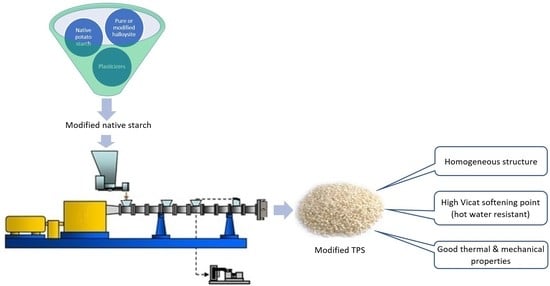
Morphology and Selected Properties of Modified Potato Thermoplastic Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Halloysite Modification and Characterization
2.3. Thermoplastic Starch Processing
2.4. Methods
3. Results and Discussion
3.1. Halloysite Characterization
3.1.1. Infrared Spectroscopy (FTIR)
3.1.2. Thermal Properties
3.2. Thermoplastic Starch
3.2.1. Chemical Structure and Molecular Interactions
3.2.2. Morphology
3.2.3. Viscoelastic Behavior
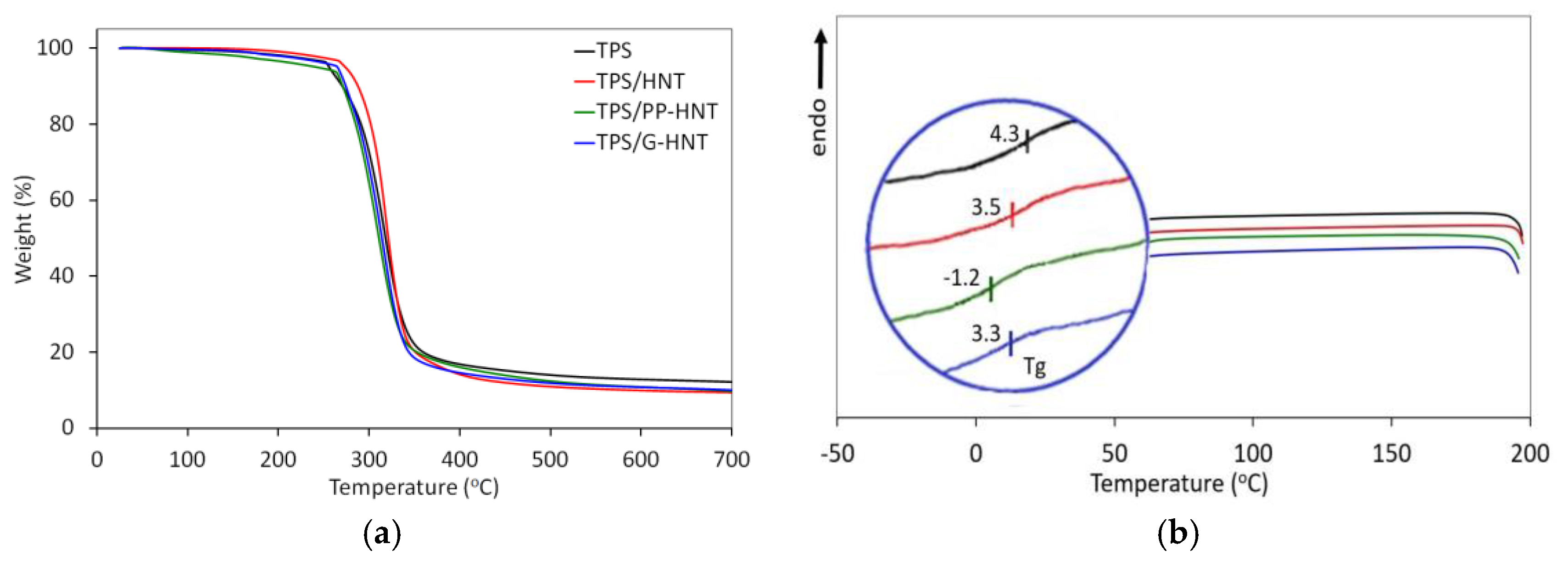
3.2.4. Thermal Properties
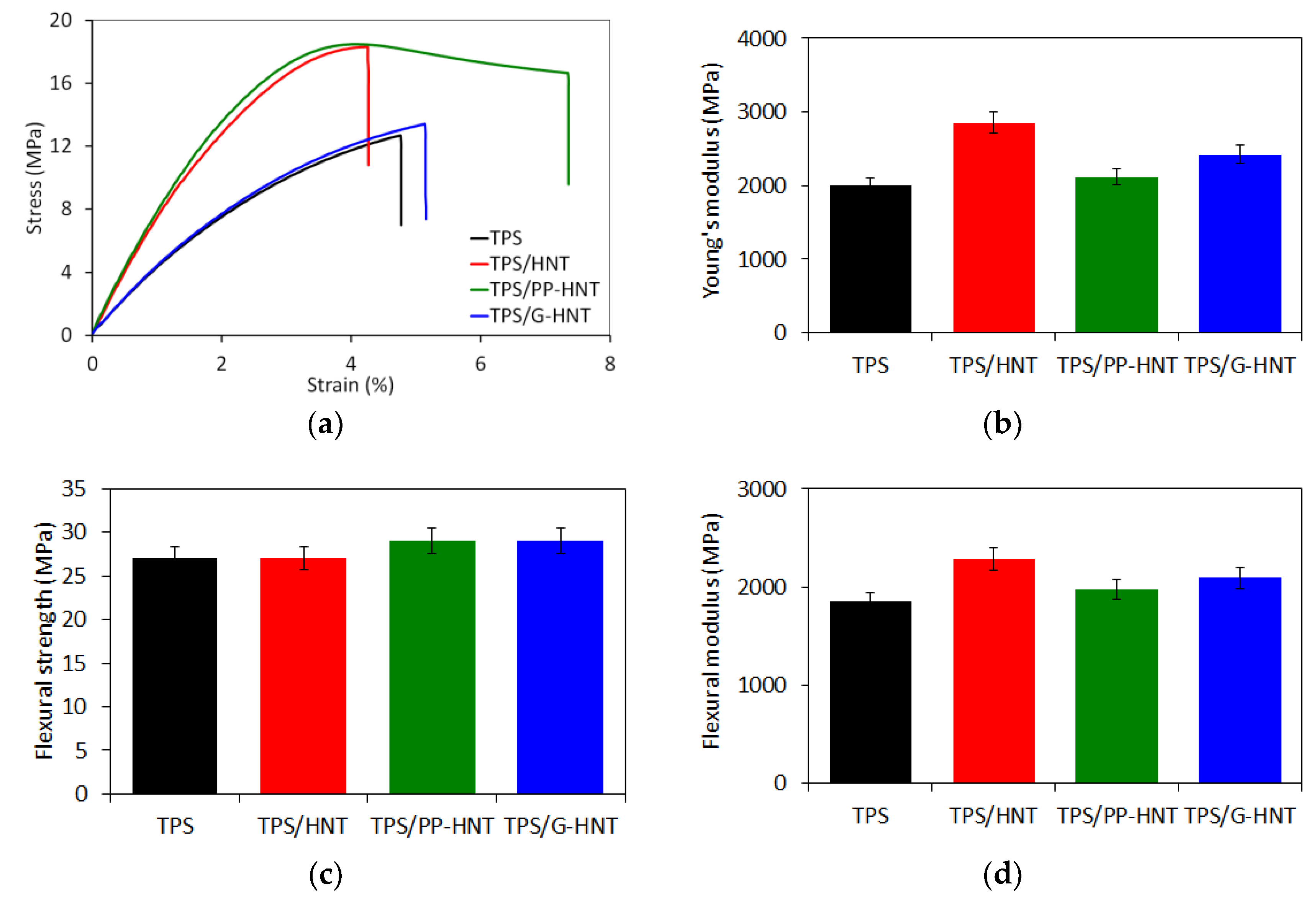
3.2.5. Mechanical Properties
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yottakot, S.; Leelavatcharamas, V. Isolation and optimization of polylactic acid (PLA)-packaging-degrading Actinomycete for PLA-packaging degradation. Pertanika J. Trop. Agric. Sci. 2019, 42, 1111–1129. [Google Scholar]
- Srivastava, A.; Srivasatva, A.K.; Singh, A.; Singh, P.; Verma, S.; Vats, M.; Sagadevan, S. Biopolymers as renewable polymeric materials for sustainable development-an overview. Polimery 2022, 67, 185–197. [Google Scholar] [CrossRef]
- Halimatul, M.J.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Ilyas, R.A. Effect of sago starch and plasticizer content on the properties of thermoplastic films: Mechanical testing and cyclic soaking-drying. Polimery 2019, 64, 422–431. [Google Scholar] [CrossRef]
- Borkowski, K. Pollution of seas and oceans with plastic waste as an accelerator of new legal regulations in this area (in Polish). Polimery 2019, 64, 759–763. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. Shelf Life 2021, 30, 1007–1043. [Google Scholar] [CrossRef]
