Mosses on Geopolymers: Preliminary Durability Study and Chemical Characterization of Metakaolin-Based Geopolymers Filled with Wood Ash
Abstract
:1. Introduction
2. Raw Materials and Test Methods
2.1. Raw Materials
2.2. Chemical Composition of Raw Materials
2.2.1. ED-XRF
2.2.2. TOC
2.3. Experimental Process
2.4. Physicochemical Stability
2.5. FT-IR Analysis
2.6. Antibacterial Activity
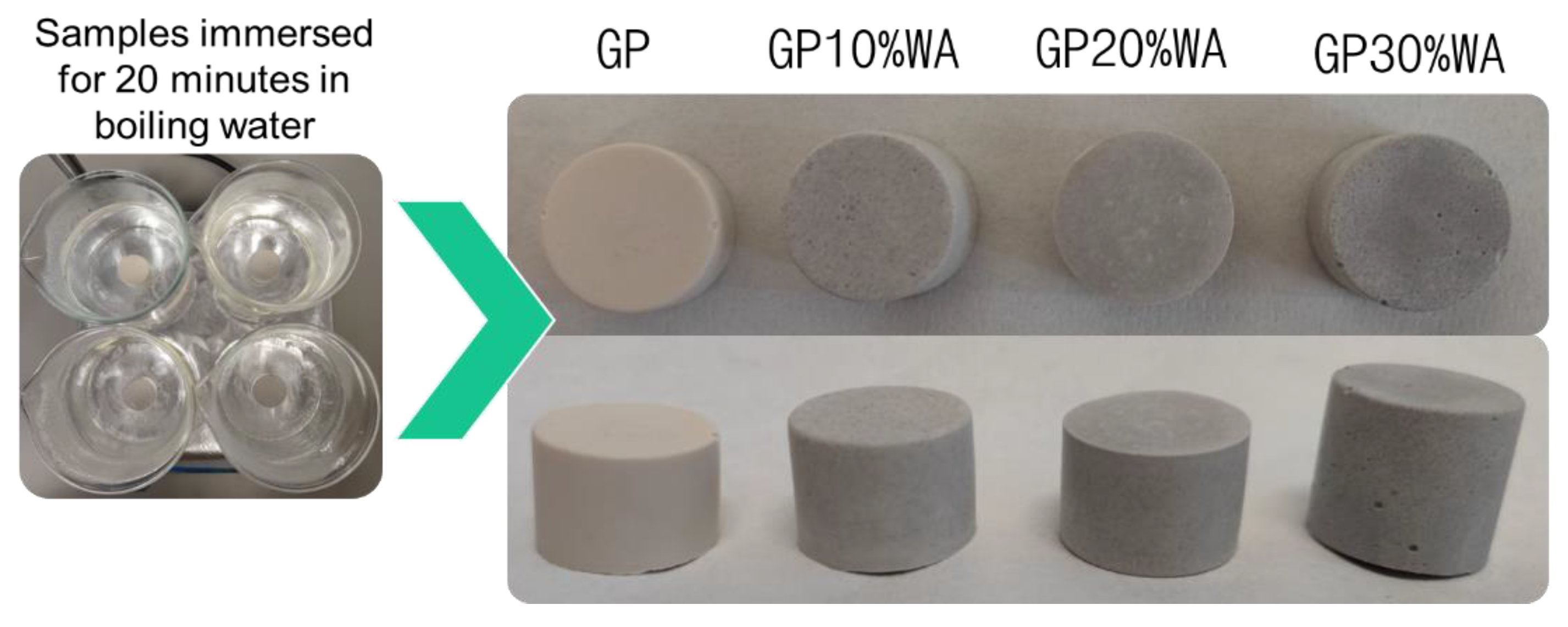
2.7. Boiling Water Test
2.8. Mechanical Strength
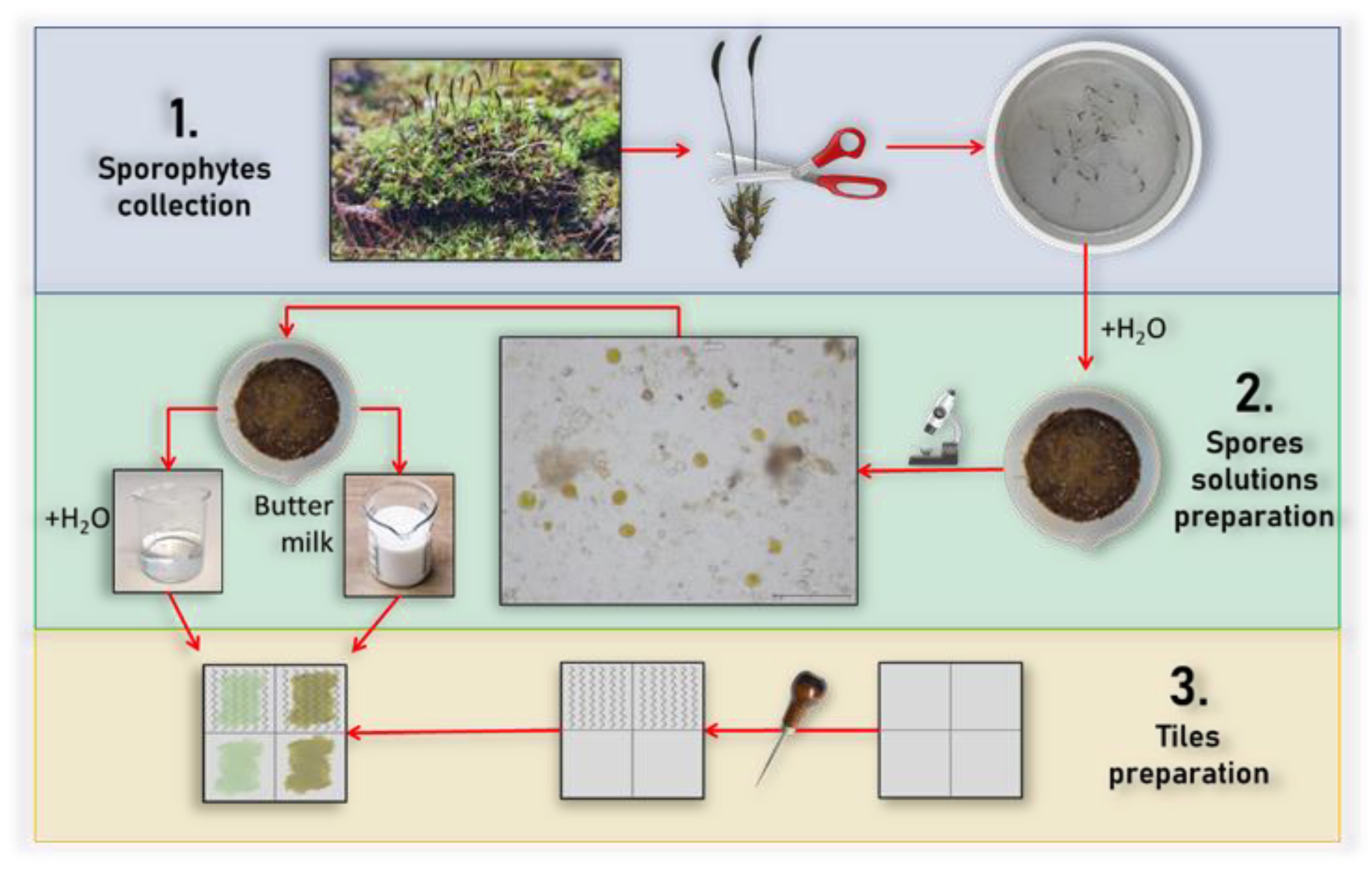
2.9. Moss Growing Test
- Sporophytes collection. Once collected, the mosses were placed in a container and sprayed with H2O to increase the vitality of the spores. After 30 min of wetting them, the sporophytes containing the spores were removed using tweezers.
