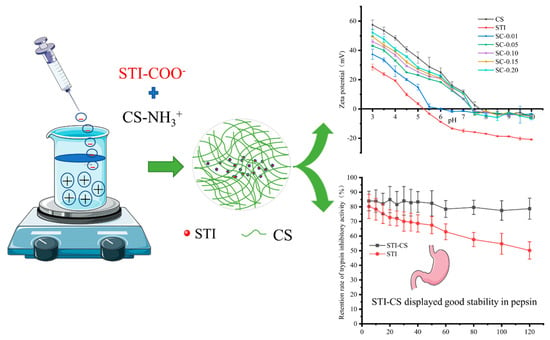
Interactions between Soybean Trypsin Inhibitor and Chitosan in an Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstocks and Reagents
2.2. STI Preparation
2.3. Preparation of STI–CS Mixture System
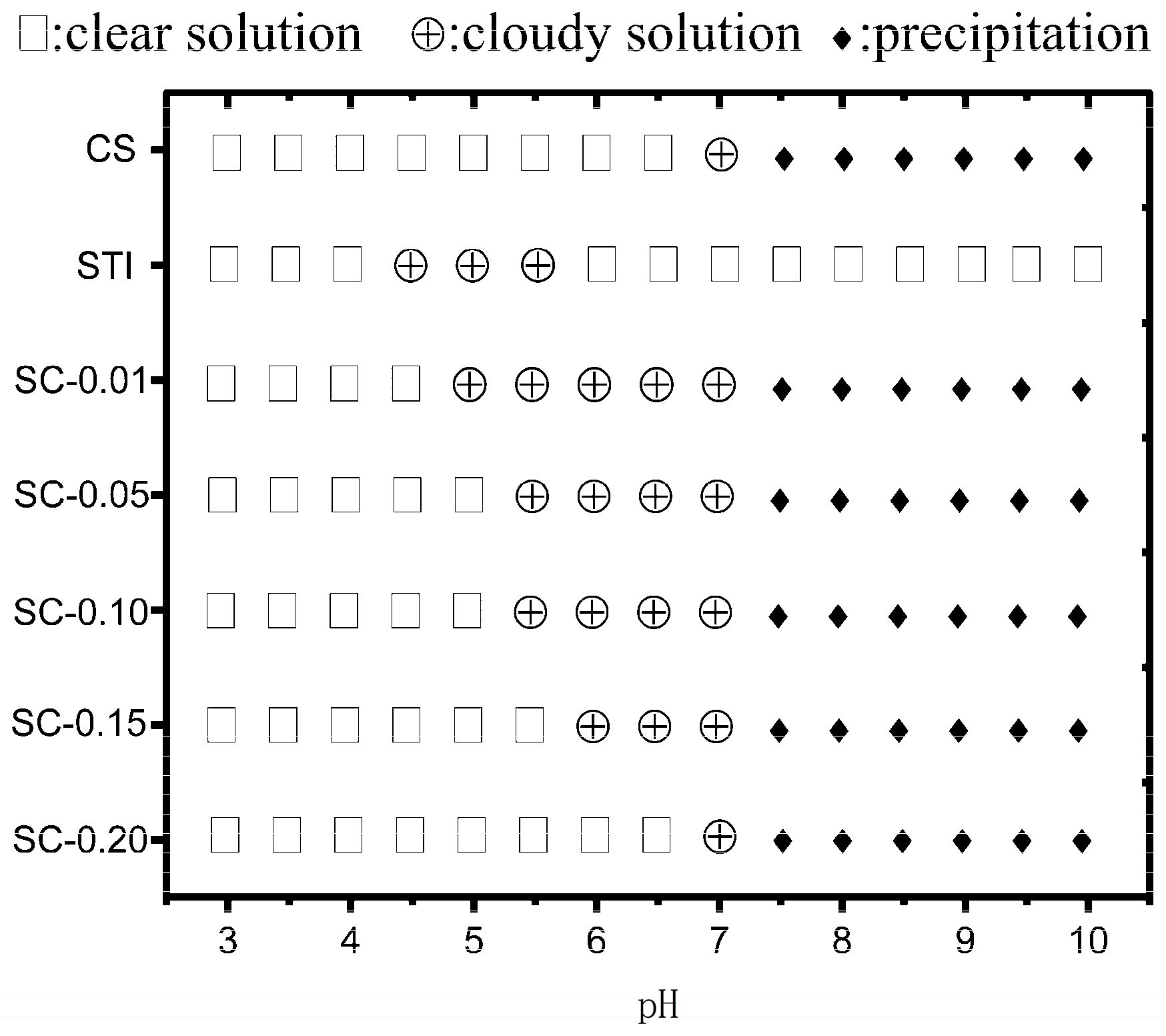
2.4. Phase Diagram
2.5. Determination of Turbidity [30]
2.6. Determination of Zeta Potential
2.7. Fluorescence Spectroscopy
2.8. Far-UV Circular Dichroism (CD) Spectroscopy
2.9. Fourier Transform Infrared (FTIR) Spectroscopy [33]
2.10. Microstructure Analysis
2.11. Examination of the STI–CS Stability
2.12. Statistical Analysis
3. Results and Discussion
3.1. Phase Diagram of CS–STI Mixed Dispersions
3.2. Turbidimetric Analysis
3.3. Zeta Potential Measurements
3.4. Fluorescence Spectroscopy
3.5. Circular Dichroism Spectroscopy
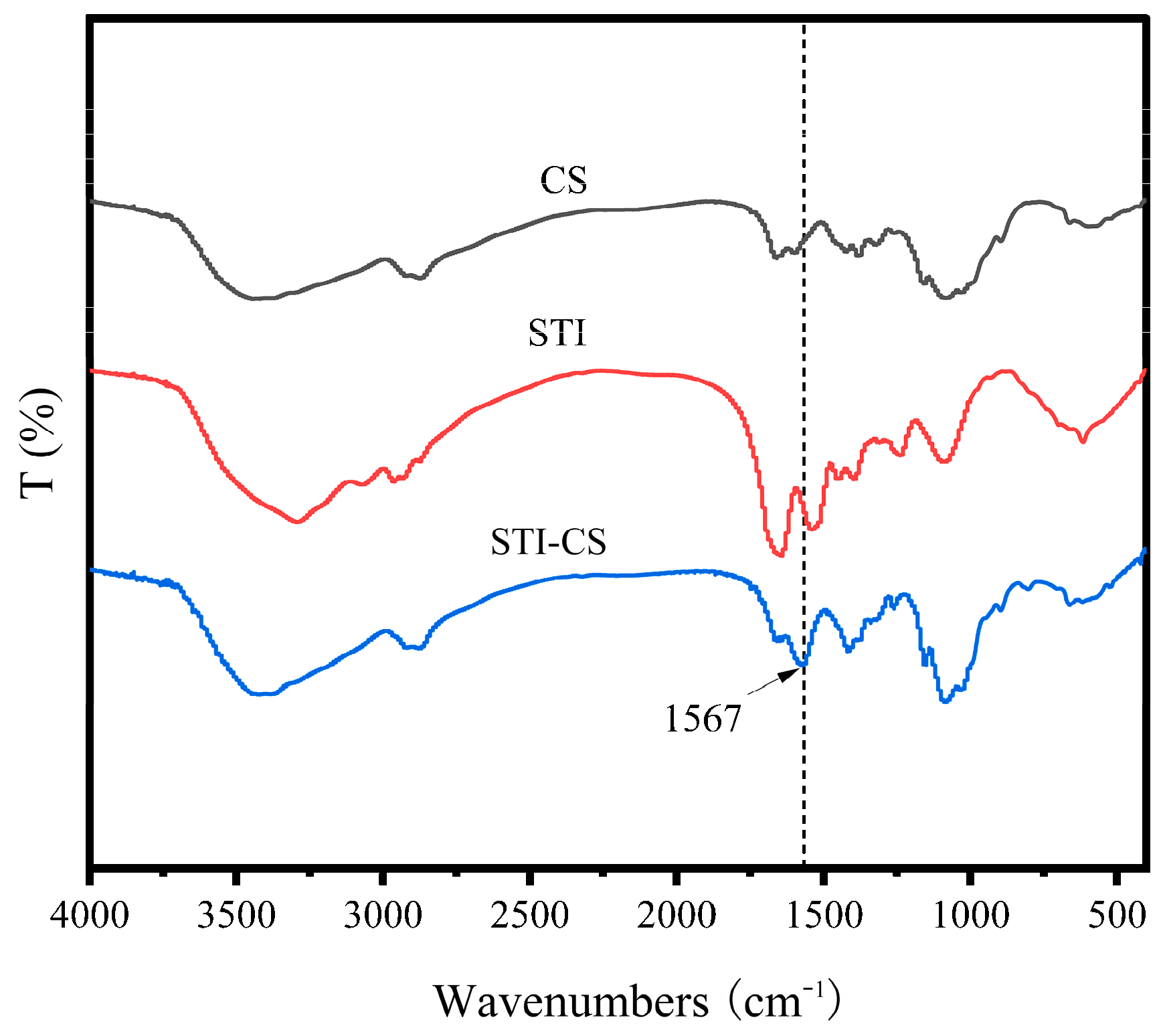
3.6. Fourier Transform Infrared Spectroscopy
3.7. Morphological Characteristics of STI–CS Complex
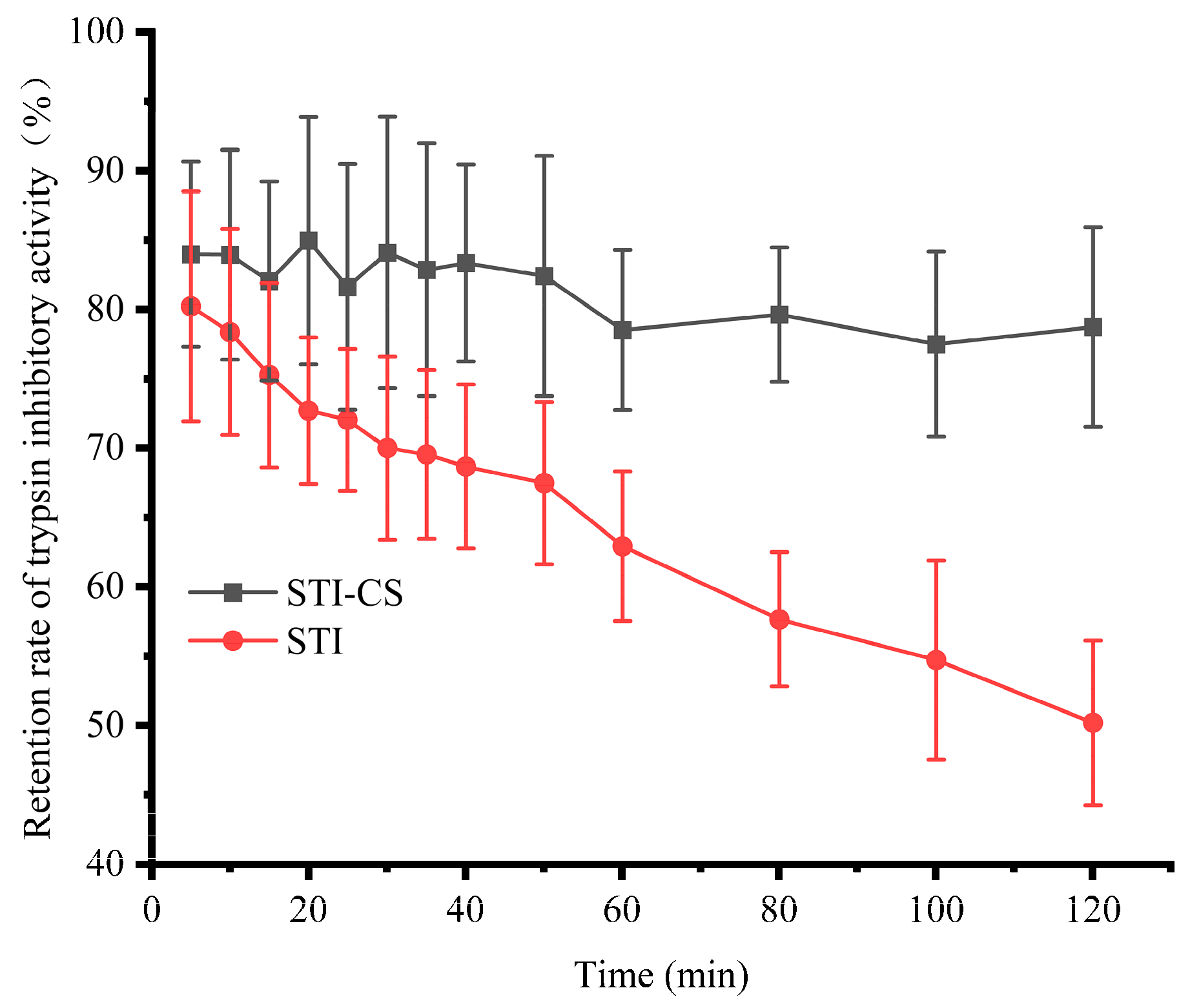
3.8. The Protective Effect of STI–CS Complex Coacervates on STI Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm. Sin. B 2019, 9, 902–922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, Z.; Paul, P.K.; Lu, Y.; Wu, W.; Qi, J. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm. Sin. B 2021, 11, 2416–2448. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-j.; Lv, Z.-q.; Guo, S.; Jiang, G.-p.; Liu, H.-l. An update—Prolonging the action of protein and peptide drugs. J. Drug Deliv. Sci. Technol. 2021, 61, 102124. [Google Scholar] [CrossRef]
- Salar, S.; Jafari, M.; Kaboli, S.F.; Mehrnejad, F. The role of intermolecular interactions on the encapsulation of human insulin into the chitosan and cholesterol-grafted chitosan polymers. Carbohydr. Polym. 