Utilizing Robust Design to Optimize Composite Bioadhesive for Promoting Dermal Wound Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Robust Design Experiment
2.3. Preparation of the Composite Adhesive
2.4. Characterization of the Composite Adhesive
2.5. Full Thickness Dermal Wound Repair Model
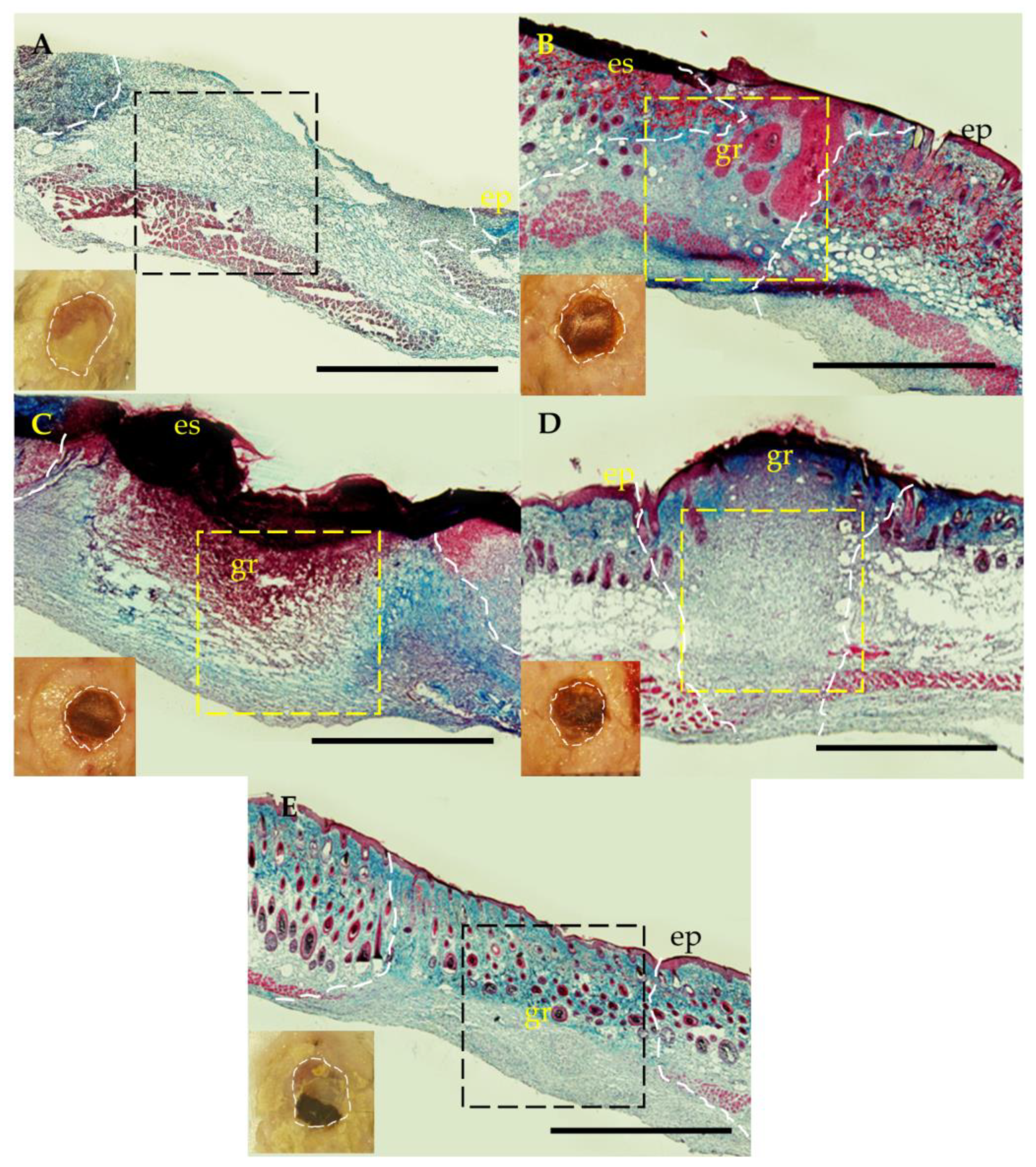
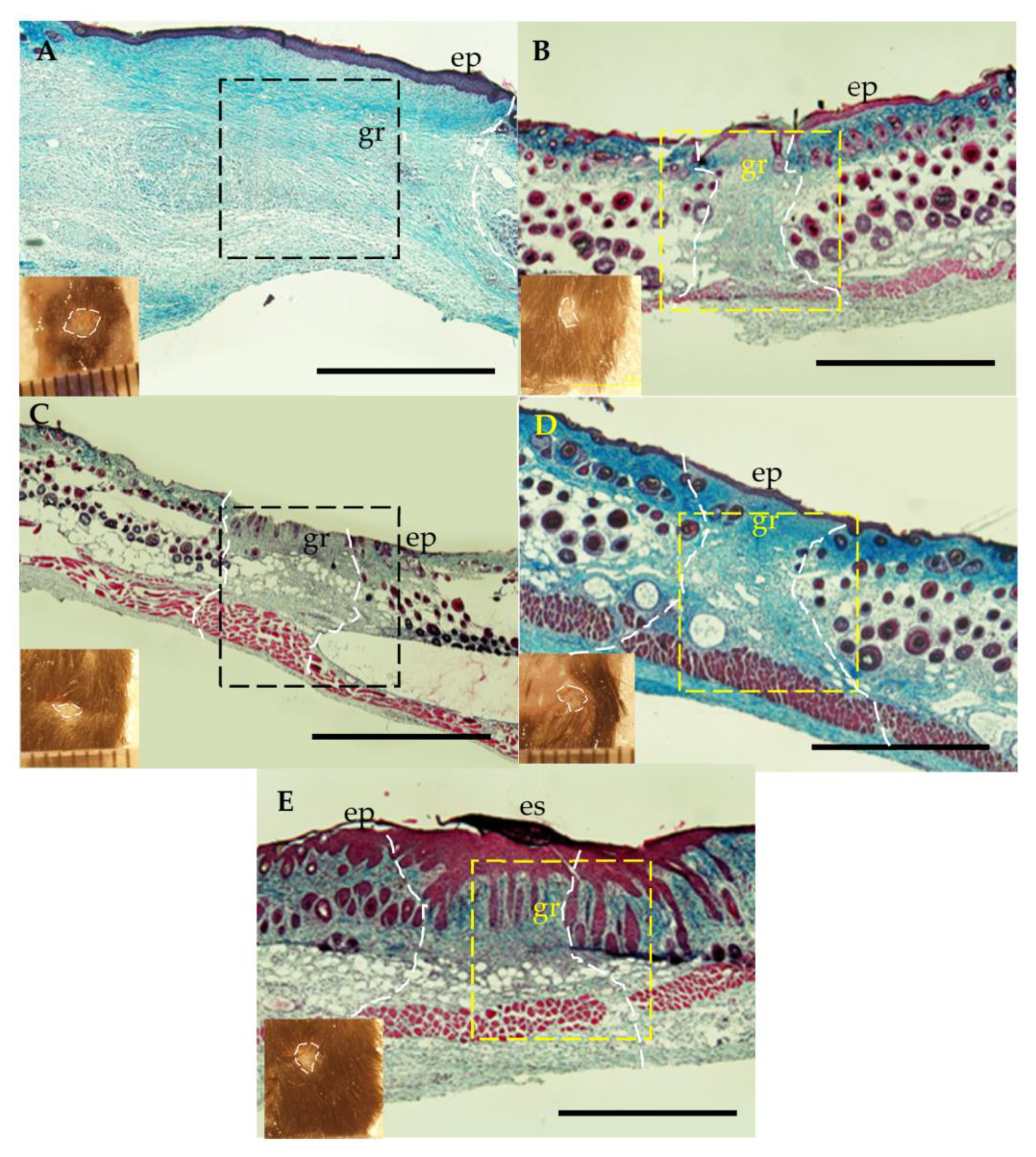
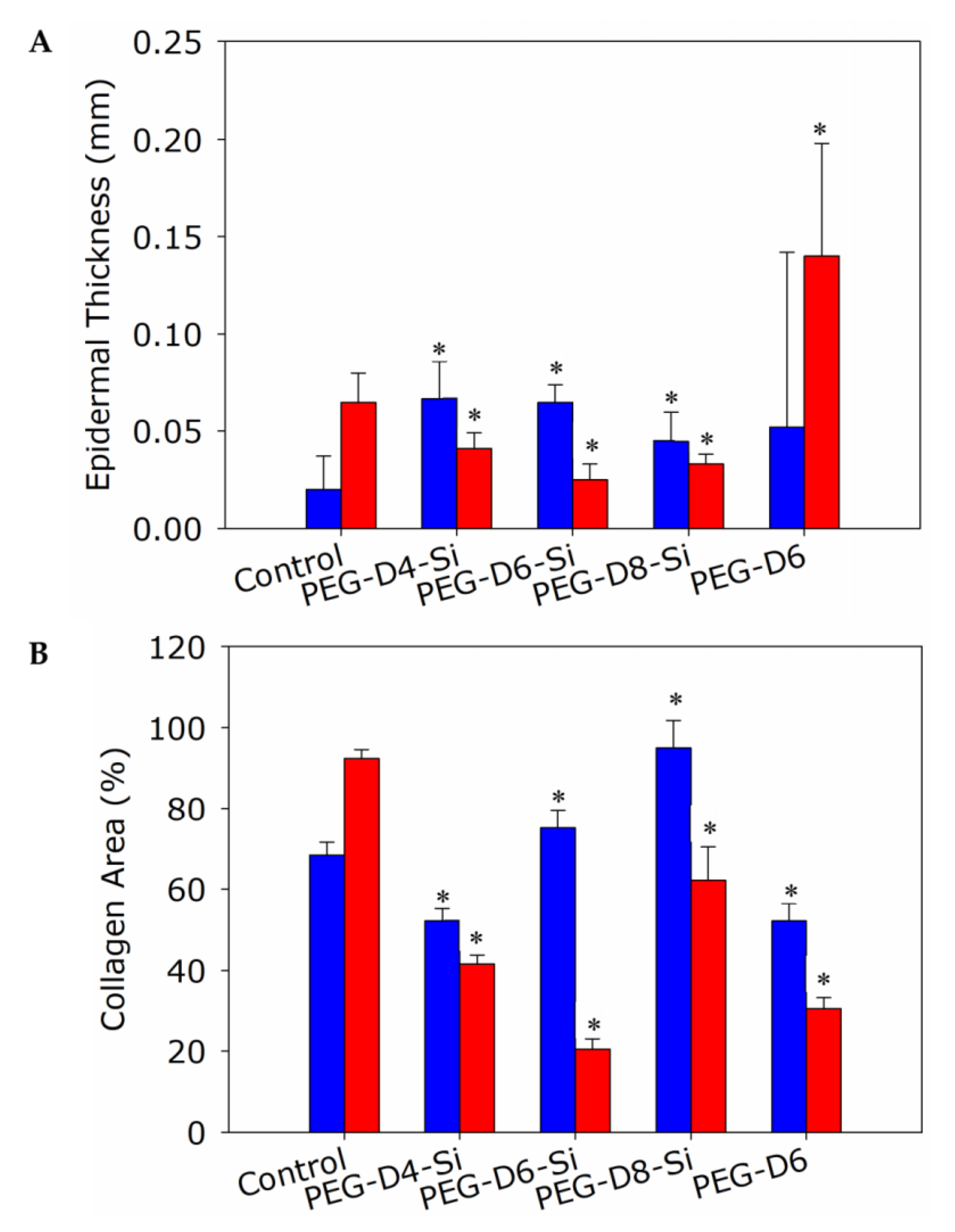
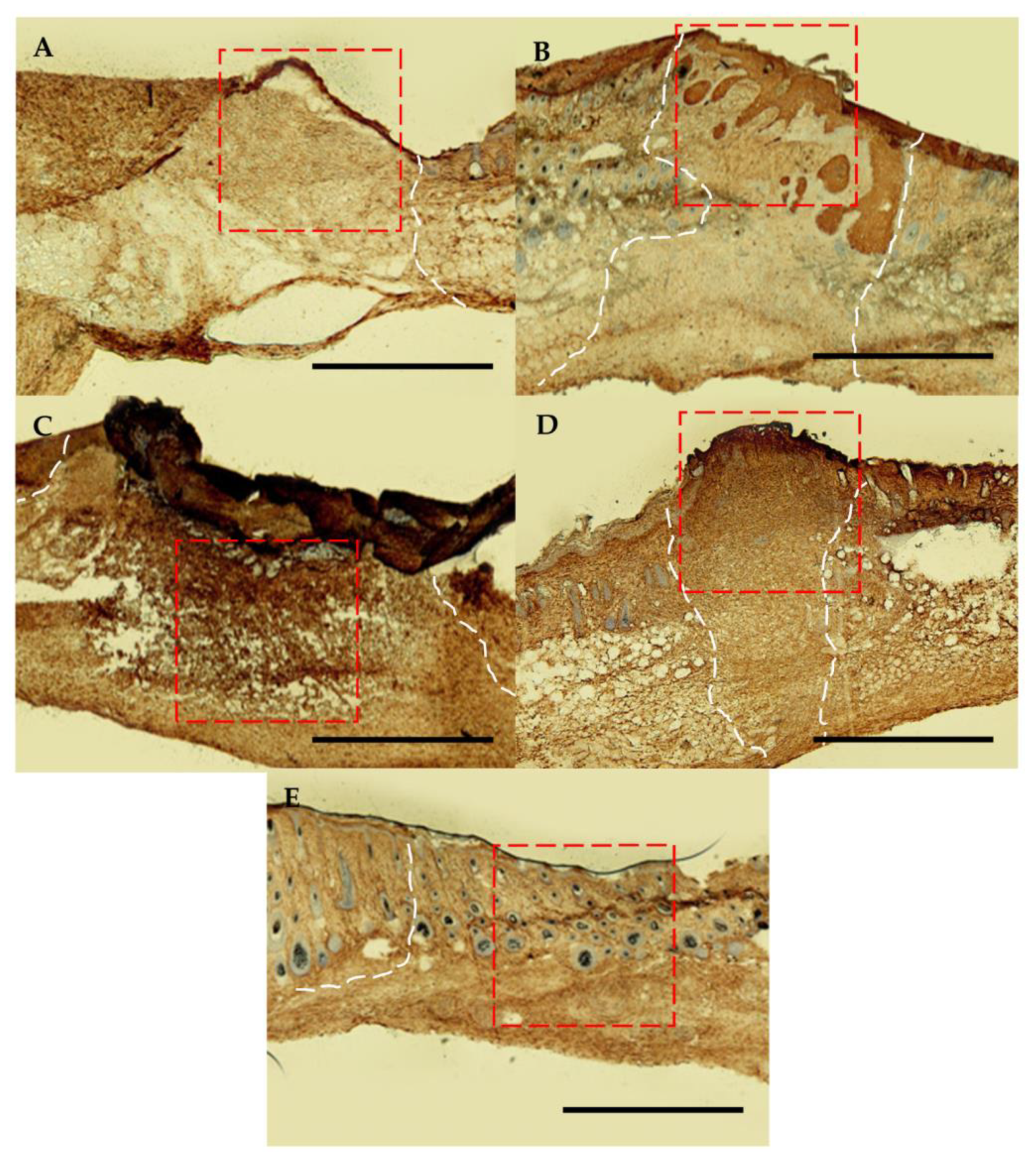
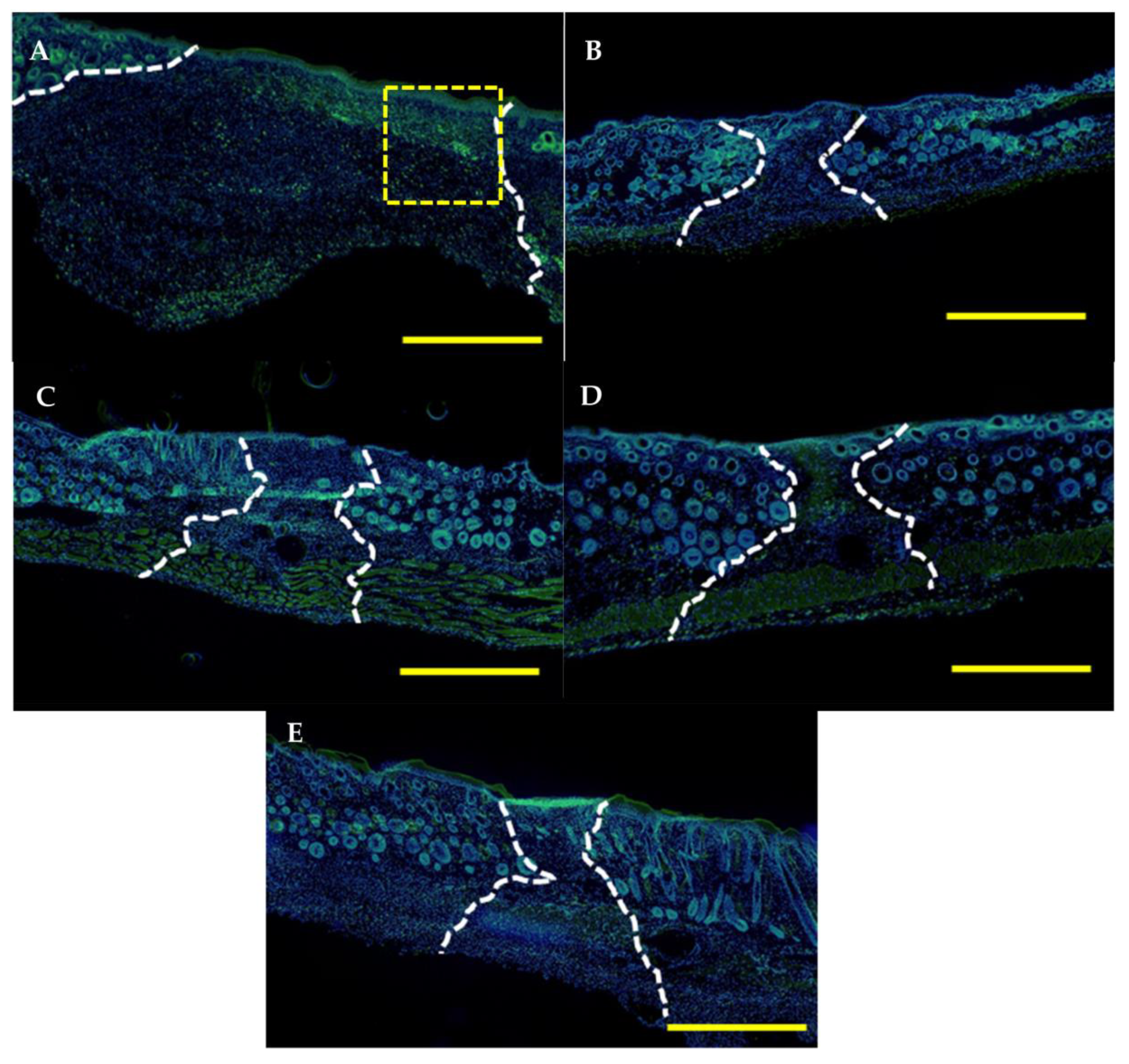
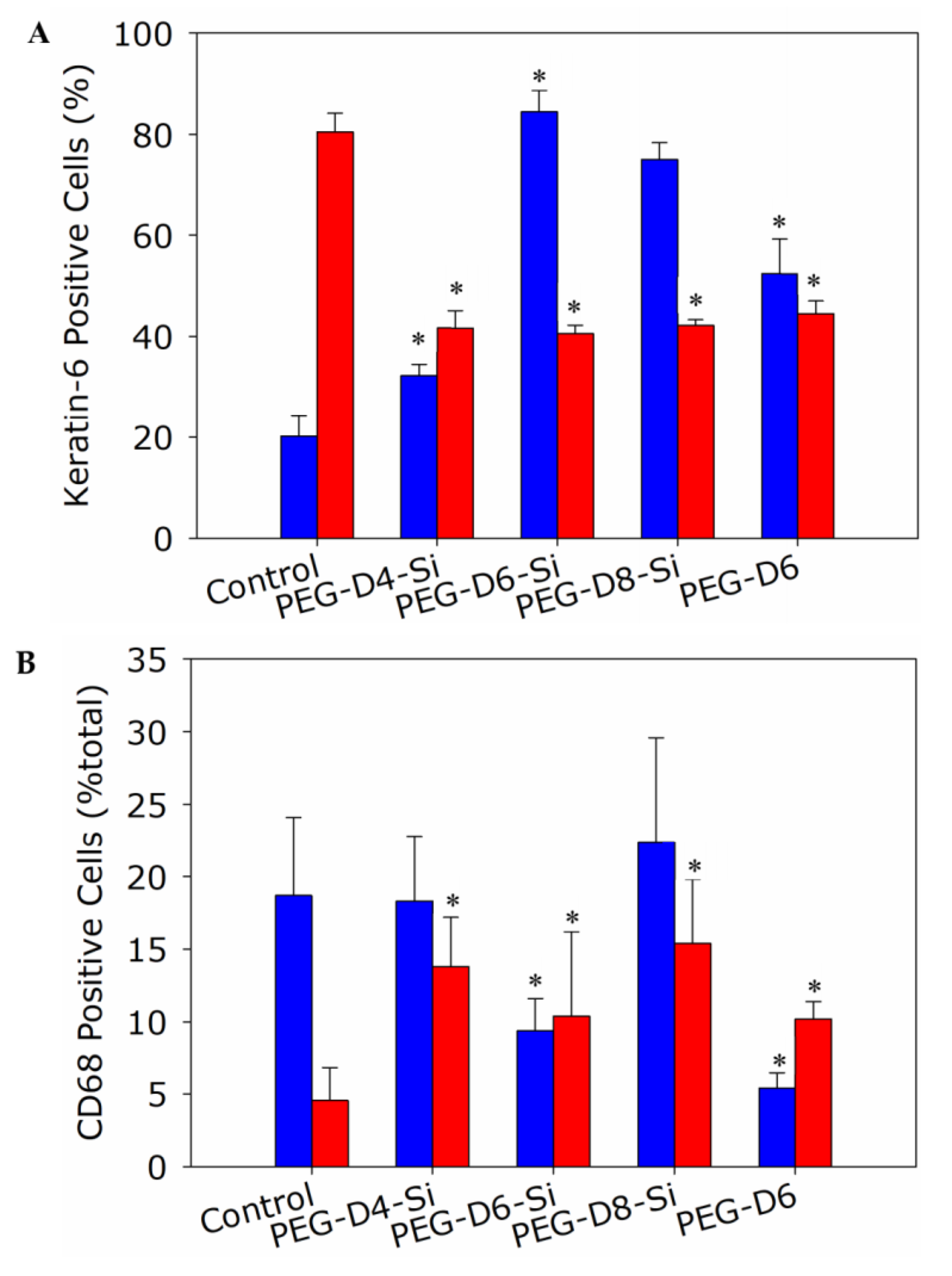
2.6. Histological and Immunological Analysis of Dermal Wounds
2.7. Statistical Analysis
3. Results
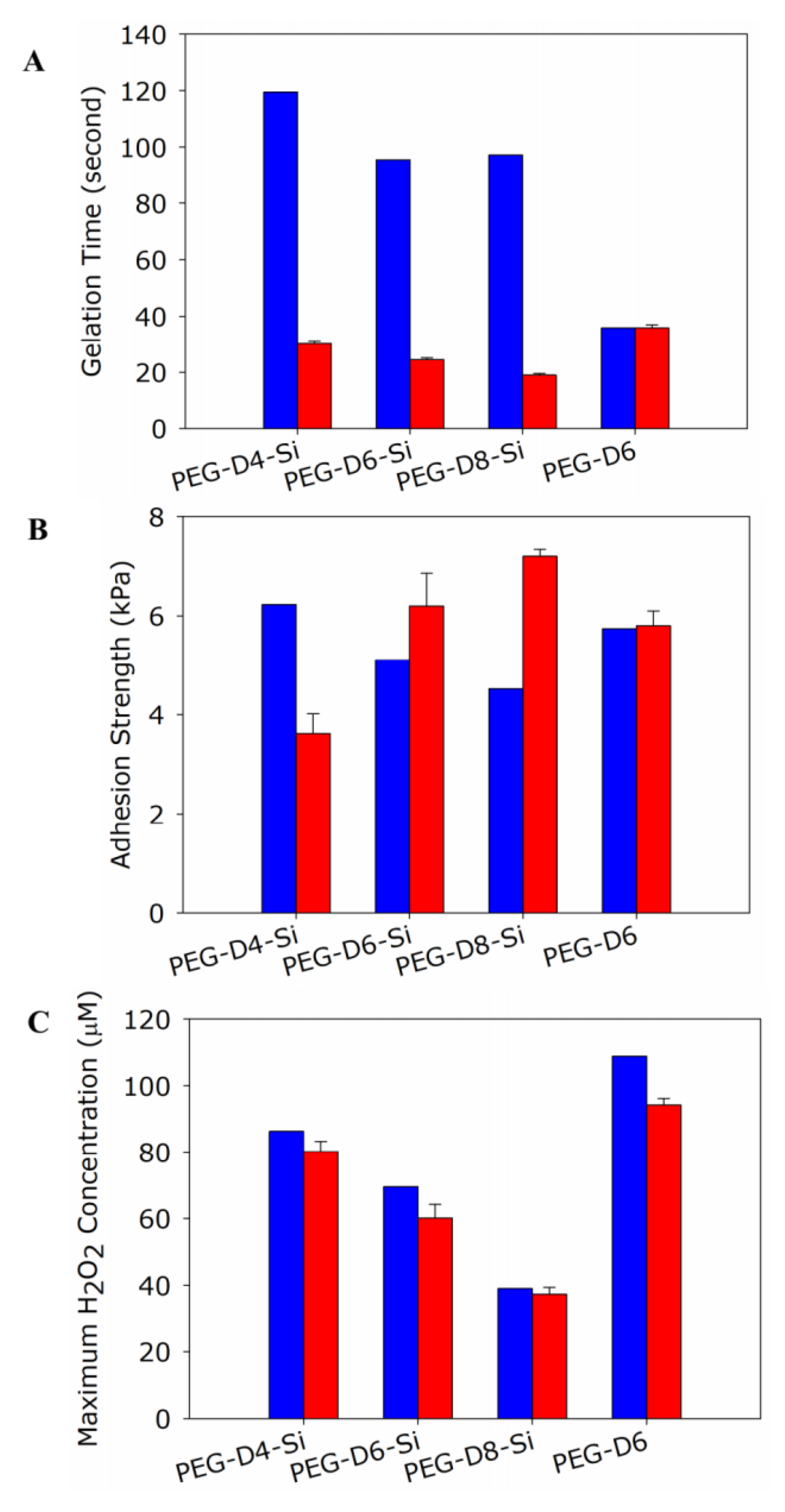
3.1. Robust Design Experiments
3.2. Prediction Based on Robust Design Experiment
3.3. Validating Results from Robust Design Experiment
3.4. Dermal Wound Closure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal Wound Healing Is Subject to Redox Control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Babior, B.M.; Hunt, T.K.; Ellison, E.C.; Roy, S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J. Biol. Chem. 2002, 277, 33284–33290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.