- Spores’ solutions preparation. The collected sporophytes were put into a mortar containing 20 mL of water to help the pestling process and the sporophytes’ opening. The mixture was analyzed with an optical microscope Zeiss Standard 25, Germany, to confirm the presence of the spores. The amalgamated mixture was then divided into two halves: in one half another 10 mL of water was added, while in the second half, 10 mL of buttermilk was added. Buttermilk was made by whipping fresh cream for about 20 min and separating the solid part (the butter) from the liquid part (the buttermilk).
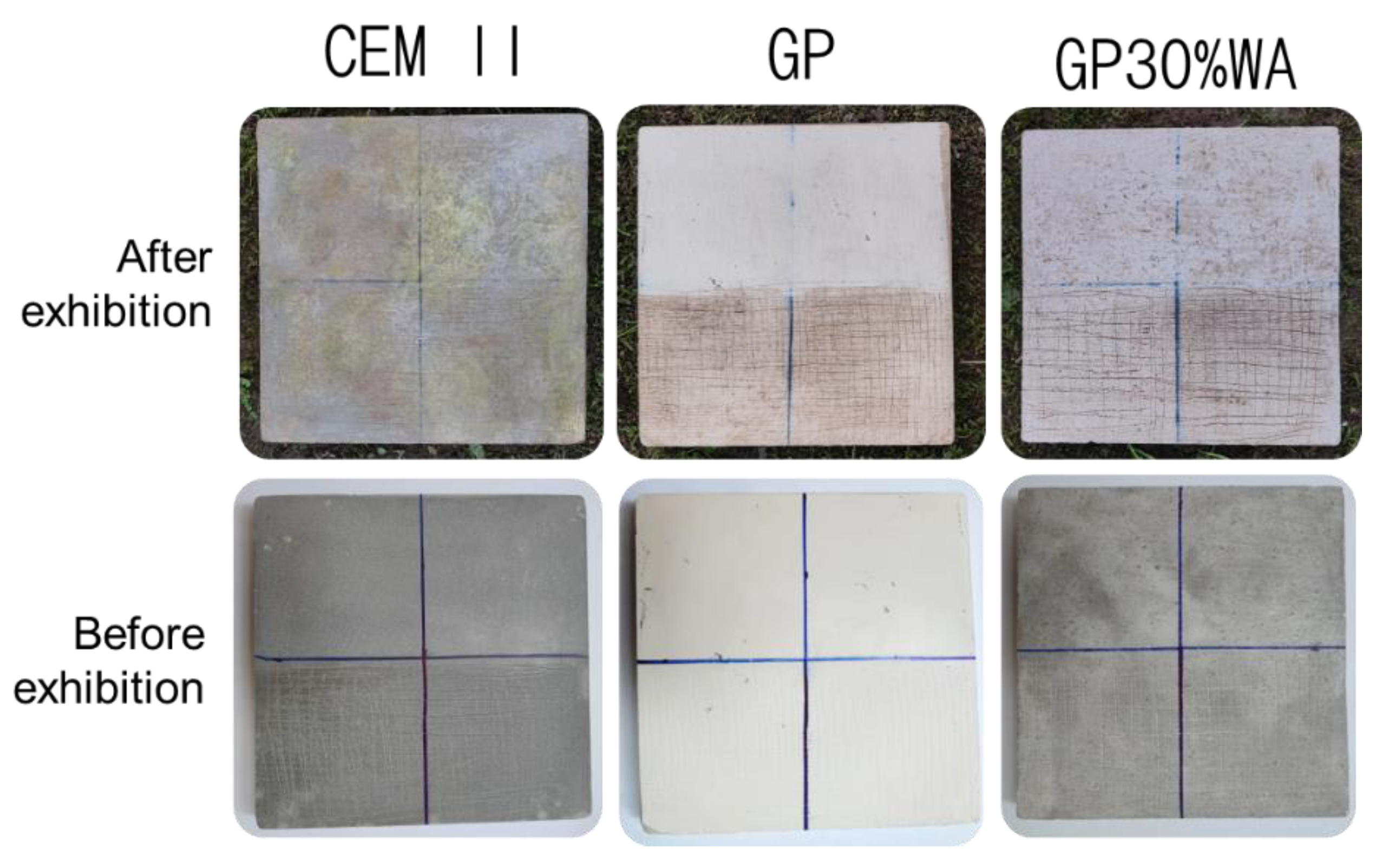
- Tiles preparation. The tiles were realized and divided into four sections: two were left unaltered, while the other two were scratched to make the surface rough and simulate aging. The two mixtures realized in the previous step were brushed, as shown in the flowchart in Figure 2.
