Disinfection and Isotonic Drinks’ Influence on Hardness and Color Stability of Ethylene-Vinyl-Acetate Copolymer Mouthguards Used in Martial Arts: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Sample Disinfection Protocol
2.3. Protocol for Immersion of Samples in Isotonic Drinks
2.4. Hardness and Color Change Measurements
2.5. Statistical Analysis
3. Results
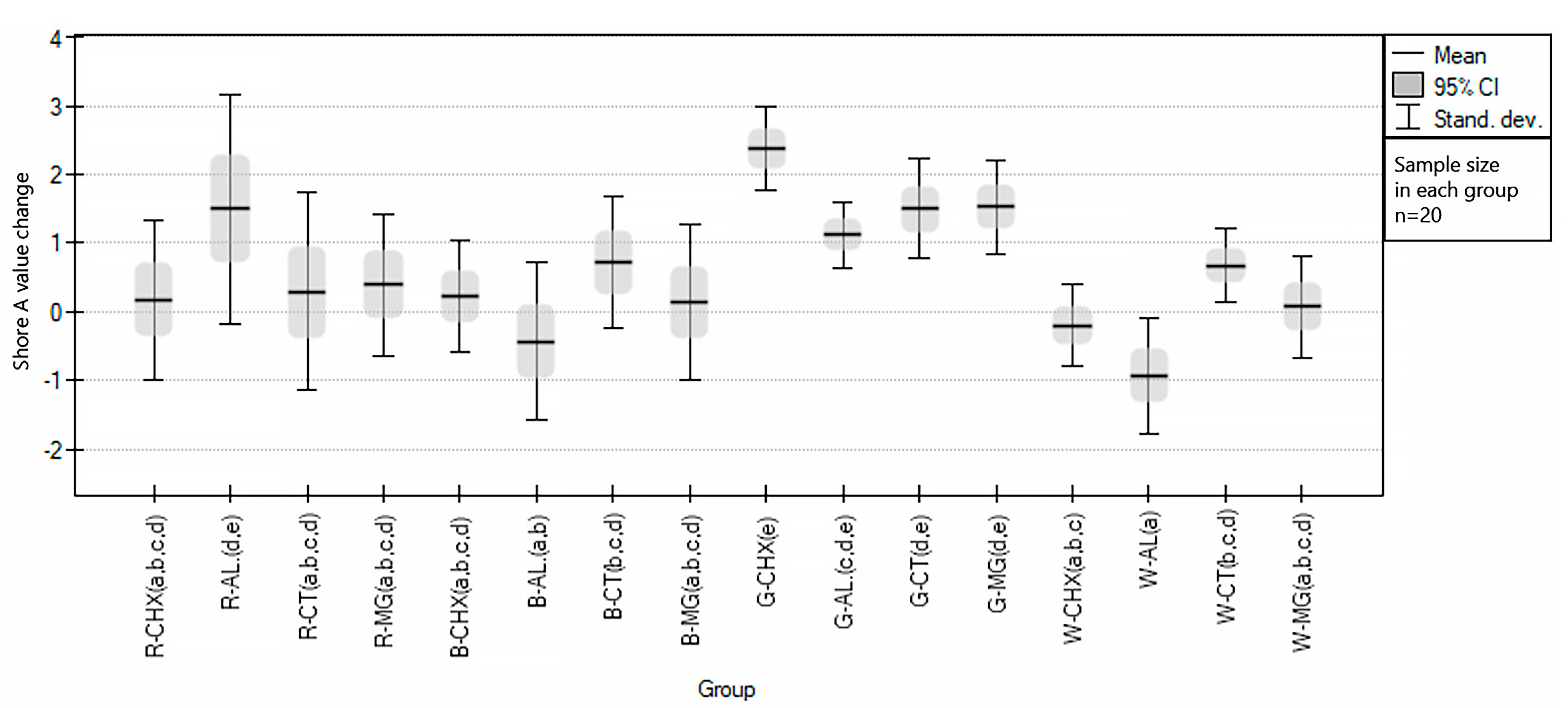
3.1. Influence of Cleaning Agents on Color and Hardness Changes of EVA
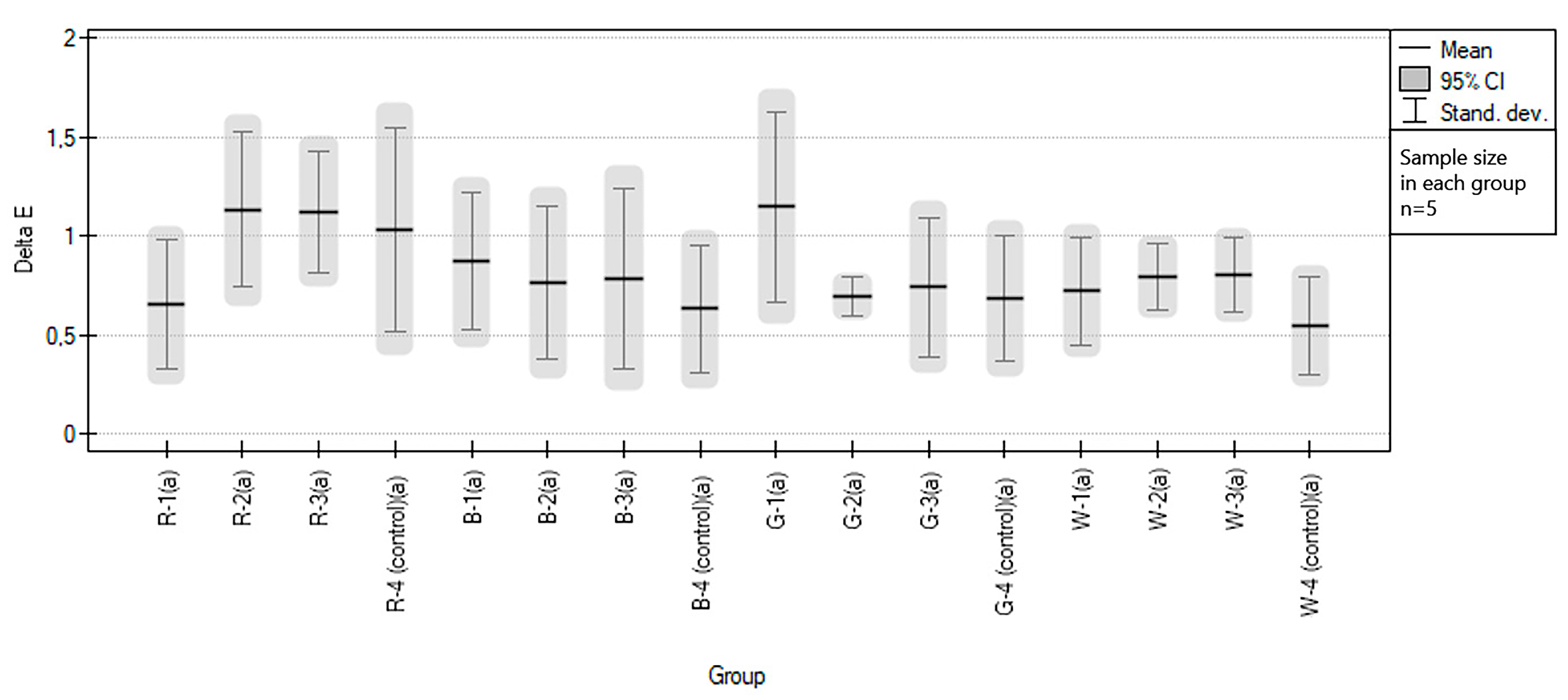
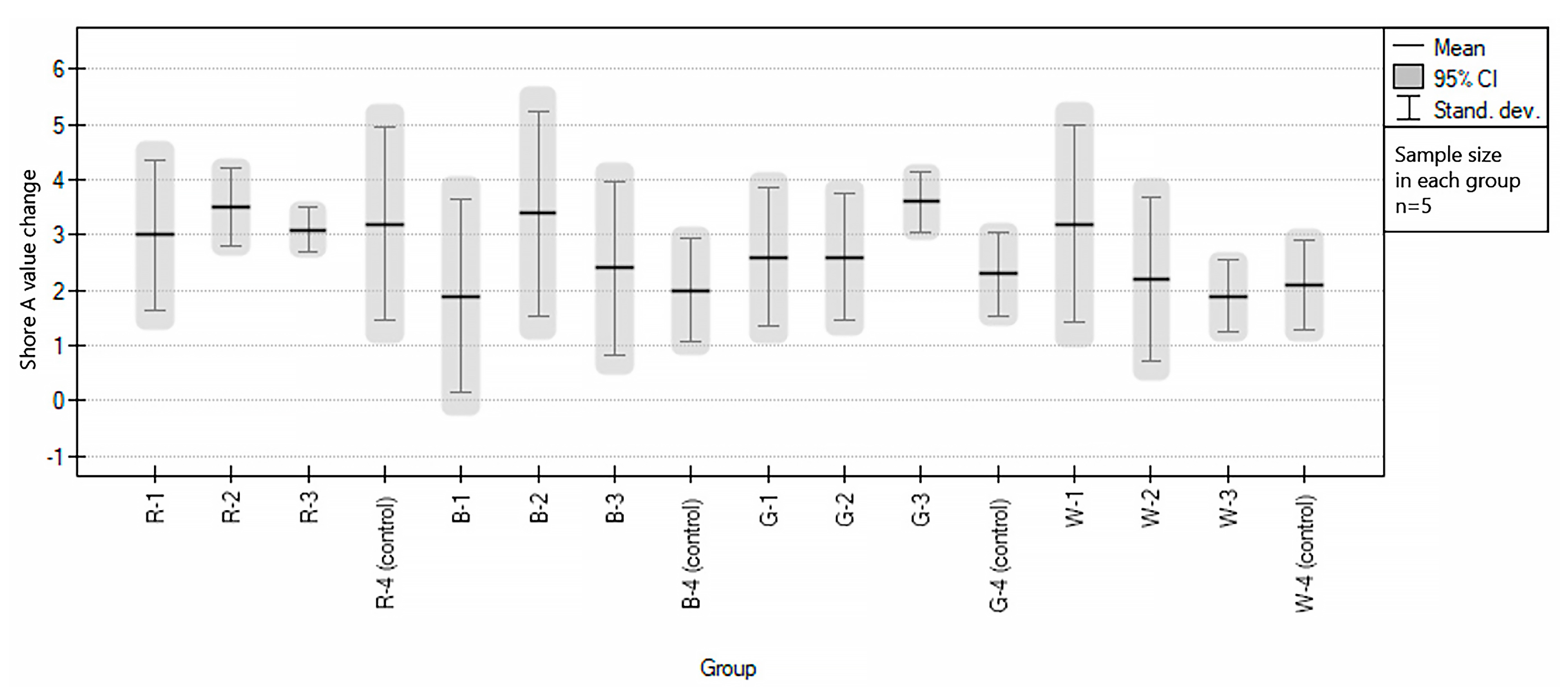
3.2. Influence of Sport Drinks on Color and Hardness Changes of EVA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knapik, J.; Mashall, S.; Lee, R.; Darakjy, S.; Jones, S. Mouthguards in sports activities history, physical properties, and injury prevention effectiveness. Sport Med. 2007, 37, 117–144. [Google Scholar] [CrossRef]
- Craig, R.G.; Godwin, W.C. Properties of athletic mouth protectors and materials. J. Oral. Rehab. 2002, 29, 146–150. [Google Scholar] [CrossRef]
- Gawlak, D.; Mańka-Malara, K.; Mierzwińska-Nastalska, E.; Waśniewski, B.; Ryszkowska, J. Comparison of the hardness, energy absorption and water absorbability of polymeric materials used in the manufacture of mouthguards. Dent. Med. Probl. 2015, 52, 78–85. [Google Scholar]
- ADA Council on Access, Prevention and Interprofessional Relations; ADA Council on Scientific Affairs. Using mouthguards to reduce the incidence and severity of sports-related oral injuries. J. Am. Dent. Assoc. 2006, 137, 1712–1720; quiz 1731. [Google Scholar] [CrossRef] [PubMed]
- Spinas, E.; Aresu, M.; Gianetti, L. Use of mouth guards in basketball: Observational study of a group of teenagers with and without motivational reinforcement. Eur. J. Paediatr. Dent. 2014, 15, 392–396. [Google Scholar]
- Gawlak, D.; Mańka-Malara, T.; Kamiński, T. Assessment of the usage of custom mouthguards prepared using pressure injection—Preliminary examination. Dent. Med. Probl. 2014, 51, 218–224. [Google Scholar]
- Bochnig, M.S.; Oh, M.J.; Nagel, T.; Ziegler, F.; Brinkmann, P.G.J. Comparison of the shock absorption capacities of different mouthguards. Dent. Traumatol. 2017, 33, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Guerard, S.; Barou, J.L.; Petit, J.; Poisson, P. Characterization of mouth-formed mouthguards: Thermal performance. Dent. Mater. 2014, 33, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Mańka-Malara, K.; Gawlak, D. Comparison of mouthguards used in combat sports. Dent. Med. Probl. 2013, 50, 205–209. [Google Scholar]