2019, 208, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toala, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of bioactive peptides: A strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Yue, W.; Qin, W.; Dong, H.; Vasanthan, T. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation of bioactive compounds. Trends Food Sci. Technol. 2021, 109, 169–196. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Hu, Y.; Wu, J.; Van der Meeren, P. Designing delivery systems for functional ingredients by protein/polysaccharide interactions. Trends Food Sci. Technol. 2022, 119, 272–287. [Google Scholar] [CrossRef]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019, 279, 223–230. [Google Scholar] [CrossRef]
- Leite Milião, G.; Souza Soares, L.d.; Balbino, D.F.; Almeida Alves Barbosa, É.d.; Bressan, G.C.; Carvalho Teixeira, A.V.N.d.; dos Reis Coimbra, J.S.; Oliveira, E.B.d. pH influence on the mechanisms of interaction between chitosan and ovalbumin: A multi-spectroscopic approach. Food Hydrocoll. 2022, 123, 107137. [Google Scholar] [CrossRef]
- Kedir, W.M.; Abdi, G.F.; Goro, M.M.; Tolesa, L.D. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: A review. Heliyon 2022, 8, e10196. [Google Scholar] [CrossRef]
- Barbosa, F.C.; Silva, M.C.D.; Silva, H.N.D.; Albuquerque, D.; Gomes, A.A.R.; Silva, S.M.L.; Fook, M.V.L. Progress in the Development of Chitosan Based Insulin Delivery Systems: A Systematic Literature Review. Polymers 2020, 12, 2499. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xu, Y.; Xu, L.; Bai, Y.; Xu, X. Interactions of water-soluble myofibrillar protein with chitosan: Phase behavior, microstructure and rheological properties. Innov. Food Sci. Emerg. Technol. 2022, 78, 103013. [Google Scholar] [CrossRef]
