Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
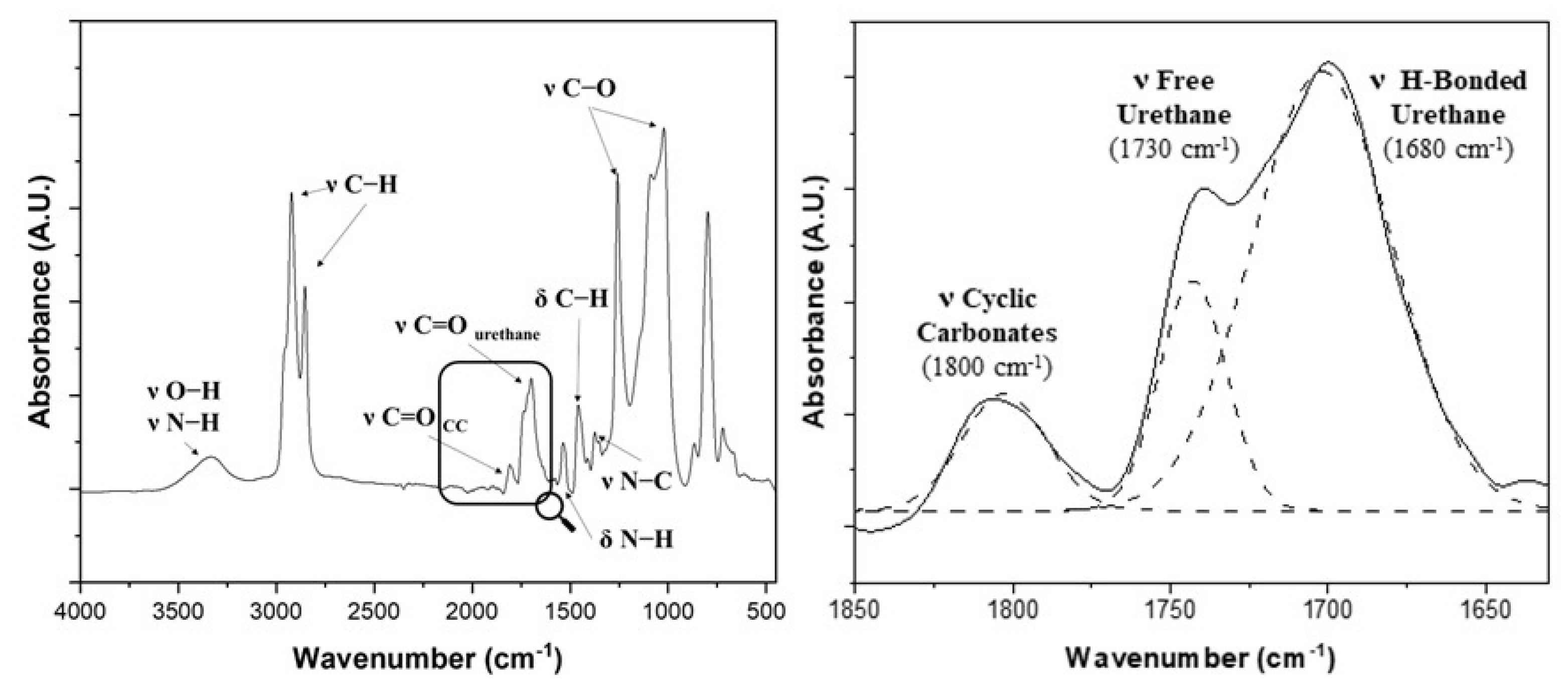
2.3. Characterization Techniques
- Nuclear Magnetic Resonance (1H-NMR) Spectrometry
- Infrared Spectrometry (FT-IR)
- Swelling Index (S. I.)
- Differential Scanning Calorimetry (DSC)
- –
- Nitrogen flow rate: 40 mL/min;
- –
- Heating temperature range: 50–300 °C;
- –
- Heating rate: 3 °C/min.
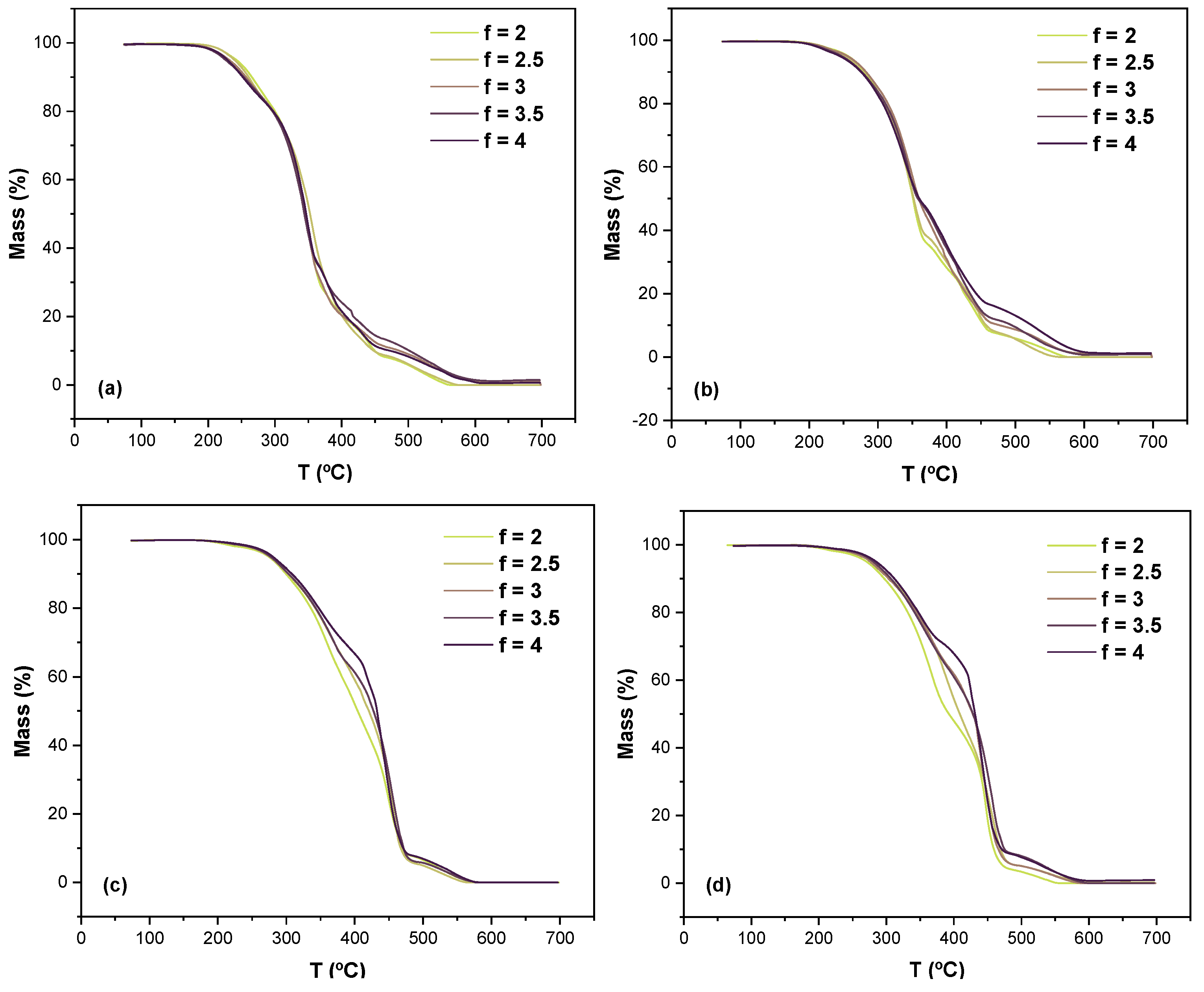
- Thermogravimetric Analysis (TGA)
- –
- Nitrogen flow rate: 50 mL/min;
- –
- Heating temperature range: 70–700 °C;
- –
- Heating rate: 5 K/min.
