Novel Airflow-Field-Driven Melt Spinning 3D Printing of Tubular Scaffolds Based on Polycaprolactone Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
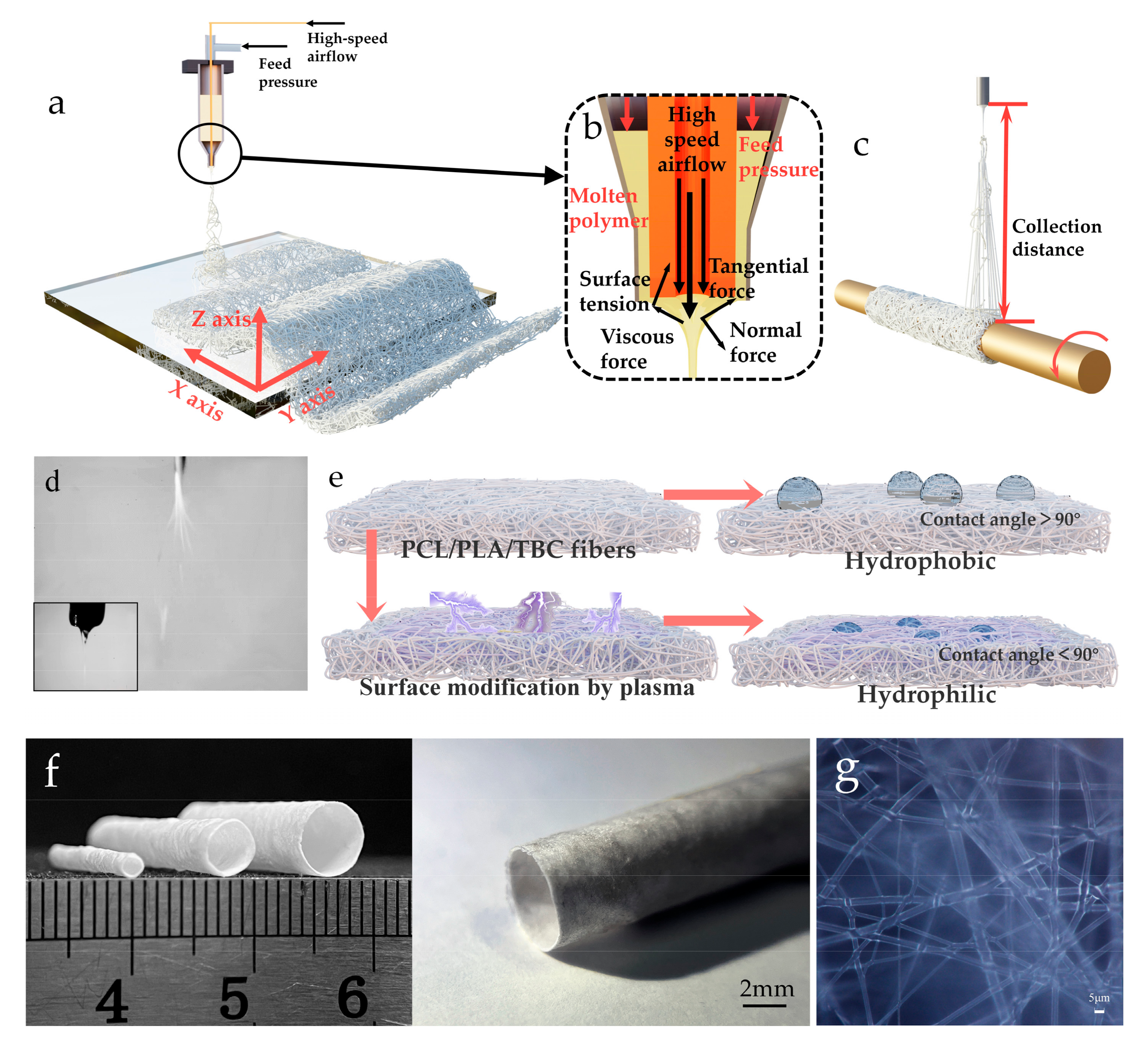
2.2. Preparation of PCL/PLA/TBC Fibers
2.3. Microscopic Morphological Characterization
2.4. Fourier-Transform Infrared Spectrophotometer Spectra
2.5. Wetting Performance and Mechanical Properties Testing
2.6. In Vitro Degradation
3. Results and Discussions
3.1. Fourier-Transform Infrared Spectroscopy
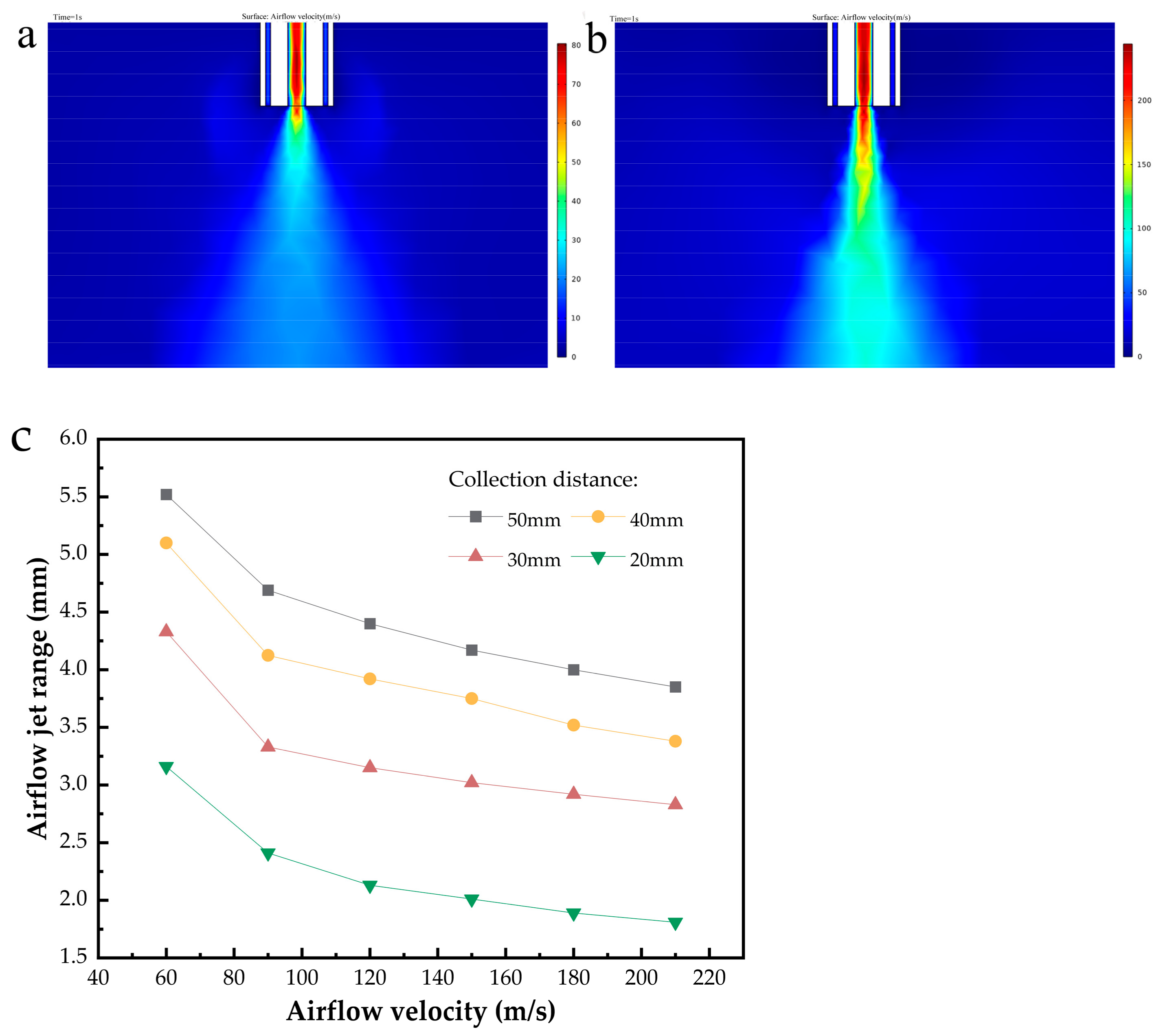
3.2. Airflow Field Simulation
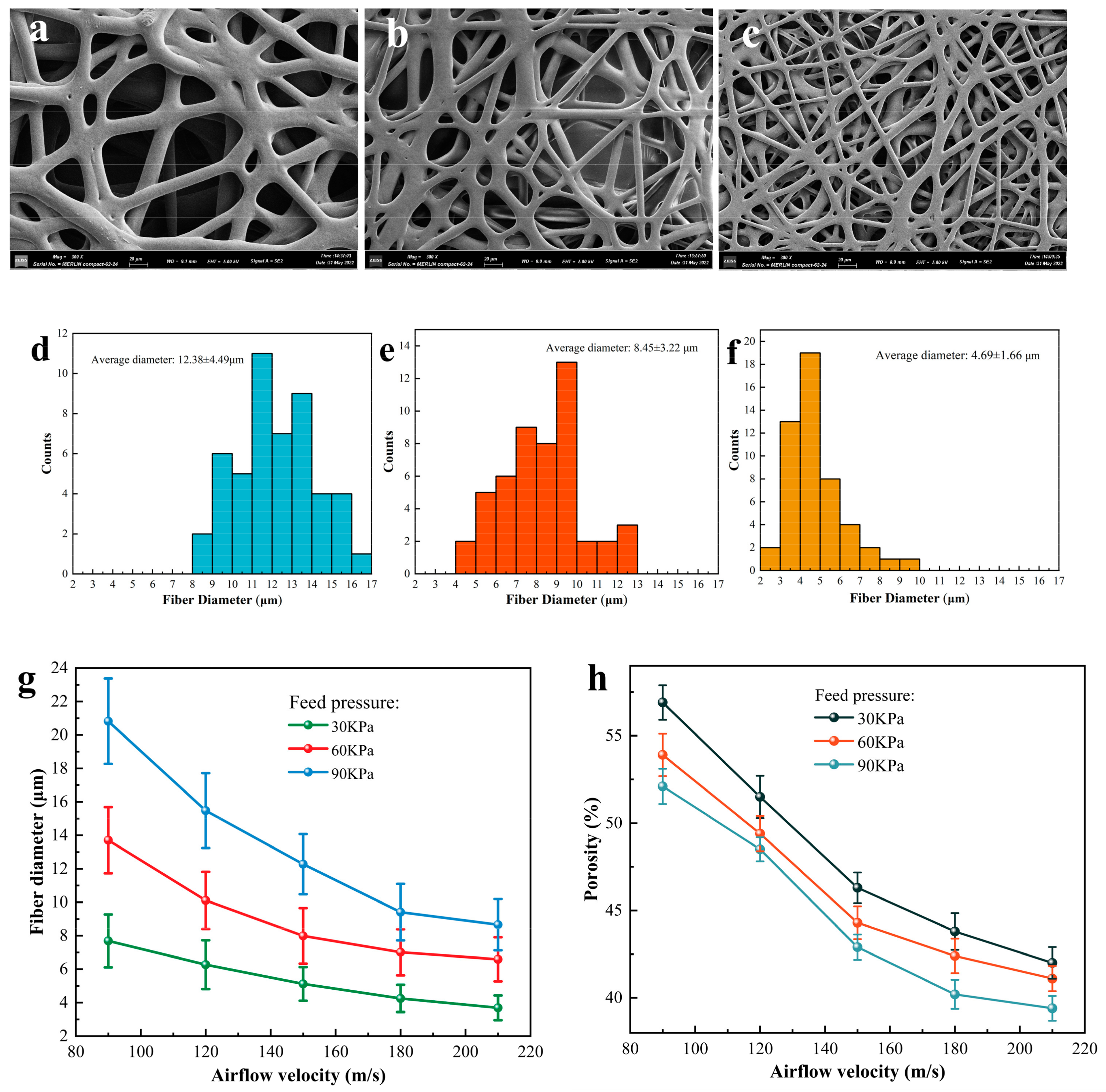
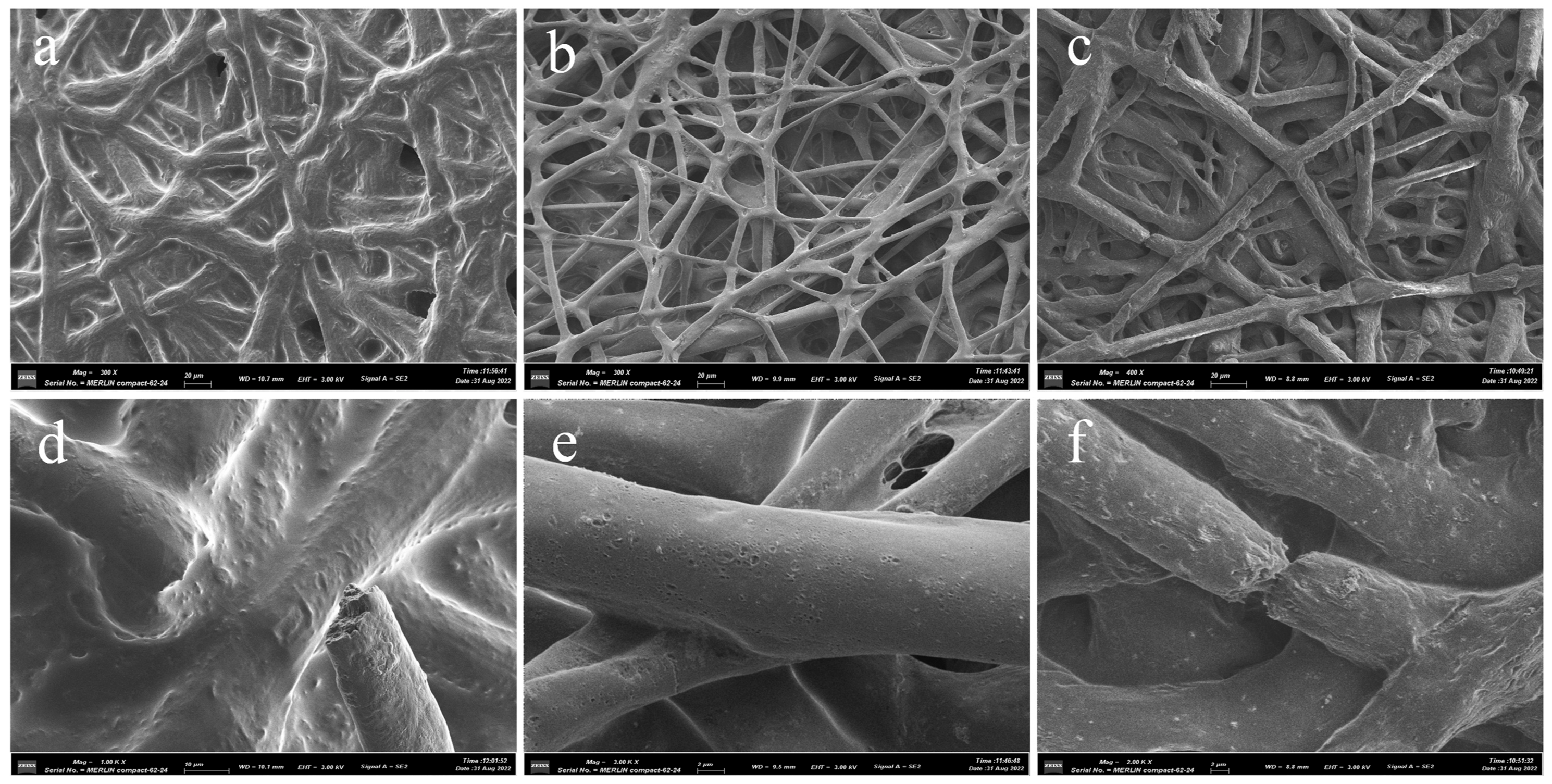
3.3. Fibers’ Morphology Characterization of PCL/PLA/TBC
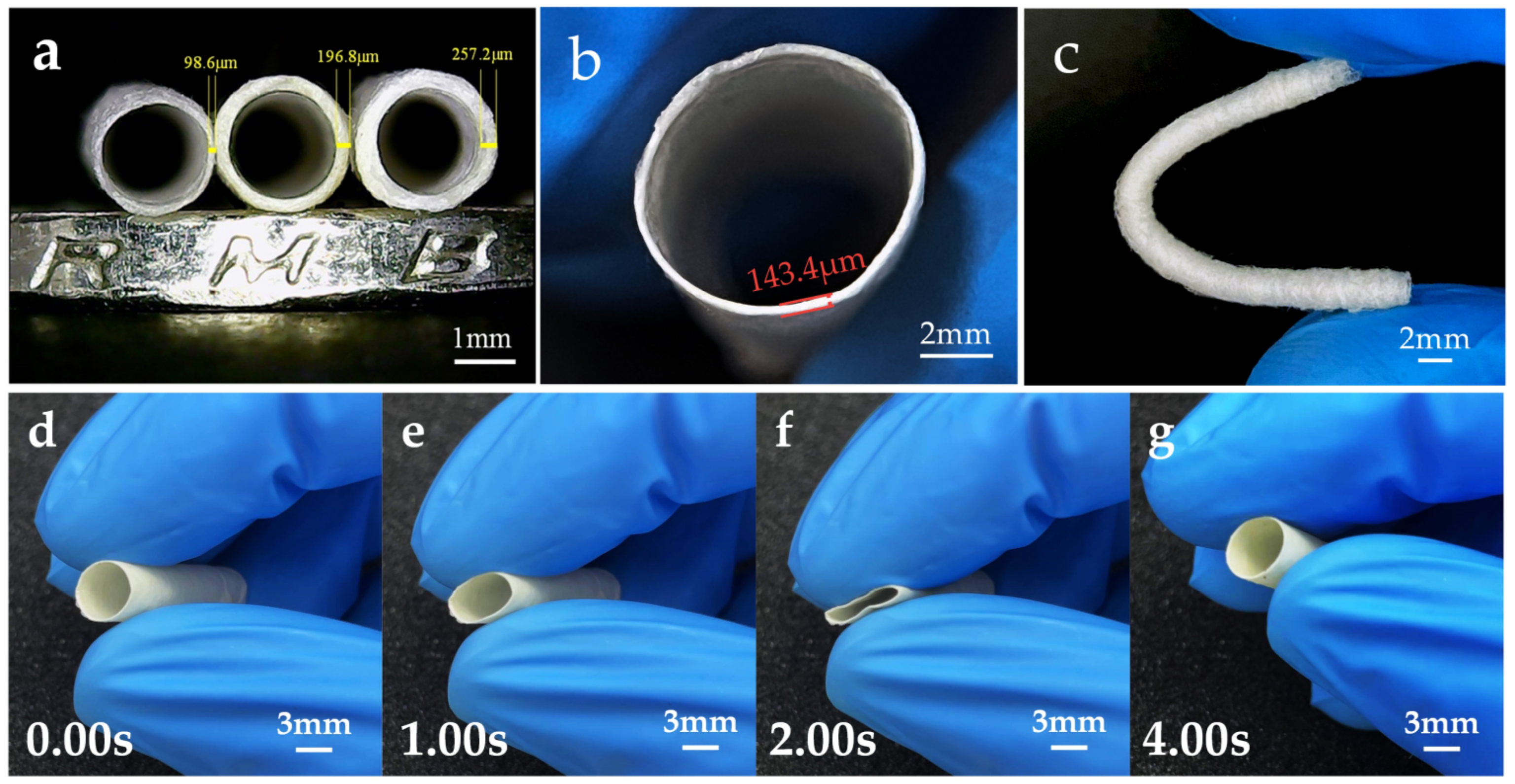
3.4. Tubular Microfiber Scaffolds and Characterization of Their Properties
3.5. In Vitro Degradation Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Chen, B.; Zhang, X.; Huang, S.; Wa, Q. 3D printed concentrated alginate/GelMA hollow-fibers-packed scaffolds with nano apatite coatings for bone tissue engineering. Int. J. Biol. Macromol. 2022, 202, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Bin, S.; Yun, L.; Hong, Z.; Meng, L.; Jean, D.; Xing, J.; Hai, Y. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar]