- de Kock, L.; Sadan, Z.; Arp, R.; Upadhyaya, P. A Circular economy response to plastic pollution: Current policy landscape and consumer perception. S. Afr. J. Sci. 2020, 116, 1–2. [Google Scholar] [CrossRef]
- Razak, N.S.; Mohamed, R. Antimicrobial sustainable biopolymers for biomedical plastics applications–an overview. Polimery 2021, 66, 574–583. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 1005–1051. [Google Scholar] [CrossRef]
- Swierz-Motysia, B.; Jeziórska, R.; Szadkowska, A.; Piotrowska, M. Preparation and properties of biodegradable polylactide and thermoplastic starch blends (in Polish). Polimery 2011, 56, 271–280. [Google Scholar] [CrossRef]
- Sin, L.T.; Tueen, B.S. Polylactic Acid: A Practical Guide for the Processing, Manufacturing, and Applications of PLA, 2nd ed.; William Andrew: Oxford, UK, 2019. [Google Scholar]
- Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Available online: https://eur-lex.europa.eu/eli/dir/2019/904/oj (accessed on 7 March 2023).
- Ai, Y.; Jane, J. Understanding starch properties and functionality. In Starch Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Oxford, UK, 2018; pp. 151–178. [Google Scholar]
- Agarwal, S. Major factors affecting the characteristics of starch based biopolymer films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Mlynarczyk, K.; Longwic, F.; Podkoscielna, B.; Klepka, T. Influence on natural fillers on the thermal and mechanical properties of epoxy resin composites. Polimery 2022, 67, 102–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; McLaren, D. Thermoplastic starch. In Innovation in Food Packaging, 2nd ed.; Han, J., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 391–412. [Google Scholar]
- Ross, A.S. Starch in foods. In Food Carbohydrate Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons Ltd.: Oxford, UK, 2012; pp. 107–133. [Google Scholar]