- Mizuhashi, F.; Koide, K. Formation of vacuum-formed and pressure-formed mouthguards. Dent. Traumatol. 2017, 33, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Shewe, P.; Roehler, A.; Spintzyk, S.; Huettig, F. Shock absorption behavior of elastic polymers for sports mouthguards: An In Vitro comparison of thermoplastic forming and additive manufacturing. Materials 2022, 15, 2928. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Coto, N.; Dias, B.; Costa, R.; Antoniazzi, T.; Carvalho, E. Mechanical behavior of Ethylene Vinyl Acetate copolymer (EVA) used for fabrication of mouthguards and interocclusal splints. Braz. Dent. J. 2007, 18, 324–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugada, D.C.; Rudin, A. Molecular structure and melting behaviour of ethylene-vinyl acetate copolymers. Eur. Polym. J. 1992, 28, 219–227. [Google Scholar] [CrossRef]
- Dias, R.B.; Coto, N.; Batalha, G.; Driemeier, L. Systematic Study of Ethylene-Vinyl Acetate (EVA) in the Manufacturing of Protector Devices for the Orofacial System. Arch. Mater. Sci. Eng. 2018, 1, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Bando, Y. Effective thermoforming method for maintaining mouthguard thickness with a circular sheet using a circular frame. Dent. Traumatol. 2022, 38, 325–331. [Google Scholar] [CrossRef]
- Takahashi, M.; Bando, Y. Effect of acute angle model on mouthguard thickness with the thermoforming method and moving the model position just before fabrication. Dent. Traumatol. 2021, 37, 138–144. [Google Scholar] [CrossRef]
- Mizuhashi, F.; Mizuhashi, R.; Koide, K. Basic research to propose a new design of laminated mouthguard—Effect of lamination order on thickness. Dent. Traumatol. 2022, 38, 238–243. [Google Scholar] [CrossRef]
- Park, J.; Shaull, K.; Overton, B.; Donly, K. Improving mouthguards. J. Prosthet. Dent. 1994, 72, 373–380. [Google Scholar] [CrossRef]
- Takeda, T.; Ishigami, K.; Kawamura, S.; Nakajima, K.; Shimada, A.; Sumii, T.; Swain, M. Adhesive strength and its improvement referring to the laminated-type mouthguard. Dent. Traumatol. 2006, 22, 205–214. [Google Scholar] [CrossRef]
- Del Rossi, G.; Lisman, P.; Signorile, J. Fabricating a better mouthguard. Part II: The effect of color on adaptation and fit. Dent. Traumatol. 2008, 24, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, R.; Tomita, R.; Ogawa, N.; Nakajima, K.; Takeda, T.; Uehara, H.; Yamanobe, T. Crystallization and hardening of poly(ethylene-co-vinyl-acetate) mouthguards during routine use. Sci. Rep. 2017, 15, 44672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benli, M.; Gümüs, B.E.; Kahraman, Y.; Yagci, Ö.; Erdogan, D.; Huck, O.; Özcan, M. Thermal, structural and morphological characterization of dental polymers for clinical applications. J. Prosthodont. Res. 2021, 30, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, U.; Yildiz, E.; Eren, M.M.; Ozel, S. Surface hardness of different restorative materials after long-term immersion in sports and energy drinks. Dent. Mater. J. 2012, 31, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Owens, B.M.; Kitchens, M. The erosive potential of soft drinks on enamel surface substrate: An in vitro scanning electron microscopy investigation. J. Contemp. Dent. Pr 2007, 8, 11–20. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chang, C.C.; Chi, C.W.; Chang, H.H.; Chiang, Y.C.; Chuang, Y.C.; Chang, H.H.; Huang, G.F.; Liao, Y.S.; Lin, C.P. Erosive potential of soft drinks on human enamel: And in vitro study. J. Med. Assoc. 2014, 113, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Ostrowska, A.; Szymański, W.; Kołodziejczyk, Ł.; Bołtacz-Rzepkowska, E. Evaluation of the erosive potential of selected isotonic drinks: In vitro studies. Adv. Clin. Exp. Med. 2016, 25, 1313–1319. [Google Scholar] [CrossRef] [Green Version]