- Wei, L.; Liu, C.; Mao, X.; Dong, J.; Fan, W.; Zhi, C.; Qin, X.; Sun, R. Multiple-Jet Needleless Electrospinning Approach via a Linear Flume Spinneret. Polymers 2019, 11, 2052. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Cooper-White, J. The use of an electrostatic lens to enhance the efficiency of the electrospinning process. Cell Tissue Res. 2012, 347, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Orafa, Z.; Irani, S.; Zamanian, A.; Bakhshi, H.; Nikukar, H.; Ghalandari, B. Coating of laponite on PLA nanofibrous for bone tissue engineering application. Macromol. Res. 2021, 29, 191–198. [Google Scholar] [CrossRef]
- Peng, Z.; Gou, N.; Wei, Z.; Zhao, J.; Wang, F.; Yang, J.; Li, Y.; Lan, H. Fabrication of a Large-Area, Fused Polymer Micromold Based on Electric-Field-Driven (EFD) μ-3D Printing. Polymers 2019, 11, 1902. [Google Scholar] [CrossRef]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, W.; Jiang, Y.; Guo, C. Gradient degradable nerve guidance conduit with multilayer structure prepared by electrospinning. Mater. Lett. 2020, 276, 128238. [Google Scholar] [CrossRef]
- Aminatun; Huriah, R.; Hikmawati, D.; Hadi, S.; Amrillah, T.; Abdullah, C.A. Nanofiber Scaffold Based on Polylactic Acid-Polycaprolactone for Anterior Cruciate Ligament Injury. Polymers 2022, 14, 