-G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [Green Version]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, V.; Yanes, O.; Aguilar, E.; Wang, M.; Friedlander, D.; Moreno, S.; Storm, K.; Zhan, M.; Naccache, S.; Nemerow, G.; et al. Differential Macrophage Polarization Promotes Tissue Remodeling and Repair in a Model of Ischemic Retinopathy. Sci. Rep. 2011, 1, 76. [Google Scholar] [CrossRef] [Green Version]
- Kessler, L.; Bilbault, P.; Ortega, F.; Grasso, C.; Passemard, R.; Stephan, D.; Pinget, M.; Schneider, F. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: A prospective randomized study. Diabetes Care 2003, 26, 2378–2382. [Google Scholar] [CrossRef] [Green Version]
- Baldry, M.G.C. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 1983, 54, 417–423. [Google Scholar] [CrossRef]
- Loo, A.E.K.; Wong, Y.T.; Ho, R.; Wasser, M.; Du, T.; Ng, W.T.; Halliwell, B. Effects of Hydrogen Peroxide on Wound Healing in Mice in Relation to Oxidative Damage. PLoS ONE 2012, 7, e49215. [Google Scholar] [CrossRef] [Green Version]
- Brian, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Reactive Oxygen Species (ROS)-A Family of Fate Deciding Molecules Pivotal in Constructive Inflammation and Wound