Performance Optimization of FA-GGBS Geopolymer Based on Response Surface Methodology
Abstract
:1. Introduction
2. Raw Materials
3. Experimental Design
4. Specimen Preparation and Test Methods
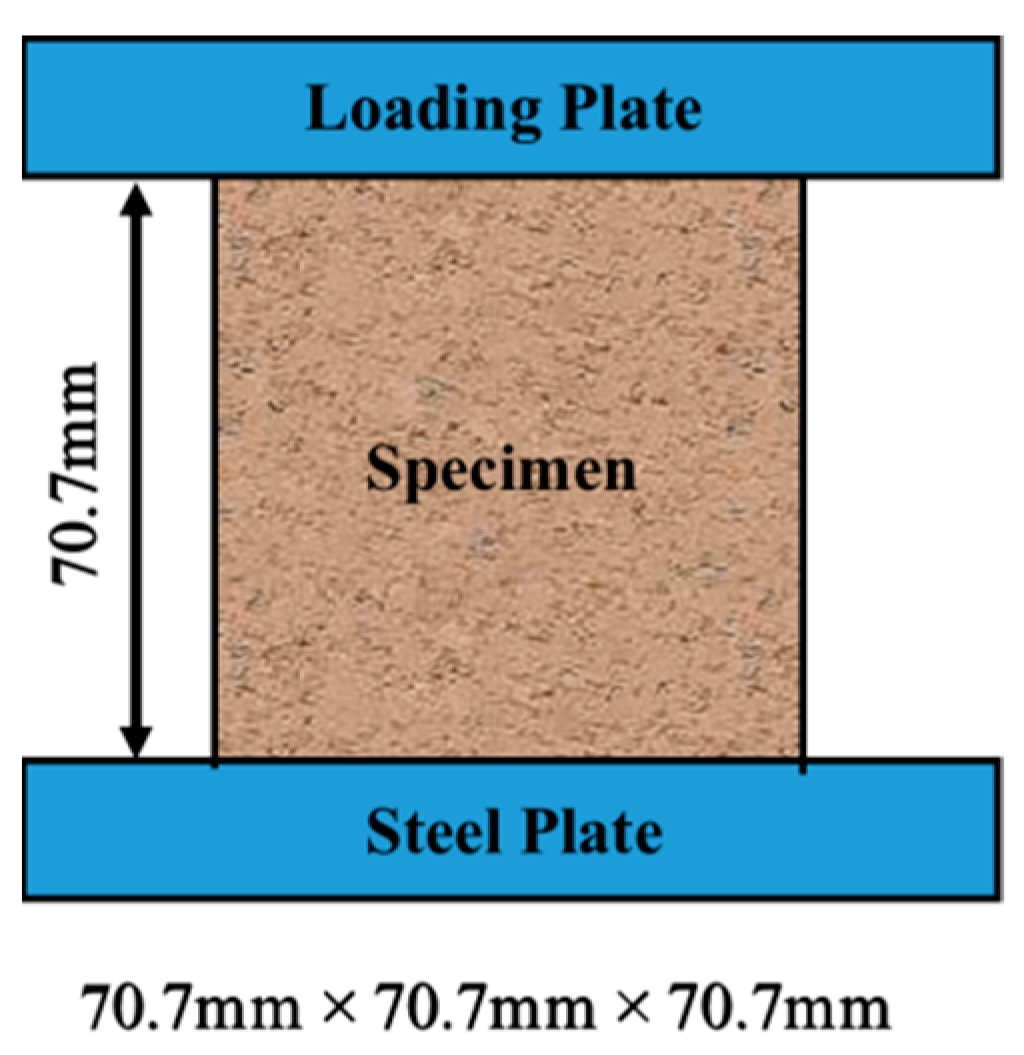
4.1. Specimen Preparation
4.2. Test Methodology
5. Results and Analysis
5.1. Experimental Results and Model Analysis
5.2. Establishment of the Regression Equation and Merit Search Test
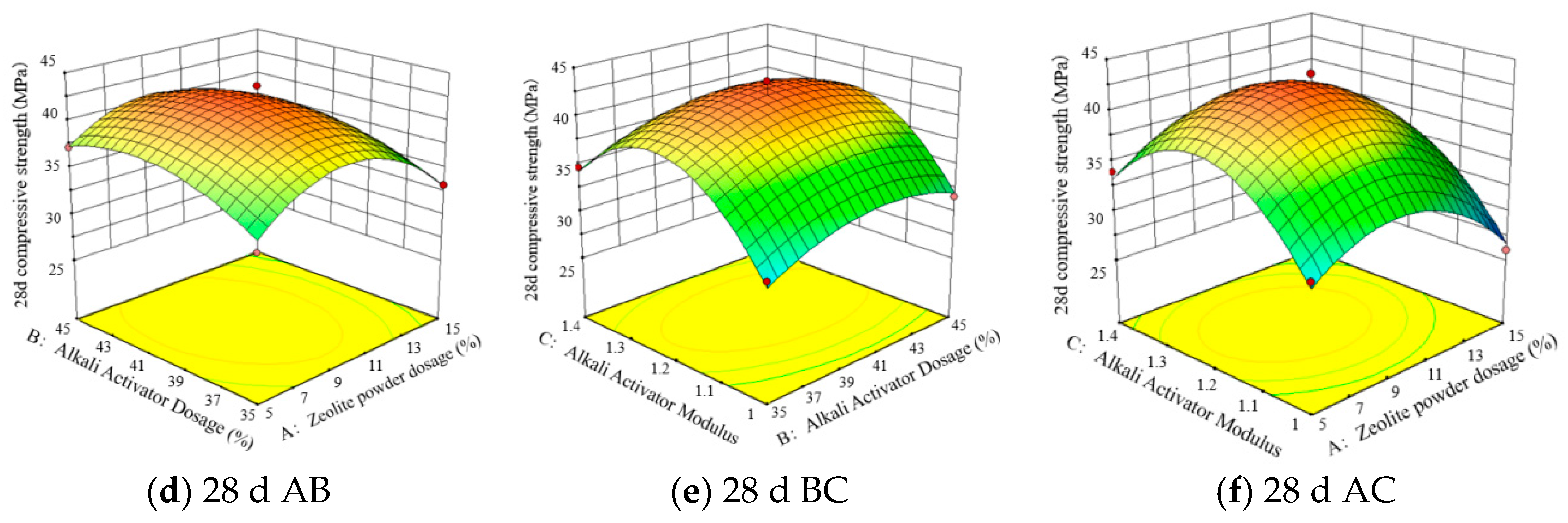
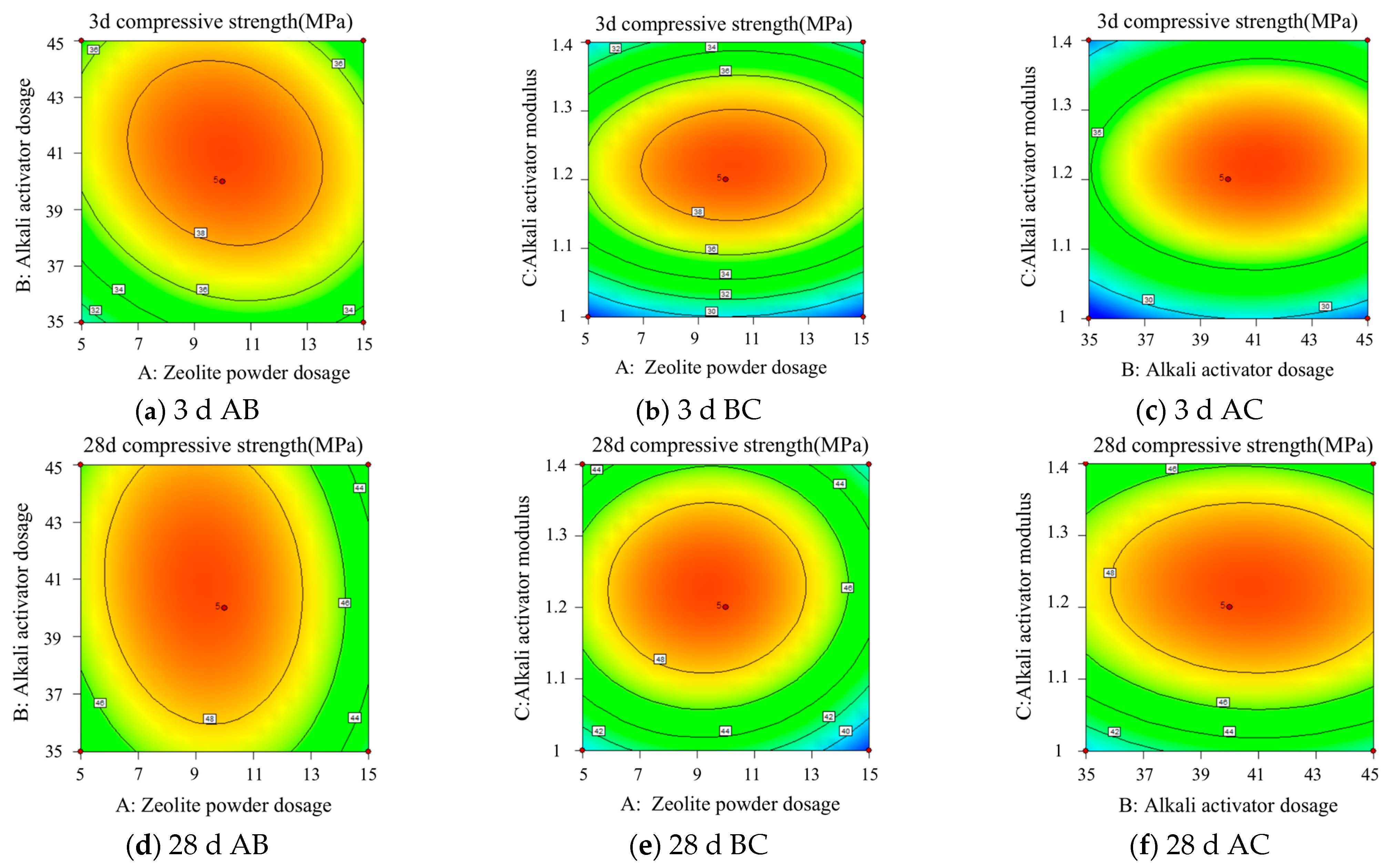
5.3. Response Surface Interaction Analysis
6. Micro-Mechanical Analysis
6.1. Scanning Electron Microscopy Analysis
6.2. X-ray Diffraction Analysis
6.3. Fourier Transform Infrared Spectroscopy
6.4. 29Si Nuclear Magnetic Resonance Analysis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghanbari, M.; Hadian, A.M.; Nourbakhsh, A.A.; MacKenzie, K.J.D. Modeling and optimization of compressive strength and bulk density of metakaolin-based geopolymer using central composite design: A numerical and experimental study. Ceram. Int. 2017, 43, 324–335. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Guo, X.; Pan, X. Effects of steel slag on mechanical properties and mechanism of fly ash–based geopolymer. J. Mater. Civ. Eng. 2020, 32, 04019348. [Google Scholar] [CrossRef]
- Parathi, S.; Nagarajan, P.; Pallikkara, S.A. Ecofriendly geopolymer concrete: A comprehensive review. Clean Technol. Env. Policy 2021, 23, 1701–1713. [Google Scholar] [CrossRef]
