Phormidium ambiguum and Leptolyngbya ohadii Exopolysaccharides under Low Water Availability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cyanobacteria Origin and Growth Conditions
2.2. Inoculation and Biocrust Sampling
2.3. Biofilm Formation in Liquid Culture Medium
2.4. Biocrusts and Biofilm Characterization
2.4.1. Growth Measurements through Chlorophyll a
2.4.2. EPS Isolation, Quantification and Characterization
2.5. Data Analysis
3. Results
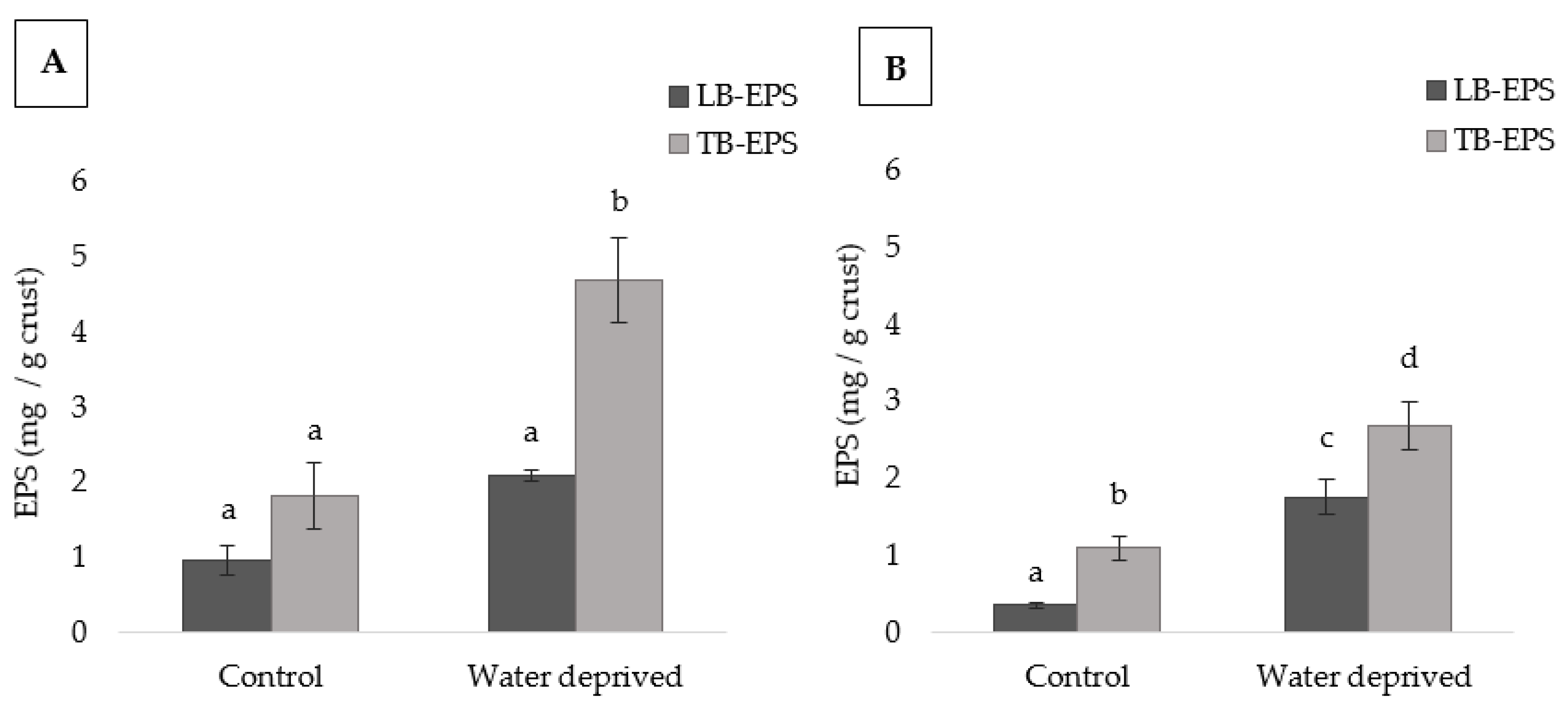
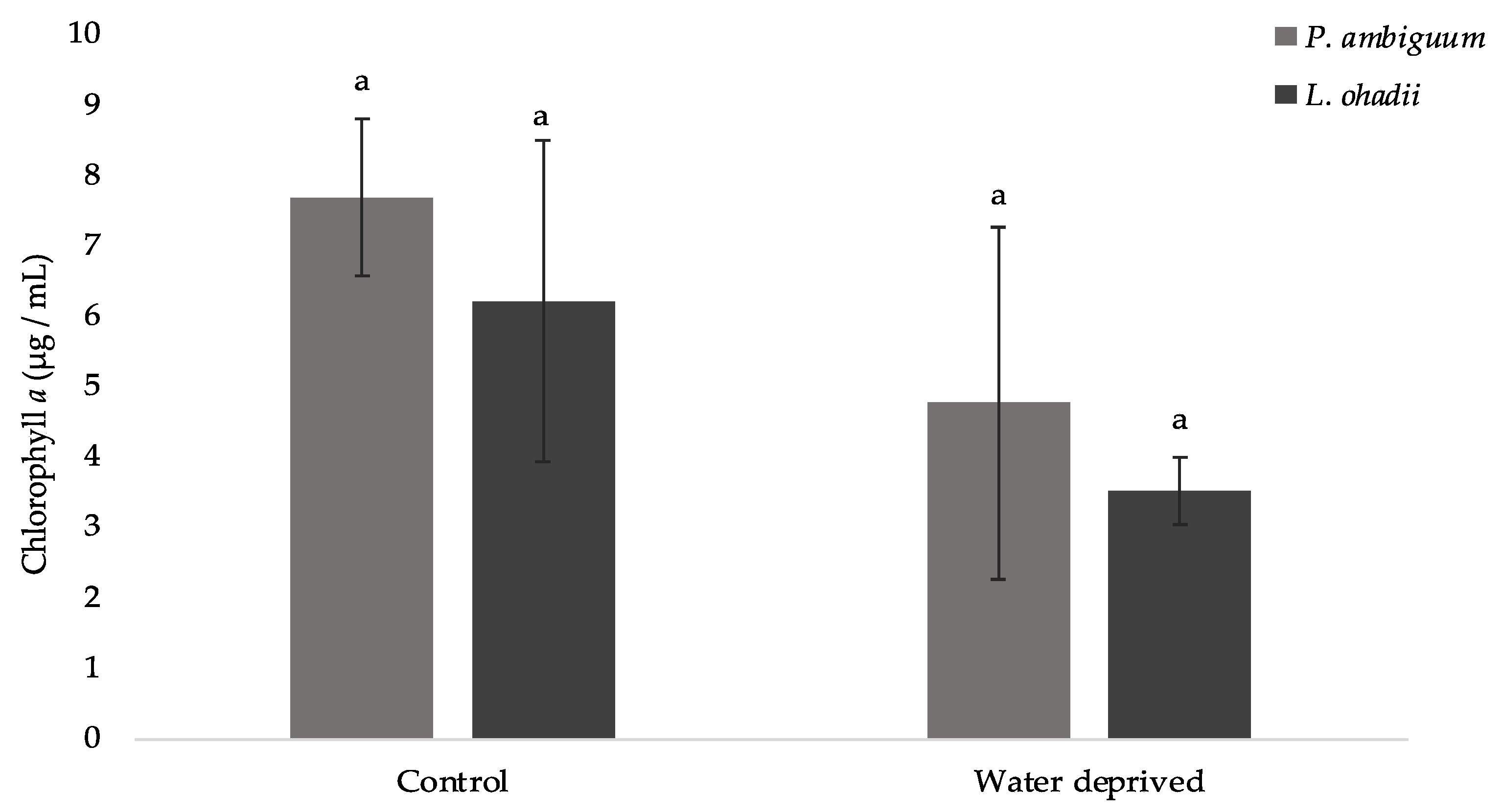
3.1. Effect of Water Deprivation on Chlorophyll a and EPS Production in Sandy Microcosms
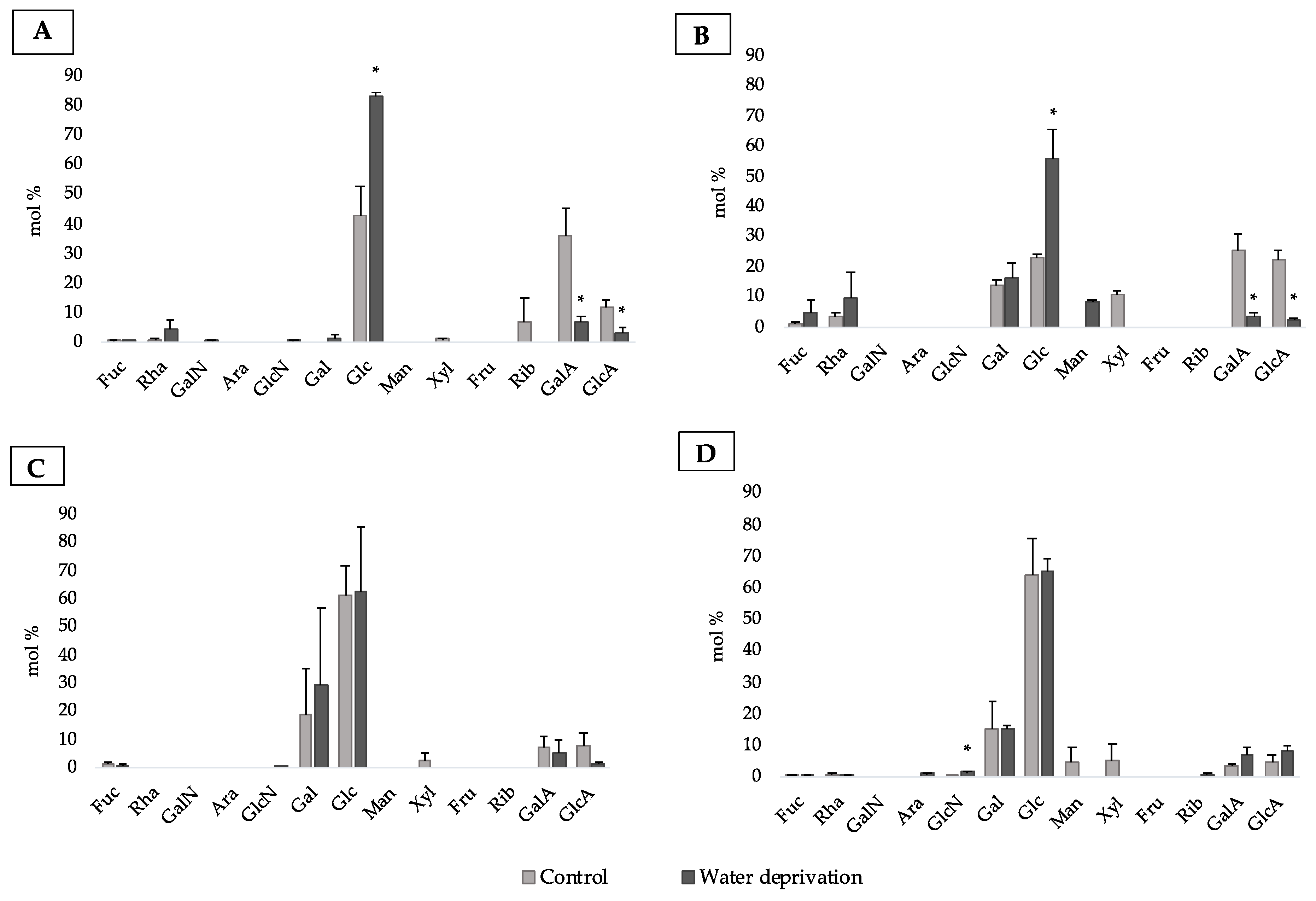
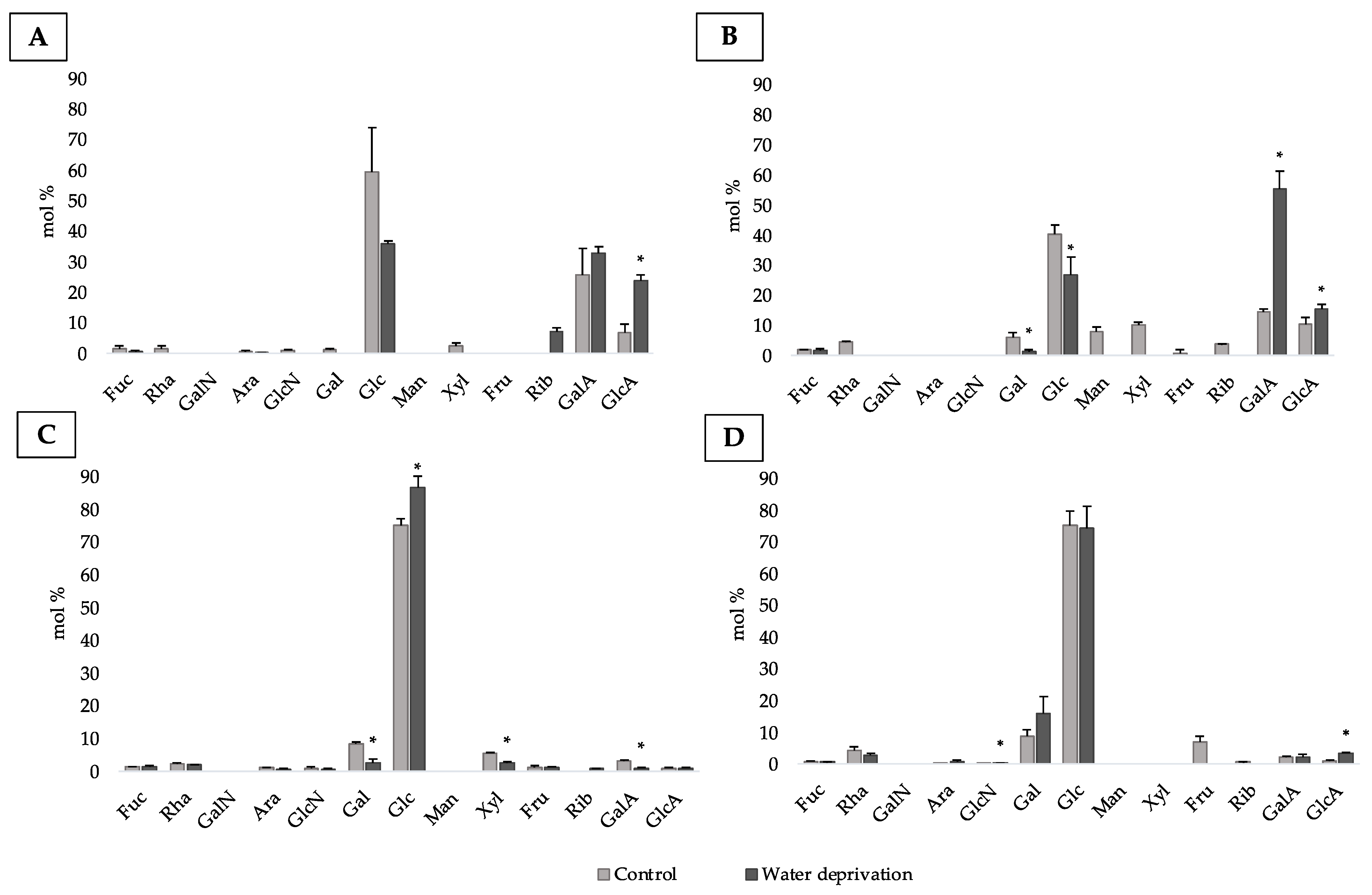
3.2. Changes in Monosaccharidic Composition and Molecular Weight Distribution of Microcosms EPSs
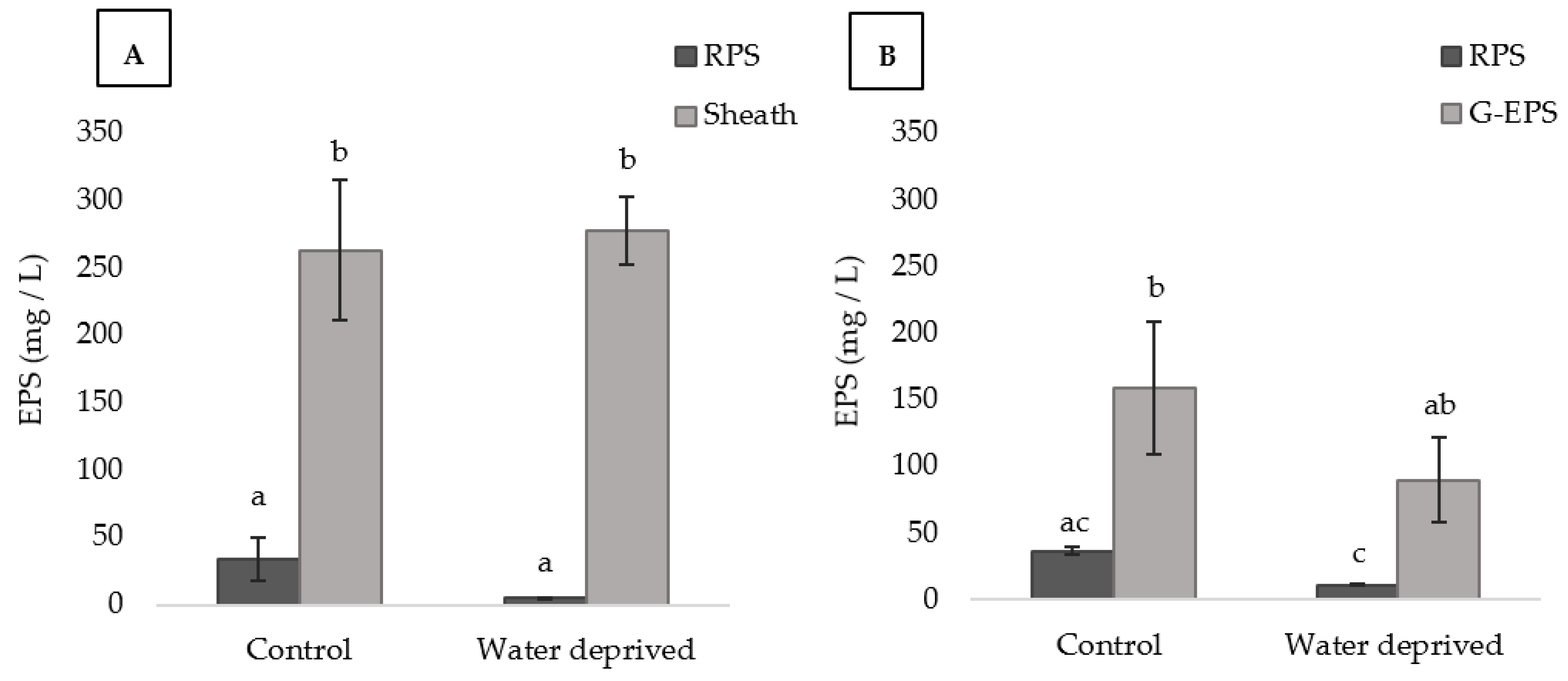
3.3. Effects of Water Deprivation in Chlorophyll a and EPS Production in Biofilms
3.4. Changes in Monosaccharidic Composition and Molecular Weight Distribution of Biofilms EPSs
4. Discussion
4.1. EPS and Growth Profile in Biocrust-Forming Cyanobacteria
4.2. Biocrusts and Biofilms EPSs, Similarities and Differences
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gaysina, L.A.; Saraf, A.; Singh, P. Cyanobacteria in diverse habitats. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–28. [Google Scholar]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef]
- Lan, S.; Zhang, Q.; Wu, L.; Liu, Y.; Zhang, D.; Hu, C. Artificially accelerating the reversal of desertification: Cyanobacterial inoculation facilitates the succession of vegetation communities. Environ. Sci. Technol. 2014, 48, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Jafarpoor, A.; Sadeghi, S.H.; Darki, B.Z.; Homaee, M. Changes in hydrologic components from a mid-sized plots induced by rill erosion due to cyanobacterization. ISWCR 2022, 10, 143–148. [Google Scholar] [CrossRef]
- Rossi, F.; Mugnai, G.; De Philippis, R. Cyanobacterial biocrust induction: A comprehensive review on soil rehabilitation-effective biotechnology. Geoderma 2022, 415, 115766. [Google Scholar] [CrossRef]