- Glass, R.; Bullard, J.; Conrad, R. The contamination of protective mouthguards: A characterization of the microbiota found in football players’ protective mouthguards as compared to oral microbiota found in first-year medical students. Am. Dent. Inst. Cont. Educ. J. 2006, 93, 23–28. [Google Scholar]
- Glass, R.; Wood, C.; Bullard, J.; Conrad, S. Possible disease transmission by contaminated mouthguards in two football players. Gen. Dent. 2007, 55, 436–440. [Google Scholar]
- Glass, R.; Conrad, R.S.; Kühler, G.A.; Warren, A.J.; Bullard, J.W. Microbiota found in protective athletic mouthguards. Sport Health 2011, 3, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Glass, R.T.; Conrad, R.S.; Wood, R.C.; Warren, A.J.; Kühler, G.A.; Bullard, J.W.; Benson, G.; Gulden, J.M. Protective athletic mouthguards: Do they cause harm? Sport Health 2009, 1, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, W.; Guo, L.; Finnegan, M.; Bradshaw, D.J.; Webster, P.; Loewy, Z.G.; Zhou, X.; Shi, W.; Lux, R. Development of a new model system to study microbial contamination on dentures. J. Prosthodont. 2013, 22, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.T.; Bullard, J.W.; Hadley, C.S.; Mix, E.W.; Conrad, R.S. Partial spectrum of microorganisms found in dentures and possible implications. J. Am. Osteopath. Assoc. 2001, 101, 92–94. [Google Scholar]
- Kumar, A.; Seenivasan, M.K.; Inbarajan, A. A literature review on biofilm formation on silicone and poymethyl methacrylate used for maxillofacial prostheses. Cureus 2021, 13, e20029. [Google Scholar] [CrossRef]
- D’Ercole, S.; Martinelli, D.; Tripodi, D. Influence of sport mouthguards on the ecological factors of the children oral cavity. BMC Oral Health 2014, 14, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ifkovits, T.; Kühl, S.; Connert, T.; Krastl, G.; Dagassan-Berndt, D.; Filippi, A. Prevention of dental accidents in Swiss boxing clubs. Swiss. Dent. J. SSO 2015, 125, 1322–1329. [Google Scholar]
- Mańka-Malara, K.; Łuniewska, J.; Łuniewska, M.; Hovhannisyan, A.; Gawlak, D. Assessment of intraoral mouthguards: Usage and hygiene issues. Protet. Stomatol. 2017, 67, 182–196. [Google Scholar] [CrossRef]
- Tripodi, D.; Cosi, A.; Fulco, D.; D’Erkole, S. The impact of sport training on oral health in athletes. Dent. J. 2021, 9, 51. [Google Scholar] [CrossRef]
- Suzuki, H.; Kawara, M.; Matsubara, Y.; Toshikazu, K.; Komiyama, O.; Asano, T.; Takashi, I. Development of mouthguard cleaning solution. IADR General Session 2010. [Google Scholar]