- Tensile Strength
- Yield Calculations
3. Results and Discussion
3.1. Preliminary Experiments
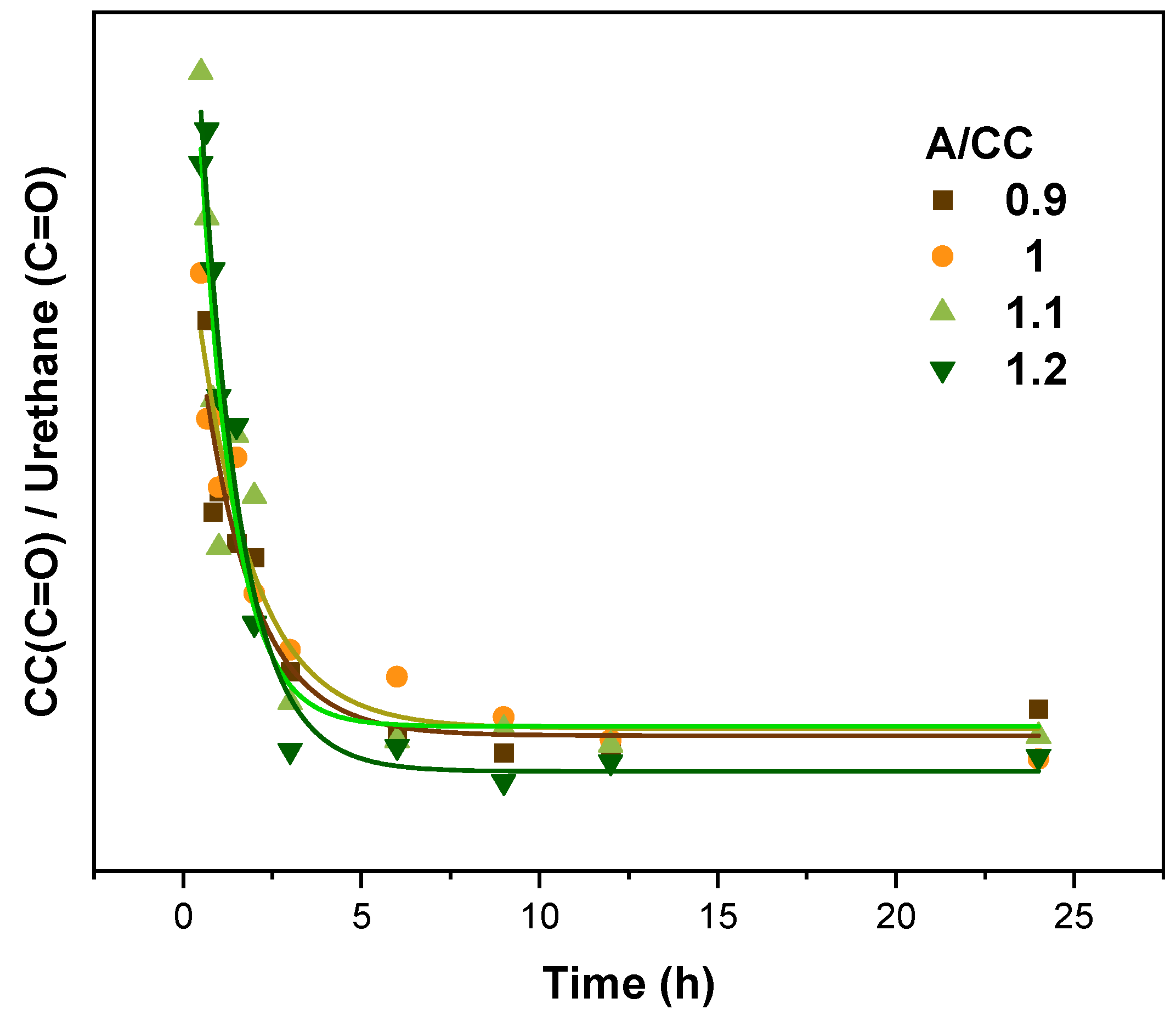
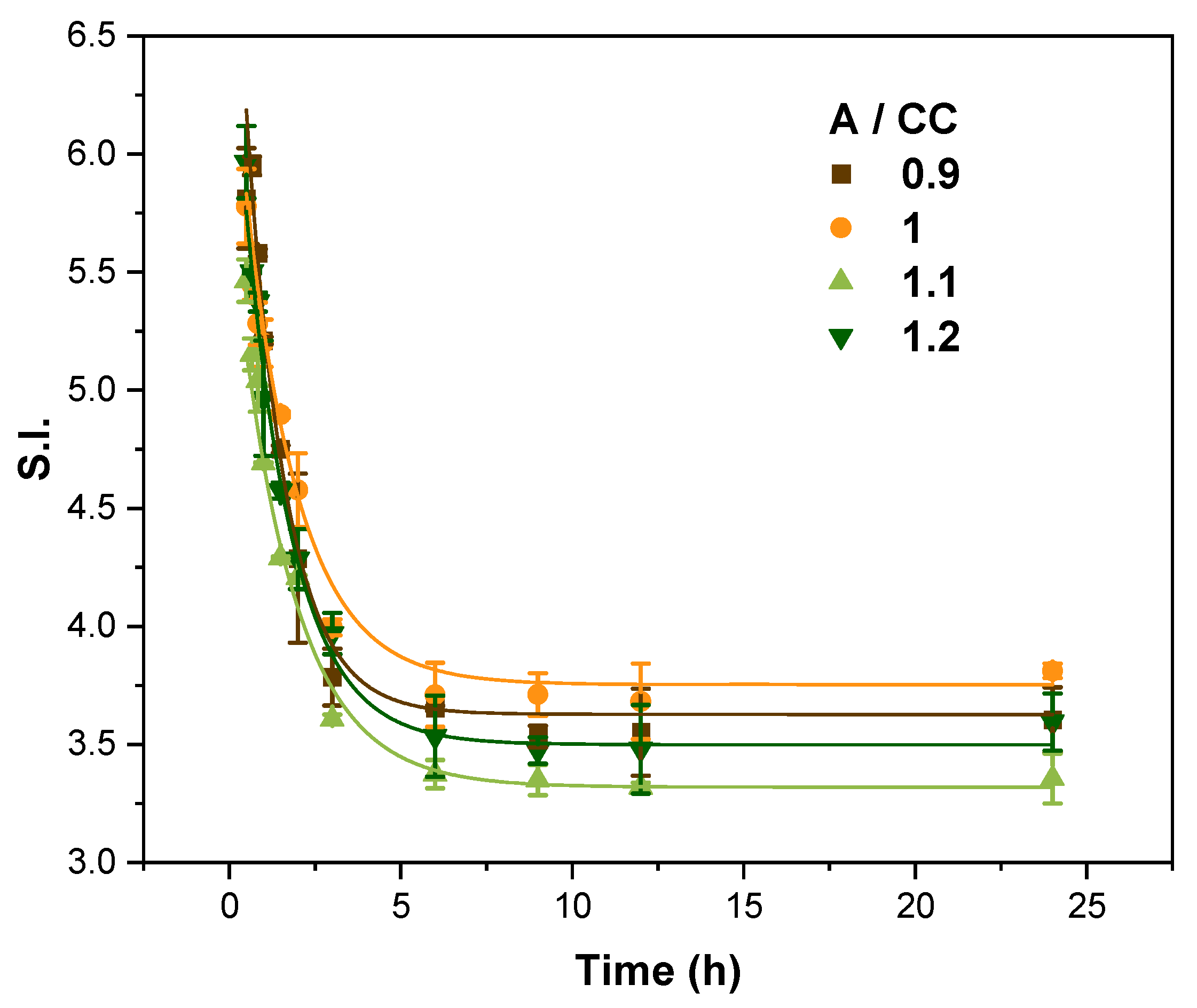
3.1.1. Influence of the Amine/Cyclic Carbonates Molar Ratio
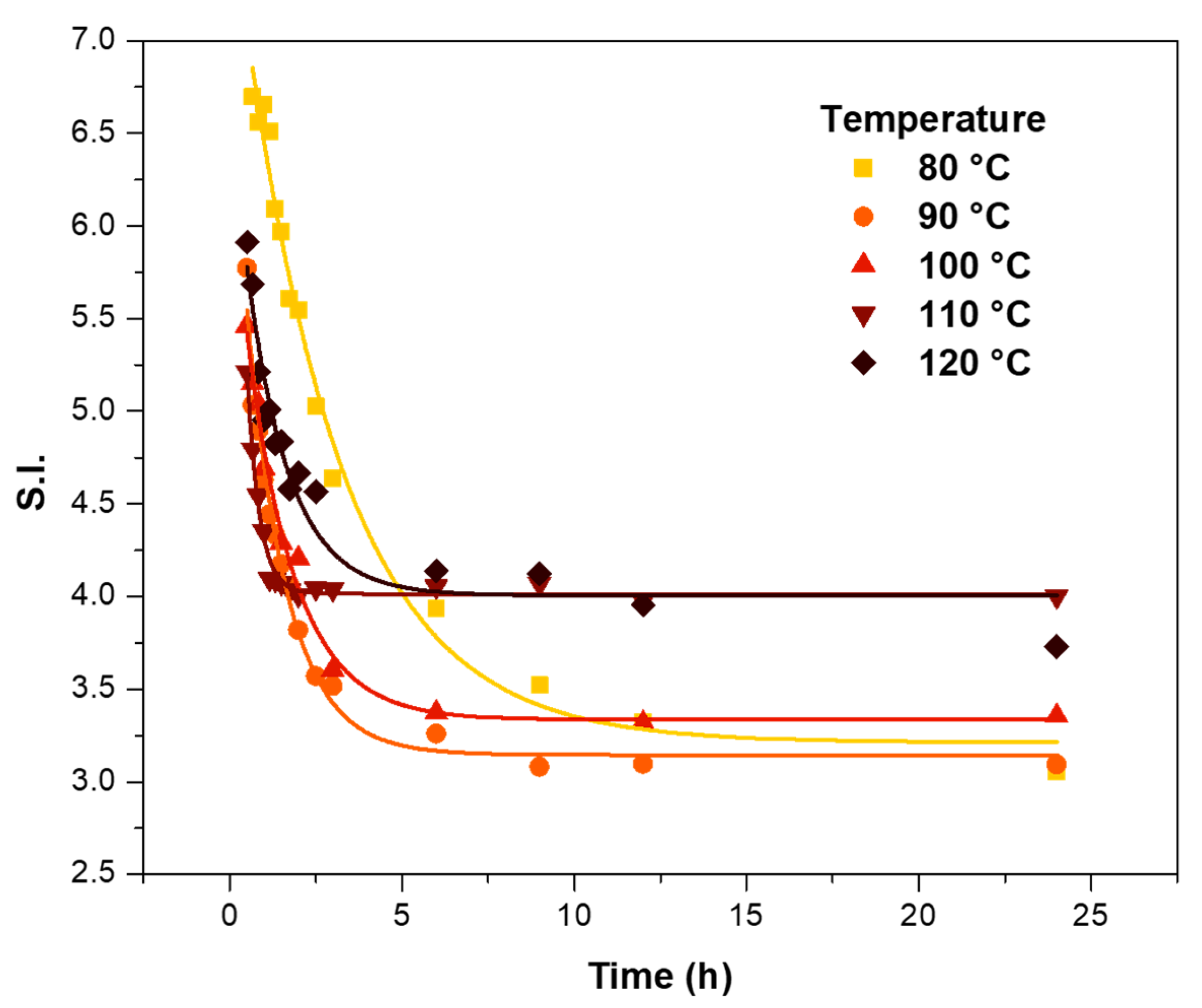