- Perez, S.; Baldwin, P.M.; Gallant, D.J. Structural features of starch granules. In Starch Chemistry and Technology; BeMiller, J., Whistler, R., Eds.; Academic Press: New York, NY, USA, 2009; pp. 149–192. [Google Scholar]
- Zhou, Y.; Zhao, D.; Winkworth-Smith, C.G.; Wang, Y.; Liang, J.; Foster, T.J.; Cheng, Y. Effect of a small amount of sodium carbonate on konjac glucomannan-induced changes in wheat starch gel. Carbohydr. Polym. 2014, 114, 357–364. [Google Scholar] [CrossRef]
- Ma, X.F.; Yu, J.G.; Wang, N. Fly ash reinforced thermoplastic starch composites. Carbohydr. Polym. 2007, 67, 32–39. [Google Scholar] [CrossRef]
- Stepto, R.F.T. The processing of starch as a thermoplastic. Macromol. Symp. 2003, 201, 203–212. [Google Scholar] [CrossRef]
- Tabassi, N.; Moghbeli, M.R.; Ghasemi, I. Thermoplastic starch/cellulose nanocrystal green composites prepared in an internal mixer. Iran. Polym. J. 2016, 25, 45–57. [Google Scholar] [CrossRef]
- Huang, L.; Han, X.; Chen, H.; An, S.; Zhao, H.; Xu, H.; Huang, C.; Wang, S.; Liu, Y. Preparation and barrier performance of layer-modified soil-stripping/cassava starch composite films. Polymers 2020, 12, 1611. [Google Scholar] [CrossRef] [PubMed]
- Mansour, G.; Zoumaki, M.; Marinopoulou, A.; Tzetzis, D.; Prevezanos, M.; Raphaelides, S.N. Characterization and properties of non-granular thermoplastic starch-Clay biocomposite films. Carbohydr. Polym. 2020, 245, 116629. [Google Scholar] [CrossRef]
- Ashaduzzaman, M.; Saha, D.; Rashid, M.M. Mechanical and thermal properties of self-assembled kaolin-doped starch-based environment-friendly nanocomposite films. J. Compos. Sci. 2020, 4, 38. [Google Scholar] [CrossRef]