- Buyondo, K.A.; Olupot, P.W.; Kirabira, J.B.; Yusuf, A.A. Optimization of the production parameters of Rice Husk Ash geopolymer cement using Response Surface Methodology. Case Stud. Constr. Mater. 2020, 13, e00461. [Google Scholar] [CrossRef]
- Shehata, N.; Mohamed, O.A.; Sayed, E.T.; Abdelkareem, M.A.; Olabi, A.G. Geopolymer concrete as green building materials: Recent applications, sustainable development and circular economy potentials. Sci. Total Environ. 2022, 836, 155577. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Xia, J.; Wu, R.-J.; Shen, X.-Y.; Chen, J.-J.; Zhao, Y.-X.; Jin, W.-L. An overview on the influence of various parameters on the fabrication and engineering properties of CO2-cured cement-based composites. J. Clean. Prod. 2022, 366, 132968. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, R.; Lin, Z.W.; Zhang, M.; Fu, Z.S. Experimental study on setting time and mechanical properties of polymer mortar based on fly ash-slag base. New Build. Mater. 2021, 48, 138–144. [Google Scholar]
- Chen, Y.L.; Zeng, H.; Li, T.Y.; Liu, Z.F. Development and application of metakaolin, fly ash and slag geopolymer grouting materials. Highw. Eng. 2021, 46, 142–147. [Google Scholar]
- Lei, J.; Fu, J.; Yang, E.-H. Alkali-silica reaction resistance and pore solution composition of low-calcium fly ash-based geopolymer concrete. Infrastructures 2020, 5, 96. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Chen, H.; Zhang, G.; Yao, R.; Pan, C.; Zhang, Z. Development of low-calcium fly ash-based geopolymer mortar using nanosilica and hybrid fibers. Ceram. Int. 2021, 47, 21791–21806. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Lu, M.; Chen, B.; Gao, S.; Bai, J.; Zhang, H.; Yang, Y. Study on the effect of calcium and sulfur content on the properties of fly ash based geopolymer. Constr. Build. Mater. 2022, 314, 125650. [Google Scholar] [CrossRef]