2983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, Q.; Li, H.; Liu, M.; Li, Z.; Yang, K.; Zhao, J.; Qian, L.; Peng, Z.; Zhang, G.; et al. Fabrication of high-performance silver mesh for transparent glass heaters via electric-field-driven microscale 3D printing and UV-assisted microtransfer. Adv. Mater. 2019, 31, 1902479. [Google Scholar] [CrossRef]
- Can-Herrera, L.A.; Oliva, A.L.; Dzul-Cervantes, M.A.; Pacheco-Salazar, O.F.; Cervantes-Uc, J.M. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers 2021, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Lipik, V.T.; Widjaja, L.K.; Liow, S.S.; Abadie, M.J.; Venkatraman, S.S. Effects of transesterification and degradation on properties and structure of polycaprolactone-polylactide copolymers. Polym. Degrad. Stab. 2010, 95, 2596–2602. [Google Scholar] [CrossRef]
- Hoidy, W.H.; Amad, M.B.; Al-Mulla, E.A.; Ibrahim, N.A. Preparation and characterization of polylactic Acid/Polycaprolactone clay nanocomposites. J. Appl. Sci. 2010, 10, 97–106. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, M.; Lv, H.; Zhang, J.; Li, Y.; Wu, J.; Zhang, S.; Wang, F.; Zhang, G.; Zhu, X.; et al. Electric field-driven microscale 3D printing of flexible thin-walled tubular mesh structures of molten polymers. Mater. Des. 2023, 225, 111433. [Google Scholar] [CrossRef]
