. Polymer 2004, 45, 2977–2980. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Xia, Y.; Yang, H. Magnetic-field-assisted electrospinning of aligned straight and wavy polymeric nanofibers. Adv. Mat. 2010, 22, 2454–2457. [Google Scholar] [CrossRef]
- Petrik, S.; Maly, M. Production nozzle-less electrospinning nanofiber technology. MRS. Online. Proceedings. Libr. OPL 2009, 1240, 1240-WW03. [Google Scholar] [CrossRef]
- Montero, R.B.; Vial, X.; Nguyen, D.T.; Farhand, S.; Reardon, M.; Pham, S.M.; Tsechpenakis, G.; Andreopoulos, F.M. bFGF-containing electrospun gelatin scaffolds with controlled nano-architectural features for directed angiogenesis. Acta. Biomater. 2012, 8, 1778–1791. [Google Scholar] [CrossRef]
- Liu, S.; Qin, M.; Hu, C.; Wu, F.; Cui, W.; Jin, T.; Fan, C. Tendon healing and anti-adhesion properties of electrospun fibrous membranes containing bFGF loaded nanoparticles. Biomaterials 2013, 34, 4690–4701. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, J.; Dong, S.; Huangfu, X.; Li, B.; Yang, H.; Zhao, J.; Cui, W. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int. J. Nanomed. 2014, 9, 2373–2385. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Yang, Q.Q.; Zhang, L.; Tang, J.B. Nanoparticle-coated sutures providing sustained growth factor delivery to improve the healing strength of injured tendons. Acta. Biomater. 2021, 124, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, F.; Gu, S.; Wu, T.; Chen, S.; Chen, S.; Wang, C.; Huang, G.; Jin, T.; Cui, W.; et al. Gene Silencing via PDA/ERK2-siRNA-Mediated Electrospun Fibers for Peritendinous Antiadhesion. Adv. Sci. 2018, 6, 1801217. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Wang, W.; Wang, B.; Zhang, P.; Zhou, G.; Zhang, W.J.; Cao, Y.; Liu, W. Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials 2014, 35, 8801–8809. [Google Scholar] [CrossRef] [PubMed]
- Chirieleison, S.M.; Feduska, J.M.; Schugar, R.C.; Askew, Y.; Deasy, B.M. Human Muscle-Derived Cell Populations Isolated by Differential Adhesion Rates: Phenotype and Contribution to Skeletal Muscle Regeneration in Mdx/SCID Mice. Tissue. Eng. Part A 2012, 18, 232–241. [Google Scholar] [CrossRef]
- Chen, B.; Wang, B.; Zhang, W.J.; Zhou, G.; Cao, Y.; Liu, W. In vivo tendon engineering with skeletal muscle derived cells in a mouse model. Biomaterials 2012, 33, 6086–6097. [Google Scholar] [CrossRef]
- Rinoldi, C.; Kijeńska, E.; Chlanda, A.; Choinska, E.; Khenoussi, N.; Tamayol, A.; Khademhosseini, A.; Swieszkowski, W. Nanobead-on-string composites for tendon tissue engineering. J. Mater. Chem. B 2018, 6, 3116–3127. [Google Scholar] [CrossRef]
- Wu, G.; Deng, X.; Song, J.; Chen, F. Enhanced biological properties of biomimetic apatite fabricated polycaprolactone/chitosan nanofibrous bio-composite for tendon and ligament regeneration. J. Photochem. Photobiol. B 2018, 178, 27–32. [Google Scholar] [CrossRef]