3.1.2. Curing Temperature Influence
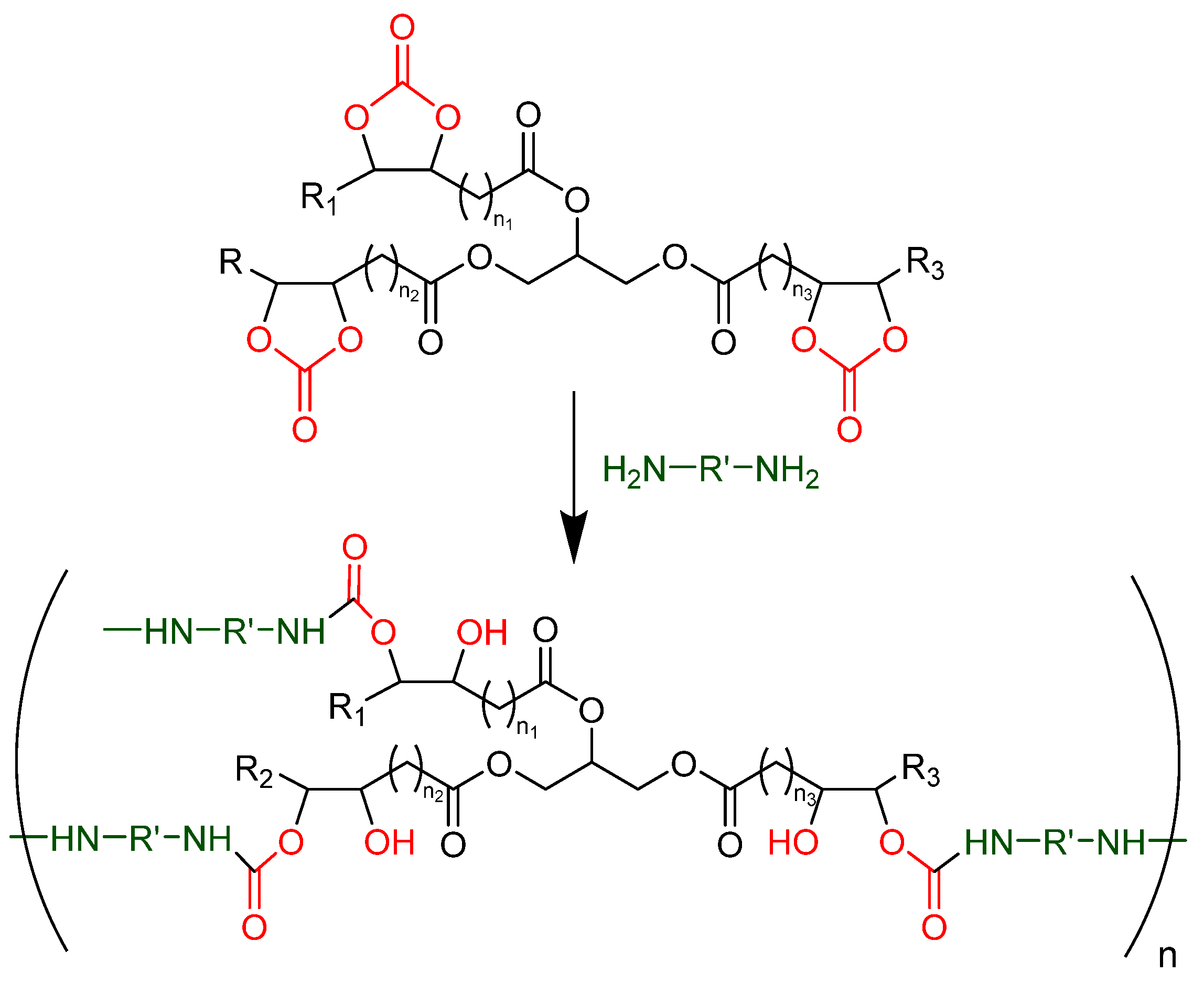
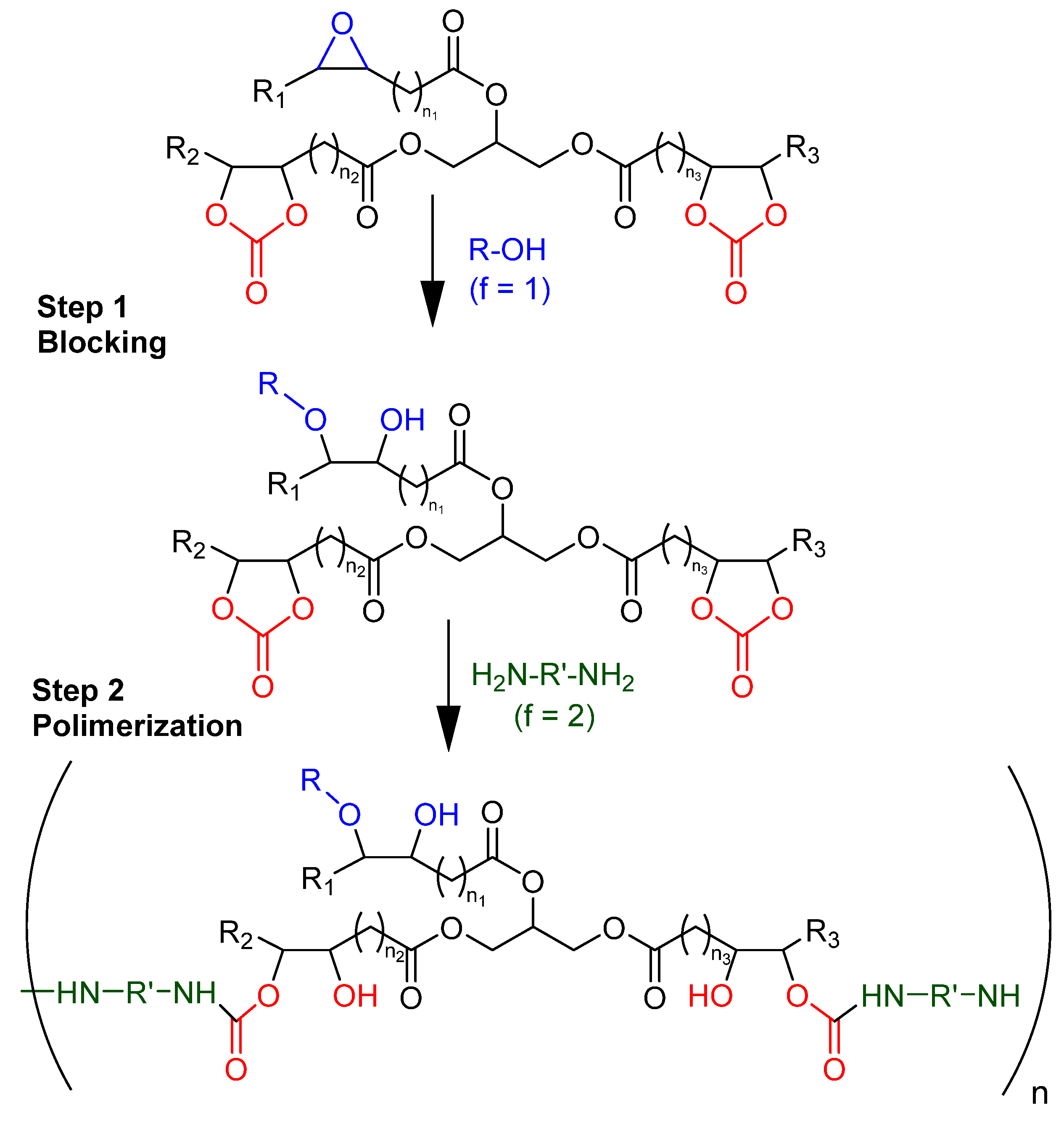
3.2. Tailor-Made Non-Isocyanate Polyurethane (Monoamine Blocking Method)
- Carbonated soybean oil (CSBO).