- Bubakir, M.M.; Li, H.; Wu, W.; Li, X.; Ma, S.; Yang, W. Applications of web produced by hot air assisted melt differential electrospinning method. Mater. Sci. Eng. 2014, 64, 012052. [Google Scholar] [CrossRef]
- Niu, H.; Zhou, H.; Yan, G.; Wang, H.; Fu, S.; Zhou, X.; Shao, H.; Lin, T. Enhancement of Coil Electrospinning using Two-level Coil Structure. Ind. Eng. Chem. Res. 2018, 57, 15473–15478. [Google Scholar] [CrossRef]
- Varabhas, J.S.; Chase, G.G.; Reneker, D.H. Electrospun nanofibers from a porous hollow tube. Polymers 2008, 49, 4226–4229. [Google Scholar] [CrossRef]
- He, J.; Qi, K.; Wang, L.; Zhou, Y.; Liu, R.; Cui, S. Combined Application of Multinozzle Air-jet Electrospinning and Airflow Twisting for the Efficient Preparation of Continuous-Twisted Nanofiber Yarn. Fibers Polym. 2015, 16, 1319–1326. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W. Effect of experimental parameters on needleless electrospinning from a conical wire coil. J. Appl. Polym. Sci. 2012, 123, 3703–3709. [Google Scholar] [CrossRef]
- Liu, Z.; Ang, K.K.; He, J. Needle-disk electrospinning inspired by natural point discharge. J. Mater. Sci. 2017, 52, 1823–1830. [Google Scholar] [CrossRef]
- Liu, X.; Tan, J.; Yu, S.; Yousefzadeh, M.; Lyu, T.T.; Jiao, Z.; Liu, H.; Ramakrishna, S. High-efficiency preparation of polypropylene nanofiber by melt differential centrifugal electrospinning. J. Appl. Polym. Sci. 2020, 137, 48299. [Google Scholar] [CrossRef]