- Tay, C.H.; Mazlan, N.; Wayayok, A.; Basri, M.S.; Mustafa, M.; Abdullah, A. Nanocellulose reinforced zeolite based geopolymer concrete: Density analysis through response surface methodology. Mater. Today Proc. 2022, 66, 2873–2882. [Google Scholar] [CrossRef]
- Shahmansouri, A.A.; Nematzadeh, M.; Behnood, A. Mechanical properties of GGBFS-based geopolymer concrete incorporating natural zeolite and silica fume with an optimum design using response surface method. J. Build. Eng. 2021, 36, 102138. [Google Scholar] [CrossRef]
- Özen, S.; Alam, B. Compressive strength and microstructural characteristics of natural zeolite-based geopolymer. Period. Polytech. Civ. Eng. 2018, 62, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhu, H.; Li, H.; Zhu, H.; Gao, M. Application of response surface methodology in the optimization of fly ash geopolymer concrete. Rev. Romana De Mater. 2018, 48, 45–52. [Google Scholar]
- Oyebisi, S.O.; Ede, A.N.; Olutoge, F.A. Optimization of design parameters of slag-corncob ash-based geopolymer concrete by the central composite design of the response surface methodology. Iran. J. Sci. Technol. Trans. Civ. Eng. 2021, 45, 27–42. [Google Scholar] [CrossRef]