- Propylamine (PA) and dibutylamine (DBA) as monofunctional amines. Both primary and secondary amines are selected in order to study their influence on the blocking step.
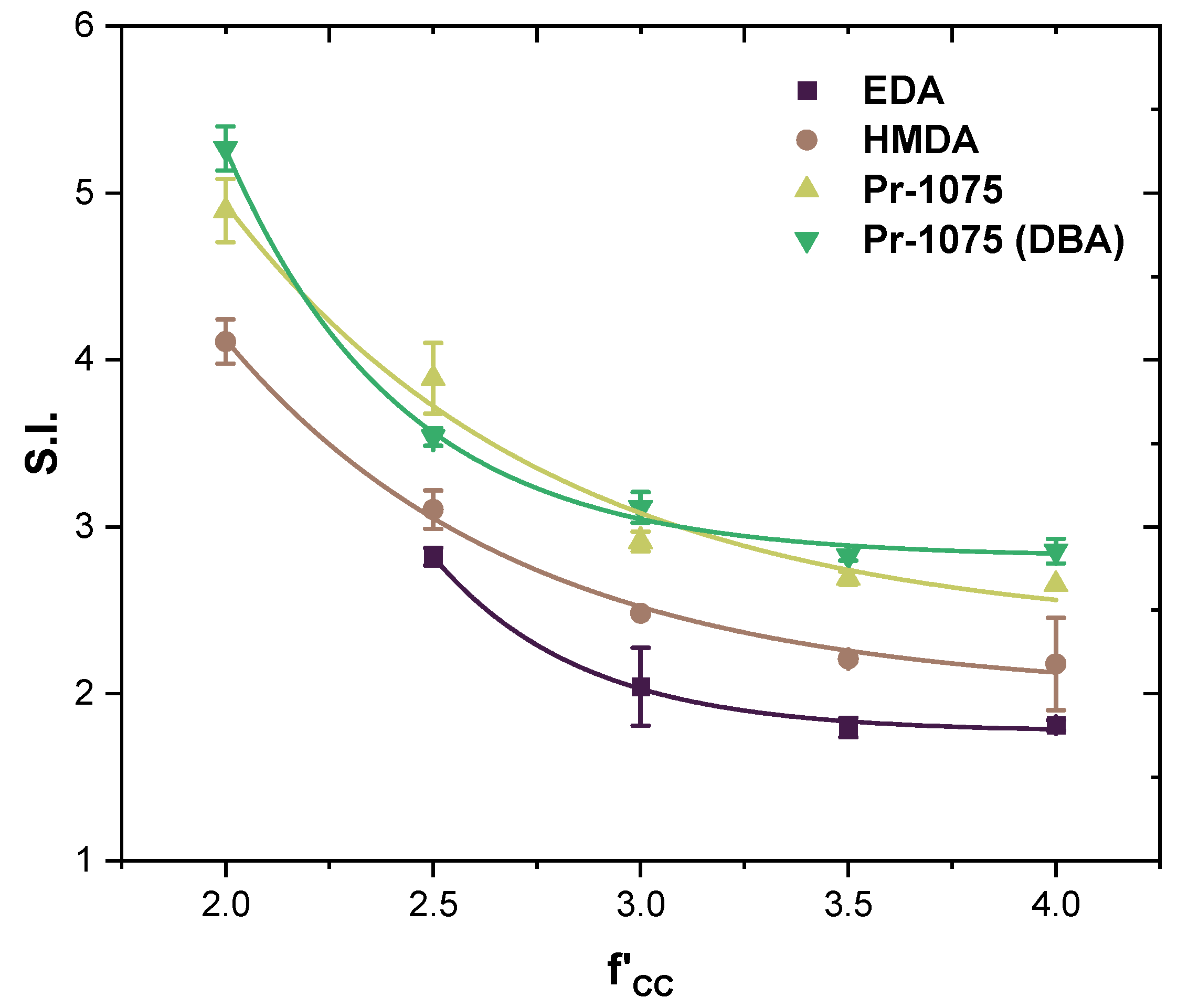
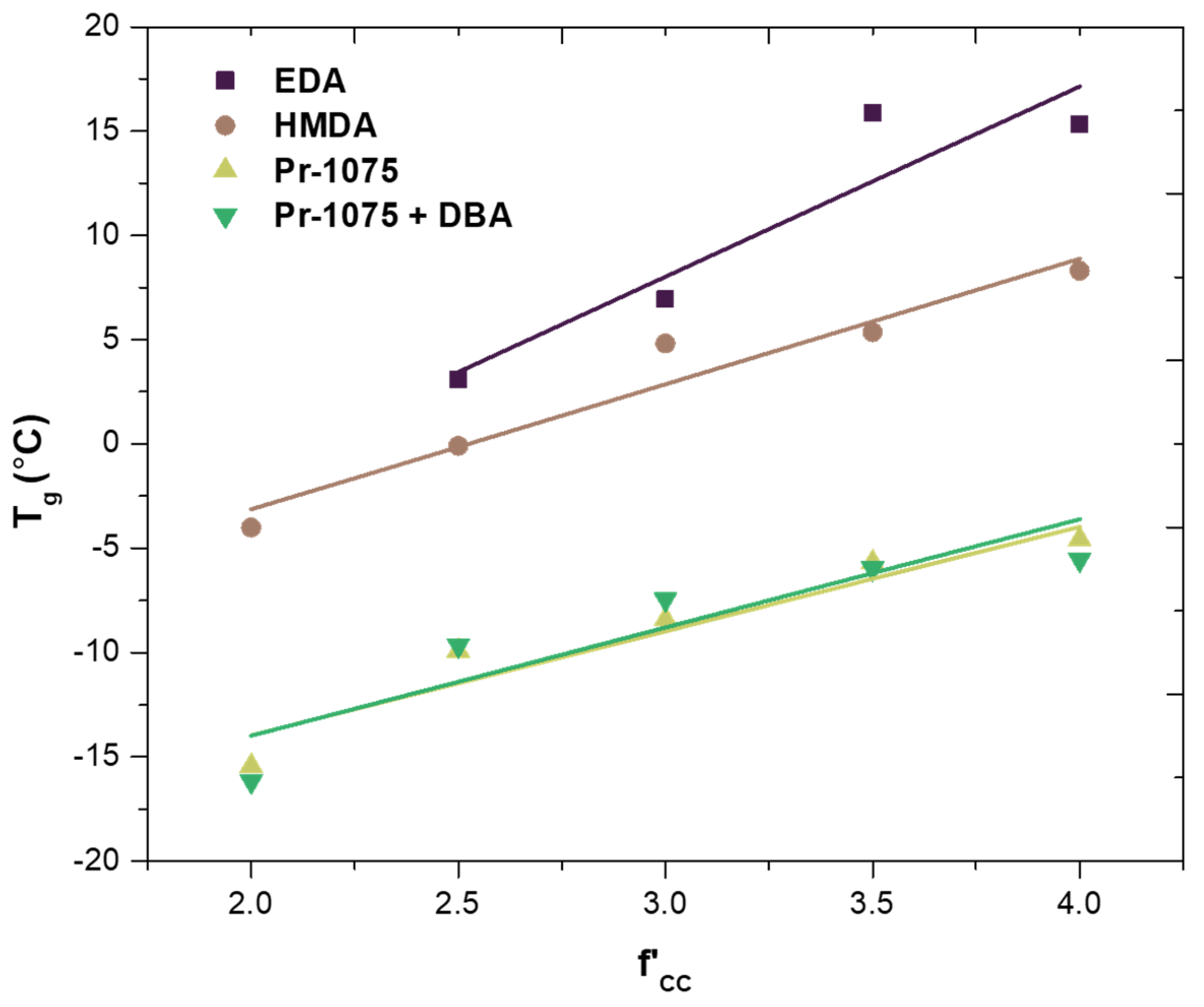
- Ethylenediamine (EDA), hexamethylenediamine (HMDA), and Priamine™ 1075 (Pr-1075) as difunctional amines acting as chain extenders. The difference between each of them lies in their molecular weight and thus in the length of the chains, allowing the study of their effect on the final properties of the material.