Healing. Eur. Cells Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, F.; Roy, S.; Sen, C.K.; Guan, J. Injectable, Highly Flexible, and Thermosensitive Hydrogels Capable of Delivering Superoxide Dismutase. Biomacromolecules 2009, 10, 3306–3316. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.; Zhou, Y.; Zhou, F.; Liu, W.; Wang, Z. Mussel-inspired hydrogels: From design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.; Zhao, B.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Melrose, J. High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired. Molecules 2022, 27, 8982. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D.-A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef]
- Cencer, M.; Murley, M.; Liu, Y.; Lee, B.P. Effect of nitro-functionalization on the cross-linking and bioadhesion of biomimetic adhesive moiety. Biomacromolecules 2015, 16, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Cencer, M.M.; Liu, Y.; Winter, A.; Murley, M.; Meng, H.; Lee, B.P. Effect of pH on the rate of curing and bioadhesive properties of dopamine functionalized poly (ethylene glycol) hydrogels. Biomacromolecules 2014, 15, 2861–2869. [Google Scholar] [CrossRef] [Green Version]
- Meredith, H.J.; Jenkins, C.L.; Wilker, J.J. Enhancing the Adhesion of a Biomimetic Polymer Yields Performance Rivaling Commercial Glues. Adv. Funct. Mater. 2014, 24, 3259–3267. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Faust, M.; Konst, S.; Lee, B.P. Hydrogen peroxide generation and biocompatibility of hydrogel-bound mussel adhesive moiety. Acta Biomater. 2015, 17, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.; Liu, Y.; Lee, B.P. Model polymer system for investigating the generation of hydrogen peroxide and its biological responses during the crosslinking of mussel adhesive moiety. Acta Biomater. 2017, 48, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinnaratip, R.; Meng, H.; Rajachar, R.M.; Lee, B.P. Effect of incorporating clustered silica nanoparticles on the performance and biocompatibility of catechol-containing PEG-based bioadhesive. Biomed. Mater. 2018, 13, 025003. [Google Scholar] [CrossRef] [PubMed]
- Żeglin’ski, J.; Piotrowski, G.P.; Piękos, R. A study of interaction between hydrogen peroxide and silica gel by FTIR spectroscopy and quantum chemistry. J. Mol. Struct. 2006, 794, 83–91. [Google Scholar] [CrossRef]