- Hagemann, M.; Henneberg, M.; Felde, V.J.M.N.L.; Berkowicz, S.M.; Raanan, H.; Pade, N.; Felix-Henningsen, P.; Kaplan, A. Cyanobacterial populations in biological soil crusts of the northwest Negev desert, Israel—effects of local conditions and disturbance. FEMS Microbiol. 2017, 93, fiw228. [Google Scholar] [CrossRef]
- Weber, B.; Belnap, J.; Büdel, B.; Antoninka, A.J.; Barger, N.N.; Chaudhary, V.B.; Darrouzet-Nardi, A.; Eldridge, D.J.; Faist, A.M.; Ferrenberg, S.; et al. What is a biocrust? A refined, contemporary definition for a broadening research community. Biol. Rev. 2022, 97, 1768–1785. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rossi, F.; Deng, S.; Liu, Y.; Wang, G.; Adessi, A.; De Philippis, R. Macromolecular and chemical features of the excreted extracellular polysaccharides in induced biological soil crusts of different ages. Soil Biol. Biochem. 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Colica, G.; Li, H.; Rossi, F.; Li, D.; Liu, Y.; De Philippis, R. Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol. Biochem. 2014, 68, 62–70. [Google Scholar] [CrossRef]
- Mugnai, G.; Rossi, F.; Chamizo, S.; Adessi, A.; De Philippis, R. The role of grain size and inoculum amount on biocrust formation by Leptolyngbya ohadii. Catena 2020, 184, 104248. [Google Scholar] [CrossRef]
- Swenson, T.L.; Couradeau, E.; Bowen, B.P.; De Philippis, R.; Rossi, F.; Mugnai, G.; Northen, T.R. A novel method to evaluate nutrient retention by biological soil crust exopolymeric matrix. Plant Soil 2018, 429, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Hasnain, S. Phytostimulation and biofertilization in wheat by cyanobacteria. J. Ind. Microbiol. Biotechnol. 2011, 38, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gr, S.; Yadav, R.K.; Chatrath, A.; Gerard, M.; Tripathi, K.; Govindsamy, V.; Abraham, G. Perspectives on the potential application of cyanobacteria in the alleviation of drought and salinity stress in crop plants. J. Appl. Phycol. 