- Wesołowski, P.; Mańka-Malara, K.; Tokarska, P.; Wojtowicz, A. The assessment of the frequency of mouthguards usage in combat sports. Nowa Stom. 2012, 17, 156–160. [Google Scholar]
- Szerszeń, M.; Cierech, M.; Wojnarowicz, J.; Górski, B.; Mierzwińska-Nastalska, E. Color stability of zinc oxide poly(methyl methacrylate) nanocomposite—A new biomaterial for denture bases. Polymers 2022, 14, 4982. [Google Scholar] [CrossRef] [PubMed]
- Cierech, M.; Szerszeń, M.; Wojnarowicz, J.; Łojkowski, W.; Kostrzewa-Janicka, J.; Mierzwińska-Nastalska, E. Colorimetric study of zinc oxide poly(methyl methacrylate) nanocomposite—New material for denture bases. Protet. Stomatol. 2020, 70, 335–351. [Google Scholar] [CrossRef]
- Gawlak, D.; Mańka-Malara, K.; Mierzwińska-Nastalska, E.; Roman, G.; Kamiński, T.; Łuniewska, M. A comparison of impact force reduction by polymer materials used for mouthguards fabrication. Acta Bioeng. Biomech. 2017, 19, 89–95. [Google Scholar]
- Brogly, M.; Nardin, M.; Schultz, J. Effect of vinylactate content on crystallinity and second-order transitions in ethylene-vinylacetate copolymers. J. Appl. Polym. Sci. 1997, 64, 1903–1912. [Google Scholar] [CrossRef]
- Arsac, A.; Carrot, J.; Guilet, J. Determination of primary relaxation temperatures and melting points of ethylene vinyl acetate copolymers. J. Therm. Anal. Calorim. 2000, 61, 681–685. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, W.; Yang, G.; Chen, Q. Studies on the phase structure of ethylene-vinyl acetate copolymers by solid-state 1H and 13C NMR. J. Pol. Sci. Part B Polym. Phys. 2002, 40, 2199–2207. [Google Scholar] [CrossRef]
- Wang, L.; Fang, P.; Ye, C.; Fng, J. Solid-state NMR characterizations on phase structures and molecular dynamics of poly(ethylene-co-vinyl acetate). J. Polym. Sci. B Polym. Phys. 2002, 44, 2864–2879. [Google Scholar] [CrossRef]
- Riberio, Y.J.S.; Delgado, R.Z.R.; Paula-Silva, F.W.G.; Rematal-Valdes, B.; Feres, M.G.; Palma-Dibb, R.G.; Faraoni, J.; Segato, R.A.B.; da Silva, L.A.B.; de Queiroz, A.M.; et al. Sports mouthguards: Contamination, roughness, and chlorhexidine for disinfection—A randomized clinical trial. B. Dent. J. 2021, 32, 66–73. [Google Scholar] [CrossRef]
- D’Ercole, S.; Tieri, M.; Fulco, D.; Martinelli, D.; Tripodi, D. The use of chlorhexidine in mouthguards. J. Biol. Regul. Homeost. Agents 2017, 31, 487–493. [Google Scholar]
- Wood, N.J.; Maddocks, S.E.; Grady, H.J.; Collins, A.M.; Barbour, M.E. Functionalization of ethylene vinyl acetate with antimicrobial chlorhexidine hexametaphosphate nanoparticles. Int. J. Nanomed. 2014, 27, 4145–4152. [Google Scholar]