3. Results and Discussion
3.1. Raw Materials Characterization
3.2. Sample Characterization
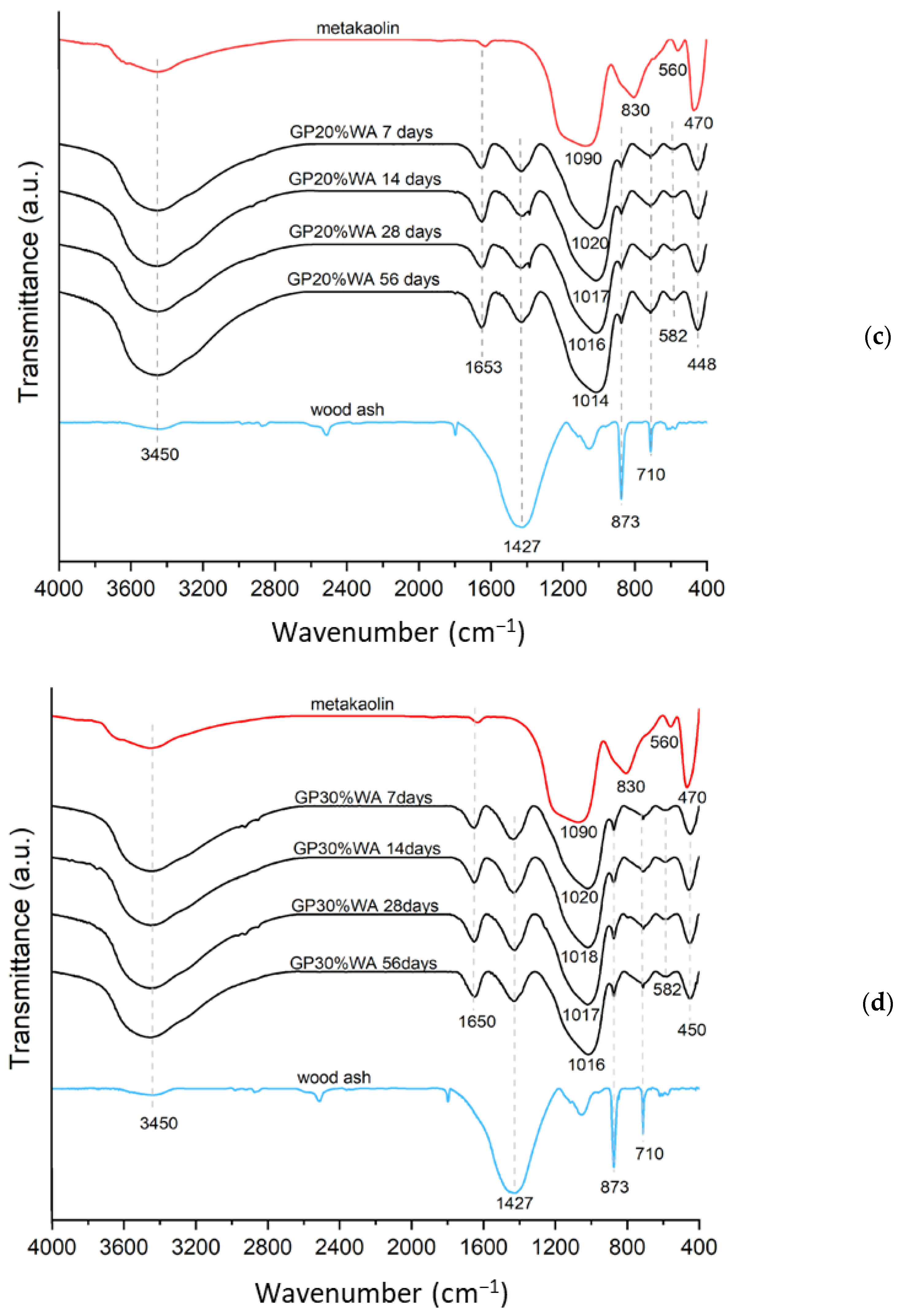
3.3. FT-IR Analysis
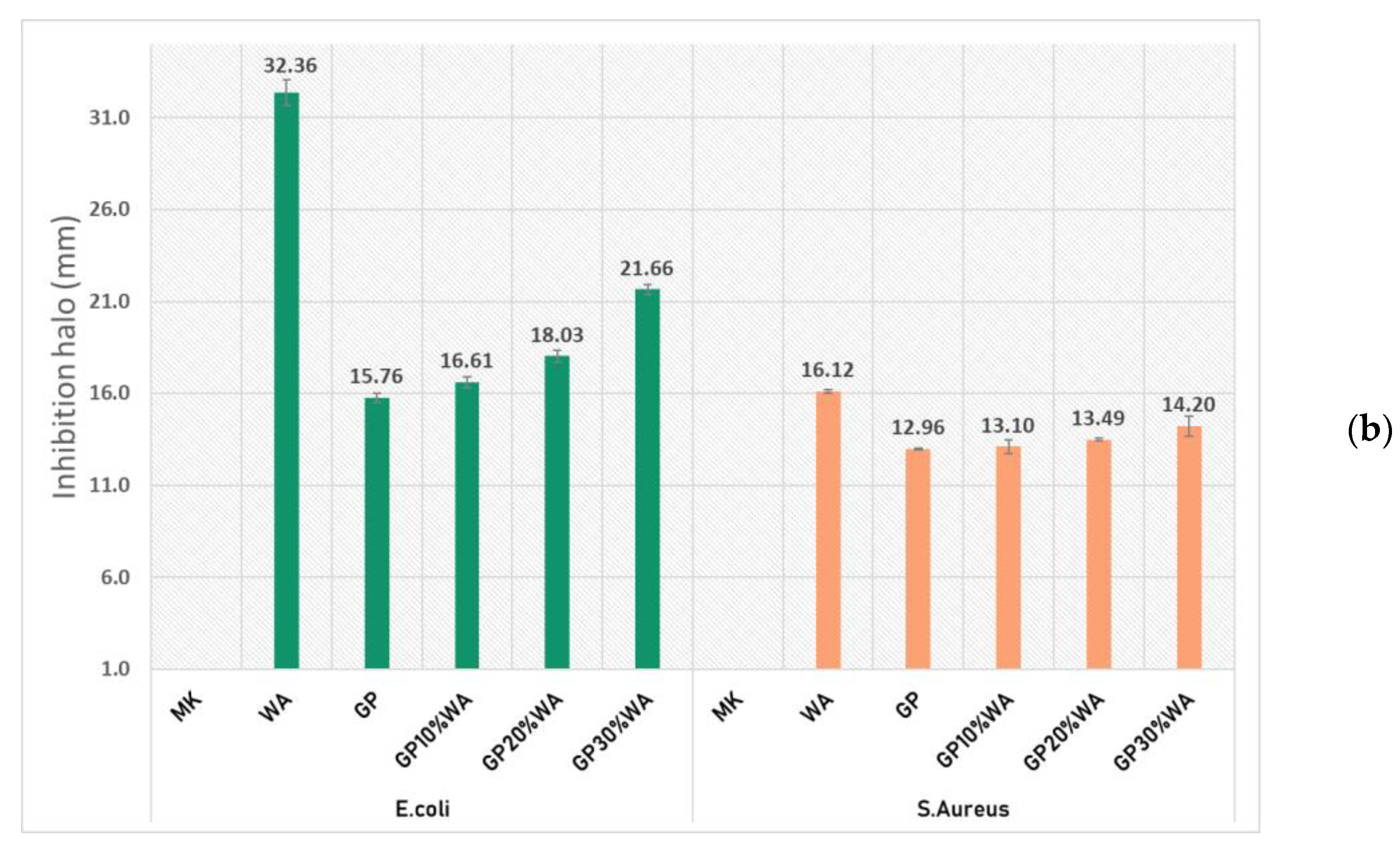