2021, 33, 3761–3778. [Google Scholar] [CrossRef]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-inflammatory activity of exopolysaccharides from Phormidium sp. ETS05, the most abundant cyanobacterium of the therapeutic Euganean thermal muds, Using the Zebrafish Model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Furrer, J.M.; Kadilak, A.L.; Hinestroza, H.F.; Gage, D.J.; Cho, Y.K.; Shor, L.M. Bacterial extracellular polymeric substances amplify water content variability at the pore scale. Front. Environ. Sci. 2018, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Nishanth, S.; Bharti, A.; Gupta, H.; Gupta, K.; Gulia, U.; Prasanna, R. Cyanobacterial extracellular polymeric substances (EPS): Biosynthesis and their potential applications. In Microbial and Natural Macromolecules Synthesis and Applications, 1st ed.; Das, S., Dash, H.R., Eds.; Academic Press: London, UK, 2021; pp. 349–369. [Google Scholar]
- Mugnai, G.; Rossi, F.; Felde, V.J.M.N.L.; Colesie, C.; Büdel, B.; Peth, S.; Kaplan, A.; De Philippis, R. The potential of the cyanobacterium Leptolyngbya ohadii as inoculum for stabilizing bare sandy substrates. Soil Biol. Biochem. 2018, 127, 318–328. [Google Scholar] [CrossRef]
- Rossi, F.; Micheletti, E.; Bruno, L.; Adhikary, S.P.; Albertano, P.; De Philippis, R. Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on stone monuments. Biofouling 2012, 28, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Mugnai, G.; De Philippis, R. Complex role of the polymeric matrix in biological soil crusts. Plant Soil 2018, 429, 19–34. [Google Scholar] [CrossRef]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Raanan, H.; Dai, G.; Oren, N.; Berkowicz, S.; Murik, O.; Kaplan, A.; Qiu, B. Reading and surviving the harsh conditions in desert biological soil crust: The cyanobacterial viewpoint. FEMS Microbiol. Rev. 2021, 45, fuab036. [Google Scholar] [CrossRef]
- Zammit, G. Phototrophic biofilm communities and adaptation to growth on ancient archaeological surfaces. Ann. Microbiol. 2019, 69, 1047–1058. [Google Scholar] [CrossRef]
- Di Pippo, F.; Ellwood, N.T.W.; Gismondi, A.; Bruno, L.; Rossi, F.; Magni, P.; De Philippis, R. Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J. Appl. Phycol. 2013, 25, 1697–1708. [Google Scholar] [CrossRef] [Green Version]