- Babich, H.; Wurzburger, B.J.; Rubin, Y.L.; Sinesky, M.C.; Blau, L. An in vivo study on the cytotoxicity of chlorhexidine digluconate to human gingival cells. Cell Biol. Toxicol. 1995, 11, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Catalanotto, F.A.; Knopfti, R.U.; Antczak, A.A. Quality-specific taste impairment following the application of chlorhexidine digluconate mouthrinses. J. Clin. Periodontol. 1988, 15, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, M.N.; Gibson, J. Chlorhexidine and hypersensitivity reactions in dentistry. Br. Dent. J. 2012, 213, 547–550. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.R.; Davies, R.; Bradshaw, B.; Ellwood, R.; Simone, A.J.; Robinson, R.; Mukerjee, C.; Petrone, M.E.; Chaknis, P.; Volpe, A.R.; et al. Efficacy of a mouthrinse containing 0.05% cetylpyridinium chloride for the control of plaque and gingivitis: A 6-month clinical study in adults. Compend. Contin. Educ. Dent. 1998, 19, 20–26. [Google Scholar] [PubMed]
- Cortelli, S.C.; Costa, F.O.; Rode Sde, M.; Haas, A.N.; Andrade, A.K.; Pannuti, C.M.; Escobar, E.C.; Almeida, E.R.; Cortelli, J.R.; Pedrazzi, V. Mouthrinse recommendation for prosthodontic patients. Braz. Oral Res. 2014, 28. [Google Scholar] [CrossRef]
- Eugene, J.W.; Naser, S.M.; Al, A.; Cemil, Y. Evaliation of hand sanitizer for on-site disinfection of mouthguards. Adv. Dent. Oral Health 2019, 10, 555796. [Google Scholar]
- Basavarajappa, S.; Al-Kheraif, A.A.; ElSharawy, M.; Vallittu, P.K. Effect of solvent/disinfectant ethanol on the micro-surface structure and properties of multiphase denture polymers. J. Mech. Behav. Biomed. Mater. 2015, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Asad, T.; Watkinson, A.C.; Huggett, R. The effect of disinfecting procedures on flexural properties of denture base acrylics. J. Prosthet. Dent. 1992, 68, 191–195. [Google Scholar] [CrossRef]
- Ogawa, T.; Yamasaki, S.; Honda, M.; Terao, Y.; Kawabata, S.; Maeda, Y. Long-term survival of salivary streptococci on dental devices made of ethylene vinyl acetate. Int. J. Oral Sci. 2012, 4, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, G.; Churei, H.; Takeuchi, Y.; Hayashi, K.; Kanasaki, A.; Yoshida, Y.; Toma, J.; Araie, Y.; Ueno, T. Antibacterial effect of a disinfectant spray for sports mouthguards on Streptococcus sobrinus. Dent. Res. J. 2021, 18, 59. [Google Scholar]
- Nagai, K.; Domon, H.; Oda, M.; Shirai, T.; Ohsumi, T.; Terao, Y.; Arai, Y. Antimicrobial activity of ethylene-vinyl acetate containing bioactive filler against bacteria. Dent. Mater. J. 2017, 36, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mańka-Malara, K.; Panasiewicz, P.; Kacprzyk, M.; Gawryszewska, M.; Mierzwińska-Nastalska, E.; Gawlak, D. The effect of decontamination procedures on elastic polymeric materials used in dental mouthguards fabrication. Acta Bioeng. Biomech. 2019, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, E.E.; Powers, J.M. Properties of custom-made mouth-protector materials. Physician Sportsmed. 1986, 14, 77–84. [Google Scholar] [CrossRef]
- Almeida, M.H.; Ceschim, G.V.; Iorio, N.L.P.P.; Póvoa, H.C.C.; Cajazeira, M.R.R.; Guimarães, G.S.; Antunes, L.S.; Antunes, L.A. Influence of thickness, color and polishing process of ethylene-vinyl-acetate sheets on surface roughness and microorganism adhesion. Dent. Traumatol. 2018, 34, 51–57. [Google Scholar] [CrossRef]