3.4. Antibacterial Activity
3.5. Boiling Water Test
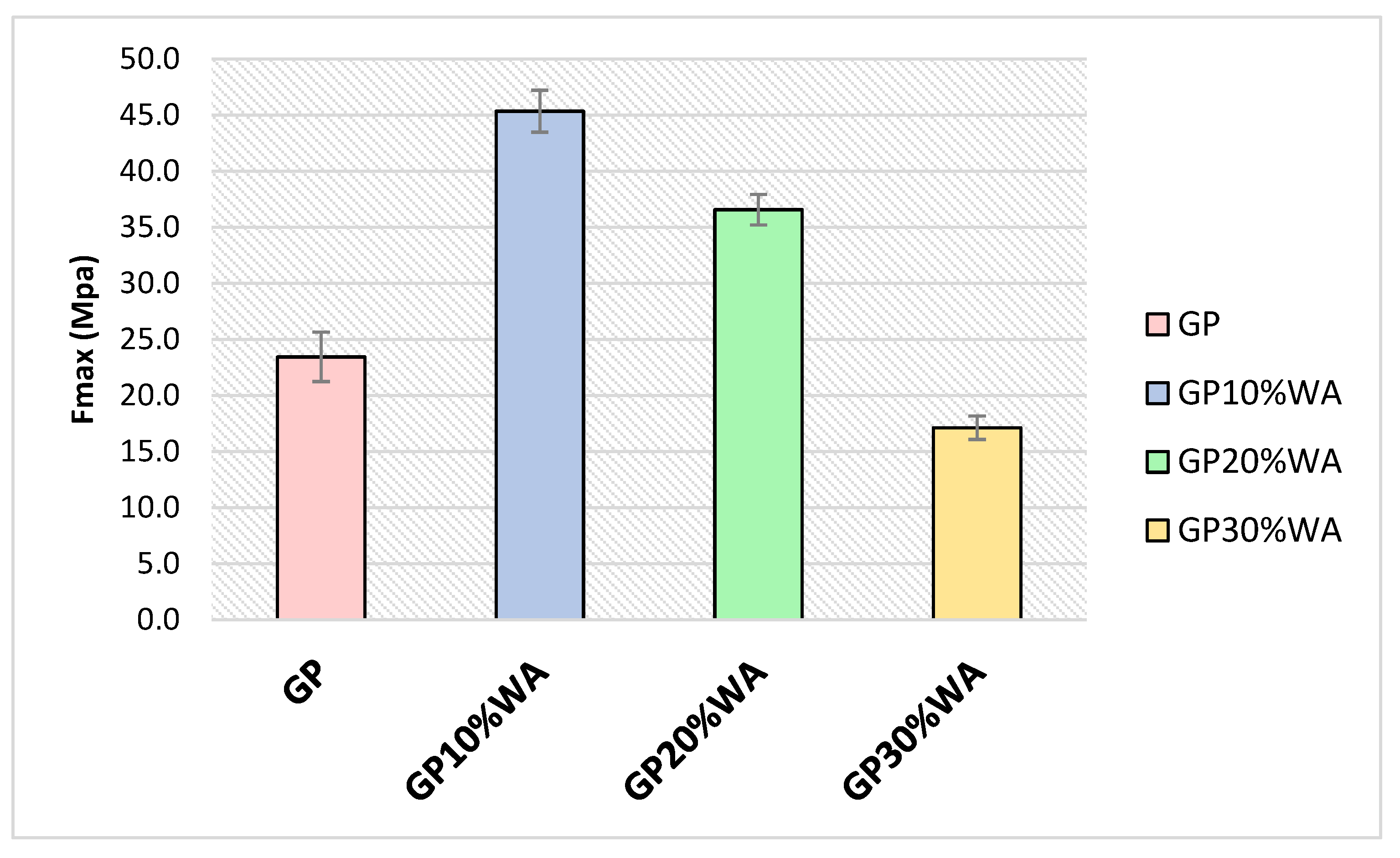
3.6. Mechanical Strength
3.7. Moss Growing Tests
4. Conclusions