- Keshari, N.; Das, S.K.; Adhikary, S.P. Colonization and survival of a stress tolerant cyanobacterium on a heritage monument of Santiniketan, India. Int. Biodeterior. Biodegrad. 2021, 164, 105294. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Mugnai, G.; Simiani, A.; De Philippis, R. Soil type and cyanobacteria species influence the macromolecular and chemical characteristics of the polysaccharidic matrix in induced biocrusts. Microb. Ecol. 2019, 78, 482–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamizo, S.; Adessi, A.; Torzillo, G.; De Philippis, R. Exopolysaccharide features influence growth success in biocrust-forming cyanobacteria, moving from liquid culture to sand microcosms. Front. Microbiol. 2020, 11, 568224. [Google Scholar] [CrossRef] [PubMed]
- Brüll, L.P.; Huang, Z.; Thomas-Oates, J.E.; Paulsen, B.S.; Cohen, E.H.; Michaelsen, T.E. Studies of polysaccharides from three edible species of Nostoc (cyanobacteria) with different colony morphologies: Structural characterization and effect on the complement system of polysaccharides from Nostoc commune. J. Phycol. 2000, 36, 871–881. [Google Scholar] [CrossRef]
- Hagemann, M.; Henneberg, M.; Felde, V.J.; Drahorad, S.L.; Berkowicz, S.M.; Felix-Henningsen, P.; Kaplan, A. Cyanobacterial diversity in biological soil crusts along a precipitation gradient, Northwest Negev Desert, Israel. Microb. Ecol. 2015, 70, 219–230. [Google Scholar] [CrossRef]
- Murik, O.; Oren, N.; Shotland, Y.; Raanan, H.; Treves, H.; Kedem, I.; Keren, N.; Hagemann, M.; Pade, N.; Kaplan, A. What distinguishes cyanobacteria able to revive after desiccation from those that cannot: The genome aspect. Environ. Microbiol. 2017, 19, 535–550. [Google Scholar] [CrossRef]
- Raanan, H.; Oren, N.; Treves, H.; Berkowicz, S.M.; Hagemann, M.; Pade, N.; Keren, N.; Kaplan, A. Simulated soil crust conditions in a chamber system provide new insights on cyanobacterial acclimation to desiccation: Simulation of BSC conditions and acclimation. Environ. Microbiol. 2016, 18, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Deng, S.; Xu, Y.; Wang, G.; Liu, Y. The response of carbohydrate metabolism to the fluctuation of relative humidity (RH) in the desert soil cyanobacterium Phormidium tenue. Eur. J. Soil Biol. 2012, 48, 11–16. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Paulsen, B.S.; Petersen, D.; Klaveness, D. Extracellular carbohydrate polymers from five desert soil algae with different cohesion in the stabilization of fine sand grain. Carbohydr. Polym. 2003, 54, 33–42. [Google Scholar] [CrossRef]