- Dodane, V.; Khan, M.A.; Merwin, J.R. Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Jacquier, J.C.; O’Riordan, E.D. Entrapment of proteins and peptides in chitosan-polyphosphoric acid hydrogel beads: A new approach to achieve both high entrapment efficiency and controlled in vitro release. Food Chem. 2018, 239, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Yin, B.C.; Zhang, W.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloids Surf. B Biointerfaces 2009, 68, 245–249. [Google Scholar] [CrossRef]
- Li, X.; Dong, D.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Soybean whey protein/chitosan complex behavior and selective recovery of kunitz trypsin inhibitor. J. Agric. Food Chem. 2014, 62, 7279–7286. [Google Scholar] [CrossRef]
- Roychaudhuri, R.; Sarath, G.; Zeece, M.; Markwell, J. Reversible denaturation of the soybean Kunitz trypsin inhibitor. Arch. Biochem. Biophys. 2003, 412, 20–26. [Google Scholar] [CrossRef]
- Karlund, A.; Paukkonen, I.; Gomez-Gallego, C.; Kolehmainen, M. Intestinal Exposure to Food-Derived Protease Inhibitors: Digestion Physiology- and Gut Health-Related Effects. Healthcare 2021, 9, 1002. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Wang, T.T. An effective and simple procedure to isolate abundant quantities of biologically active chemopreventive Lunasin Protease Inhibitor Concentrate (LPIC) from soybean. Food Chem. 2015, 177, 120–126. [Google Scholar] [CrossRef]
- Kennedy, A.R. The Bowman-Birk inhibitor from soybeans as an anticarcinogenic agent. Am. J. Clin. Nutr. 1998, 68 (Suppl. S6), 1406S–1412S. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, D.-F.; Li, H.-Y.; Xu, Y.; Zhang, L. Preparation and properties of chitosan–soybean trypsin inhibitor blend film with anti-Aspergillus flavus activity. Ind. Crops Prod. 2009, 29, 541–548. [Google Scholar] [CrossRef]