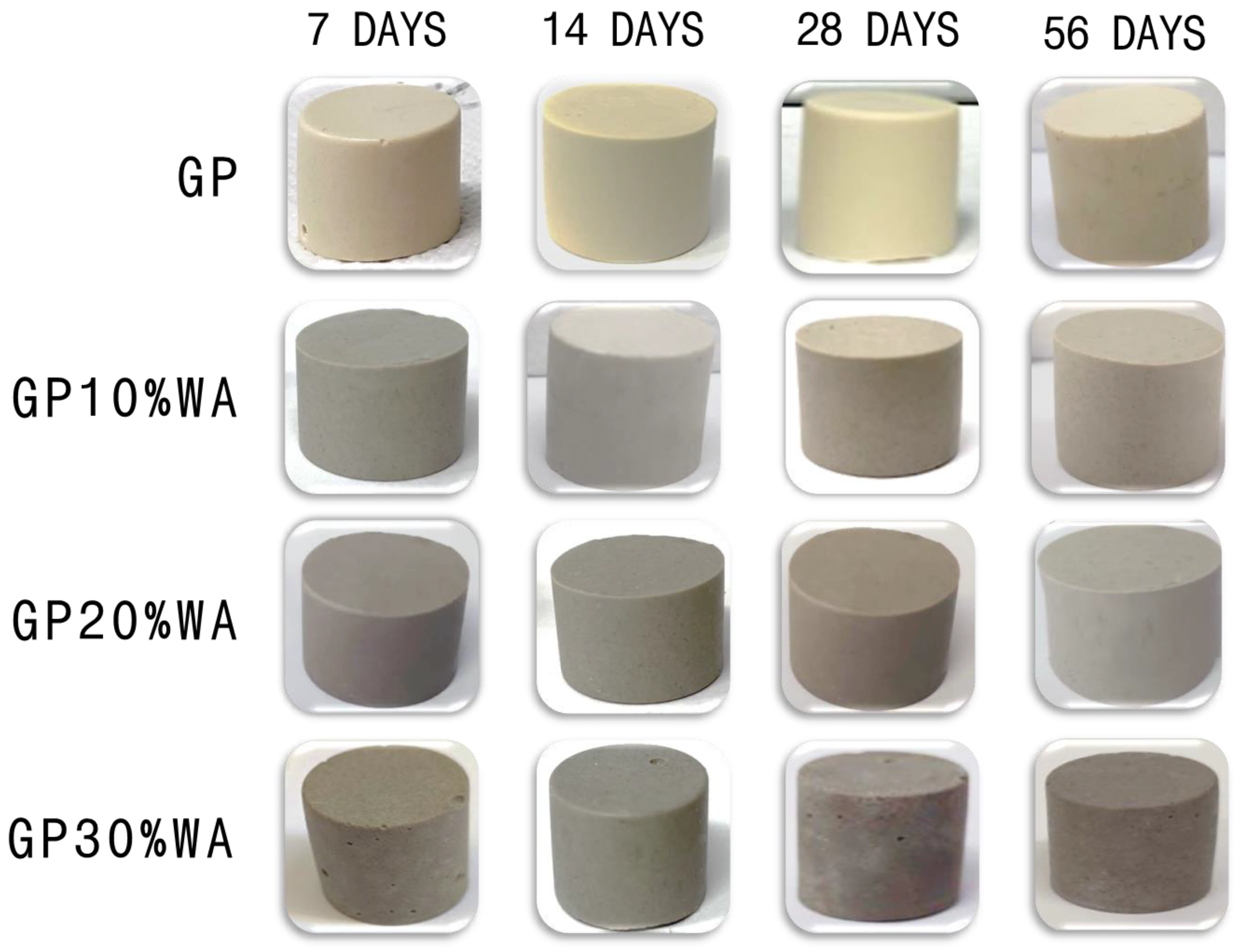
- The samples obtained with 10%, 20% and 30% of WA filler proved compact and solid, despite some surface bubbles in the GP30%WA formulation.