- Lai, D.S.; Osman, A.F.; Adnan, S.A.; Ibrahim, I.; Alrashdi, A.A.; Ahmad Salimi, M.N.; Ul-Hamid, A. On the use of OPEFB-derived microcrystalline cellulose and nano-bentonite for development of thermoplastic starch hybrid bio-composites with improved performance. Polymers 2021, 13, 897. [Google Scholar] [CrossRef]
- Jeziorska, R.; Szadkowska, A.; Spasowka, E.; Lukomska, A.; Chmielarek, M. Characteristics of biodegradable polylactide/thermoplastic starch/nanosilica composites: Effects of plasticizer and nanosilica functionality. Adv. Mater. Sci. Eng. 2018, 15. [Google Scholar] [CrossRef]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch modification for non-food, industrial applications: Market intelligence and critical review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef]
- da Costa, J.C.M.; Miki, K.S.L.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kong, W.; Wang, W.; Liu, T.; Liu, Y.; Gong, Q.; Gao, J. Modified natural halloysite/potato starch composite films. Carbohydr. Polym. 2012, 87, 2706–2711. [Google Scholar] [CrossRef]
- Xie, Y.; Chang, P.R.; Wang, S.; Yu, J.; Ma, X. Preparation, and properties of halloysite nanotubes/plasticized Dioscorea opposite Thunb. starch composites. Carbohydr. Polym. 2011, 83, 186–191. [Google Scholar] [CrossRef]
- Zhang, A.B.; Pan, L.; Zhang, H.Y.; Liu, S.T.; Ye, Y.; Xia, M.S.; Chen, X.G. Effects of acid treatment on the physico-chemical and pore characteristics of halloysite. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 182–188. [Google Scholar] [CrossRef]