- Johansen, J.R. Cryptogamic crusts of semiarid and arid lands of North America. J. Phycol. 1993, 29, 140–147. [Google Scholar] [CrossRef]
- Burezq, H. Combating wind erosion through soil stabilization under simulated wind flow condition—Case of Kuwait. Int. Soil Water Conserv. Res. 2020, 8, 154–163. [Google Scholar] [CrossRef]
- Dregne, H.E. Land degradation in the drylands. Arid Land Res. Manag. 2002, 16, 99–132. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: Gaining insights for applicability in soil restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Roncero-Ramos, B.; Muñoz-Martín, M.A.; Cantón, Y.; Chamizo, S.; Rodríguez-Caballero, E.; Mateo, P. Land degradation effects on composition of pioneering soil communities: An alternative successional sequence for dryland cyanobacterial biocrusts. Soil Biol. Biochem. 2020, 146, 107824. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stainier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Castle, S.C.; Morrison, C.D.; Barger, N.N. Extraction of chlorophyll a from biological soil crusts: A comparison of solvents for spectrophotometric determination. Soil Biol. Biochem. 2011, 43, 853–856. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Yéprémian, C.; Catherine, A.; Bernard, C.; Congestri, R.; Elersek, T.; Pilkaityte, R. Chlorophyll a extraction and determination. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, 1st ed.; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 331–334. [Google Scholar]
- Mugnai, G.; Rossi, F.; Felde, V.J.M.N.L.; Colesie, C.; Büdel, B.; Peth, S.; Kaplan, A.; De Philippis, R. Development of the polysaccharidic matrix in biocrusts induced by a cyanobacterium inoculated in sand microcosms. Biol. Fertil. Soils 2018, 54, 27–40. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Oren, N.; Raanan, H.; Murik, O.; Keren, N.; Kaplan, A. Dawn illumination prepares desert cyanobacteria for dehydration. Curr. Biol. 2017, 27, R1056–R1057. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Wu, J. Tolerance of soil algae and cyanobacteria to drought stress. J. Phycol. 2014, 50, 131–139. [Google Scholar] [CrossRef]