- Chen, S.; Wang, G.; Wu, T.; Zhao, X.; Liu, S.; Li, G.; Cui, W.; Fan, C. Silver Nanoparticles/Ibuprofen-Loaded Poly(l-lactide) Fibrous Membrane: Anti-Infection and Anti-Adhesion Effects. Int. J. Mol. Sci. 2014, 15, 14014–14025. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, S.H.; Shalumon, K.T.; Chen, J.P. Dual functional core-sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta. Biomater. 2015, 26, 225–235. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Sheu, C.; Chen, C.-H.; Chen, S.-H.; Jose, G.; Kuo, C.-Y.; Chen, J.-P. Multi-functional electrospun antibacterial core-shell nanofibrous membranes for prolonged prevention of post-surgical tendon adhesion and inflammation. Acta. Biomater. 2018, 72, 121–136. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, S.; Liu, S.; Chen, S.; Lin, Z.Y.; Pan, G.; He, F.; Li, F.; Fan, C.; Cui, W. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials 2015, 61, 61–74. [Google Scholar] [CrossRef]
- He, X.; Huang, Z.; Liu, W.; Liu, Y.; Qian, H.; Lei, T.; Hua, L.; Hu, Y.; Zhang, Y.; Lei, P. Electrospun polycaprolactone/hydroxyapatite/ZnO films as potential biomaterials for application in bone-tendon interface repair. Colloid. Surface. B. 2021, 204, 111825. [Google Scholar] [CrossRef] [PubMed]
- Rinoldi, C.; Fallahi, A.; Yazdi, I.K.; Campos Paras, J.; Kijeńska-Gawrońska, E.; Trujillo-de Santiago, G.; Tuoheti, A.; Demarchi, D.; Annabi, N.; Khademhosseini, A.; et al. Mechanical and Biochemical Stimulation of 3D Multilayered Scaffolds for Tendon Tissue Engineering. ACS. Biomater. Sci. Eng. 2019, 5, 2953–2964. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wang, W.; Liang, J.; Li, Y.; Lu, M.; Cui, W.; Fan, C.; Deng, L.; Li, Y.; Wang, F.; et al. MMP-2 Responsive Unidirectional Hydrogel-Electrospun Patch Loading TGF-β1 siRNA Polyplexes for Peritendinous Anti-Adhesion. Adv. Funct. Mater. 2020, 31, 2008364. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, X.; Li, Y.; Liu, X.; Wang, S.; Lu, M.; Yan, X.; Deng, L.; Liu, S.; Wang, F.; et al. Self-Healing Hydrogel Embodied with Macrophage-Regulation and Responsive-Gene-Silencing Properties for Synergistic Prevention of Peritendinous Adhesion. Adv. Mater. 2022, 34, e2106564. [Google Scholar] [CrossRef]
- Kim, G.; Son, J.; Park, S.; Kim, W. Hybrid Process for Fabricating 3D Hierarchical Scaffolds Combining Rapid Prototyping and Electrospinning. Macromol. Rapid. Comm. 2008, 29, 1577–1581. [Google Scholar] [CrossRef]
- Touré, A.B.R.; Mele, E.; Christie, J.K. Multi-layer Scaffolds of Poly(caprolactone), Poly(glycerol sebacate) and Bioactive Glasses Manufactured by Combined 3D Printing and Electrospinning. Nanomaterials 2020, 10, 626. [Google Scholar] [CrossRef]
- Guner, M.B.; Dalgic, A.D.; Tezcaner, A.; Yilanci, S.; Keskin, D. A dual-phase scaffold produced by rotary jet spinning and electrospinning for tendon tissue engineering. Biomed. Mater. 2020, 15, 065014. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y.; Xia, J.; Zhou, Z.; Fang, Y.; Zhang, L.; Sun, W. Multi-scale hierarchical scaffolds with aligned micro-fibers for promoting cell alignment. Biomed. Mater. 2021, 16, 045047. [Google Scholar] [CrossRef]
- Hakimi, O.; Mouthuy, P.A.; Zargar, N.; Lostis, E.; Morrey, M.; Carr, A. A layered electrospun and woven surgical scaffold to enhance endogenous tendon repair. Acta. Biomater. 2015, 26, 124–135. [Google Scholar] [CrossRef]
- Guo, M.; Bi, S.; Liu, J.; Xu, W.; Zhou, G.; Liu, Y.; Chen, C. C60(OH)n-loaded nanofibrous membranes protect HaCaT cells from ROS-associated damage. Chinese. Chem. Lett. 2017, 28, 1889–1892. [Google Scholar] [CrossRef]