- Pinnaratip, R.; Forooshani, P.K.; Li, M.; Hu, Y.H.; Rajachar, R.M.; Lee, B.P. Controlling the Release of Hydrogen Peroxide from Catechol-Based Adhesives Using Silica Nanoparticles. ACS Biomater. Sci. Eng. 2020, 6, 4502–4511. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, H.; Qian, Z.C.; Fan, N.; Choi, W.Y.; Zhao, F.; Lee, B.P. A Moldable Nanocomposite Hydrogel Composed of Roya Mussel-Inspired Polymer and a Nanosilicate as a Fit-to-Shape Tissue Sealant. Angew. Chem. Int. Ed. 2017, 56, 4224–4228. [Google Scholar] [CrossRef] [Green Version]
- Reichert, J.; Hull, H. Control of pH in peroxide solutions. Ind. Eng. Chem. Anal. Ed. 1939, 11, 311–314. [Google Scholar] [CrossRef]
- Phadke, M.S. Quality Engineering Using Robust Design; Prentice Hall: Englewood Cliffs, NJ, USA, 1989; p. 334. [Google Scholar]
- Roy, R.K. Design of Experiments Using the Taguchi Approach: 16 Steps to Product and Process Improvement; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Pinnaratip, R. Study of Silica Nanoparticle Composite on Silica-Hydrogen Peroxide Complexations and Their Effects in Catechol Based Adhesives. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2020. [Google Scholar]
- ASTM-F2255; ASTM F2255 Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading. ASTM: West Conshohocken, PA, USA, 2015.