- Zahid, M.; Shafiq, N.; Isa, M.H.; Gil, L. Statistical modeling and mix design optimization of fly ash based engineered geopolymer composite using response surface methodology. J. Clean. Prod. 2018, 194, 483–498. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Zhang, Z.; Pan, C.; Shen, X.; Xia, L.; Zang, J. Modeling and optimization of fly ash–slag-based geopolymer using response surface method and its application in soft soil stabilization. Constr. Build. Mater. 2022, 315, 125723. [Google Scholar] [CrossRef]
- Singh, P.; Kapoor, K. Development of mix design method based on statistical analysis of different factors for geopolymer concrete. Front. Struct. Civ. Eng. 2022, 16, 1315–1335. [Google Scholar] [CrossRef]
- Quiatchon, P.R.J.; Dollente, I.J.R.; Abulencia, A.B.; Libre, R.G.D.G., Jr.; Villoria, M.B.D.; Guades, E.J.; Promentilla, M.A.B.; Ongpeng, J.M.C. Investigation on the compressive strength and time of setting of low-calcium fly ash geopolymer paste using response surface methodology. Polymers 2021, 13, 3461. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, H.; Zhu, H. Preparation of fly ash geopolymer concrete based on response surface methodology. J. Saf. Environ. 2018, 18, 296–300. [Google Scholar]
- Guanji, L.V.; Tao, J.I. Mechanical Properties of Terpolymer Mortar Based on Response Surface Method. J. Build. Mater. 2021, 24, 970–976. [Google Scholar]
- Mermerdaş, K.; Algın, Z.; Oleiwi, S.M.; Nassani, D.E. Optimization of lightweight GGBFS and FA geopolymer mortars by response surface method. Constr. Build. Mater. 2017, 139, 159–171. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.; Wang, X.; Zhang, T.; Wang, Q. Response Surface Methodology for multi-objective optimization of fly ash-GGBS based geopolymer mortar. Constr. Build. Mater. 