- Xu, G.; Chen, X.; Zhu, Z.; Wu, P.; Wang, H.; Chen, X.; Gao, W.; Liu, Z. Pulse gas-assisted multi-needle electrospinning of nanofibers. Adv. Compos. Hybrid Mater. 2020, 3, 98–113. [Google Scholar] [CrossRef]
- Zheng, G.; Jiang, J.; Wang, X.; Li, W.; Zhong, W.; Guo, S. Self-cleaning threaded rod spinneret for high-efficiency needleless electrospinning. Appl. Phys. A 2018, 124, 473. [Google Scholar] [CrossRef]
- He, H.; Liu, C.; Molnar, K. A Novel Needleless Electrospinning System Using a Moving Conventional Yarn as the Spinneret. Fibers Polym. 2018, 19, 1472–1478. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Liu, A.; Wei, L.; Wang, L.; Qin, X.; Yu, J. Mass production of high-quality nanofibers via constructing pre-Taylor cones with high curvature on needleless electrospinning. Mater. Des. 2021, 197, 109247. [Google Scholar] [CrossRef]
- Wei, L.; Sun, R.; Liu, C.; Xiong, J.; Qin, X. Mass production of nanofibers from needleless electrospinning by a novel annular spinneret. Mater. Des. 2019, 179, 107885. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Li, H.; Wang, Y.; Bubakir, M.; Ding, Y.; Zhang, Y. Water Filtration Properties of Novel Composite Membranes Combining Solution Electrospinning and Needleless Melt Electrospinning Methods. J. Appl. Polym. Sci. 2015, 132, 41601. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Wu, W.; Chen, H.; Ding, Y.; Yang, W. Effect of electric field on gas-assisted melt differential electrospinning with hollow disc electrode. J. Polym. Eng. 2015, 35, 61–70. [Google Scholar] [CrossRef]
- Wan, H.; Yu, D.; Xiao, M.; Yong, Q.; Wei, Y.; Jing, T.; Hao, L. Preparation of PP/(PLA+PEG) Core-Shell Ultrafine Fiber by Melt Electrospinning. Eng. Plast. Appl. 2016, 44, 48–52. [Google Scholar]
- Li, H.; Chen, H.; Zhong, X.; Wu, W.; Ding, Y.; Yang, W. Interjet Distance in Needleless Melt Differential Electrospinning with Umbellate Nozzles. J. Appl. Polym. Sci. 2014, 131, 40515. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, Y.; Zeng, Y. Design of a novel co-electrospinning system with flat spinneret for producing helical nanofibers. J. Polym. Sci. B Polym. Phys. 2019, 57, 1496–1505. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Z.; Han, Z.; Zhao, S.; Zhao, X.; Kang, L. Stable multi-jet electrospinning with high throughput using the bead structure nozzle. RSC Adv. 2018, 8, 6069–6074. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, W.; Bubakir, M.M.; Chen, H.; Zhong, X.; Liu, Z.; Ding, Y.; Yang, W. Polypropylene Fibers Fabricated via a Needleless Melt-Electrospinning Device for Marine Oil-Spill Cleanup. J. Appl. Polym. Sci. 2014, 131, 40080. [Google Scholar] [CrossRef]