- Szerszeń, M.; Surowiecki, D.; Tyrajski, M. Influence of storage conditions of alginate mass impressions on their spatial dimensions. Protet. Stomatol. 2018, 68, 406–414. [Google Scholar] [CrossRef]
- Szerszeń, M.; Tyrajski, M.; Surowiecki, D.; Poniewierski, W.; Górski, B. Influence of disinfecting agents on the spatial dimensions of alginate mass impressions. Postep. Hig. Med. Dosw. 2020, 74, 36–41. [Google Scholar] [CrossRef]
- Huggett, R.; Brooks, S.C.; Campbell, A.M.; Satuguranathan, R.; Bell, G.A. Evalution of analytical techniques for measurement of denture-base acrylic resin glass-transition temperature. Dent. Mater. 1990, 6, 19–27. [Google Scholar] [CrossRef]
- Vilijanen, E.K.; Skrifvars, M.; Vallittu, P.K. Dendritic copolymers and particulare filler composites for dental applications: Degree of conversion and thermal properties. Dent. Mater. 2007, 23, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- D’Erkole, S.; Tripodi, D.; Ristoldo, F.; Quaranta, F.; Amaddeo, P. Analysis of oral health status and of salivary factors in young soccer players: A pilot study. Med. Dello Sport 2013, 66, 71–80. [Google Scholar]
- D’Erkole, S.; Tripodi, D. The effect of swimming on oral ecological factors. J. Biol. Regul. Homeost. Agents 2013, 2, 551–558. [Google Scholar]
- Gawlak, D.; Mierzwińska-Nastalska, E.; Mańka-Malara, K.; Kamiński, T. Assessment of custom and standard self-adapted mouthguards in terms of comfort and users subjective impressions of their protective function. Dent. Traumatol. 2015, 31, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, S.; Ye, H.; Lv, L.; Zhao, X.; Liu, Y.; Zhou, Y. Preliminary clinical application of complete workflow of digitally designed and manufactured sport mouthguards. Int. J. Prosthodont. 2020, 33, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Simone, C.; Brambilla, D.; Leroux, J.C. 3D printing of wereable personalized oral delivery device: A first-human-study. Sci. Adv. 2018, 9, eeat2544. [Google Scholar] [CrossRef] [Green Version]
- Tun, P.S.; Churei, H.; Hikita, K.; Kamijo, S.; Oki, M.; Tanabe, G.; Hayashi, K.; Aung, T.K.; Win, A.; Hlaing, S.; et al. Fabrication of Shock Absorbing Photopolymer Composite Material for 3D Printing Sports Mouthguard. J. Photopolym. Sci. Technol. 2020, 33, 615–622. [Google Scholar] [CrossRef]
- Gisolfi, C.V.; Duchman, S.M. Guidelines for optimal replacement beverages for different athletic events. Med. Sci. Sport Exerc. 1992, 24, 679–687. [Google Scholar] [CrossRef]
- Shirreffs, S.M.; Sawka, M.N. Fluid and electrolyte needs for training, competition, and recovery. J. Sport Sci. 2011, 29, 39–46. [Google Scholar] [CrossRef]
- Gonzales-Alonso, J.; Mora-Rodriguez, R.; Below, P.R.; Coyle, E.F. Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. J. Appl. Physiol. 1995, 79, 1487–1496. [Google Scholar] [CrossRef]