- Yendluri, R.; Lvov, Y.; de Villiers, M.M.; Vinokurov, V.; Naumenko, E.; Tarasova, E.; Fakhrullin, R. Paclitaxel encapsulated in halloysite clay nanotubes for intestinal and intracellular delivery. J. Pharm. Sci. 2017, 106, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Balikile, R.D.; Roy, A.S.; Nagaraju, S.C.; Ramgopal, G. Conductivity properties of hollow ZnFe2O4 nanofibers doped polyaniline nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 7368–7375. [Google Scholar] [CrossRef]
- Schmitt, H.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Preparation, and properties of novel melt-blended halloysite nanotubes/wheat starch nanocomposites. Carbohydr. Polym. 2012, 89, 920–927. [Google Scholar] [CrossRef]
- Wardzinska-Jarmulska, E.; Szczepaniak, B.; Szczepankowska, B.; Modzelewska, A.; Stanecka, J.; Badowska, A.; Potajczuk-Czaja, K.; Grzybek, R. Method of Obtaining Unplasticized Polyester Plasticizer. Polish Patent 236 221, 13 August 2020. [Google Scholar]
- Jeziorska, R.; Legocka, I.; Szadkowska, A.; Spasowka, E.; Zubrowska, M.; Studzinski, M.; Wierzbicka, E.; Dzierżawski, J.; Kolasa, J.; Rucinski, J. Method of Producing Modified Thermoplastic Starch and Biodegradable Composites Containing Modified Thermoplastic Starch. Polish Patent Application 441 782, 19 July 2022. [Google Scholar]
- Li, Y.; Yuan, X.; Guan, X.; Bai, J.; Wang, H. One-pot synthesis of siliceous ferrihydrite–coated halloysite nanorods in alkaline medium: Structure, properties, and cadmium adsorption performance. J. Colloid Interface Sci. 2023, 636, 435–444. [Google Scholar] [CrossRef]
- Sid, D.; Baitiche, M.; Bourzami, R.; Merir, R.; Djerboua, F.; Gil, A.; Boutahala, M. Experimental and theoretical studies of the interaction of ketoprofen in halloysite nanotubes. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 627, 127136. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Mellouk, S.; Cherifi, S.; Sassi, M.; Marouf-Khelifa, K.; Bengueddach, A.; Schott, J.; Khelifa, A. Intercalation of halloysite from Djebel Debagh (Algeria) and adsorption of copper ions. Appl. Clay Sci. 2009, 44, 230–236. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Schmidt, J.M.; Kloprogge, J.T. Raman spectroscopy of potassium acetate-intercalated kaolinites at liquid nitrogen temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 603–609. [Google Scholar] [CrossRef]
- Cheng, H.; Frost, R.L.; Yang, J.; Liu, Q.; He, J. Infrared, and infrared emission spectroscopic study of typical Chinese kaolinite and halloysite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 1014–1020. [Google Scholar] [CrossRef]
- Horvath, E.; Kristov, J.; Frost, R.L. Vibrational spectroscopy of intercalated kaolinites. Part I. Appl. Spectrosc. Rev. 2010, 45, 130–147. [Google Scholar] [CrossRef]
- Hendessi, S.; Sevinis, E.B.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Antibacterialsustained-release coatings from halloysite nanotubes/water borne polyurethanes. Prog. Org. Coat. 2016, 101, 253–261. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F.; Yan, W.; Fan, M.; Liu, D.; He, H. Changes in structure, morphology, porosity, and surface activity of mesoporous halloysite nanotubes under heating. Clays Clay Miner. 2012, 60, 561–573. [Google Scholar] [CrossRef]
- Kittaka, S.; Ishimaru, S.; Kuranishi, M.; Matsuda, T.; Yamaguchi, T. Enthalpy and interfacial free energy changes of water capillary condensed in mesoporous silica, MCM-41, and SBA-15. Phys. Chem. Chem. Phys. 2006, 8, 3223–3231. [Google Scholar] [CrossRef]