- Bar Eyal, L.; Eisenberg, I.; Faust, A.; Raanan, H.; Nevo, R.; Rappaport, F.; Krieger-Liszkay, A.; Sétif, P.; Thurotte, A.; Reich, Z.; et al. An easily reversible structural change underlies mechanisms enabling desert crust cyanobacteria to survive desiccation. BBA Bioenerg. 2015, 1847, 1267–1273. [Google Scholar] [CrossRef]
- Bar Eyal, L.; Ranjbar Choubeh, R.; Cohen, E.; Eisenberg, I.; Tamburu, C.; Dorogi, M.; Ünnep, R.; Appavou, M.-S.; Nevo, R.; Raviv, U.; et al. Changes in aggregation states of light-harvesting complexes as a mechanism for modulating energy transfer in desert crust cyanobacteria. Proc. Natl. Acad. Sci. USA 2017, 114, 9481–9486. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Wei, Y.; Yuasa, K.; Nishiyama, Y. Recovery of photosynthesis after long-term storage in the terrestrial cyanobacterium Nostoc commune. J. Gen. Appl. Microbiol. 2022, 68, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, L.; da Rocha, U.; Klitgord, N.; Luning, E.G.; Fortney, J.; Axen, S.D.; Shih, P.M.; Bouskill, N.J.; Bowen, B.P.; Kerfeld, C.A.; et al. Dynamic cyanobacterial response to hydration and dehydration in a desert biological soil crust. ISME J. 2013, 7, 2178–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, K.; Ai, Y.; Jiang, H.; Gao, X.; Qiu, B. Differential display analysis of cDNA fragments potentially involved in Nostoc flagelliforme response to osmotic stress. J. Appl. Phycol. 2012, 24, 1487–1494. [Google Scholar] [CrossRef]
- Lan, S.; Wu, L.; Zhang, D.; Hu, C. Desiccation provides photosynthetic protection for crust cyanobacteria Microcoleus vaginatus from high temperature. Physiol. Plant. 2014, 152, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Roberson, E.B.; Firestone, M.K. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yu, K.; Li, L.; Wang, L.; Liang, W. Enhancement of exopolysaccharides production and reactive oxygen species level of Nostoc flagelliforme in response to dehydration. Environ. Sci. Pollut. Res. 2021, 28, 34300–34308. [Google Scholar] [CrossRef]