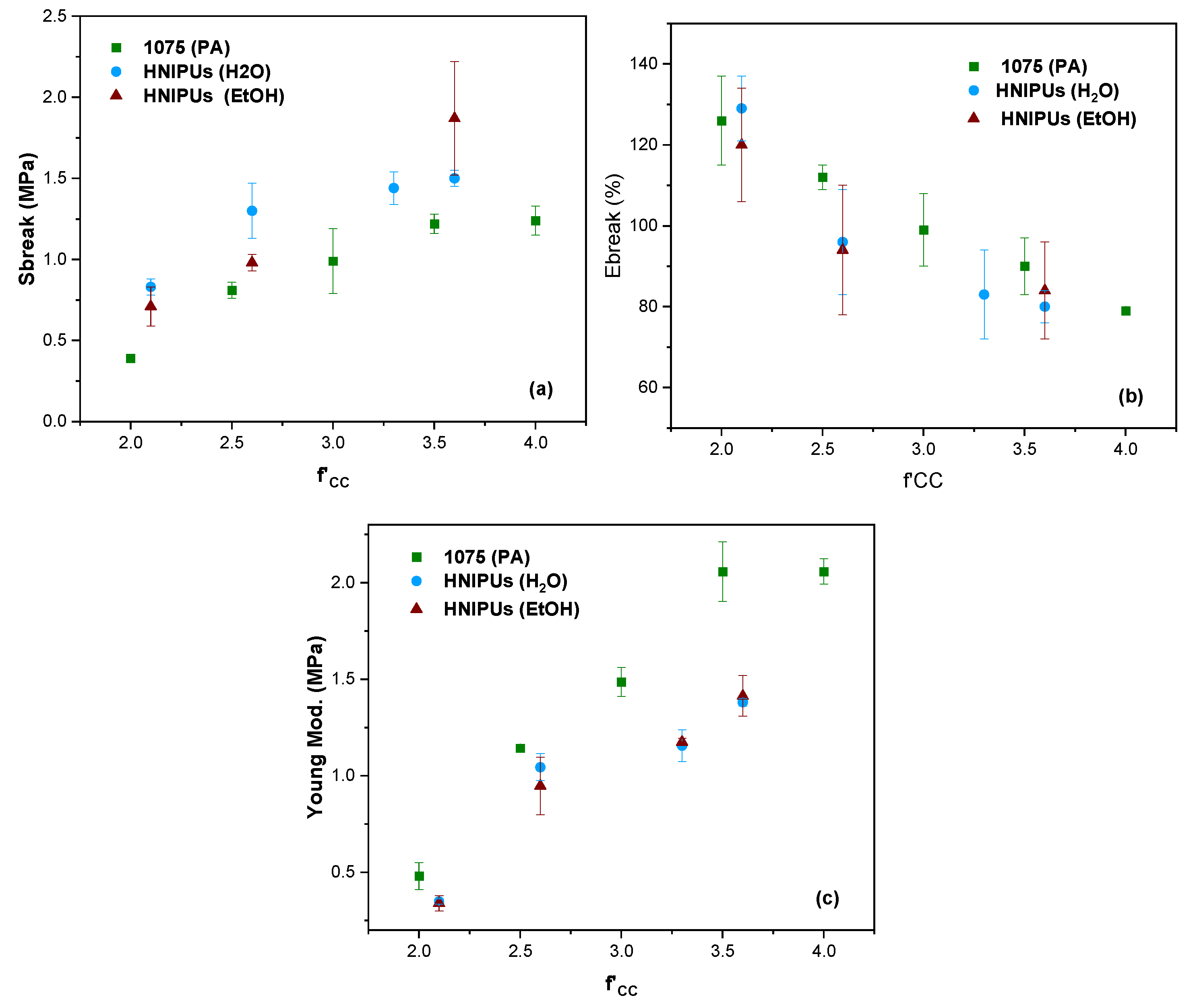
3.3. Fully Bio-Based Tailor-Made Hybrid Non-Isocyanate Polyurethane (Hydroxyl Blocking Method)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Polyurethane Market Size, Share & Trends Analysis Report by Product (Flexible Foam, Rigid Foam), by End Use (Construction, Electronics & Appliances), by Region (APAC, North America), and Segment Forecasts, 2021–2028, (n.d.). Available online: https://www.researchandmarkets.com/reports/4118824/polyurethane-market-size-share-and-trends?utm_source=GNOM&utm_medium=PressRelease&utm_code=bw9hth&utm_campaign=1552823+-+Global+Polyurethane+Market+Report+2021&utm_exec=chdo54prd (accessed on 8 April 2022).
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; Taylor & Francis: Abingdon, UK, 2013. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Martin, D.J.; Meijs, G.F.; McCarthy, S.J.; Adhikari, R. Designing biostable polyurethane elastomers for biomedical implants. Aust. J. Chem. 2003, 56, 545–557. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Martin, D.J.; Meijs, G.F.; McCarthy, S.J.; Adhikari, R.; Kanyanta, V.; Ivankovic, A. Mechanical characterisation of polyurethane elastomer for biomedical applications. J. Mech. Behav. Biomed. Mater. 2003, 3, 51–62. [Google Scholar] [CrossRef]
- Serrano, A.; Borreguero, A.M.; Catalá, J.; Rodríguez, J.F.; Carmona, M. Effect of Foaming Formulation and Operating Pressure on Thermoregulating Polyurethane Foams. Polymers 2021, 13, 2328. [Google Scholar] [CrossRef]
- Szczotok, A.M.; Madsen, D.; Serrano, A.; Carmona, M.; Van Hees, P.; Rodriguez, J.F.; Kjøniksen, A.L. Flame retardancy of rigid polyurethane foams containing thermoregulating microcapsules with phosphazene-based monomers. J. Mater. Sci. 2021, 56, 1172–1188. [Google Scholar] [CrossRef]
- Cuevas, J.M.; Cobos, R.; Germán, L.; Sierra, B.; Laza, J.M.; Vilas-Vilela, J.L. Enhanced mar/scratch resistance in automotive clear coatings by modifying crosslinked polyurethane network with branched flexible oligomers. Prog. Org. Coat. 2022, 163, 106668. [Google Scholar] [CrossRef]
- Dores, A.P.D.; Llorente, O.; Martin, L.; González, A.; Irusta, L. Polydimethylsiloxane containing waterborne hydrophobic polyurethane coatings with good adhesion to metals: Synthesis and characterization. Prog. Org. Coat. 2022, 162, 106564. [Google Scholar] [CrossRef]
- Çetin, M.E. Investigation of carbon nanotube reinforcement to polyurethane adhesive for improving impact performance of carbon fiber composite sandwich panels. Int. J. Adhes. Adhes. 2022, 112, 103002. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, J.F.; Xiao, H.L.; Pan, Y.T.; Song, Z.N. Effect of polyurethane foam adhesive on the static mechanical properties of municipal solid waste incineration bottom ash (IBA). Constr. Build Mater. 2022, 325, 126460. [Google Scholar] [CrossRef]
- European Parliament and the Council. Regulation (EC) No 1221/2009. Off. J. Eur. Union 2009, 52, 1–209. [Google Scholar]
- Reghunadhan, A.; Thomas, S. Polyurethanes: Structure, Properties, Synthesis, Characterization, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Tersac, G. Chemistry and Technology of Polyols for Polyurethanes; Milhail Ionescu. Rapra Technology: Shrewsbury, UK, 2007. [Google Scholar] [CrossRef]
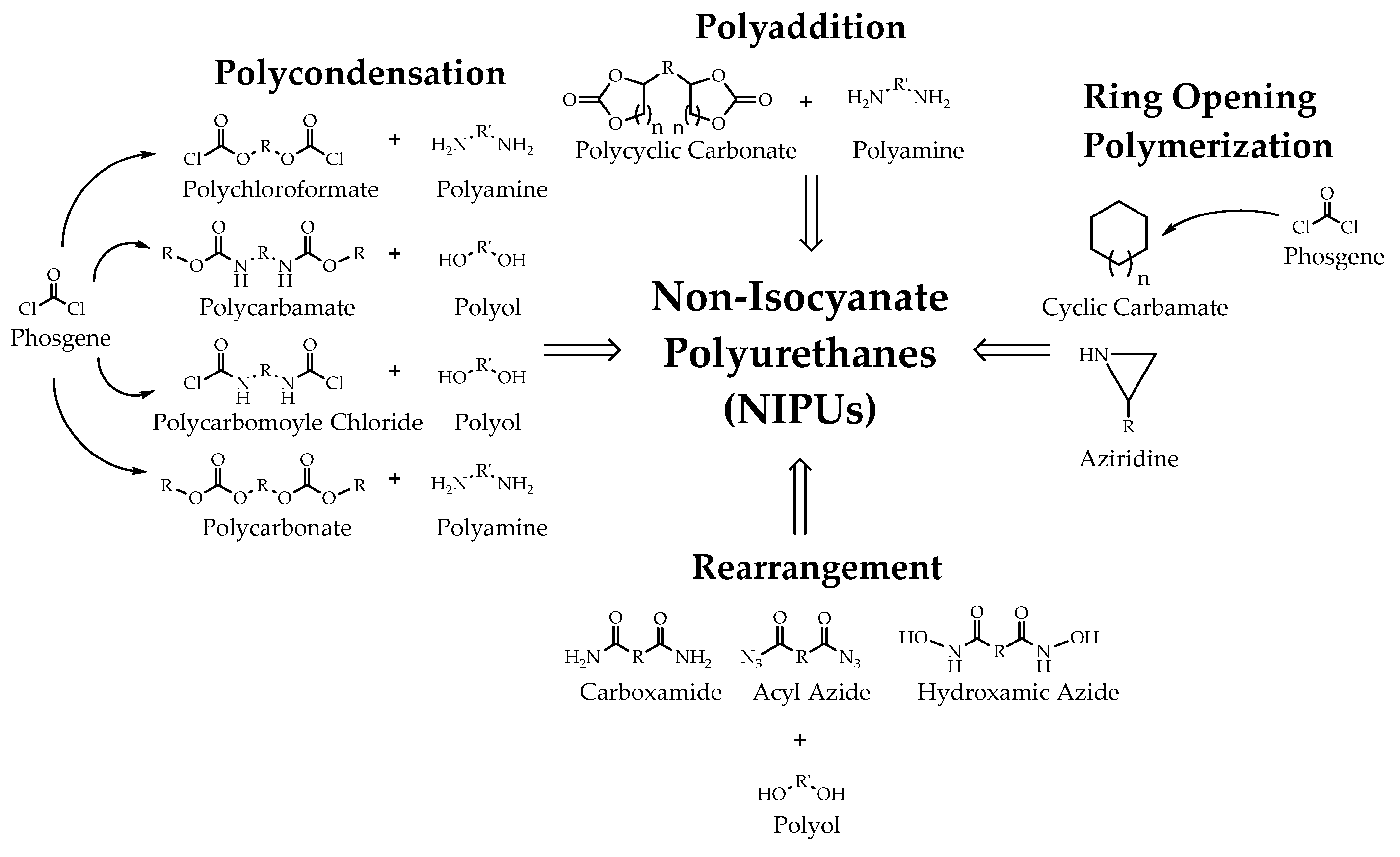
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-based routes to synthesize cyclic carbonates and polyamines precursors of non-isocyanate polyurethanes: A review. Eur. Polym. J. 2019, 118, 668–684. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog Polym Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Yagund, É.M.; Maklakov, L.I.; Stroganov, V.F.; Savchenko, V.N. Studies of hydrogen bonds in model urethan compounds obtained by the “cyclocarbonate-amine” reaction. J. Appl. Spectrosc. 1986, 45, 737–741. [Google Scholar] [CrossRef]
- Cornille, A.; Ecochard, Y.; Blain, M.; Boutevin, B.; Caillol, S. Synthesis of hybrid polyhydroxyurethanes by Michael addition. Eur. Polym. J. 2017, 96, 370–382. [Google Scholar] [CrossRef]