- Schoenenberger, A.D.; Foolen, J.; Moor, P.; Silvan, U.; Snedeker, J.G. Substrate fiber alignment mediates tendon cell response to inflammatory signaling. Acta. Biomater. 2018, 71, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Schoenenberger, A.D.; Tempfer, H.; Lehner, C.; Egloff, J.; Mauracher, M.; Bird, A.; Widmer, J.; Maniura-Weber, K.; Fucentese, S.F.; Traweger, A.; et al. Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon in vitro and in vivo. Biomaterials 2020, 249, 120034. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xu, Q.; Li, C.; Huang, J.; Zhang, Y.; Wu, D.; Wang, Z. Engineering 3D Aligned Nanofibers for Regulation of Cell Growth Behavior. Macromol. Mater. Eng. 2017, 302, 1600448. [Google Scholar] [CrossRef]
- Loiselle, A.E.; Bragdon, G.A.; Jacobson, J.A.; Hasslund, S.; Cortes, Z.E.; Schwarz, E.M.; Mitten, D.J.; Awad, H.A.; O’Keefe, R.J. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J. Orthop. Res. 2009, 27, 833–840. [Google Scholar] [CrossRef]
- Lin, D.; Alberton, P.; Caceres, M.D.; Volkmer, E.; Schieker, M.; Docheva, D. Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing. Cell Death Dis. 2017, 8, e3116. [Google Scholar] [CrossRef]
- Xu, P.; Deng, B.; Zhang, B.; Luo, Q.; Song, G. Stretch-Induced Tenomodulin Expression Promotes Tenocyte Migration via F-Actin and Chromatin Remodeling. Int. J. Mol. Sci. 2021, 22, 4928. [Google Scholar] [CrossRef]
- Yang, R.; Zheng, Y.; Zhang, Y.; Li, G.; Xu, Y.; Zhang, Y.; Xu, Y.; Zhuang, C.; Yu, P.; Deng, L.; et al. Bipolar Metal Flexible Electrospun Fibrous Membrane Based on Metal-Organic Framework for Gradient Healing of Tendon-to-Bone Interface Regeneration. Adv. Healthc. Mater. 2022, 11, e2200072. [Google Scholar] [CrossRef]
- Olvera, D.; Schipani, R.; Sathy, B.N.; Kelly, D.J. Electrospinning of highly porous yet mechanically functional microfibrillar scaffolds at the human scale for ligament and tendon tissue engineering. Biomed. Mater. 2019, 14, 035016. [Google Scholar] [CrossRef]
- Sakabe, T.; Sakai, K.; Maeda, T.; Sunaga, A.; Furuta, N.; Schweitzer, R.; Sasaki, T.; Sakai, T. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J. Bio. Chem. 2018, 293, 5766–5780. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, L.; Lin, F.; Tang, Y.; Deng, L.; Cui, W. A Biomaterial-Based Hedging Immune Strategy for Scarless Tendon Healing. Adv. Mater. 2022, 34, e2200789. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Mimpen, J.Y.; Cribbs, A.P.; Stace, E.; Philpott, M.; Dakin, S.G.; Carr, A.J.; Snelling, S.J. Electrospun Scaffold Micro-Architecture Induces an Activated Transcriptional Phenotype within Tendon Fibroblasts. Front. Bioeng. Biotech. 2021, 9, 795748. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Y.; Liu, S.; Cui, W.G. Simulated Tendinous Sheath Film and Preparation Method Thereof. CN-103071185-A, 1 May 2013. [Google Scholar]
- Laurencin, C.; Ko, F.; Cooper, J.; Lu, H.; Attawia, M. Ligament and Tendon Replacement Constructs and Methods for Production and Use Thereof. US-2015190222-A1, 9 July 2015. [Google Scholar]
- Rizk, S.; Martin, D.P.; Bernasconi, M.; Williams, S.F. Soft Suture Anchor. CA-2917427-C, 18 June 2019. [Google Scholar]
- Lu, H.H.; Antrobus, R.M.; Childs, H.R. Fiber-Based Scaffolds for Tendon Cell Migration and Regeneration. WO-2021077042-A1, 22 April 2021. [Google Scholar]