- Galiano, R.D.; Michaels, V.; Joseph, M.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef]
- Wang, X.; Ge, J.; Tredget, E.E.; Wu, Y. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat. Protoc. 2013, 8, 302–309. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T.J.N.m. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Tan, N.S.; Wahli, W. Studying Wound Repair in the Mouse. Curr. Protoc. Mouse Biol. 2013, 3, 171–185. [Google Scholar] [CrossRef]
- Baecker, V. ImageJ macro tool sets for biological image analysis. In Proceedings of the ImageJ User and Developer Conference, Luxembourg, 24–26 October 2012; pp. 24–26. [Google Scholar]
- Loo, A.E.K.; Ho, R.; Halliwell, B. Mechanism of hydrogen peroxide-induced keratinocyte migration in a scratch-wound model. Free Radical Bio Med. 2011, 51, 884–892. [Google Scholar] [CrossRef]
- Grinnell, F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994, 124, 401–404. [Google Scholar] [CrossRef]
- Klein-Szanto, A.; Slaga, T. Effects of peroxides on rodent skin: Epidermal hyperplasia and tumor promotion. J. Investig. Dermatol. 1982, 79, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Melrose, J. Glycosaminoglycans in Wound Healing. Bone Tissue Regen. Insights 2016, 7, BTRI.S38670. [Google Scholar] [CrossRef] [Green Version]
- Tammi, R.H.; Tammi, M.I. Hyaluronan Accumulation in Wounded Epidermis: A Mediator of Keratinocyte Activation. J. Investig. Dermatol. 2009, 129, 1858–1860. [Google Scholar] [CrossRef] [Green Version]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef]
- Kopcewicz, M.; Walendzik, K.; Bukowska, J.; Kur-Piotrowska, A.; Machcinska, S.; Gimble, J.M.; Gawronska-Kozak, B. Cutaneous wound healing in aged, high fat diet-induced obese female or male C57BL/6 mice. Aging 2020, 12, 7066–7111. [Google Scholar] [CrossRef]