2022, 315, 125644. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, J.; Kaur, M. Compressive strength of rice husk ash based geopolymer: The effect of alkaline activator. Constr. Build. Mater. 2018, 169, 188–192. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Elsayed, H.A.; AbdelMoied, S. Geopolymer synthesis by the alkali-activation of blastfurnace steel slag and its fire-resistance. Hbrc J. 2018, 14, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag. mechanical strength and paste characterisation. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Prusty, J.K.; Pradhan, B. Multi-response optimization using Taguchi-Grey relational analysis for composition of fly ash-ground granulated blast furnace slag based geopolymer concrete. Constr. Build. Mater. 2020, 241, 118049. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Q.; Liu, Y.; Xue, T.; Yu, Q.; Chen, S. Influence of new organic alkali activators on microstructure and strength of fly ash geopolymer. Ceram. Int. 2022, 48, 12442–12449. [Google Scholar] [CrossRef]
- Nagajothi, S.; Elavenil, S. Effect of GGBS addition on reactivity and microstructure properties of ambient cured fly ash based geopolymer concrete. Silicon 2021, 13, 507–516. [Google Scholar] [CrossRef]
- Xu, G.; Zhong, J.; Shi, X. Influence of graphene oxide in a chemically activated fly ash. Fuel 2018, 226, 644–657. [Google Scholar] [CrossRef]