- Han, P.P.; Shen, S.G.; Guo, R.J.; Zhao, D.X.; Lin, Y.H.; Jia, S.R.; Yan, R.R.; Wu, Y.K. ROS is a factor regulating the increased polysaccharide production by light quality in the edible cyanobacterium Nostoc flagelliforme. J. Agric. Food Chem. 2019, 67, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Román, J.R.; Roncero-Ramos, B.; Rodríguez-Caballero, E.; Chamizo, S.; Cantón, Y. Effect of water availability on induced cyanobacterial biocrust development. Catena 2021, 197, 104988. [Google Scholar] [CrossRef]
- Mager, D.M.; Thomas, A.D. Extracellular polysaccharides from cyanobacterial soil crusts: A review of their role in dryland soil processes. J. Arid Environ. 2011, 75, 91–97. [Google Scholar] [CrossRef]
- Kehr, J.C.; Dittmann, E. Biosynthesis and function of extracellular glycans in cyanobacteria. Life 2015, 5, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laroche, C. Exopolysaccharides from microalgae and cyanobacteria: Diversity of strains, production strategies, and applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Uhliariková, I.; Matulová, M.; Capek, P. Structural features of the bioactive cyanobacterium Nostoc sp. exopolysaccharide. Int. J. Biol. Macromol. 2020, 164, 2284–2292. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Svircev, Z.; Markovic, S.B.; Stevens, T.; Codd, G.A.; Smalley, I.; Simeunovic, J.; Obreht, I.; Dulic, T.; Pantelic, D.; Hambach, U. Importance of biological loess crusts for loess formation in semi-arid environments. Quat. Int. 2013, 296, 206–215. [Google Scholar] [CrossRef]
- Hoiczyk, E. Structural and biochemical analysis of the sheath of Phormidium uncinatum. J. Bacteriol. 1998, 180, 3923–3932. [Google Scholar] [CrossRef] [Green Version]
- Keshari, N.; Adhikary, S.P. Characterization of cyanobacteria isolated from biofilms on stone monuments at Santiniketan, India. Biofouling 2013, 29, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Olsson-Francis, K.; Watson, J.; Cockell, C. Cyanobacteria isolated from the high-intertidal zone: A model for studying the physiological prerequisites for survival in low Earth orbit. Int. J. Astrobiol. 2013, 12, 292–303. [Google Scholar] [CrossRef]
- Tirkey, J.; Adhikary, S.P. Cyanobacteria in biological soil crusts of India. Curr. Sci. 2005, 89, 515–521. [Google Scholar]