- Zheng, G.; Jiang, J.; Chen, D.; Liu, J.; Zheng, J.; Wang, X.; Li, W. Multinozzle high efficiency electrospinning with the constraint of sheath gas. J. Appl. Polym. Sci. 2019, 136, 47574. [Google Scholar] [CrossRef]
- Feng, Z.; Rong, G.; Isaac, P. Polymeric nanofibers via flat spinneret electrospinning. Polym. Eng. Sci. 2009, 49, 2475–2481. [Google Scholar]
- Scaffaro, R.; Lopresti, F.; Botta, L. Preparation, characterization and hydrolytic degradation of PLA/PCL co-mingled nanofibrous mats prepared via dual-jet electrospinning. Eur. Polym. J. 2017, 96, 266–277. [Google Scholar] [CrossRef]

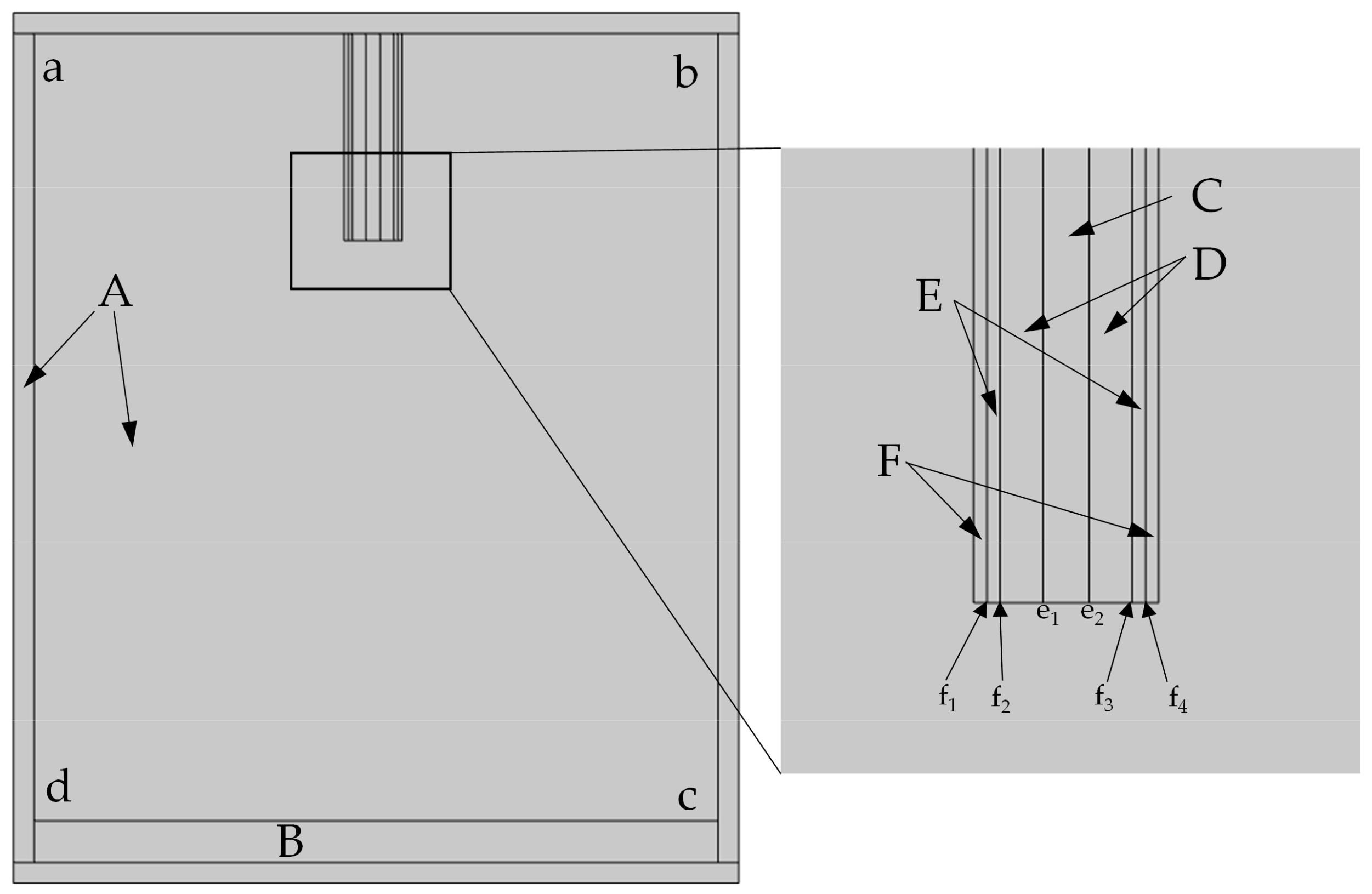



| Regional Division | Material | |
|---|---|---|
| A | Infinite metadomain | Air |
| B | Substrate | Glass |
| C | Vacant position | Air |
| D | Hollow copper pipe wall | Copper |
| E | Printed material | PCL |
| F | Nozzle | Copper |
| Position | Boundary Condition |
|---|---|
| e1–e2 | Normal inflow velocity = 60, 90, 120, 150, 180, and 210 m/s |
| f1–f2, f3–f4 | Normal inflow velocity = 20 m/s |
| Fiber Samples | pH | Fiber Diameter (μm) | |
|---|---|---|---|
| Before Degradation Test | After Degradation Test | ||