- Sawka, M.N.; Noakes, T.D. Does dehydration impair exercise performance? Med. Sci. Sport Exerc. 2007, 39, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Goulet, E.D. Effect of exercise-induced dehydration on time-trial exercise performance: A meta-analysis. Br. J. Sport Med. 2011, 45, 1149–1156. [Google Scholar] [CrossRef]
- James, L.J.; Moss, J.; Henry, J.; Papadopoulou, C.; Mears, S.A. Hypohydration impairs endurance performance: A blinded study. Physiol. Rep. 2017, 5, e13315. [Google Scholar] [CrossRef]
- Geraldini, S.; de Freitas Cruz, I.; Romero, A.; Fonseca, F.L.A.; de Campos, M.P. Isotonic sports drink promotes rehydration and decreases proteinuria following karate training. Braz. J. Nephrol. 2017, 39, 362–369. [Google Scholar] [CrossRef]
- Da Fonte Porto Carreiro, A.; Dos Santos Cruz, C.A.; Vergani, C.E. Hardness and compressive strength of indirect composite resins: Effect of immersion in distilled water. J. Oral Rehabil. 2004, 31, 1085–1089. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.I.; Chen, Y.Y.; Jiang, Q.S. Long-term hygroscopic dimensional changes of core buildup materials in deionized water and artificial saliva. Dent. Mater. J. 2021, 31, 143–149. [Google Scholar] [CrossRef]
- Goring, D.C.; Maletz, R.; Ottl, P.; Warkentin, M. Influence of artificial aging: Mechanical and physicochemical properties of dental composites under static and dynamic compression. Clin. Oral Investig. 2022, 26, 1491–1504. [Google Scholar] [CrossRef]
- Mayworm, C.D.; Camargo, S.S.; Bastian, F.L. Influence of artificial saliva on abraxive wear and microhardness of dental composites filled with nanoparticles. J. Dent. 2008, 36, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.W.; Darvell, B.W. Artificial salivas for in vitro studies of dental materials. J. Dent. 1997, 25, 475–484. [Google Scholar] [CrossRef]
- Arakawa, T.; Tomoto, K.; Nitta, H.; Toma, K.; Takeuchi, S.; Sekita, T.; Minakuchi, S.; Mitsubayashi, K. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 2020, 92, 12201–12207. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.I.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 15, 106–111. [Google Scholar] [CrossRef] [Green Version]


| Oshee Red Orange Flavour | Oshee Lemon Flavour | Oshee Multifruit Flavour |
|---|---|---|
| water | water | water |
| glucose | glucose | glucose |
| citric acid | maltodextrin | maltodextrin |
| sodium citrate | citric acid | citric acid |
| potassium citrates | trisodium citrates | sodium citrates |
| flavours | tripotassium citrates | potassium citrates |
| potassium sorbate | flavours | flavours |
| potassium benzoate | potassium sorbate | potassium sorbate |
| gum arabic | potassium benzoate | potassium benzoate |
| glycerol esters of wood rosins | gum arabic | gum arabic |
| sucralose | glycerol esters of wood rosins | glycerol esters of wood rosins |
| Allura Red AC | aspartame | aspartame |
| niacine | acesulfame K | acesulfame K |
| vitamin B | guinoline yellow | brilliant blue FCF |
| biotin | niacine | niacine |
| vitamin B | vitamin B | |
| biotin | biotin |
| Abbreviation | Group Description |
|---|---|