- Ouyang, H.; Goh, C.H.J.; Toh, S.L.; Tay, T.E. Tissue Construct and Method Thereof. WO-2006110110-A1, 19 October 2006. [Google Scholar]
- Hunter, W.L.; Gravett, D.M.; Toleikis, P.M.; Maiti, A.; Signore, P.E.; Liggins, R.T.; Guan, D. Medical Implants and Fibrosis-Inducing Agents. WO-2005065079-A2, 21 July 2005. [Google Scholar]
- Downes, S.; Bosworth, L.A. Tissue Repair Scaffold. US-9770529-B2, 26 September 2017. [Google Scholar]
- Binette, F.; Hwang, J.; Zimmerman, M.; Melican, M.C. Biocompatible Scaffold for Ligament or Tendon Repair. US-8637066-B2, 28 January 2014. [Google Scholar]
- Cartmell, S.; Bosworth, L.; Wong, J.; McGrouther, D.A. Tissue Repair Scaffold and Device. US-2022176014-A1, 9 June 2022. [Google Scholar]
- Francis, M.; Kemper, N.; Wriggers, H. Biopolymer Compositions, Scaffolds and Devices. US-2020338232-A1, 14 September 2021. [Google Scholar]
- Han, B. Functional Scaffold for Tissue Repair and Regeneration. US-2017182080-A1, 29 June 2017. [Google Scholar]
- Laurencin, C.T.; Yu, X.; Valmikinathan, C.M.; Wang, J. Synergetic Functionalized Spiral-In-Tubular Bone Scaffolds. US-2010310623-A1, 9 December 2010. [Google Scholar]
- Hakimi, O.; Mouthuy, P.-A.; Baboldashti, N.Z.; Carr, A. Scaffold. US-11517646-B2, 6 December 2022. [Google Scholar]
- Gravett, D.; Acampora, K.B.; Saini, P. Functionalized and Crosslinked Polymers. US-11440976-B2, 13 September 2022. [Google Scholar]
- Buschmann, J.; Evrova, O.; Vogel, V. Device for Repair Surgery of Cylindrical Organs, Particularly Ruptured Tendons, Comprising a Therapeutic Agent for Stimulating Regrowth, and Method of Producing Such Device. US-10653819-B2, 19 May 2020. [Google Scholar]
- Lu, H.H.; Spalazzi, J.; Moffat, K.L.; Levine, W.N. Biomimmetic Nanofiber Scaffold for Soft Tissue and Soft Tissue-To-Bone Repair, Augmentation and Replacement. US-10265155-B2, 23 April 2019. [Google Scholar]
- Leonard, A. A Device for the Repair of Ruptured Tendons Comprises One or Two Needles and 60–80 cm of a Supple Thread in a Resilient Biodegradable Polymer, Preferably Polylactic Acid or Polyglycolic Acid. FR2867380-A1, 16 March 2007. [Google Scholar]
- Buschmann, J. A Device for Repair Surgery of Cylindrical Organs, Particularly Ruptured Tendons. EP-2747796-B1, 26 December 2018. [Google Scholar]
- Ahmet, H.; Mao, H.Q. Biological Tissue Connection and Repair Devices. US-9539009-B2, 10 January 2017. [Google Scholar]
- Katsuro, T.; Hiroyuki, T.; Katsuhiko, K.; Junsuke, N.; Keigo, H.; Kunio, M. Promoter for Regeneration of Tendon-Bone Junction Tissue or Ligament-Bone Junction Tissue. EP-2351574-B1, 24 August 2016. [Google Scholar]
- Lu, H.H.; Jeffrey, S. Fully Synthetic Implantable Multi-Phased Scaffold. EP-2155112-A1, 1 March 2017. [Google Scholar]
- Zhang, N.Z.; Xu, S.S.; Han, Z.C. Tissue Repair Sleeve. CN-210433516-U, 1 May 2020. [Google Scholar]
- Zhang, N.Z.; Xu, S.S.; Han, Z.C. A Kind of Tissue Repair Casing and Its Preparation Method and Application. CN-109758197-A, 17 May 2019. [Google Scholar]
- Sengan, M.Z.; Wittac, G.R.; Cleveland, B.; Balti, S.; Diab, T.; Parisu, W.R.; Tice, R.A. Tissue Enhancement Scaffold for Use with Soft Tissue Fixation Repair Systems and Methods. CN-114601516-A, 10 June 2022. [Google Scholar]