- Gupta, R.; Tomar, A.S.; Mishra, D.; Sanghi, S.K. Multinuclear MAS NMR Characterization of Fly-Ash-Based Advanced Sodium Aluminosilicate Geopolymer: Exploring Solid-State Reactions. ChemistrySelect 2020, 5, 4920–4927. [Google Scholar] [CrossRef]
- Moutaoukil, G.; Sobrados, I.; Alehyen, S. Study of thermomechanical properties of foamed and unfoamed fly ash-based geopolymer using FT Raman spectroscopy and 29Si MAS NMR. Mater. Lett. 2023, 330, 133261. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; He, X.; Su, Y.; Zeng, J.; Xiong, L.; Zeng, L.; Yu, X.; Tan, H. Low-carbon wet-ground fly ash geopolymer activated by single calcium carbide slag. Constr. Build. Mater. 2022, 353, 129084. [Google Scholar] [CrossRef]
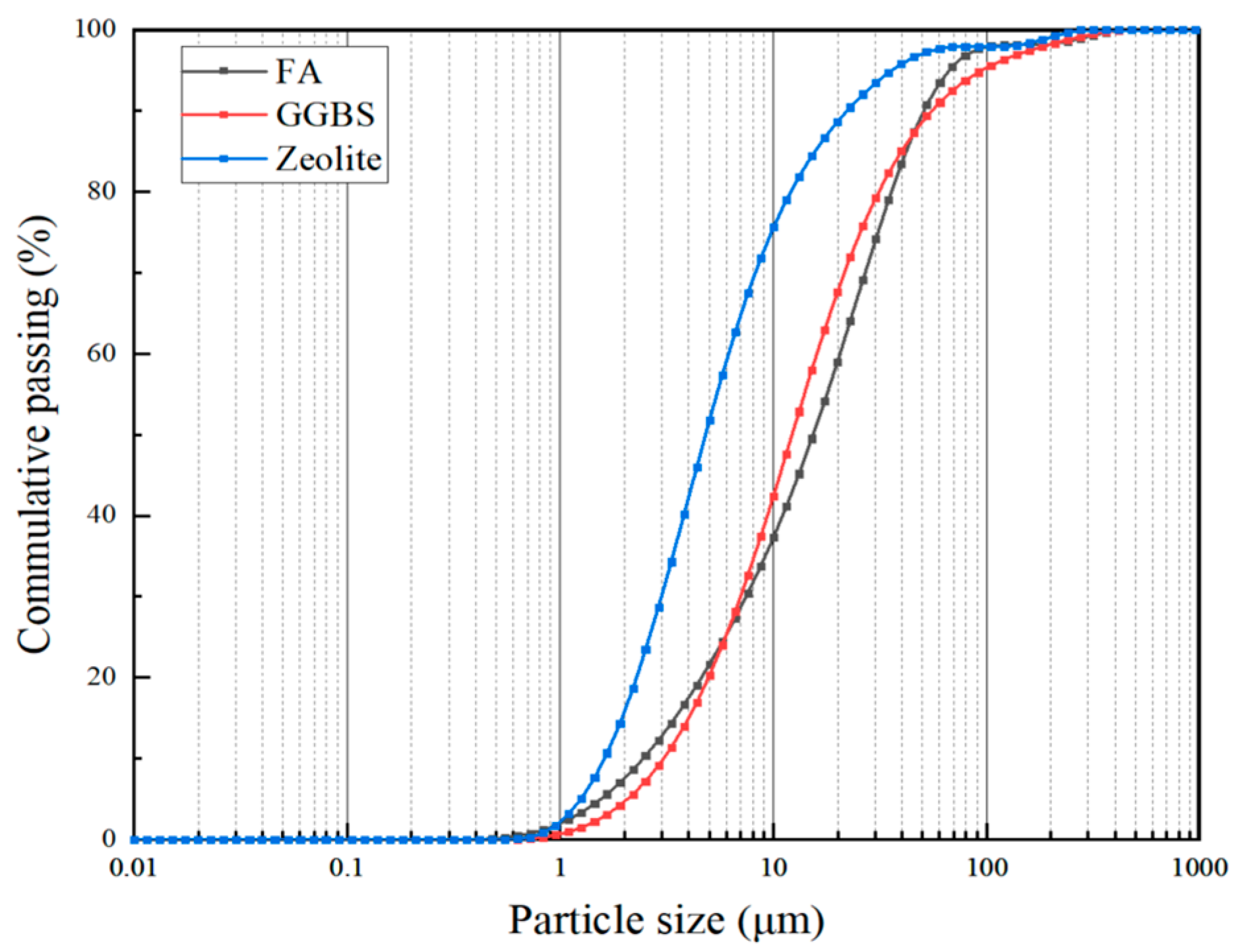





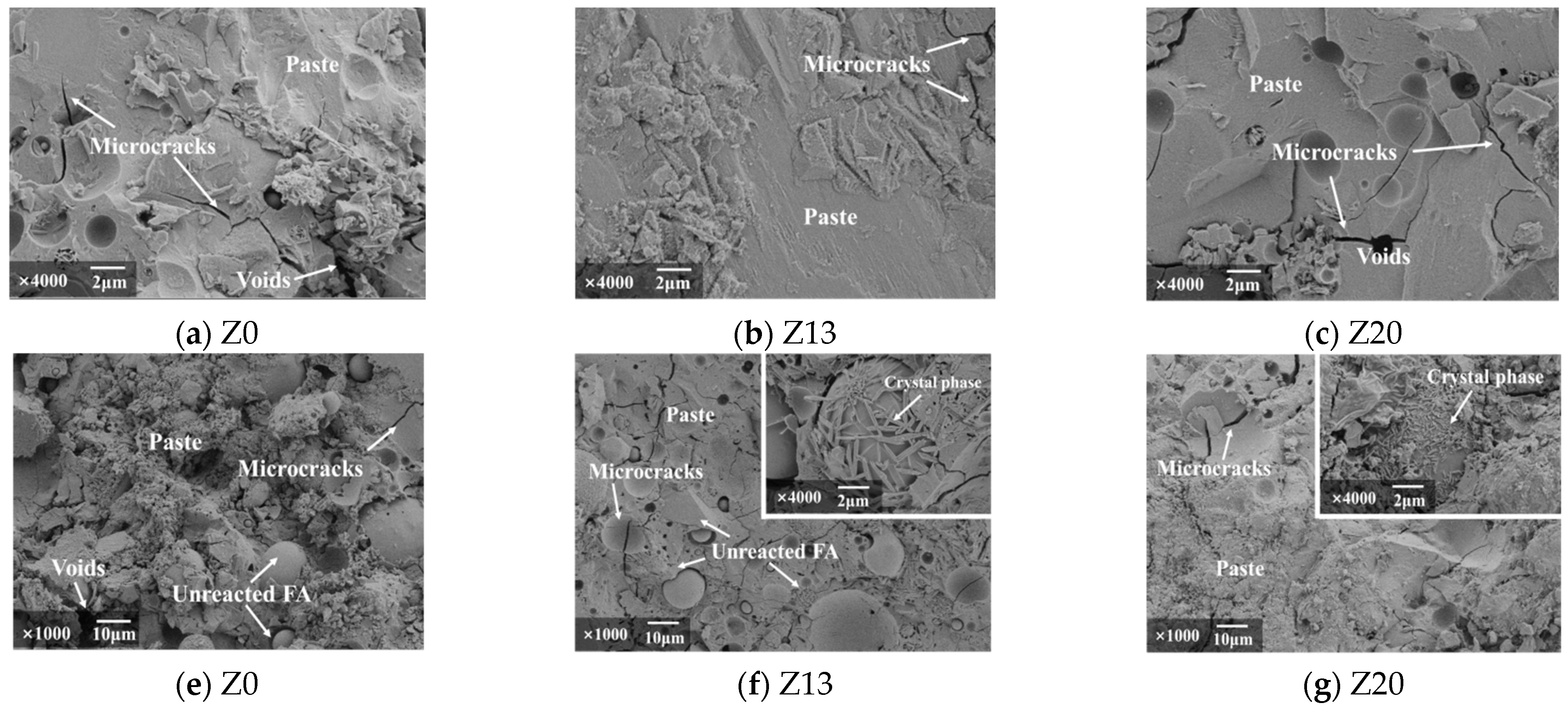
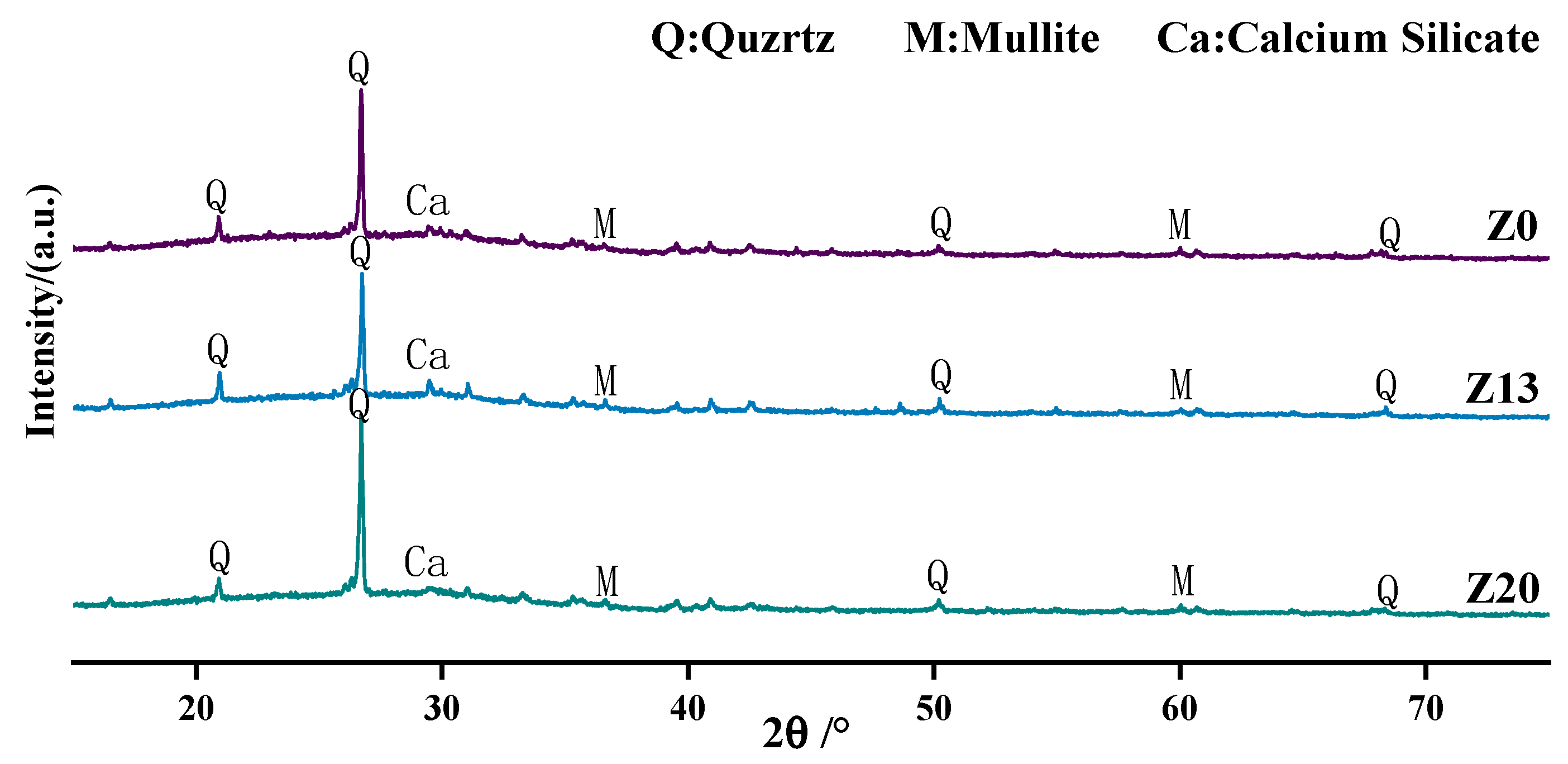

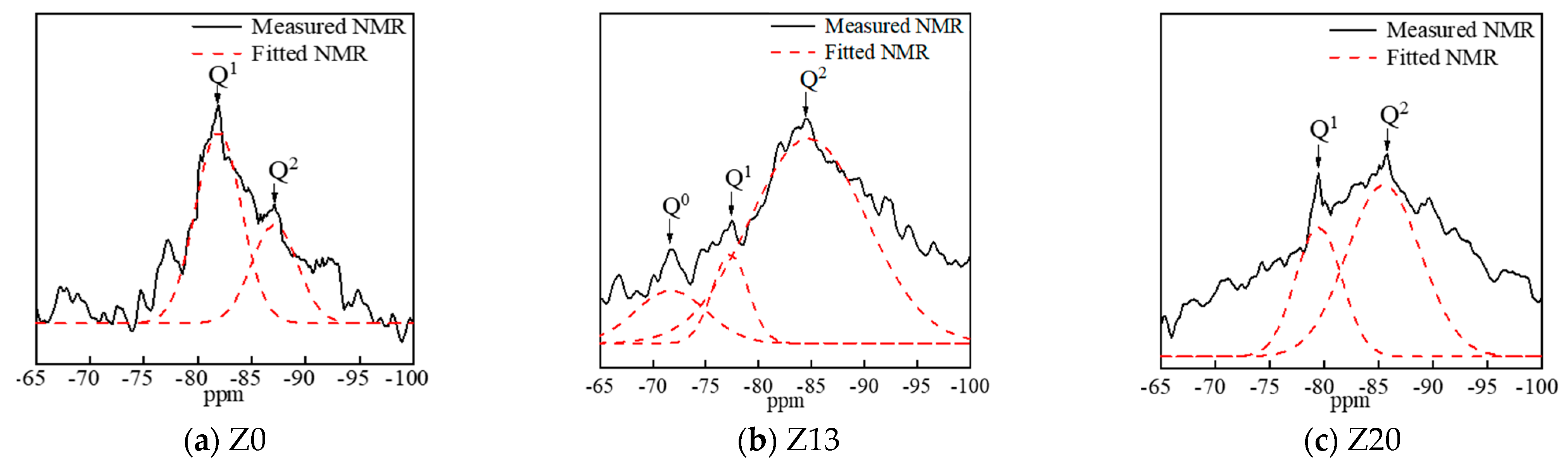
| Chemical Component | Al2O3 | SiO2 | Fe2O3 | CaO | MgO | TiO2 | K2O | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|
| FA | 23.67 | 56.96 | 4.63 | 1.50 | 1.50 | 2.86 | 1.73 | 7.15 |
| GGBS | 16.32 | 36.10 | 1.28 | 30.58 | 9.32 | 2.94 | 0.53 | 2.93 |
| Zeolite | 15.47 | 68.90 | 0.95 | 2.32 | 1.95 | 0.09 | 2.16 | 8.16 |
| Modulus | Na2O (wt%) | SiO2 (wt%) | H2O (wt%) |
|---|---|---|---|
| 3.3 | 8.3 | 26.5 | 65.2 |
| Sample | Independent Variable | Compressive Strength (MPa) | |||
|---|---|---|---|---|---|
| A | B | C | 3 d | 28 d | |
| 1 | 5 | 0.35 | 1.2 | 29.5 | 42.8 |
| 2 | 15 | 0.35 | 1.2 | 32.3 | 42.6 |
| 3 | 5 | 0.45 | 1.2 | 36.2 | 45.8 |
| 4 | 15 | 0.45 | 1.2 | 35.5 | 43.7 |
| 5 | 5 | 0.40 | 1 | 27.4 | 40.6 |
| 6 | 15 | 0.40 | 1 | 26.8 | 36.8 |