- Nieto-Veloza, A.; Wang, Z.; Zhong, Q.; D’Souza, D.; Krishnan, H.B.; Dia, V.P. Lunasin protease inhibitor concentrate decreases pro-inflammatory cytokines and improves histopathological markers in dextran sodium sulfate-induced ulcerative colitis. Food Sci. Hum. Wellness 2022, 11, 1508–1514. [Google Scholar] [CrossRef]
- Burshtein, G.; Itin, C.; Tang, J.C.Y.; Galitzer, H.; Fraser, W.D.; Schwartz, P. The combined effect of permeation enhancement and proteolysis inhibition on the systemic exposure of orally administrated peptides: Salcaprozate sodium, soybean trypsin inhibitor, and teriparatide study in pigs. Int. J. Pharm. X 2021, 3, 100097. [Google Scholar] [CrossRef] [PubMed]
- Chuang, E.Y.; Lin, K.J.; Su, F.Y.; Chen, H.L.; Maiti, B.; Ho, Y.C.; Yen, T.C.; Panda, N.; Sung, H.W. Calcium depletion-mediated protease inhibition and apical-junctional-complex disassembly via an EGTA-conjugated carrier for oral insulin delivery. J. Control. Release 2013, 169, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Plati, F.; Ritzoulis, C.; Pavlidou, E.; Paraskevopoulou, A. Complex coacervate formation between hemp protein isolate and gum Arabic: Formulation and characterization. Int. J. Biol. Macromol. 2021, 182, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Ohm, J.B.; Chen, B.; Rao, J. Phase behavior and complex coacervation of concentrated pea protein isolate-beet pectin solution. Food Chem. 2020, 307, 125536. [Google Scholar] [CrossRef]
- Elmer, C.; Karaca, A.C.; Low, N.H.; Nickerson, M.T. Complex coacervation in pea protein isolate–chitosan mixtures. Food Res. Int. 2011, 44, 1441–1446. [Google Scholar] [CrossRef]
- Huang, G.Q.; Sun, Y.T.; Xiao, J.X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ying, Z.; Li, W.; Li, H.; Liu, X. Trypsin Inhibitor from Soybean Whey Wastewater: Isolation, Purification and Stability. Appl. Sci. 2022, 12, 10084. [Google Scholar] [CrossRef]