- Whitaker, G.R.; Segon, M.Z.; Cleveland, B.; Cranson, C.; Theis, R.A. Tissue-Reinforcing Scaffold for Use in Soft Tissue Fixation Repair. CN-112107340-A, 22 December 2020. [Google Scholar]
- Zhao, P.; Gu, H.B.; He, Y.; Fu, J.Z. A Kind of Preparation Method of Multiple Dimensioned Three Dimensional Biological Tissue Engineering Bracket. CN-106039417-B, 29 January 2019. [Google Scholar]
- Mathis, B. For Ligament or the Method for Tendon Repair. CN-104254351-B, 1 December 2017. [Google Scholar]
- Weiss, A.S.; Aghaei-Ghareh-Bolagh, B. Tissue Engineering Scaffolds. CA-3165939-A1, 12 August 2021. [Google Scholar]
- Francis, M.P.; Maghdouri-white, Y.; Wriggers, H.; Nardos, S.; Petrova, S.; Seth, P.; Thayer, N. Biopolymer Scaffold Implants and Methods for Their Production. CA-3079958-A1, 2 May 2019. [Google Scholar]
- Tamim, D.; Hester, D.L.; Sengun, M.Z. Systems and Methods for Using Structured Tissue Augmentation Constructs in Soft Tissue Fixation Repair. AU-2022202795-A1, 17 November 2022. [Google Scholar]
- Park, Y.H.; Kim, W.; Choi, J.W.; Kim, H.J. Absorbable versus nonabsorbable sutures for the Krackow suture repair of acute Achilles tendon rupture: A prospective randomized controlled trial. Bone. Joint. J. 2022, 104-B, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Wiig, M.E.; Dahlin, L.B.; Fridén, J.; Hagberg, L.; Larsen, S.E.; Wiklund, K.; Mahlapuu, M. PXL01 in Sodium Hyaluronate for Improvement of Hand Recovery after Flexor Tendon Repair Surgery: Randomized Controlled Trial. PLoS ONE 2014, 9, e110735. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, M.; Hoss, H.; Keppler, P.; Ebinger, T.; Kinzl, L.; Wachter, N.J. The Effectiveness of ADCON-T/N, a New Anti-Adhesion Barrier Gel, in Fresh Divisions of the Flexor Tendons in Zone II. J. Hand. Surg. 2000, 25, 590–592. [Google Scholar] [CrossRef]
- Liew, S.H.; Potokar, T.; Bantick, G.L.; Morgan, I.; Ford, C.; Murison, M.S.C. The use of ADCON-T/N after repair of zone II flexor tendons. Chirurgie. De La Main. 2001, 20, 384–387. [Google Scholar] [CrossRef]
- de Freitas Dutra Junior, E.; Hidd, S.; Amaral, M.M.; Filho, A.; Assis, L.; Ferreira, R.S., Jr.; Barraviera, B.; Martignago, C.C.S.; Figueredo-Silva, J.; de Oliveira, R.A.; et al. Treatment of partial injury of the calcaneus tendon with heterologous fibrin biopolymer and/or photobiomodulation in rats. Lasers. Med. Sci. 2022, 37, 971–981. [Google Scholar] [CrossRef]
- Liu, C.; Bai, J.; Yu, K.; Liu, G.; Tian, S.; Tian, D. Biological Amnion Prevents Flexor Tendon Adhesion in Zone II: A Controlled, Multicentre Clinical Trial. Biomed Res. Int. 2019, 2019, 2354325. [Google Scholar] [CrossRef]
- Kocaoglu, B.; Ulku, T.K.; Gereli, A.; Karahan, M.; Turkmen, M. Evaluation of Absorbable and Nonabsorbable Sutures for Repair of Achilles Tendon Rupture with a Suture-Guiding Device. Foot. Ankle. Int. 2015, 36, 691–695. [Google Scholar] [CrossRef]
- Adolfsson, L.; SÖDerberg, G.; Larsson, M.; Karlander, L.E. The Effects of a Shortened Postoperative Mobilization Programme after Flexor Tendon Repair in Zone 2. J. Hand. Surg. 1996, 21, 67–71. [Google Scholar] [CrossRef]
- Ozgenel, G.Y.; Etöz, A. Effects of repetitive injections of hyaluronic acid on peritendinous a dhesions after flexor tendon repair: A preliminary randomized, placebo -controlled clinical trial. Ulus. Travma. Acil. Cer. 2012, 18, 11–17. [Google Scholar] [CrossRef]
