Reinforcement of Nanocomposite Hydrogel with Dialdehyde Cellulose Nanofibrils via Physical and Double Network Crosslinking Synergies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
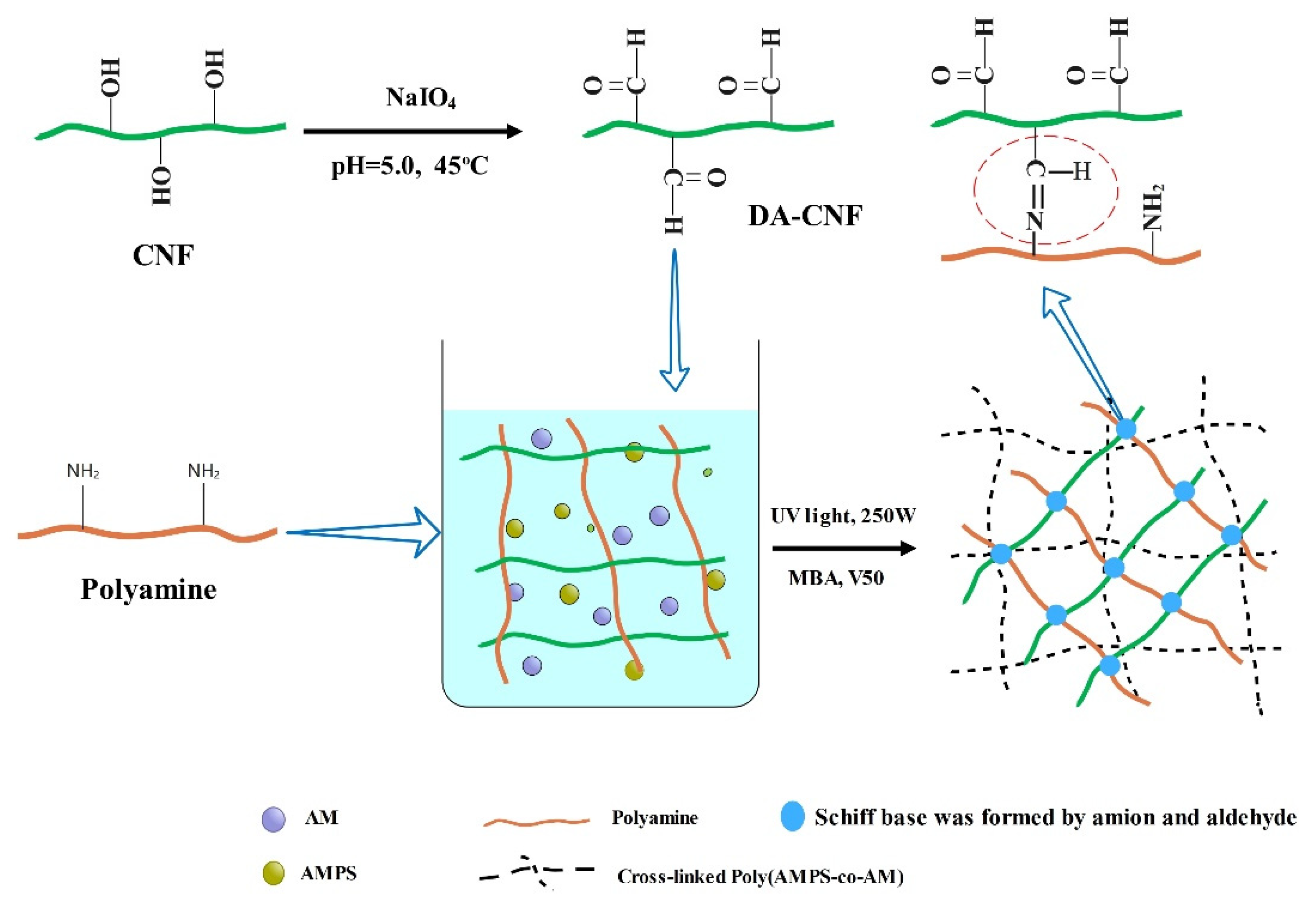
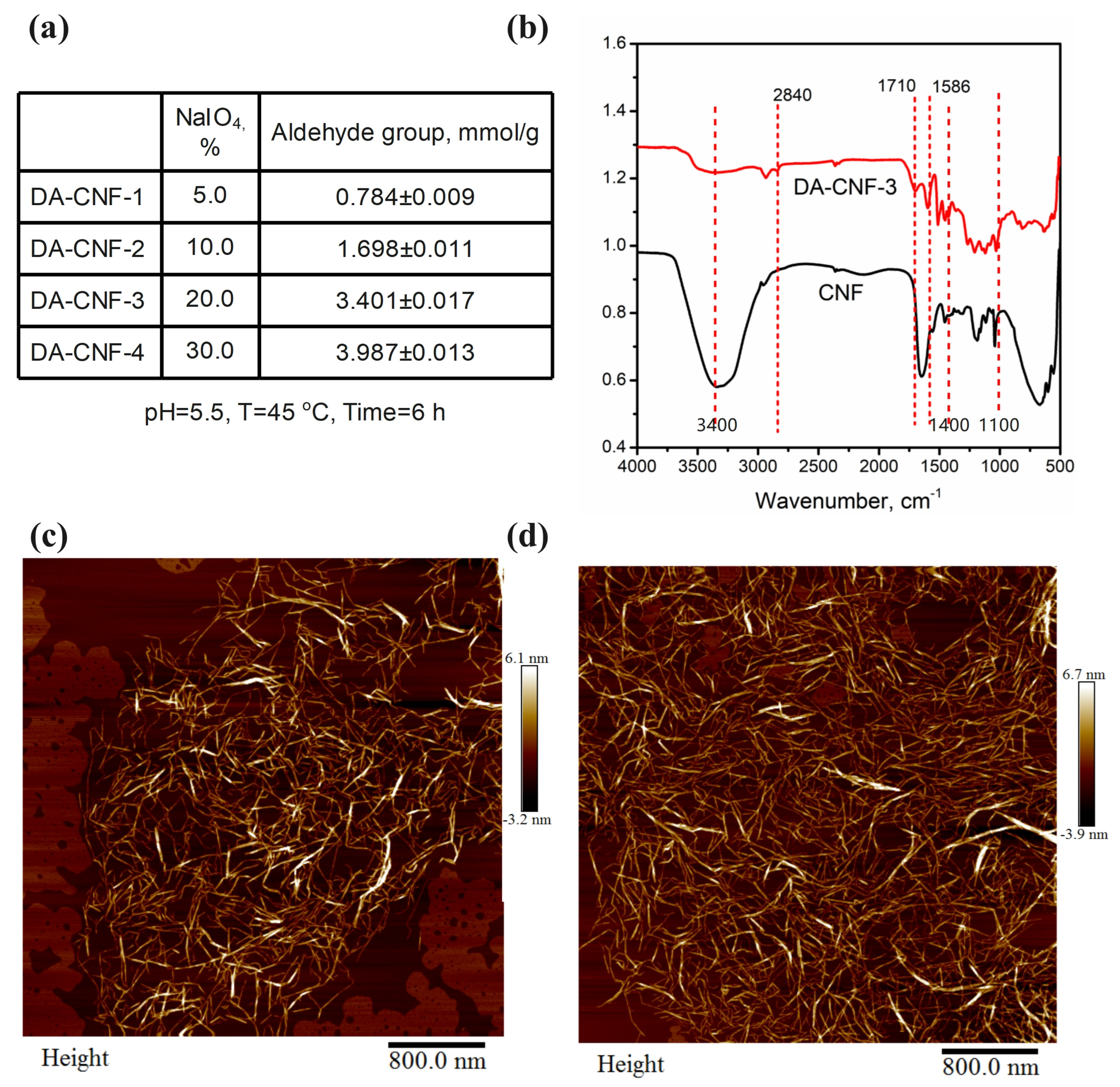
2.2. Preparation of Dialdehyde Cellulose Nanofibrils (DA-CNFs)
2.3. Preparation of Nanocomposite Hydrogel
2.4. Characterization of Hydrogel
2.5. Analytical Methods
3. Results and Discussion
3.1. Synthesis of Hydrogels
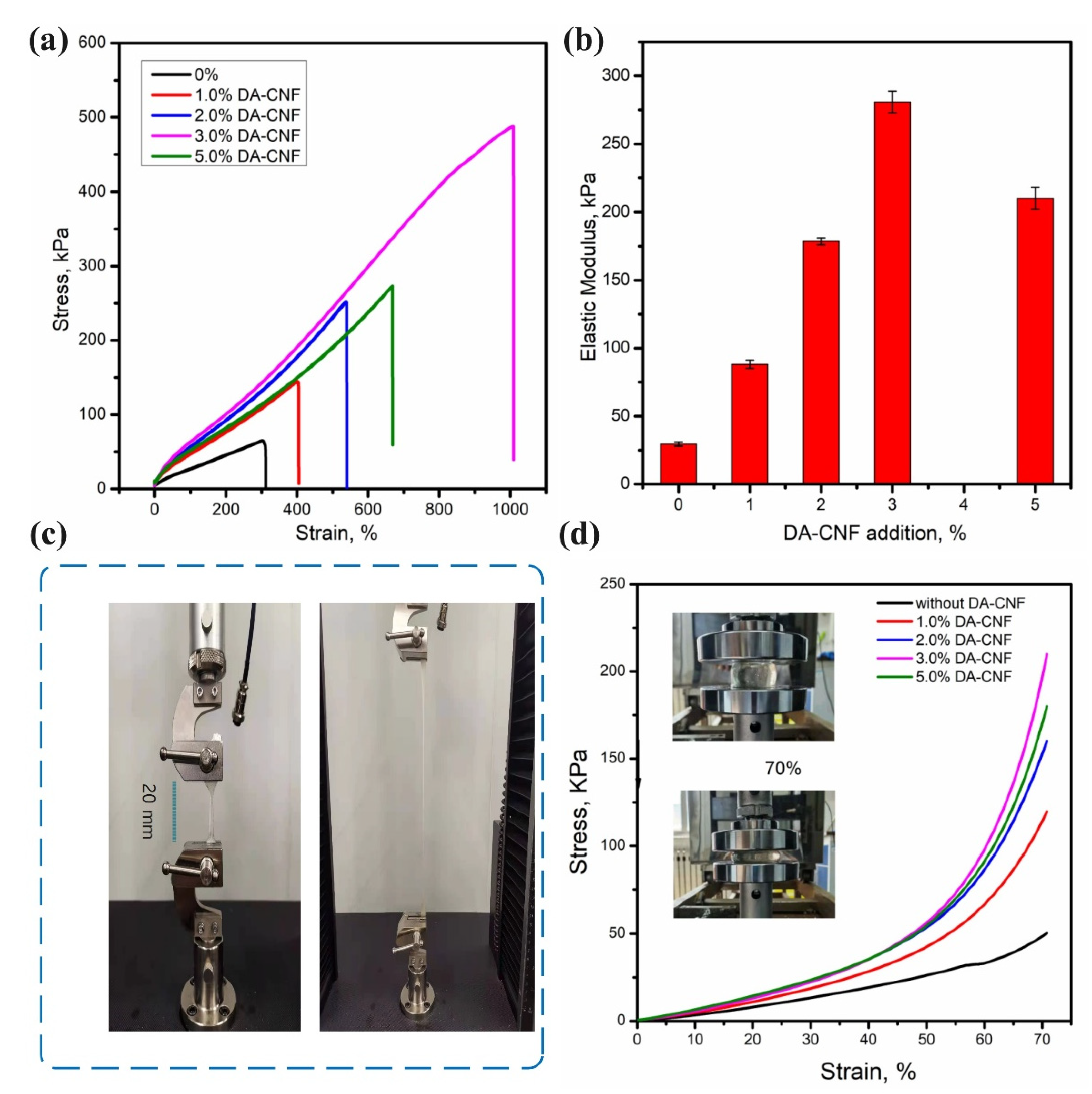
3.2. Mechanical Properties of the Hydrogels
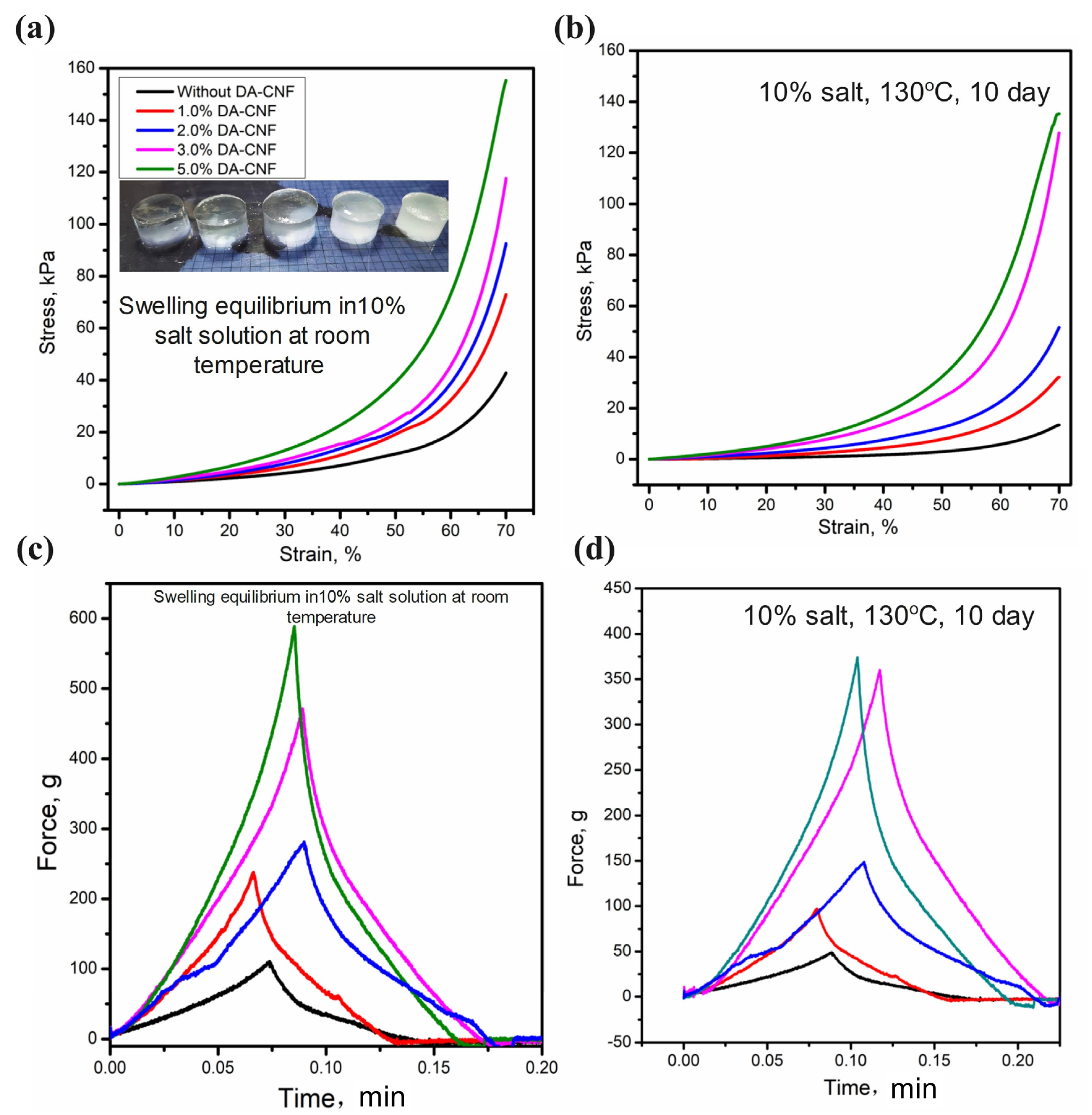
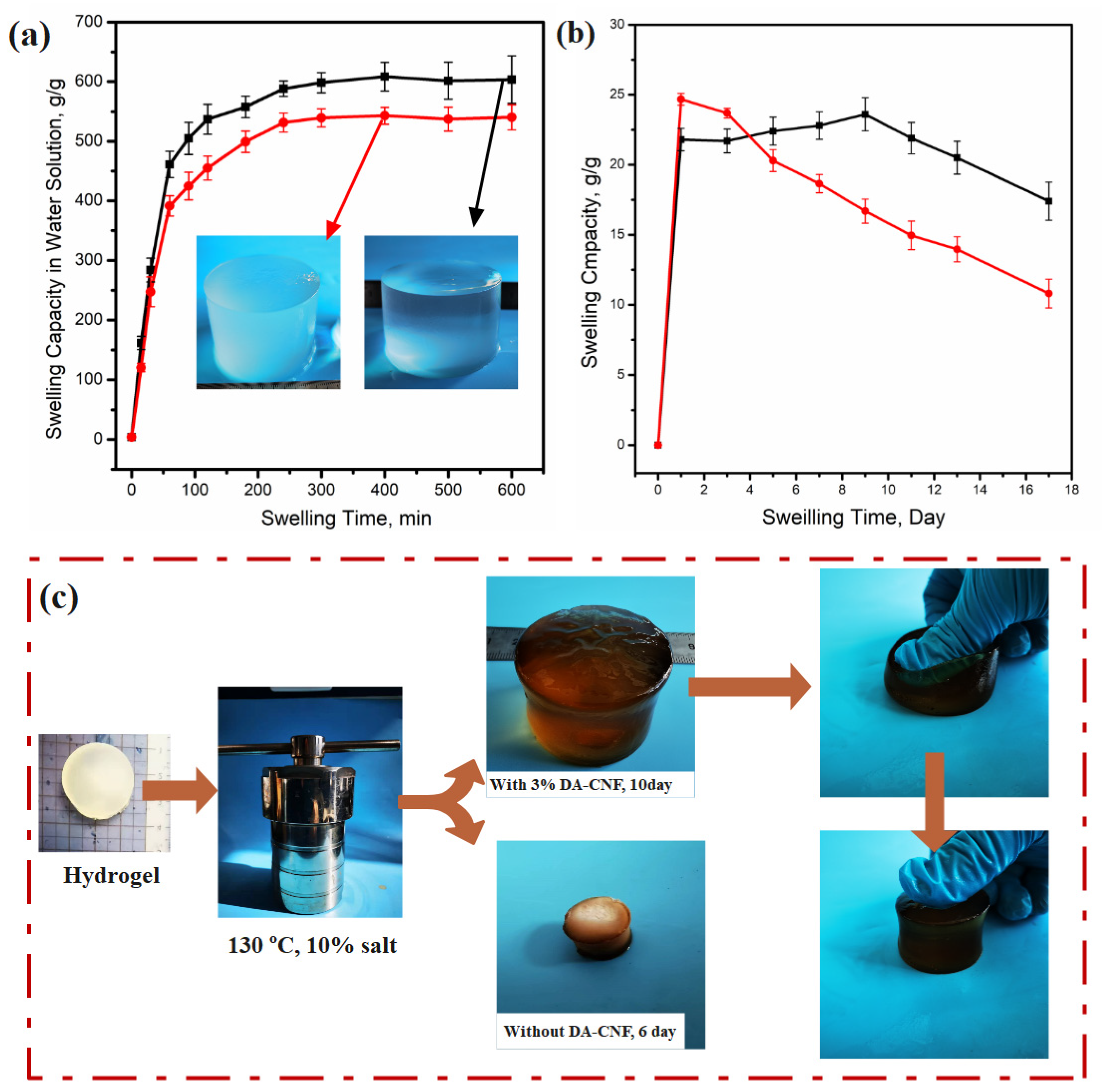
3.3. Swelling Behavior and Texture Intensity in High Salinity Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bashari, A.; Shirvan, A.R.; Shakeri, M. Cellulose-based hydrogels for personal care products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Liu, C.; Lei, F.; Li, P.; Jiang, J.; Wang, K. Borax crosslinked fenugreek galactomannan hydrogel as potential water-retaining agent in agriculture. Carbohydr. Polym. 2020, 236, 116100. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, Y.; Yu, Q.; Yan, B.; Qian, Y.; Wen, J.; Ma, C.; Jiang, S.; Wang, X.; Wang, N. Bio-inspired antibacterial cellulose paper–poly (amidoxime) composite hydrogel for highly efficient uranium (vi) capture from seawater. Chem. Commun. 2020, 56, 3935–3938. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Zareie, C.; Bahramian, A.R.; Sefti, M.V.; Salehi, M.B. Network-gel strength relationship and performance improvement of polyacrylamide hydrogel using nano-silica; with regards to application in oil wells conditions. J. Mol. Liq. 2019, 278, 512–520. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Wang, L.; Long, Y.; Ding, H.; Geng, J.; Bai, B. Mechanically robust re-crosslinkable polymeric hydrogels for water management of void space conduits containing reservoirs. Chem. Eng. J. 2017, 317, 952–960. [Google Scholar] [CrossRef] [Green Version]
- Pu, J.; Zhou, J.; Chen, Y.; Bai, B. Development of thermos-transformable controlled hydrogel for enhancing oil recovery. Energy Fuels 2017, 31, 13600–13609. [Google Scholar] [CrossRef]
- Gu, Z.; Huang, K.; Luo, Y.; Zhang, L.; Kuang, T.; Chen, Z.; Liao, G. Double network hydrogel for tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1520. [Google Scholar] [CrossRef]
- Schexnailder, P.; Schmidt, G. Nanocomposite polymer hydrogels. Colloid Polym. Sci. 2009, 287, 1–11. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Biswal, D.; Hilt, J.Z. Hydrogel nanocomposites: A review of applications as remote controlled biomaterials. Soft Matter 2010, 6, 2364–2371. [Google Scholar] [CrossRef]
- Haraguchi, K. Nanocomposite hydrogels. Curr. Opin. Solid State Mater. Sci. 2007, 11, 47–54. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite hydrogels: A unique organic–inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, B.S.; Kim, H.S.; Lee, Y.J.; Min, S.K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, D.M.; Nunes, Y.L.; Figueirêdo, M.C.; de Azeredo, H.M.; Aouada, F.A.; Feitosa, J.P.; Dufresne, A.; Rosa, M.F. Nanocellulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zhao, Z.; Feng, C.; Mayes, E.; Yang, J. Nanocellulose reinforced P (AAm-co-AAc) hydrogels with improved mechanical properties and biocompatibility. Compos. Part A Appl. Sci. Manuf. 2018, 112, 395–404. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Qin, S.; Pei, Y.; Zheng, X.; Tang, K. High mechanical strength gelatin composite hydrogels reinforced by cellulose nanofibrils with unique beads-on-a-string morphology. Int. J. Biol. Macromol. 2020, 164, 1776–1784. [Google Scholar] [CrossRef]
- Madivoli, E.S.; Kareru, P.G.; Gachanja, A.N.; Mugo, S.M.; Makhanu, D.S. Synthesis and characterization of dialdehyde cellulose nanofibers from O. sativa husks. SN Appl. Sci. 2019, 1, 723. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xiao, G.; Peng, Y.; Chen, L.; Fu, S. Effects of cellulose nanofibrils on dialdehyde carboxymethyl cellulose based dual responsive self-healing hydrogel. Cellulose 2019, 26, 8813–8827. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.; Liu, Z.; Ni, Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose nanofibrils enhanced, strong, stretchable, freezing-tolerant ionic conductive organohydrogel for multi-functional sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Liu, Z.; Wang, Q. Facile fabrication of tough photocrosslinked polyvinyl alcohol hydrogels with cellulose nanofibrils reinforcement. Polymer 2019, 173, 103–109. [Google Scholar] [CrossRef]
- Liu, S.; Oderinde, O.; Hussain, I.; Yao, F.; Fu, G. Dual ionic cross-linked double network hydrogel with self-healing, conductive, and force sensitive properties. Polymer 2018, 144, 111–120. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Y.; Yang, Q.; Zhou, Q.; Hui, L.; Liu, Z.; Ding, D. One-pot freezing-thawing preparation of cellulose nanofibrils reinforced polyvinyl alcohol based ionic hydrogel strain sensor for human motion monitoring. Carbohydr. Polym. 2022, 275, 118697. [Google Scholar] [CrossRef]
- Gao, H.; Mao, J.; Cai, Y.; Li, S.; Fu, Y.; Liu, X.; Liang, H.; Zhao, T.; Liu, M.; Jiang, L. Euryhaline hydrogel with constant swelling and salinity-enhanced mechanical Strength in a Wide Salinity Range. Adv. Funct. Mater. 2021, 31, 2007664. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Poly (acrylic acid-co-acrylamide)/cellulose nanofibrils nanocomposite hydrogels: Effects of CNFs content on the hydrogel properties. Cellulose 2016, 23, 3691–3701. [Google Scholar] [CrossRef]