- Gilbert, S.F. The Epidermis and the Origin of Cutaneous Structures, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Patel, G.K.; Wilson, C.H.; Harding, K.G.; Finlay, A.Y.; Bowden, P.E. Numerous Keratinocyte Subtypes Involved in Wound Re-Epithelialization. J. Investig. Dermatol. 2006, 126, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Quignard, S.; Coradin, T.; Powell, J.J.; Jugdaohsingh, R. Silica nanoparticles as sources of silicic acid favoring wound healing in vitro. Colloids and Surf. B 2017, 155, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Wojcik, S.M.; Bundman, D.S.; Roop, D.R. Delayed Wound Healing in Keratin 6a Knockout Mice. Mol. Cell. Biol. 2000, 20, 5248–5255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, A.; Lichti, U.F.; Carlson, B.A.; Ryscavage, A.O.; Gladyshev, V.N.; Yuspa, S.H.; Hatfield, D.L. Selenoproteins Are Essential for Proper Keratinocyte Function and Skin Development. PLoS ONE 2010, 5, e12249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grotheer, V.; Goergens, M.; Fuchs, P.C.; Dunda, S.; Pallua, N.; Windolf, J.; Suschek, C.V. The performance of an orthosilicic acid-releasing silica gel fiber fleece in wound healing. Biomaterials 2013, 34, 7314–7327. [Google Scholar] [CrossRef] [PubMed]
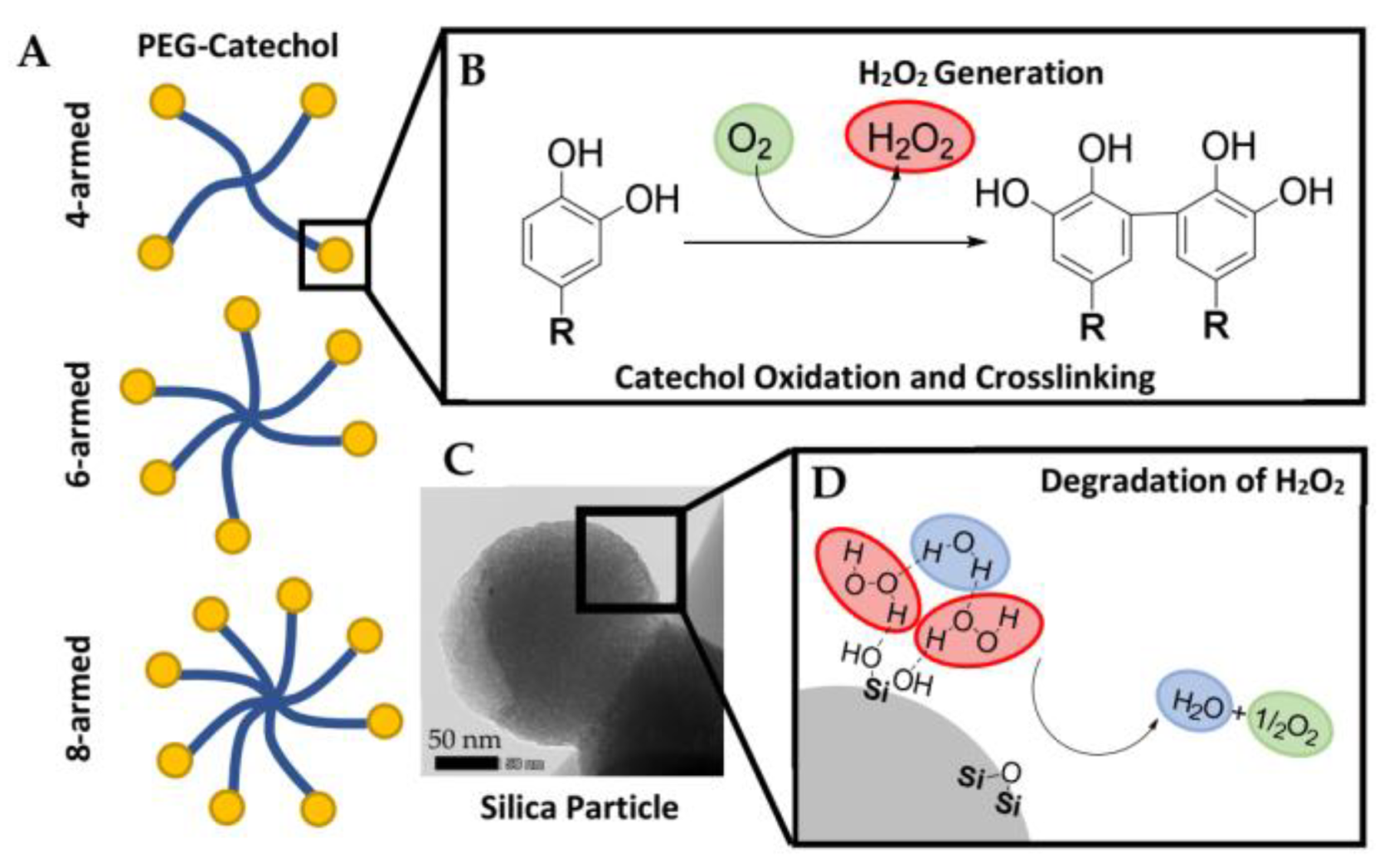
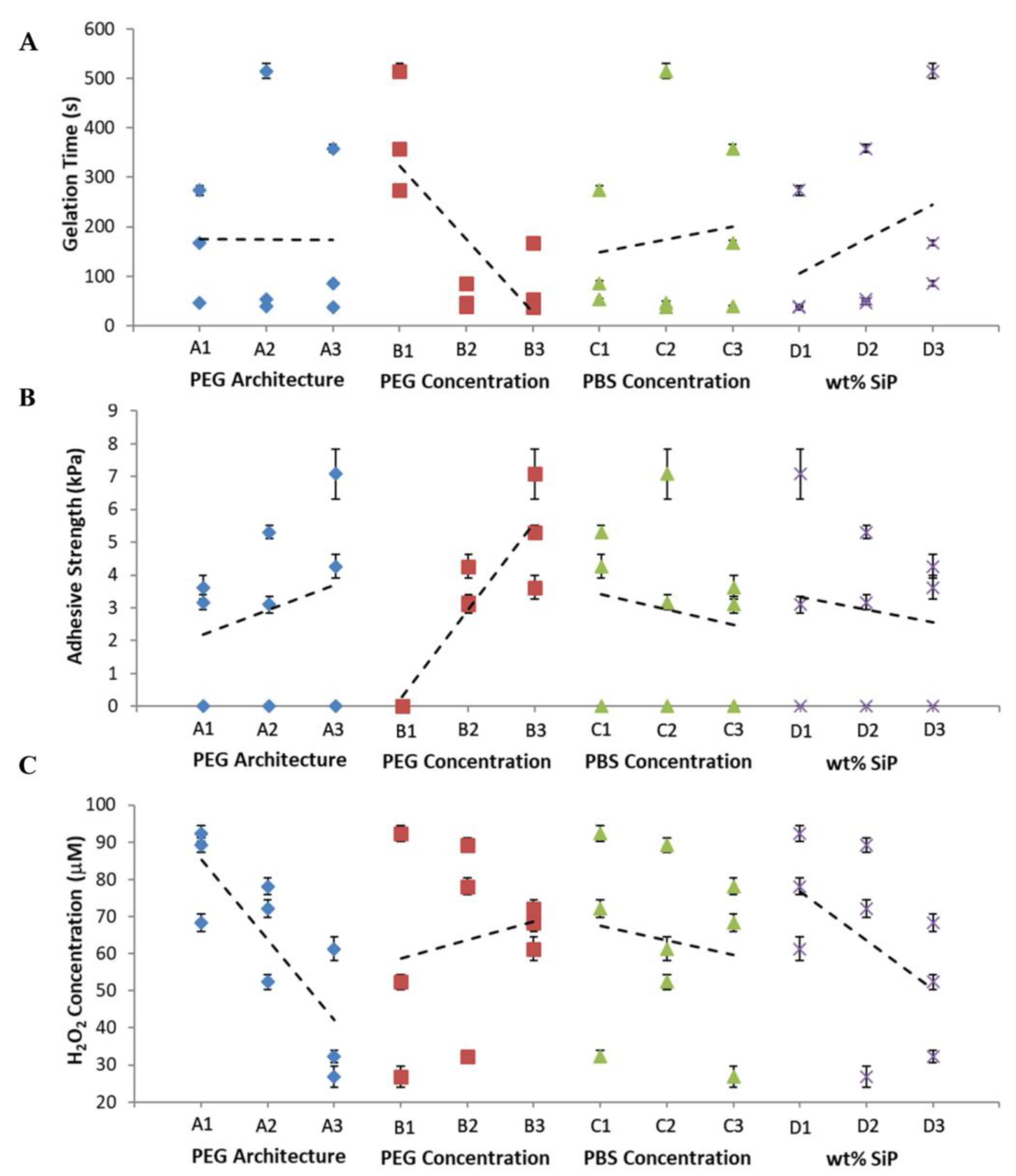
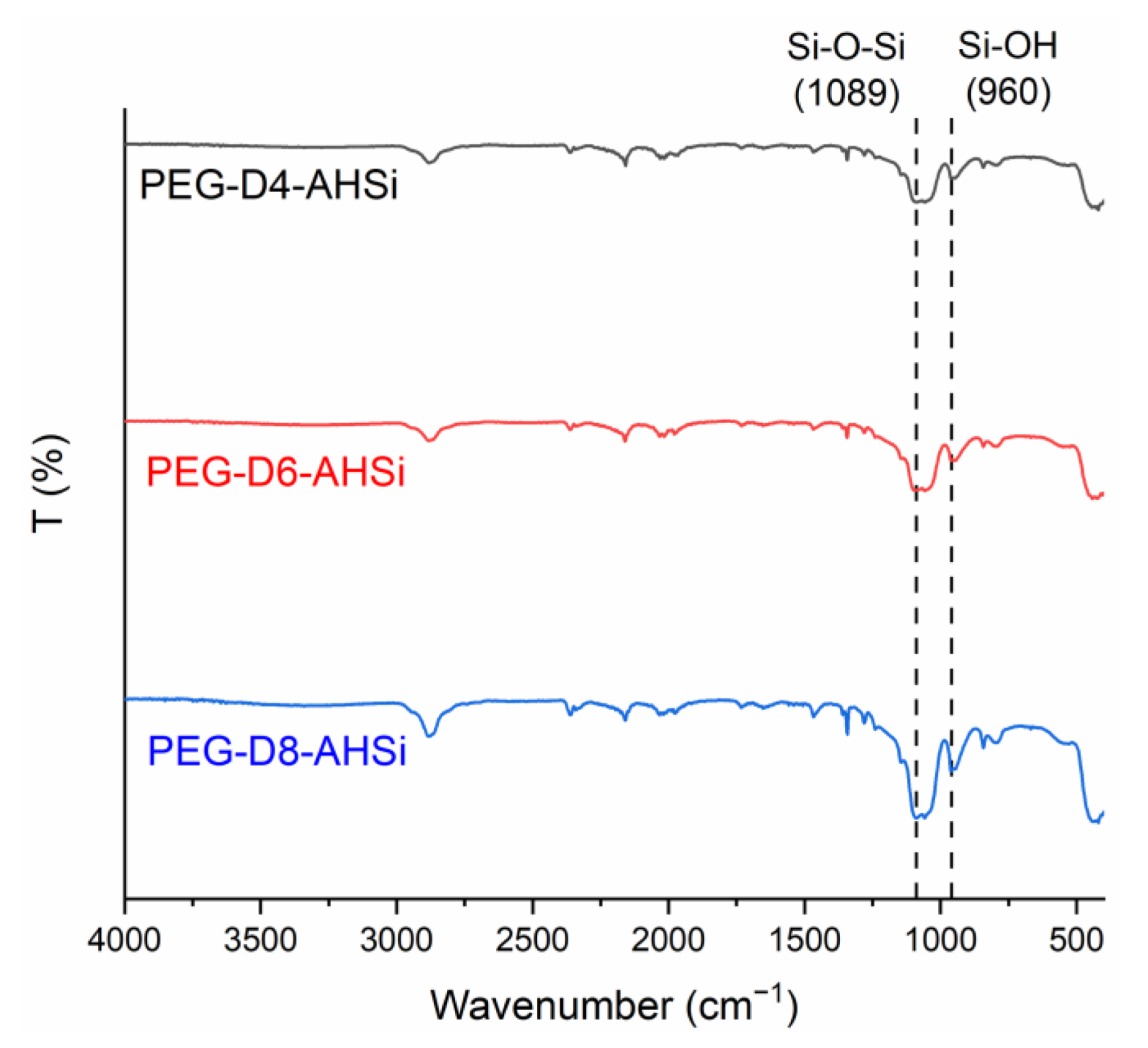
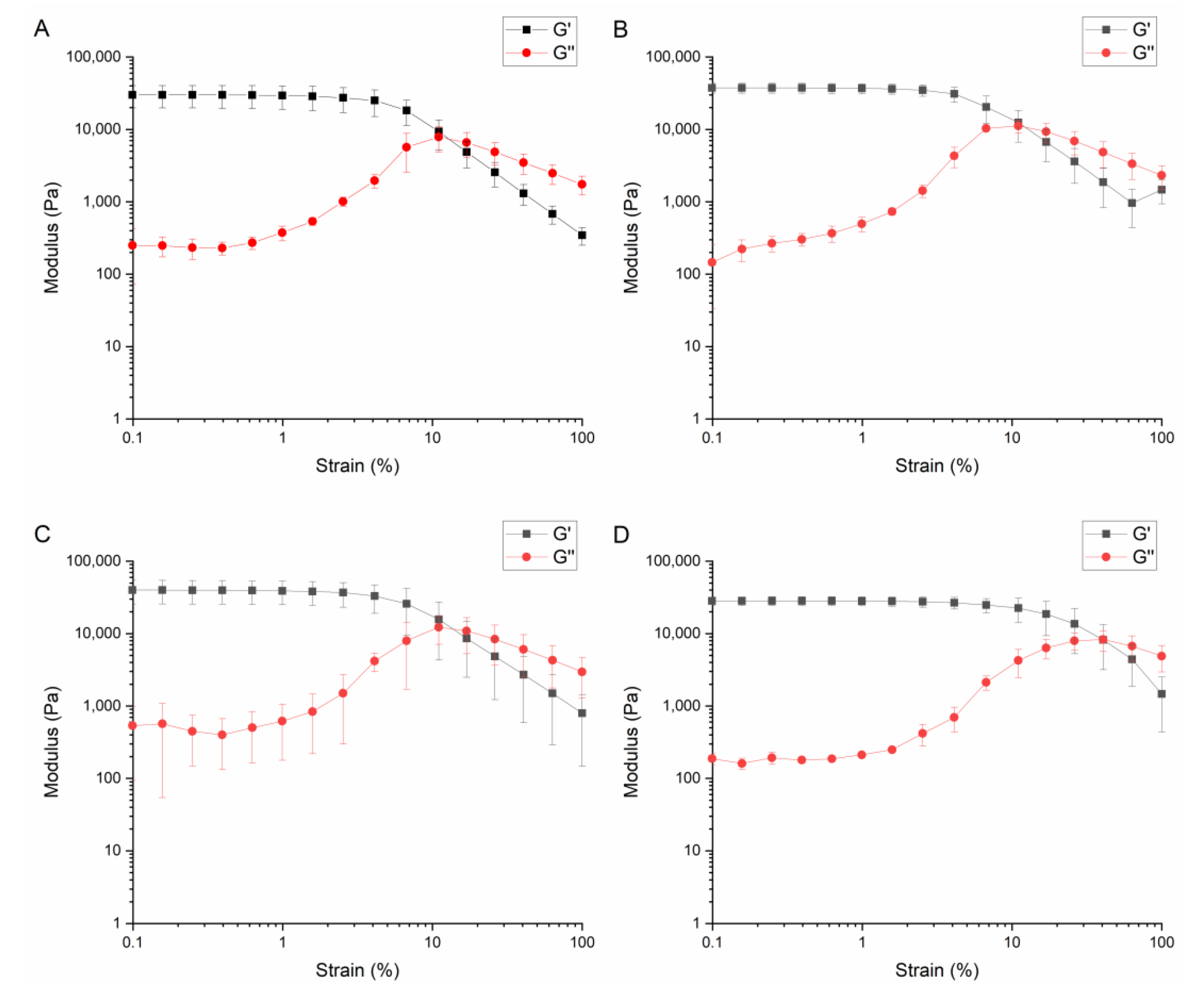




| Factor | |||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| PEG Architecture | PEG Conc. (mg/mL) | PBS Conc. | wt% SiP | ||
| Factor Level | 1 | 4-arm | 75 | 0.5× | 0 |
| 2 | 6-arm | 113 | 1× | 5 | |
| 3 | 8-arm | 150 | 2× | 10 | |
| Formulations | Factor | |||