- US6120905A—Hybrid Nonisocyanate Polyurethane Network Polymers and Composites Formed Therefrom—Google Patents, (n.d.). Available online: https://patents.google.com/patent/US6120905A/en (accessed on 6 June 2022).
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef]
- Bähr, M.; Mülhaupt, R. Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion. Green Chem. 2012, 14, 483–489. [Google Scholar] [CrossRef]
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. [Google Scholar] [CrossRef]
- Yuen, A.; Bossion, A.; Gómez-Bengoa, E.; Ruipérez, F.; Isik, M.; Hedrick, J.L.; Mecerreyes, D.; Yang, Y.Y.; Sardon, H. Room temperature synthesis of non-isocyanate polyurethanes (NIPUs) using highly reactive N-substituted 8-membered cyclic carbonates. Polym. Chem. 2016, 7, 2105–2111. [Google Scholar] [CrossRef] [Green Version]
- Pathak, R.; Kathalewar, M.; Wazarkar, K.; Sabnis, A. Non-isocyanate polyurethane (NIPU) from tris-2-hydroxy ethyl isocyanurate modified fatty acid for coating applications. Prog. Org. Coat. 2015, 89, 160–169. [Google Scholar] [CrossRef]
- Cornille, A.; Dworakowska, S.; Bogdal, D.; Boutevin, B.; Caillol, S. A new way of creating cellular polyurethane materials: NIPU foams. Eur. Polym. J. 2015, 66, 129–138. [Google Scholar] [CrossRef]
- Rix, E.; Grau, E.; Chollet, G.; Cramail, H. Synthesis of fatty acid-based non-isocyanate polyurethanes, NIPUs, in bulk and mini-emulsion. Eur. Polym. J. 2016, 84, 863–872. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Xu, X.; Wan, Q.; Bo, G.; Yan, Y. Solvent- and catalyst-free synthesis, hybridization and characterization of biobased nonisocyanate polyurethane (NIPU). Polymers 2019, 11, 1026. [Google Scholar] [CrossRef] [Green Version]
- Gerbase, A.E.; Gregório, J.R.; Martinelli, M.; Brasil, M.C.; Mendes, A.N.F. Epoxidation of soybean oil by the methyltrioxorhenium-CH2CL2/H2O2 catalytic biphasic system, JAOCS. J. Am. Oil Chem. Soc. 2002, 79, 179–181. [Google Scholar] [CrossRef]
- Bouh, A.O.; Espenson, J.H. Epoxidation reactions with urea-hydrogen peroxide catalyzed by methyltrioxorhenium(VII) on niobia. J. Mol. Catal. A Chem. 2003, 200, 43–47. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxide with organometallic compounds. Die Makromol. Chem. 1969, 130, 210–220. [Google Scholar] [CrossRef]
- Mann, N.; Mendon, S.K.; Rawlins, J.W.; Thames, S.F. Synthesis of carbonated vernonia oil, JAOCS. J. Am. Oil Chem. Soc. 2008, 85, 791–796. [Google Scholar] [CrossRef]
- Pouladi, J.; Mirabedini, S.M.; Mohammadloo, H.E.; Rad, N.G. Synthesis of novel plant oil-based isocyanate-free urethane coatings and study of their anti-corrosion properties. Eur. Polym. J. 2021, 153, 110502. [Google Scholar] [CrossRef]
- Zheng, J.L.; Tolvanen, P.; Taouk, B.; Eränen, K.; Leveneur, S.; Salmi, T. Synthesis of carbonated vegetable oils: Investigation of microwave effect in a pressurized continuous-flow recycle batch reactor. Chem. Eng. Res. Des. 2018, 132, 9–18. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Heredia, S.; Muñoz, M.G.; Samson, T.; Oger, A.; Verove, A.; Eränen, K.; Leveneur, S.; Mikkola, J.P.; et al. Epoxidation of Fatty Acids and Vegetable Oils Assisted by Microwaves Catalyzed by a Cation Exchange Resin. Ind. Eng. Chem. Res. 2018, 57, 3876–3886. [Google Scholar] [CrossRef]
- Drobny, J.G. Thermoplastic Polyurethane Elastomers. In Handbook of Thermoplastic Elastomers; Plastics Design Library; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ordon, K.; Szlachta, M.; Szatkowski, P.; Pielichowska, K. Examining the effect of starch and hydroxyapatite crosslinking on the thermal properties of polyurethane-based biomaterials. Thermochim. Acta. 2019, 682, 178414. [Google Scholar] [CrossRef]
- Gouveia, J.R.; de Sousa Júnior, R.R.; Ribeiro, A.O.; Saraiva, S.A.; Santos, D.J.D. Effect of soft segment molecular weight and NCO:OH ratio on thermomechanical properties of lignin-based thermoplastic polyurethane adhesive. Eur. Polym. J. 2020, 131, 109690. [Google Scholar] [CrossRef]