- The similar trend of pH and conductivity tests for all the samples highlighted the well-formed geopolymers. All species passed the integrity test, remaining stable to treatment in water for 24 h. This behavior was also confirmed by weight loss tests, whose percentage values do not exceed 1% after 56 days of curing.
- FT-IR analysis, as well as the boiling water test, confirmed the occurrence of the geopolymerization process in all the samples.
- The antibacterial analysis, as well as the moss growing test, demonstrated how geopolymers, with and without wood, can create a hostile environment for the formation of the bacterial layer and consequently the growth of mosses. This suggests their higher durability, compared to ordinary cement, over time, and their application in outdoor environments. The mechanical tests have shown how adding wood ash forms a stronger geopolymer than ash-free ones.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddique, R. Wood Ash. In Waste Materials and By-Products in Concrete; Springer: Berlin/Heidelberg, Germany, 2008; pp. 303–321. ISBN 978-3-540-74293-7. [Google Scholar]
- Siddique, R. Utilization of Wood Ash in Concrete Manufacturing. Resour. Conserv. Recycl. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Van Ryssen, J.B.J.; Hloniphile, N. Wood ash in livestock nutrition: Factors affecting the mineral composition of wood ash. Appl. Anim. Husb. Rural. Dev. 2018, 11, 53–61. [Google Scholar]
- Olanders, B.; Steenari, B.-M. Characterization of Ashes From Wood And Straw. Biomass Bioenergy 1995, 8, 105–115. [Google Scholar] [CrossRef]
- Lambert, M.J. Inorganic Constituents in Wood and Bark of New South Wales Forest Tree Species; Research Note/Forestry Commission of New South Wales; Forestry Commission of New South Wales: Sydney, Australia, 1981; ISBN 978-0-7240-6263-8. [Google Scholar]
- Eliche-Quesada, D.; Felipe-Sesé, M.A.; López-Pérez, J.A.; Infantes-Molina, A. Characterization and Evaluation of Rice Husk Ash and Wood Ash in Sustainable Clay Matrix Bricks. Ceram. Int. 2017, 43, 463–475. [Google Scholar] [CrossRef]
- Ayobami, A.B. Performance of Wood Bottom Ash in Cement-Based Applications and Comparison with Other Selected Ashes: Overview. Resour. Conserv. Recycl. 2021, 166, 105351. [Google Scholar] [CrossRef]
- Erich, M.S. Agronomic Effectiveness of Wood Ash as a Source of Phosphorus and Potassium. J. Environ. Qual. 1991, 20, 576–581. [Google Scholar] [CrossRef]
- Kuba, T.; Tschöll, A.; Partl, C.; Meyer, K.; Insam, H. Wood Ash Admixture to Organic Wastes Improves Compost and Its Performance. Agric. Ecosyst. Environ. 2008, 127, 43–49. [Google Scholar] [CrossRef]
- Adekayode, F.O.; Olojugba, M.R. The Utilization of Wood Ash as Manure to Reduce the Use of Mineral Fertilizer for Improved Performance of Maize (Zea Mays L.) as Measured in the Chlorophyll Content and Grain Yield. J. Soil Sci. Environ. Manag. 2010, 1, 40–45. [Google Scholar]
- Srisuwan, A.; Sompech, S.; Saengthong, C.; Thaomola, S.; Chindraprasirt, P.; Phonphuak, N. Preparation and Properties of Fired Clay Bricks with Added Wood Ash. J. Met. Mater. Miner. 2020, 30, 84–89. [Google Scholar] [CrossRef]
- Naik, T.R.; Kraus, R.N.; McCormick, S. Recycling of wood ash in cement-based construction material. In Proceedings of the 4. biennial residue to revenue residual wood conference 2001, Richmond, BC, Canada, 4–6 November 2001. [Google Scholar]
- Caserini, S.; Fraccaroli, A.; Monguzzi, A.; Moretti, M.; Angelino, E. Stima Dei Consumi Di Legna Da Ardere per Il Riscaldamento Ed Uso Domestico in Italia; ARPA: Lombardia, Italy, 2008; pp. 1–60. ISBN 978-88-448-0346-9. [Google Scholar]
- Comitato Termotecnico Italiano. Gestione e Valorizzazione Delle Ceneri Di Combustione Nella Filiera Legno-Energia. 2004. Available online: https://www.cti2000.it/solidi.htm (accessed on 2 January 2023).