- Borg, A.J.E.; Dennig, A.; Weber, H.; Nidetzky, B. Mechanistic characterization of UDP-glucuronic acid 4-epimerase. FEBS J. 2021, 288, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.E.; Mosimann, S.C.; van De Rijn, I.; Tanner, M.E.; Strynadka, N.C. The first structure of UDP-glucose dehydrogenase reveals the catalytic residues necessary for the two-fold oxidation. Biochemistry 2000, 13, 7012–7023. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: A review. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Kranner, I.; Birtic, S. A modulating role for antioxidants in desiccation tolerance. Integr. Comp. Biol. 2005, 45, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, P.; Martins, N.M.; Santos, M.; Couto, N.A.; Wright, P.C.; Tamagnini, P. The Anabaena sp. PCC 7120 exoproteome: Taking a peek outside the box. Life 2015, 5, 130–163. [Google Scholar] [CrossRef]
- Shirkey, B.; Kovarcik, D.P.; Wright, D.J.; Wilmoth, G.; Prick, T.F.; Helm, R.F.; Gregory, E.M.; Potts, M. Active Fe-containing superoxide dismutase and abundant sodF mRNA in Nostoc commune(cyanobacteria) after years of desiccation. J. Bacteriol. 2000, 182, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 2013, 3, 463–483. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moia, I.C.; Pereira, S.B.; Domizio, P.; De Philippis, R.; Adessi, A. Phormidium ambiguum and Leptolyngbya ohadii Exopolysaccharides under Low Water Availability. Polymers 2023, 15, 1889. https://doi.org/10.3390/polym15081889
Moia IC, Pereira SB, Domizio P, De Philippis R, Adessi A. Phormidium ambiguum and Leptolyngbya ohadii Exopolysaccharides under Low Water Availability. Polymers. 2023; 15(8):1889. https://doi.org/10.3390/polym15081889
Chicago/Turabian StyleMoia, Isabela C., Sara B. Pereira, Paola Domizio, Roberto De Philippis, and Alessandra Adessi. 2023. "Phormidium ambiguum and Leptolyngbya ohadii Exopolysaccharides under Low Water Availability" Polymers 15, no. 8: 1889. https://doi.org/10.3390/polym15081889
APA StyleMoia, I. C., Pereira, S. B., Domizio, P., De Philippis, R., & Adessi, A. (2023). Phormidium ambiguum and Leptolyngbya ohadii Exopolysaccharides under Low Water Availability. Polymers, 15(8), 1889. https://doi.org/10.3390/polym15081889