- Jiang, S.; Yuan, C.; Guo, Z.; Bai, X. Effect of crosslink on tribological performance of polyurethane bearing material. Tribol. Int. 2019, 136, 276–284. [Google Scholar] [CrossRef]
- Martínez, J.; De La Cruz-Martínez, F.; De Sarasa Buchaca, M.M.; Caballero, M.P.; Ojeda-Amador, R.M.; Salvador, M.D.; Fregapane, G.; Tejeda, J.; Castro-Osma, J.A.; Lara-Sánchez, A. Valorization of agricultural waste and CO2 into bioderived cyclic carbonates. J. Env. Chem. Eng. 2021, 9, 105464. [Google Scholar] [CrossRef]
- Catalá, J.; Caballero, M.P.; de la Cruz-Martínez, F.; Tejeda, J.; Castro-Osma, J.A.; Lara-Sánchez, A.; García-Vargas, J.M.; García, M.T.; Ramos, M.J.; Gracia, I.; et al. Carbonation of epoxidized soybean oil in supercritical CO2 assisted by imidazole-based organocatalysts. J. CO2 Utilization. 2022, 61, 102060. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Datta, J.; Kamila, B. Diamine derivatives of dimerized fatty acids and bio-based polyether polyol as sustainable platforms for the synthesis of non-isocyanate polyurethanes. Polymer 2020, 205, 122768. [Google Scholar] [CrossRef]
- Ivdre, A.; Abolins, A.; Sevastyanova, I.; Kirpluks, M.; Cabulis, U.; Merijs-Meri, R. Rigid Polyurethane Foams with Various Isocyanate Indices Based on Polyols from Rapeseed Oil and Waste PET. Polymers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Trzebiatowska, P.J.; Echart, A.S.; Correas, T.C.; Eceiza, A.; Datta, J. The changes of crosslink density of polyurethanes synthesised with using recycled component. Chemical structure and mechanical properties investigations. Prog. Org. Coat. 2018, 115, 41–48. [Google Scholar] [CrossRef]
- Naheed, S.; Zuber, M.; Barikani, M.; Salman, M. Molecular engineering and morphology of polyurethane elastomers containing various molecular weight of macrodiol. Mater. Sci. Eng. B. 2021, 264, 114960. [Google Scholar] [CrossRef]
- Cui, S.; Borgemenke, J.; Liu, Z.; Li, Y. Recent advances of “soft” bio-polycarbonate plastics from carbon dioxide and renewable bio-feedstocks via straightforward and innovative routes. J. CO2 Util. 2019, 34, 40–52. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Schoen, P.E. Effects of Crosslinking on Thermal and Mechanical Properties of Polyurethanes. J. Appl. Polym. Sci. 2002, 83, 212–223. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]




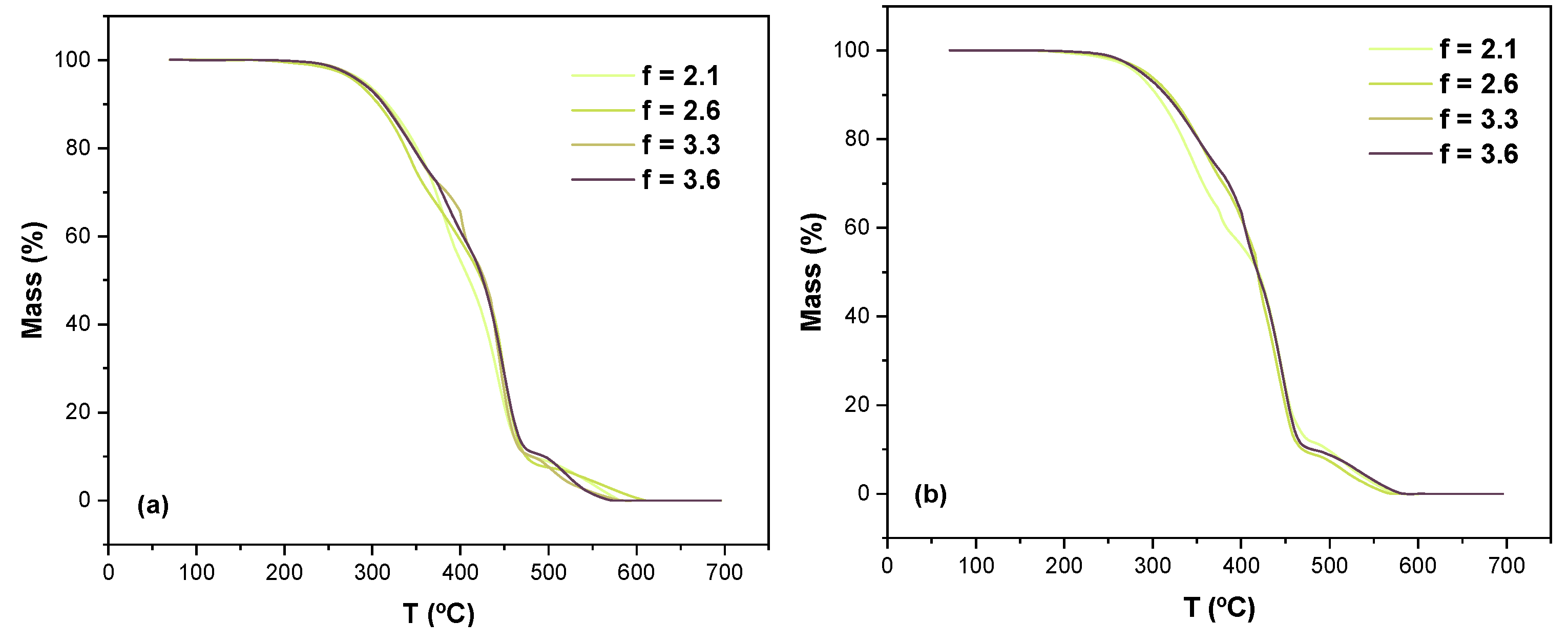

| Sample | CC Conversion (1H-NMR) | f’CC |
|---|---|---|
| 1 | 48.9 | 2.1 |
| 2 | 60.0 | 2.6 |
| 3 | 74.9 | 3.3 |
| 4 | 82.2 | 3.6 |
| Bio-Based Origin (%wt) | ||||
|---|---|---|---|---|
| f′CC | 1075 (PA) | 1075 (DBA) | HMDA | EDA |
| 4.37 | 100.00% | 100.00% | 81.00% | 89.18% |
| 4 | 99.01% | 97.87% | 80.97% | 88.39% |
| 3.5 | 97.56% | 94.81% | 80.94% | 87.36% |
| 3 | 95.95% | 91.54% | 80.91% | 86.34% |
| 2.5 | 94.15% | 88.03% | 80.88% | 85.35% |
| 2 | 92.12% | 84.37% | 80.85% | 84.39% |
| H-NIPUs | 100.00% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalá, J.; Guerra, I.; García-Vargas, J.M.; Ramos, M.J.; García, M.T.; Rodríguez, J.F. Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs). Polymers 2023, 15, 1589. https://doi.org/10.3390/polym15061589
Catalá J, Guerra I, García-Vargas JM, Ramos MJ, García MT, Rodríguez JF. Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs). Polymers. 2023; 15(6):1589. https://doi.org/10.3390/polym15061589
Chicago/Turabian StyleCatalá, Juan, Irene Guerra, Jesús Manuel García-Vargas, María Jesús Ramos, María Teresa García, and Juan Francisco Rodríguez. 2023. "Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs)" Polymers 15, no. 6: 1589. https://doi.org/10.3390/polym15061589
APA StyleCatalá, J., Guerra, I., García-Vargas, J. M., Ramos, M. J., García, M. T., & Rodríguez, J. F. (2023). Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs). Polymers, 15(6), 1589. https://doi.org/10.3390/polym15061589