- Jahnert, S.; Vaca Chavez, F.; Schaumann, G.E.; Schreiber, A.; Schonhoff, M.; Findenegg, G.H. Melting and freezing of water in cylindrical silica nanopores. Phys. Chem. Chem. Phys. 2008, 10, 6039–6051. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.H. Plasticization of pea starch films with monosaccharides and polyols. J. Food Sci. 2006, 71, 253–261. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Zullo, J.R.; Iannace, S. The effects of different starch sources and plasticizers on film blowing of thermoplastic starch: Correlation among process, elongational properties and macromolecular structure. Carbohydr. Polym. 2009, 77, 376–383. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Thermoplastic starch blown films with improved mechanical and barrier properties. Int. J. Biol. Macromol. 2021, 188, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Tiefenbacher, K.F. Chapter six-wafer sheet manufacturing: Technology and products. In Wafer and Waffle Processing and Manufacturing, 1st ed.; Tiefenbacher, K.F., Ed.; Academic Press: London, UK, 2017; pp. 405–486. [Google Scholar]
- Zhou, W.Y.; Guo, B.; Liu, M.; Liao, R.; Bakr, A.; Jia, D. Poly(vinyl alcohol)/halloysite nanotubes bionanocomposite films: Properties and in vitro osteoblasts and fibroblasts response. J. Biomed. Mater. Res. Part A 2009, 93A, 1574–1587. [Google Scholar] [CrossRef]
- Guimaraes, L.; Enyashin, A.N.; Seifert, G.; Duarte, H.A. Structural, electronic, and mechanical properties of single-walled halloysite nanotube models. J. Phys. Chem. C 2010, 114, 11358–11363. [Google Scholar] [CrossRef]

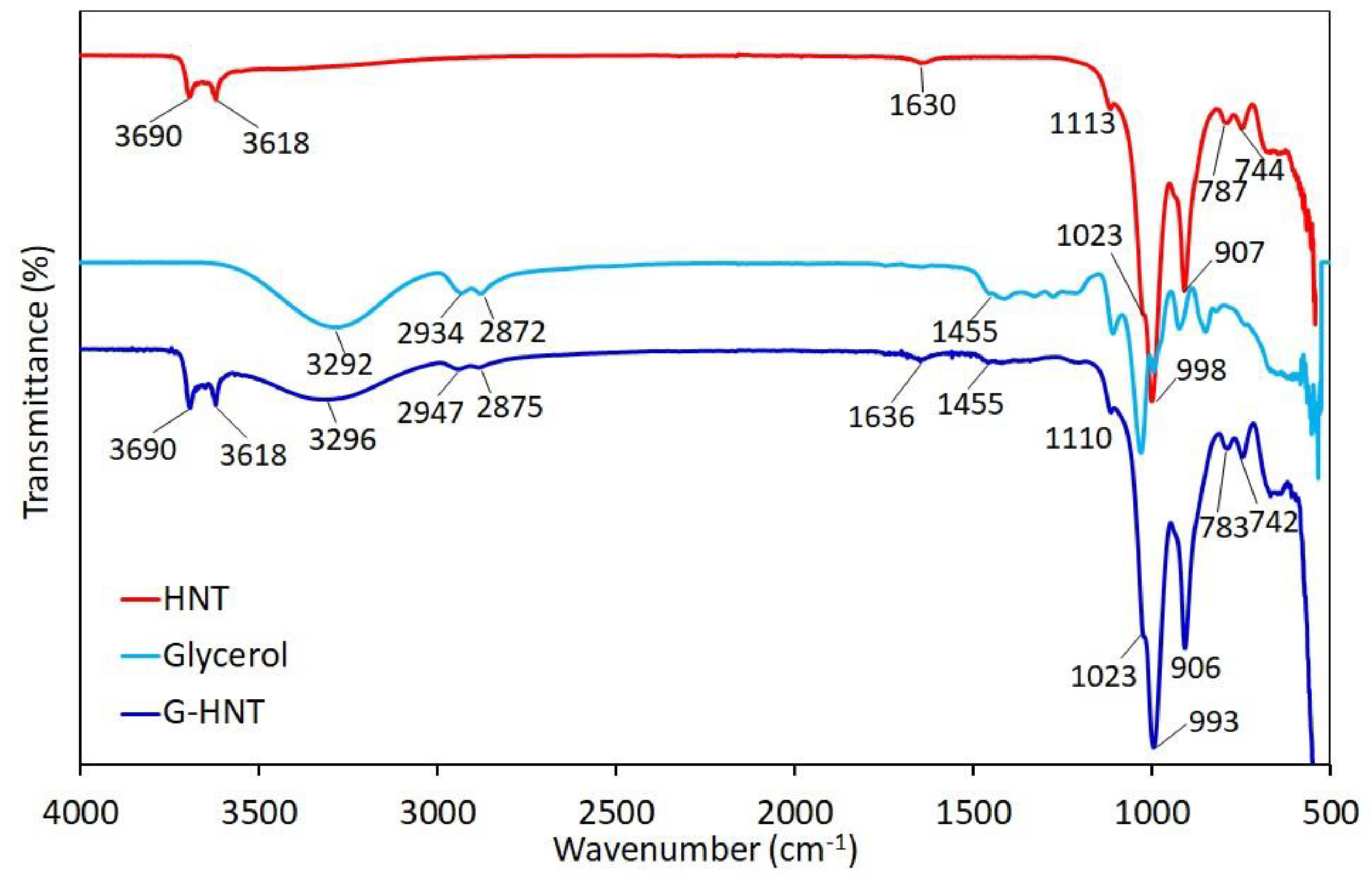


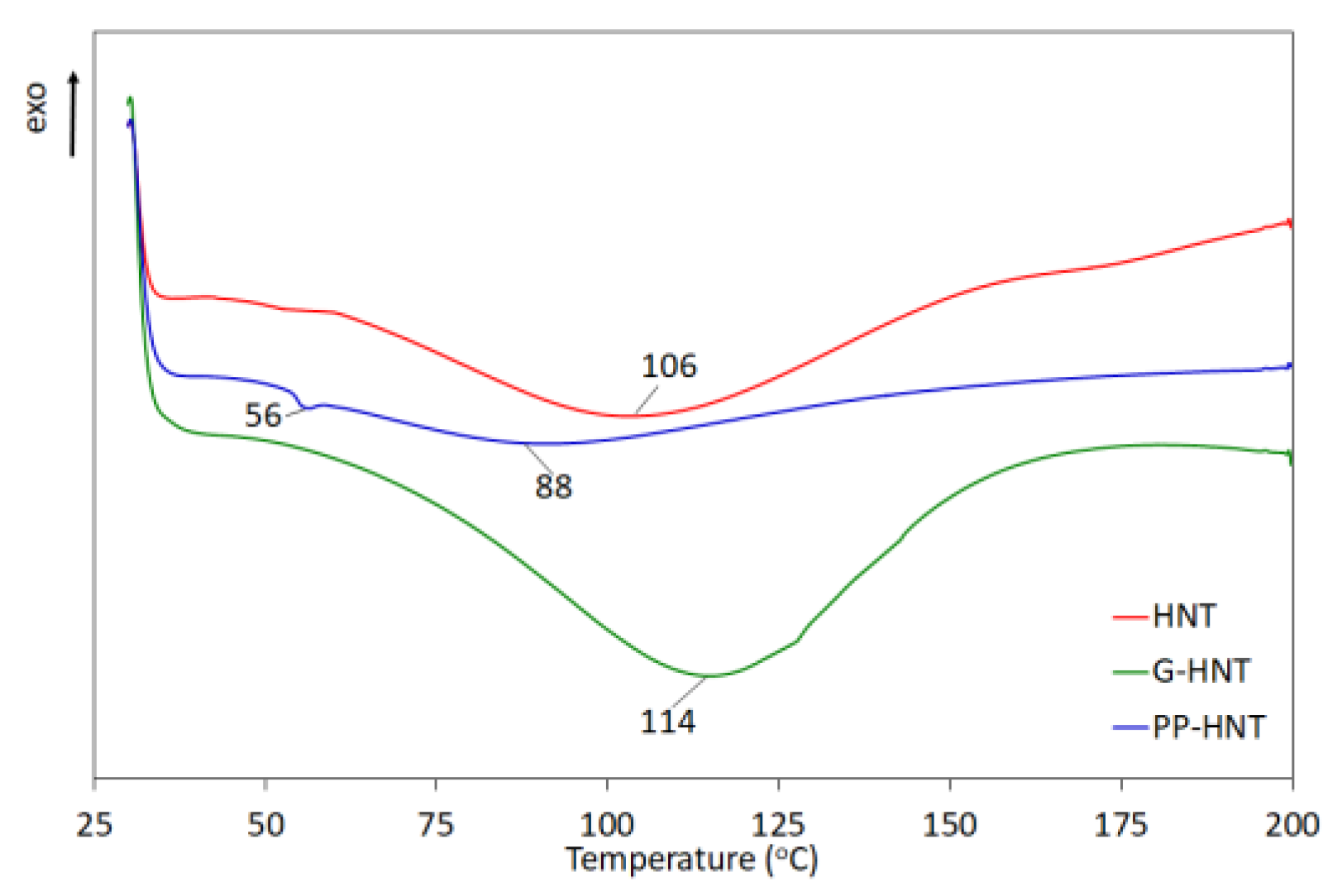



| Halloysite | BET Surface Area (m2/g) | Average Pore Size (nm) | Average Particle Size (nm) |
|---|---|---|---|
| Pure HNT | 61 | 11 | 93 |
| HNT after ultra-sonication | 35 | 17 | 173 |
| PP-HNT | 15 | 24 | 414 |
| G-HNT | 8 | 37 | 774 |
| Halloysite | T5% (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|