- Rousi, Z.; Malhiac, C.; Fatouros, D.G.; Paraskevopoulou, A. Complex coacervates formation between gelatin and gum Arabic with different arabinogalactan protein fraction content and their characterization. Food Hydrocoll. 2019, 96, 577–588. [Google Scholar] [CrossRef]
- Xu, F.Y.; Lin, J.W.; Wang, R.; Chen, B.R.; Li, J.; Wen, Q.H.; Zeng, X.A. Succinylated whey protein isolate-chitosan core-shell composite particles as a novel carrier: Self-assembly mechanism and stability studies. Food Res. Int. 2022, 160, 111695. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Niu, G.; Long, H.; Zhang, C.; Liu, X. Elucidation of interactions between gelatin aggregates and hsian-tsao gum in aqueous solutions. Food Chem. 2020, 319, 126532. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Dadmohammadi, Y.; Davachi, S.M.; Torabi, H.; Li, P.; Pomon, B.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Improvement of lactoferrin thermal stability by complex coacervation using soy soluble polysaccharides. Food Hydrocoll. 2022, 131, 107736. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Guzey, D.; McClements, D.J. Characterization of β-lactoglobulin–chitosan interactions in aqueous solutions: A calorimetry, light scattering, electrophoretic mobility and solubility study. Food Hydrocoll. 2006, 20, 124–131. [Google Scholar] [CrossRef]
- Novák, P.; Havlíček, V. Protein Extraction and Precipitation. In Proteomic Profiling and Analytical Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 51–62. [Google Scholar] [CrossRef]
- Wang, Y.; Serventi, L. Sustainability of dairy and soy processing: A review on wastewater recycling. J. Clean. Prod. 2019, 237, 117821. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeganathan, B.; Dong, H.; Chen, L.; Vasanthan, T. Effect of sodium chloride on the thermodynamic, rheological, and microstructural properties of field pea protein isolate/chitosan complex coacervates. Food Chem. 2021, 344, 128569. [Google Scholar] [CrossRef]