|---|---|---|---|---|
| PEG Architecture | PEG Conc. (mg/mL) | PBS Conc. | wt% SiP | |
| 1 | 4-arm | 75 | 0.5× | 0 |
| 2 | 4-arm | 113 | 1× | 5 |
| 3 | 4-arm | 150 | 2× | 10 |
| 4 | 6-arm | 75 | 1× | 10 |
| 5 | 6-arm | 113 | 2× | 0 |
| 6 | 6-arm | 150 | 0.5× | 5 |
| 7 | 8-arm | 75 | 2× | 5 |
| 8 | 8-arm | 113 | 0.56× | 10 |
| 9 | 8-arm | 150 | 1× | 0 |
| Factor | % Relative Variation | ||
|---|---|---|---|
| Gelation Time | Adhesive Strength | H2O2 Concentration | |
| PEG Architecture | 1.1% | 3.4% | 65.6% |
| PEG Concentration | 78.6% | 93.8% | 8.2% |
| PBS Concentration | 2.1% | 2.4% | 5.5% |
| SiP wt% | 18.2% | 0.47% | 20.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinnaratip, R.; Zhang, Z.; Smies, A.; Forooshani, P.K.; Tang, X.; Rajachar, R.M.; Lee, B.P. Utilizing Robust Design to Optimize Composite Bioadhesive for Promoting Dermal Wound Repair. Polymers 2023, 15, 1905. https://doi.org/10.3390/polym15081905
Pinnaratip R, Zhang Z, Smies A, Forooshani PK, Tang X, Rajachar RM, Lee BP. Utilizing Robust Design to Optimize Composite Bioadhesive for Promoting Dermal Wound Repair. Polymers. 2023; 15(8):1905. https://doi.org/10.3390/polym15081905
Chicago/Turabian StylePinnaratip, Rattapol, Zhongtian Zhang, Ariana Smies, Pegah Kord Forooshani, Xiaoqing Tang, Rupak M Rajachar, and Bruce P. Lee. 2023. "Utilizing Robust Design to Optimize Composite Bioadhesive for Promoting Dermal Wound Repair" Polymers 15, no. 8: 1905. https://doi.org/10.3390/polym15081905
APA StylePinnaratip, R., Zhang, Z., Smies, A., Forooshani, P. K., Tang, X., Rajachar, R. M., & Lee, B. P. (2023). Utilizing Robust Design to Optimize Composite Bioadhesive for Promoting Dermal Wound Repair. Polymers, 15(8), 1905. https://doi.org/10.3390/polym15081905