- Ge, G.; Mandal, K.; Haghniaz, R.; Li, M.; Xiao, X.; Carlson, L.; Jucaud, V.; Dokmeci, M.R.; Ho, G.W.; Khademhosseini, A. Deep Eutectic Solvents-Based Ionogels with Ultrafast Gelation and High Adhesion in Harsh Environments. Adv. Funct. Mater. 2023, 2023, 2207388. [Google Scholar] [CrossRef]
- Ge, G.; Wang, Q.; Zhang, Y.Z.; Alshareef, H.N.; Dong, X. 3D printing of hydrogels for stretchable ionotronic devices. Adv. Funct. Mater. 2021, 31, 2107437. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, Y.Z.; Zhang, W.; Yuan, W.; El-Demellawi, J.K.; Zhang, P.; Alshareef, H.N. Ti3C2TxMXene-Activated Fast Gelation of Stretchable and Self-Healing Hydrogels: A Molecular Approach. ACS Nano 2021, 15, 2698–2706. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, Y.Z.; Fang, S.; Sun, Y.; Niu, J.; Lai, W.Y. Wireless Human–Machine Interface Based on Artificial Bionic Skin with Damage Reconfiguration and Multisensing Capabilities. ACS Appl. Mater. Interfaces 2022, 14, 47300–47309. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, Y.Z.; Fang, S.; Liu, C.; Niu, J.; Li, G.; Lai, W.Y. Artificial intelligent optoelectronic skin with anisotropic electrical and optical responses for multi-dimensional sensing. Appl. Phys. Rev. 2022, 9, 021403. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, J.; Du, B.; Wang, M.; Lai, W.Y.; Huang, W. Design, synthesis, and postvapor treatment of neutral fulleropyrrolidine electron-collecting interlayers for high-efficiency inverted polymer solar cells. ACS Appl. Electron. Mater. 2019, 1, 854–861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Guo, J.; Kang, C.; Song, H. Reinforcement of Nanocomposite Hydrogel with Dialdehyde Cellulose Nanofibrils via Physical and Double Network Crosslinking Synergies. Polymers 2023, 15, 1765. https://doi.org/10.3390/polym15071765
Li L, Guo J, Kang C, Song H. Reinforcement of Nanocomposite Hydrogel with Dialdehyde Cellulose Nanofibrils via Physical and Double Network Crosslinking Synergies. Polymers. 2023; 15(7):1765. https://doi.org/10.3390/polym15071765
Chicago/Turabian StyleLi, Liang, Jixiang Guo, Chuanhong Kang, and Hanxuan Song. 2023. "Reinforcement of Nanocomposite Hydrogel with Dialdehyde Cellulose Nanofibrils via Physical and Double Network Crosslinking Synergies" Polymers 15, no. 7: 1765. https://doi.org/10.3390/polym15071765