- Barber, F.A.; Hrnack, S.A.; Snyder, S.J.; Hapa, O. Rotator Cuff Repair Healing Influenced by Platelet-Rich Plasma Construct Augmentation. Arthroscopy 2011, 27, 1029–1035. [Google Scholar] [CrossRef]
- Liem, M.D.; Zegel, H.G.; Balduini, F.C.; Turner, M.L.; Becker, J.M.; Caballero-Saez, A. Repair of Achilles tendon ruptures with a polylactic acid implant: Assessment with MR imaging. Am. J. Roentgenol. 1991, 156, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.R.; Weiss, A.B.; Schenk, R.S.; Alexander, H.; Pavlisko, F. Long-Term Follow-up of Achilles Tendon Repair with an Absorbable Polymer Carbon Fiber Composite. Foot. Ankle. 1989, 9, 179–184. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug. Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lawrence, J.G.; Bhaduri, S.B. Fabrication aspects of PLA-CaP/PLGA-CaP composites for orthopedic applications: A review. Acta. Biomater. 2012, 8, 1999–2016. [Google Scholar] [CrossRef]
- Kuang, H.; Wang, Y.; Shi, Y.; Yao, W.; He, X.; Liu, X.; Mo, X.; Lu, S.; Zhang, P. Construction and performance evaluation of Hep/silk-PLCL composite nanofiber small-caliber artificial blood vessel graft. Biomaterials 2020, 259, 120288. [Google Scholar] [CrossRef]
- Maghdouri-White, Y.; Sori, N.; Petrova, S.; Wriggers, H.; Kemper, N.; Dasgupta, A.; Coughenour, K.; Polk, S.; Thayer, N.; Mario, R.D.V.M.; et al. Biomanufacturing organized collagen-based microfibers as a Tissue ENgineered Device (TEND) for tendon regeneration. Biomed. Mater. 2021, 16, 025025. [Google Scholar] [CrossRef]
- GTR® Medical Collagen Repair Membrane. Available online: https://www.gtrbio.cn (accessed on 3 March 2023).
- TenoMend® Collagen Tendon Wrap. Available online: https://www.exac.com (accessed on 3 March 2023).
- BioBlanket® Mesh. Available online: https://www.tregistry.com (accessed on 3 March 2023).
- BioBrace® Implant. Available online: https://biorez.com, (accessed on 3 March 2023).
- CuffPatch® Rotator Cuff Repair Patch. Available online: https://www.orthopaediclist.com (accessed on 3 March 2023).
- OrthADAPT® Bioimplant. Available online: https://pegasusbio.com (accessed on 3 March 2023).
- OrthoWrap® Bioresorbable Sheet. Available online: https://mastbio.com (accessed on 3 March 2023).
- TenoGlide® Biointegrated Implant. Available online: https://www.integranerve.com (accessed on 3 March 2023).
- Han, S.S.; Nie, K.X.; Li, J.C.; Sun, Q.Q.; Wang, X.F.; Li, X.M.; Li, Q. 3D Electrospun Nanofiber-Based Scaffolds: From Preparations and Properties to Tissue Regeneration Applications. Stem. Cells. Int. 2021, 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, X.; Chen, H.; Liu, X.; Li, J.; Luo, J.; He, A.; Han, C.C.; Liu, Y.; Xu, S. Molecular Interaction, Chain Conformation, and Rheological Modification during Electrospinning of Hyaluronic Acid Aqueous Solution. Membranes 2020, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, Y.; Wang, C.; Xia, Q.; Saif Ur Rahman, M.; Chen, H.; Han, C.; Liu, Y.; Xu, S. Mechanisms Affecting Physical Aging and Swelling by Blending an Amphiphilic Component. Int. J. Mol. Sci. 2022, 23, 2185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).