| 7 | 5 | 0.40 | 1.4 | 30.2 | 43.2 |
| 8 | 15 | 0.40 | 1.4 | 30.9 | 40.5 |
| 9 | 10 | 0.35 | 1 | 27.3 | 40.4 |
| 10 | 10 | 0.45 | 1 | 26.8 | 41.3 |
| 11 | 10 | 0.35 | 1.4 | 29.6 | 43.8 |
| 12 | 10 | 0.45 | 1.4 | 30.3 | 43.7 |
| 13 | 10 | 0.40 | 1.2 | 39.3 | 49.5 |
| 14 | 10 | 0.40 | 1.2 | 37.9 | 49.3 |
| 15 | 10 | 0.40 | 1.2 | 38.7 | 49.5 |
| 16 | 10 | 0.40 | 1.2 | 39.7 | 50.9 |
| 17 | 10 | 0.40 | 1.2 | 40.2 | 49.7 |
| Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R-Squared | Predicted R-Squared | Evaluate | |
|---|---|---|---|---|---|---|
| 3 d | Linear | 0.7531 | 0.0010 | −0.1260 | −0.4209 | |
| 2FI | 0.9903 | 0.0005 | −0.4481 | −1.5958 | ||
| Quadratic | <0.0001 | 0.0729 | 0.9194 | 0.6828 | Suggested | |
| Cubic | 0.0729 | 0.9676 | ||||
| 28 d | Linear | 0.6495 | 0.0006 | −0.0895 | −0.3220 | |
| 2FI | 0.9958 | 0.0003 | −0.4077 | −1.3132 | ||
| Quadratic | <0.0001 | 0.1644 | 0.9556 | 0.7772 | Suggested | |
| Cubic | 0.1644 | 0.9756 |
| Source | DOF | Mean Square | F Value | p Value | |||
|---|---|---|---|---|---|---|---|
| 3d | 28d | 3d | 28d | 3d | 28d | ||
| Model | 9 | 42.00 | 29.41 | 18.84 | 39.24 | 0.0004 | <0.0001 |
| A | 1 | 0.61 | 9.68 | 0.27 | 12.92 | 0.6185 | 0.0088 |
| B | 1 | 12.75 | 3.00 | 5.72 | 4.01 | 0.0481 | 0.0855 |
| C | 1 | 20.16 | 18.30 | 9.04 | 24.42 | 0.0197 | 0.0017 |
| AB | 1 | 3.06 | 0.90 | 1.37 | 1.20 | 0.2795 | 0.3088 |
| AC | 1 | 0.42 | 0.30 | 0.19 | 0.40 | 0.6764 | 0.5454 |
| BC | 1 | 0.36 | 0.25 | 0.16 | 0.33 | 0.6998 | 0.5816 |
| A2 | 1 | 31.38 | 68.72 | 14.08 | 91.71 | 0.0072 | <0.0001 |