| Glycerol | 182 | 258 | 0 |
| Polyester plasticizer | 261 | 275, 395, 407 | 3 |
| Pure HNT | 403 | 58, 491 | 86 |
| PP-HNT | 262 | 56, 268, 483 | 79 |
| G-HNT | 164 | 58, 203, 482 | 72 |
| Sample | Storage Modulus (MPa) | Relaxation Temperature (°C) | |||
|---|---|---|---|---|---|
| −21 °C | 23 °C | Tα | Tβ | Tβ1 | |
| TPS | 2820 | 900 | 50 | −22 | – |
| TPS/HNT | 3400 | 1380 | 70 | −27 | 12 |
| TPS/PP-HNT | 3100 | 1040 | 69 | −25 | – |
| TPS/G-HNT | 3090 | 1120 | 72 | −25 | – |
| Sample | T5% (°C) | T10% (°C) | Residue (%) | Tmax (°C) | Tg (°C) | Vicat (°C) |
|---|---|---|---|---|---|---|
| TPS | 253 | 268 | 12 | 313 | 4.3 | 65 |
| TPS/HNT | 272 | 285 | 10 | 315 | 3.5 | 76 |
| TPS/PP-HNT | 243 | 268 | 10 | 307 | –1.2 | 72 |
| TPS/G-HNT | 262 | 271 | 10 | 313 | 3.3 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeziorska, R.; Szadkowska, A.; Studzinski, M.; Chmielarek, M.; Spasowka, E. Morphology and Selected Properties of Modified Potato Thermoplastic Starch. Polymers 2023, 15, 1762. https://doi.org/10.3390/polym15071762
Jeziorska R, Szadkowska A, Studzinski M, Chmielarek M, Spasowka E. Morphology and Selected Properties of Modified Potato Thermoplastic Starch. Polymers. 2023; 15(7):1762. https://doi.org/10.3390/polym15071762
Chicago/Turabian StyleJeziorska, Regina, Agnieszka Szadkowska, Maciej Studzinski, Michal Chmielarek, and Ewa Spasowka. 2023. "Morphology and Selected Properties of Modified Potato Thermoplastic Starch" Polymers 15, no. 7: 1762. https://doi.org/10.3390/polym15071762
APA StyleJeziorska, R., Szadkowska, A., Studzinski, M., Chmielarek, M., & Spasowka, E. (2023). Morphology and Selected Properties of Modified Potato Thermoplastic Starch. Polymers, 15(7), 1762. https://doi.org/10.3390/polym15071762