| R-CHX | red samples disinfected with Perio-AID |
| R-AL | red samples disinfected with Bioseptol AMF |
| R-CT | red samples control group (without disinfection) |
| R-MG | red samples disinfected with Safe JAWZ spray |
| B-CHX | blue samples disinfected with Perio-AID |
| B-AL | blue samples disinfected with Bioseptol AMF |
| B-CT | blue samples control group (without disinfection) |
| B-MG | blue samples disinfected with Safe JAWZ spray |
| G-CHX | green samples disinfected with Perio-AID |
| G-AL | green samples disinfected with Bioseptol AMF |
| G-CT | green samples control group (without disinfection) |
| G-MG | green samples disinfected with Safe JAWZ spray |
| W-CHX | white samples disinfected with Perio-AID |
| W-AL | white samples disinfected with Bioseptol AMF |
| W-CT | white samples control group (without disinfection) |
| W-MG | white samples disinfected with Safe JAWZ spray |
| Abbreviation | Group Description |
|---|---|
| R-1 | red samples immersed in Oshee Red Orange Flavour |
| R-2 | red samples immersed in Oshee Lemon Flavour |
| R-3 | red samples immersed in Oshee Multifruit Flavour |
| R-4 (control) | red samples immersed in water (control group) |
| B-1 | blue samples immersed in Oshee Red Orange Flavour |
| B-2 | blue samples immersed in Oshee Lemon Flavour |
| B-3 | blue samples immersed in Oshee Multifruit Flavour |
| B-4 (control) | blue samples immersed in water (control group) |
| G-1 | green samples immersed in Oshee Lemon Flavour |
| G-2 | green samples disinfected with Bioseptol AMF |
| G-3 | green samples immersed in Oshee Multifruit Flavour |
| G-4 (control) | green samples immersed in water (control group) |
| W-1 | white samples immersed in Oshee Red Orange Flavour |
| W-2 | white samples immersed in Oshee Lemon Flavour |
| W-3 | white samples immersed in Oshee Multifruit Flavour |
| W-4 (control | white samples immersed in water (control group) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mańka-Malara, K.; Szerszeń, M.; Górski, B.; Tanabe, G.; Ueno, T.; Mierzwińska-Nastalska, E. Disinfection and Isotonic Drinks’ Influence on Hardness and Color Stability of Ethylene-Vinyl-Acetate Copolymer Mouthguards Used in Martial Arts: An In Vitro Study. Polymers 2023, 15, 1822. https://doi.org/10.3390/polym15081822
Mańka-Malara K, Szerszeń M, Górski B, Tanabe G, Ueno T, Mierzwińska-Nastalska E. Disinfection and Isotonic Drinks’ Influence on Hardness and Color Stability of Ethylene-Vinyl-Acetate Copolymer Mouthguards Used in Martial Arts: An In Vitro Study. Polymers. 2023; 15(8):1822. https://doi.org/10.3390/polym15081822
Chicago/Turabian StyleMańka-Malara, Katarzyna, Marcin Szerszeń, Bartłomiej Górski, Gen Tanabe, Toshiaki Ueno, and Elżbieta Mierzwińska-Nastalska. 2023. "Disinfection and Isotonic Drinks’ Influence on Hardness and Color Stability of Ethylene-Vinyl-Acetate Copolymer Mouthguards Used in Martial Arts: An In Vitro Study" Polymers 15, no. 8: 1822. https://doi.org/10.3390/polym15081822