- Favero, M.; Pettenella, D. Italian Import Flows of Woody Biomasses for Energy Use: A Sustainable Supply? New Medit 2014, 13, 54–64. [Google Scholar]
- Pra, A.; Pettenella, D. Consumption of Wood Biomass for Energy in Italy: A Strategic Role Based on Weak Knowledge. L Ital. For. E Mont. 2016, 71, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Falciano, A.; Masi, P.; Moresi, M. Performance Characterization of a Traditional Wood-fired Pizza Oven. J. Food Sci. 2022, 87, 4107–4118. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J. Hydration, Reinforcing Mechanism, and Macro Performance of Multi-Layer Graphene-Modified Cement Composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Cui, K.; Liang, K.; Chang, J.; Lau, D. Investigation of the Macro Performance, Mechanism, and Durability of Multiscale Steel Fiber Reinforced Low-Carbon Ecological UHPC. Constr. Build. Mater. 2022, 327, 126921. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in Geopolymer Materials: A Comprehensive Review. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Manikandan, P.; Vasugi, V. Potential Utilization of Waste Glass Powder as a Precursor Material in Synthesizing Ecofriendly Ternary Blended Geopolymer Matrix. J. Clean. Prod. 2022, 355, 131860. [Google Scholar] [CrossRef]
- Churata, R.; Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Torres-Almirón, J.; Ortiz-Valdivia, Y.; Velasco, F. Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing. Buildings 2022, 12, 1118. [Google Scholar] [CrossRef]
- Esparham, A.; Moradikhou, A.B.; Andalib, F.K.; Avanaki, M.J. Strength Characteristics of Granulated Ground Blast Furnace Slag-Based Geopolymer Concrete. Adv. Concr. Constr. 2021, 11, 219–229. [Google Scholar] [CrossRef]
- Cioffi, R.; Pernice, P.; Aronne, A.; Catauro, M.; Quattroni, G. Glass-Ceramics from Fly Ash with Added Li2O. J. Eur. Ceram. Soc. 1994, 13, 143–148. [Google Scholar] [CrossRef]
- European Committee for Standardization. SIST EN 15936:2012—Sludge, Treated Biowaste, Soil and Waste—Determination of Total Organic Carbon (TOC) by Dry Combustion; European Committee for Standardization: Bruxelles, Belgium, 2012. [Google Scholar]
- Sá Ribeiro, M.G.; Sá Ribeiro, M.G.; Keane, P.F.; Sardela, M.R.; Kriven, W.M.; Sá Ribeiro, R.A. Acid Resistance of Metakaolin-Based, Bamboo Fiber Geopolymer Composites. Constr. Build. Mater. 2021, 302, 124194. [Google Scholar] [CrossRef]
- D’Angelo, A.; Dal Poggetto, G.; Piccolella, S.; Leonelli, C.; Catauro, M. Characterisation of White Metakaolin-Based Geopolymers Doped with Synthetic Organic Dyes. Polymers 2022, 14, 3380. [Google Scholar] [CrossRef]
- Sgarlata, C.; Formia, A.; Ferrari, F.; Leonelli, C. Effect of the Introduction of Reactive Fillers and Metakaolin in Waste Clay-Based Materials for Geopolymerization Processes. Molecules 2021, 26, 1325. [Google Scholar] [CrossRef] [PubMed]
- Davidovits, J. Geopolymer: Chemistry & Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020; ISBN 978-2-9544531-1-8. [Google Scholar]
- Cortini Pedrotti, C. Flora dei Muschi d’Italia; A. Delfino: Roma, Italy, 2001; ISBN 978-88-7287-250-5. [Google Scholar]
- Koleżyński, A.; Król, M.; Żychowicz, M. The Structure of Geopolymers—Theoretical Studies. J. Mol. Struct. 2018, 1163, 465–471. [Google Scholar] [CrossRef]
- Yao, X.; Yang, T.; Zhang, Z. Fly Ash-Based Geopolymers: Effect of Slag Addition on Efflorescence. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2016, 31, 689–694. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, S.; Zhang, J.; Tan, X.; Chen, D. Relationship between Moisture Transportation, Efflorescence and Structure Degradation in Fly Ash/Slag Geopolymer. Materials 2020, 13, 5550. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, L.; Deng, H.; Zheng, Z.; Xiang, Y.; Li, Y.; Ma, X. Characteristics and Mechanism of Efflorescence in Fly Ash-Based Geopolymer Mortars under Quasi-Natural Condition. J. Build. Eng. 2022, 55, 104708. [Google Scholar] [CrossRef]
- Li, W.; Dong, B.; Yang, Z.; Xu, J.; Chen, Q.; Li, H.; Xing, F.; Jiang, Z. Recent Advances in Intrinsic Self-Healing Cementitious Materials. Adv. Mater. 2018, 30, 1705679. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mishra, M.; Suganya, O. The Incorporation of Wood Waste Ash as a Partial Cement Replacement Material for Making Structural Grade Concrete: An Overview. Ain Shams Eng. J. 2015, 6, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; He, Y.; Zhu, Y.; Liu, L.; Cui, X. Novel Procedure of CO 2 Capture of the CaO Sorbent Activator on the Reaction of One-Part Alkali-Activated Slag. RSC Adv. 2021, 11, 12476–12483. [Google Scholar] [CrossRef]
- Catauro, M.; D’Angelo, A.; Piccolella, S.; Leonelli, C.; Dal Poggetto, G. Thermal Influence on Physico-Chemical Properties of Geopolymers Based on Metakaolin and Red Tomato Waste. Macromol. Symp. 2022, 404, 2100295. [Google Scholar] [CrossRef]