| Sample A | 3 | 5.52 ± 1.42 | 8.58 ± 1.67 |
| Sample B | 7.4 | 5.31 ± 1.31 | 4.51 ± 1.04 |
| Sample C | 10 | 5.36 ± 1.37 | 5.09 ± 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Peng, Z.; Wang, M.; Li, Y.; Wu, J.; Jiang, Y.; Liu, C.; Li, G.; Xu, L.; Lan, H. Novel Airflow-Field-Driven Melt Spinning 3D Printing of Tubular Scaffolds Based on Polycaprolactone Blends. Polymers 2023, 15, 1755. https://doi.org/10.3390/polym15071755
Zhang J, Peng Z, Wang M, Li Y, Wu J, Jiang Y, Liu C, Li G, Xu L, Lan H. Novel Airflow-Field-Driven Melt Spinning 3D Printing of Tubular Scaffolds Based on Polycaprolactone Blends. Polymers. 2023; 15(7):1755. https://doi.org/10.3390/polym15071755
Chicago/Turabian StyleZhang, Junyuan, Zilong Peng, Mengjie Wang, Yinan Li, Jinyin Wu, Yifan Jiang, Chaolong Liu, Guqiang Li, Lin Xu, and Hongbo Lan. 2023. "Novel Airflow-Field-Driven Melt Spinning 3D Printing of Tubular Scaffolds Based on Polycaprolactone Blends" Polymers 15, no. 7: 1755. https://doi.org/10.3390/polym15071755
APA StyleZhang, J., Peng, Z., Wang, M., Li, Y., Wu, J., Jiang, Y., Liu, C., Li, G., Xu, L., & Lan, H. (2023). Novel Airflow-Field-Driven Melt Spinning 3D Printing of Tubular Scaffolds Based on Polycaprolactone Blends. Polymers, 15(7), 1755. https://doi.org/10.3390/polym15071755