- Ding, L.; Huang, Y.; Cai, X.; Wang, S. Impact of pH, ionic strength and chitosan charge density on chitosan/casein complexation and phase behavior. Carbohydr. Polym. 2019, 208, 133–141. [Google Scholar] [CrossRef]
- Ji, Y.; Han, C.; Liu, E.; Li, X.; Meng, X.; Liu, B. Pickering emulsions stabilized by pea protein isolate-chitosan nanoparticles: Fabrication, characterization and delivery EPA for digestion in vitro and in vivo. Food Chem. 2022, 378, 132090. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Sun, Y.; Gao, X.; Liang, J. Controlled release and antioxidant activity of chitosan and beta-lactoglobulin complex nanoparticles loaded with epigallocatechin gallate. Colloids Surf. B. Biointerfaces 2020, 188, 110802. [Google Scholar] [CrossRef]
- Zeng, Q.-Z.; Li, M.-F.; Li, Z.-Z.; Zhang, J.-L.; Wang, Q.; Feng, S.-L.; Su, D.-X.; He, S.; Yuan, Y. Formation of gliadin-chitosan soluble complexes and coacervates through pH-induced: Relationship to encapsulation and controlled release properties. LWT 2019, 105, 79–86. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Liu, X.; Zhao, M. Interactions between hsian-tsao gum and chitosan in aqueous solution. Food Hydrocoll. 2018, 79, 428–438. [Google Scholar] [CrossRef]
- Tranter, G.E. Protein Structure Analysis by CD, FTIR, and Raman Spectroscopies. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 740–758. [Google Scholar] [CrossRef]
- Li, B.; Gu, W.; Bourouis, I.; Sun, M.; Huang, Y.; Chen, C.; Liu, X.; Pang, Z. Lubrication behaviors of core-shell structured particles formed by whey proteins and xanthan gum. Food Hydrocoll. 2022, 127, 107512. [Google Scholar] [CrossRef]





| Code | The Solid Content in the Mixed Aqueous Solution | |
|---|---|---|
| STI (wt%) | CS (wt%) | |