- Finocchiaro, C.; Barone, G.; Mazzoleni, P.; Leonelli, C.; Gharzouni, A.; Rossignol, S. FT-IR Study of Early Stages of Alkali Activated Materials Based on Pyroclastic Deposits (Mt. Etna, Sicily, Italy) Using Two Different Alkaline Solutions. Constr. Build. Mater. 2020, 262, 120095. [Google Scholar] [CrossRef]
- Feriancová, A.; Pajtášová, M.; Moricová, K.; Pecušová, B. Using of Wood Ash as the Alternative Filler for Preparation of Rubber Mixtures. IOP Conf. Ser. Mater. Sci. Eng. 2020, 776, 012087. [Google Scholar] [CrossRef]
- Abdul Karim, M.R.; Ul Haq, E.; Hussain, M.A.; Khan, K.I.; Nadeem, M.; Atif, M.; Ul Haq, A.; Naveed, M.; Alam, M.M. Experimental Evaluation of Sustainable Geopolymer Mortars Developed from Loam Natural Soil. J. Asian Archit. Build. Eng. 2020, 19, 637–646. [Google Scholar] [CrossRef]
- Dal Poggetto, G.; D’Angelo, A.; Blanco, I.; Piccolella, S.; Leonelli, C.; Catauro, M. FT-IR Study, Thermal Analysis, and Evaluation of the Antibacterial Activity of a MK-Geopolymer Mortar Using Glass Waste as Fine Aggregate. Polymers 2021, 13, 2970. [Google Scholar] [CrossRef]
- Ivanković, T.; Hrenović, J.; Itskos, G.; Koukouzas, N.; Kovačević, D.; Milenković, J. Alkaline Disinfection of Urban Wastewater and Landfill Leachate by Wood Fly Ash. Arch. Ind. Hyg. Toxicol. 2014, 65, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawai, J. Quantitative Evaluation of Antibacterial Activities of Metallic Oxide Powders (ZnO, MgO and CaO) by Conductimetric Assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Gedda, G.; Pandey, S.; Lin, Y.-C.; Wu, H.-F. Antibacterial Effect of Calcium Oxide Nano-Plates Fabricated from Shrimp Shells. Green Chem. 2015, 17, 3276–3280. [Google Scholar] [CrossRef]
- Rusdaryanti, A.F.; Amalia, U.; Suharto, S. Antibacterial Activity of CaO from Blood Cockle Shells (Anadara Granosa) Calcination against Escherichia Coli. Biodiversitas 2020, 21, 2826–2830. [Google Scholar] [CrossRef]
- Bae, D.-H.; Yeon, J.-H.; Park, S.-Y.; Lee, D.-H.; Ha, S.-D. Bactericidal Effects of CaO (Scallop-Shell Powder) on Foodborne Pathogenic Bacteria. Arch. Pharm. Res. 2006, 29, 298–301. [Google Scholar] [CrossRef]
- Liang, X.; Dai, R.; Chang, S.; Wei, Y.; Zhang, B. Antibacterial Mechanism of Biogenic Calcium Oxide and Antibacterial Activity of Calcium Oxide/Polypropylene Composites. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129446. [Google Scholar] [CrossRef]
- King, M.M.; Kayastha, B.B.; Franklin, M.J.; Patrauchan, M.A. Calcium Regulation of Bacterial Virulence. In Calcium Signaling; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1131, pp. 827–855. ISBN 978-3-030-12456-4. [Google Scholar]
- Tararushkin, E.V.; Shchelokova, T.N. Daily Strength Testing of the Portland Cement Mortars. IOP Conf. Ser. Mater. Sci. Eng. 2020, 862, 022031. [Google Scholar] [CrossRef]
- Verma, N.K.; Rao, M.C.; Kumar, S. Effect of Curing Regime on Compressive Strength of Geopolymer Concrete. IOP Conf. Ser. Earth Environ. Sci. 2022, 982, 012031. [Google Scholar] [CrossRef]
- Srivastava, V.; Kumar, R.; Agarwal, V.C.; Mehta, P.K. Effect of Silica Fume on Workability and Compressive Strength of OPC Concrete. J. Environ. Nanotechnol. 2014, 3, 32–35. [Google Scholar] [CrossRef]
- Abdullahi, M. Characteristics of Wood ASH/OPC Concrete. Leonardo Electron. J. Pract. Technol. 2006, 8, 9–16. [Google Scholar]
- Spiess, D.; Lippincott, B.; Lippincott, J. Facilitation Of Moss Growth And Development By Bacteria. J. Hattori Bot. Lab. 1984, 55, 67–77. [Google Scholar]
- Westhoff, L. Spontaneous Moss Growth on Concrete. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2020. [Google Scholar]




| Sample | Metakaolin | Wood Ash | W/S |
|---|---|---|---|
| GP | 100% | 0% | 0.360 |
| GP10%WA | 90% | 10% | 0.358 |
| GP20%WA | 80% | 20% | 0.328 |
| GP30%WA | 70% | 30% | 0.309 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catauro, M.; Viola, V.; D’Amore, A. Mosses on Geopolymers: Preliminary Durability Study and Chemical Characterization of Metakaolin-Based Geopolymers Filled with Wood Ash. Polymers 2023, 15, 1639. https://doi.org/10.3390/polym15071639
Catauro M, Viola V, D’Amore A. Mosses on Geopolymers: Preliminary Durability Study and Chemical Characterization of Metakaolin-Based Geopolymers Filled with Wood Ash. Polymers. 2023; 15(7):1639. https://doi.org/10.3390/polym15071639
Chicago/Turabian StyleCatauro, Michelina, Veronica Viola, and Alberto D’Amore. 2023. "Mosses on Geopolymers: Preliminary Durability Study and Chemical Characterization of Metakaolin-Based Geopolymers Filled with Wood Ash" Polymers 15, no. 7: 1639. https://doi.org/10.3390/polym15071639