| B2 | 1 | 39.30 | 17.10 | 17.63 | 22.81 | 0.0040 | 0.0020 |
| C2 | 1 | 243.52 | 125.75 | 109.24 | 167.81 | <0.0001 | <0.0001 |
| Residual | 7 | 2.23 | 0.75 | ||||
| Lack of Fit | 3 | 4.14 | 1.20 | 5.18 | 2.91 | 0.0729 | 0.1644 |
| Pure Error | 4 | 0.80 | 0.41 | ||||
| Group | Std. Dev. (MPa) | Mean /MPa | R2 | Adjusted R2 | Predicted R2 | Press | C.V. (%) | Adeq Precision |
|---|---|---|---|---|---|---|---|---|
| 3 d | 1.49 | 32.86 | 0.9604 | 0.9194 | 0.6828 | 198.74 | 4.05 | 11.536 |
| 28 d | 0.87 | 44.36 | 0.9806 | 0.9556 | 0.7772 | 60.14 | 1.95 | 18.665 |
| Sample | A (%) | B (%) | C |
|---|---|---|---|
| Z0 | 0 | 40 | 1.2 |
| Z13 | 13 | 40 | 1.2 |
| Z20 | 20 | 40 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Wang, J.; Miao, T.; Chen, K.; Zhang, Z. Performance Optimization of FA-GGBS Geopolymer Based on Response Surface Methodology. Polymers 2023, 15, 1881. https://doi.org/10.3390/polym15081881
Wu D, Wang J, Miao T, Chen K, Zhang Z. Performance Optimization of FA-GGBS Geopolymer Based on Response Surface Methodology. Polymers. 2023; 15(8):1881. https://doi.org/10.3390/polym15081881
Chicago/Turabian StyleWu, Dazhi, Junyi Wang, Tong Miao, Keyu Chen, and Zilong Zhang. 2023. "Performance Optimization of FA-GGBS Geopolymer Based on Response Surface Methodology" Polymers 15, no. 8: 1881. https://doi.org/10.3390/polym15081881
APA StyleWu, D., Wang, J., Miao, T., Chen, K., & Zhang, Z. (2023). Performance Optimization of FA-GGBS Geopolymer Based on Response Surface Methodology. Polymers, 15(8), 1881. https://doi.org/10.3390/polym15081881