| SC–0.01 | 0.10 | 0.01 |
| SC–0.05 | 0.10 | 0.05 |
| SC–0.10 | 0.10 | 0.10 |
| SC–0.15 | 0.10 | 0.15 |
| SC–0.20 | 0.10 | 0.20 |
| Sample | pH 4.0 | pH 6.0 | ||||||
|---|---|---|---|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | α-Helix | β-Sheet | β-Turn | Random Coil | |
| STI | 0 | 42.07 ± 1.65 | 14.45 ± 0.40 | 43.48 ± 2.01 | 0 | 38.67 ± 0.29 cd | 14.06 ± 0.33 bc | 47.20 ± 0.55 bc |
| CH−0.01 | 0 | 42.50 ± 2.33 | 14.55 ± 0.83 | 42.85 ± 3.29 | 0 | 42.66 ± 0.59 a | 15.06 ± 0.04 a | 42.27 ± 0.62 d |
| CH–0.05 | 0 | 41.90 ± 2.02 | 14.43 ± 0.93 | 43.68 ± 2.93 | 0 | 40.36 ± 0.04 bc | 14.53 ± 0.39 ab | 45.46 ± 0.23 c |
| CH–0.10 | 0 | 42.13 ± 1.94 | 14.60 ± 0.55 | 43.25 ± 2.48 | 0 | 40.83 ± 0.04 ab | 13.97 ± 0.40 bc | 45.20 ± 0.39 c |
| CH–0.15 | 0 | 41.73 ± 1.17 | 14.08 ± 0.37 | 44.18 ± 1.56 | 0 | 36.50 ± 1.48 e | 13.13 ± 0.53 c | 50.33 ± 1.96 a |
| CH–0.20 | 0 | 40.08 ± 2.07 | 13.73 ± 0.78 | 46.20 ± 2.84 | 0 | 36.87 ± 0.78 de | 13.70 ± 1.12 bc | 49.43 ± 0.75 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, R.; Li, H.; Li, Y.; Liu, X. Interactions between Soybean Trypsin Inhibitor and Chitosan in an Aqueous Solution. Polymers 2023, 15, 1594. https://doi.org/10.3390/polym15071594
Zhang Y, Liu R, Li H, Li Y, Liu X. Interactions between Soybean Trypsin Inhibitor and Chitosan in an Aqueous Solution. Polymers. 2023; 15(7):1594. https://doi.org/10.3390/polym15071594
Chicago/Turabian StyleZhang, Yihao, Ruijia Liu, He Li, You Li, and Xinqi Liu. 2023. "Interactions between Soybean Trypsin Inhibitor and Chitosan in an Aqueous Solution" Polymers 15, no. 7: 1594. https://doi.org/10.3390/polym15071594
APA StyleZhang, Y., Liu, R., Li, H., Li, Y., & Liu, X. (2023). Interactions between Soybean Trypsin Inhibitor and Chitosan in an Aqueous Solution. Polymers, 15(7), 1594. https://doi.org/10.3390/polym15071594