Wood−Derived Polymers from Olefin−Functionalized Lignin and Ethyl Cellulose via Thiol–Ene Click Chemistry
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of Olefin−Functionalized Ethyl Cellulose (OFE)
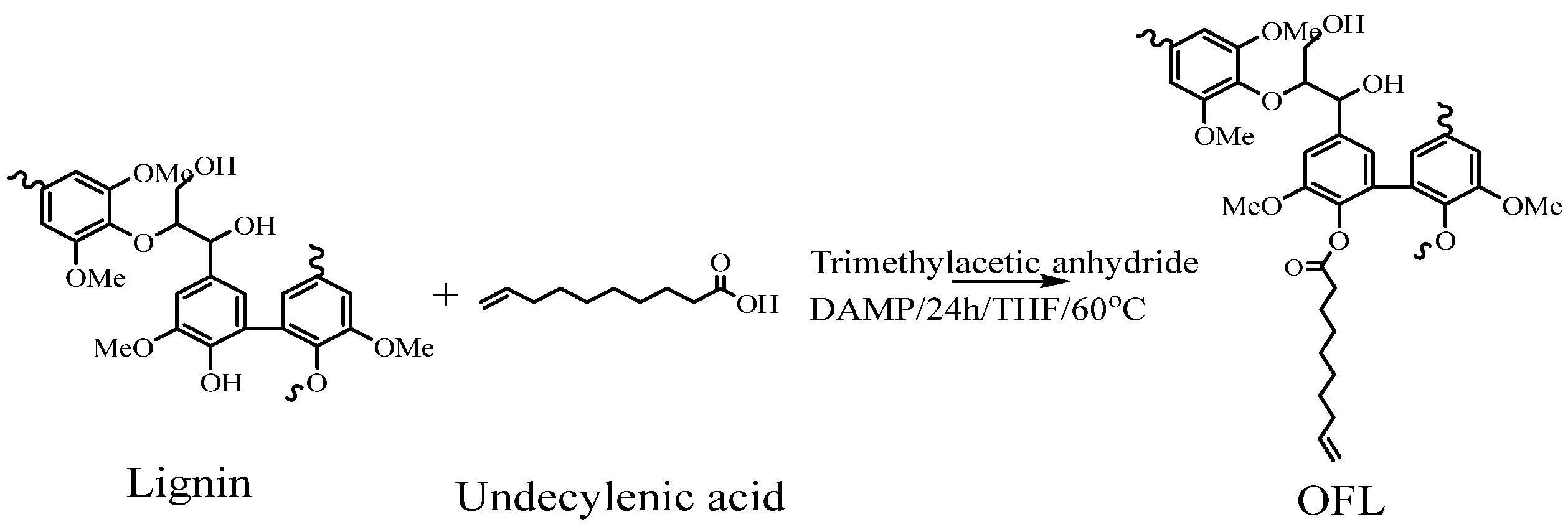
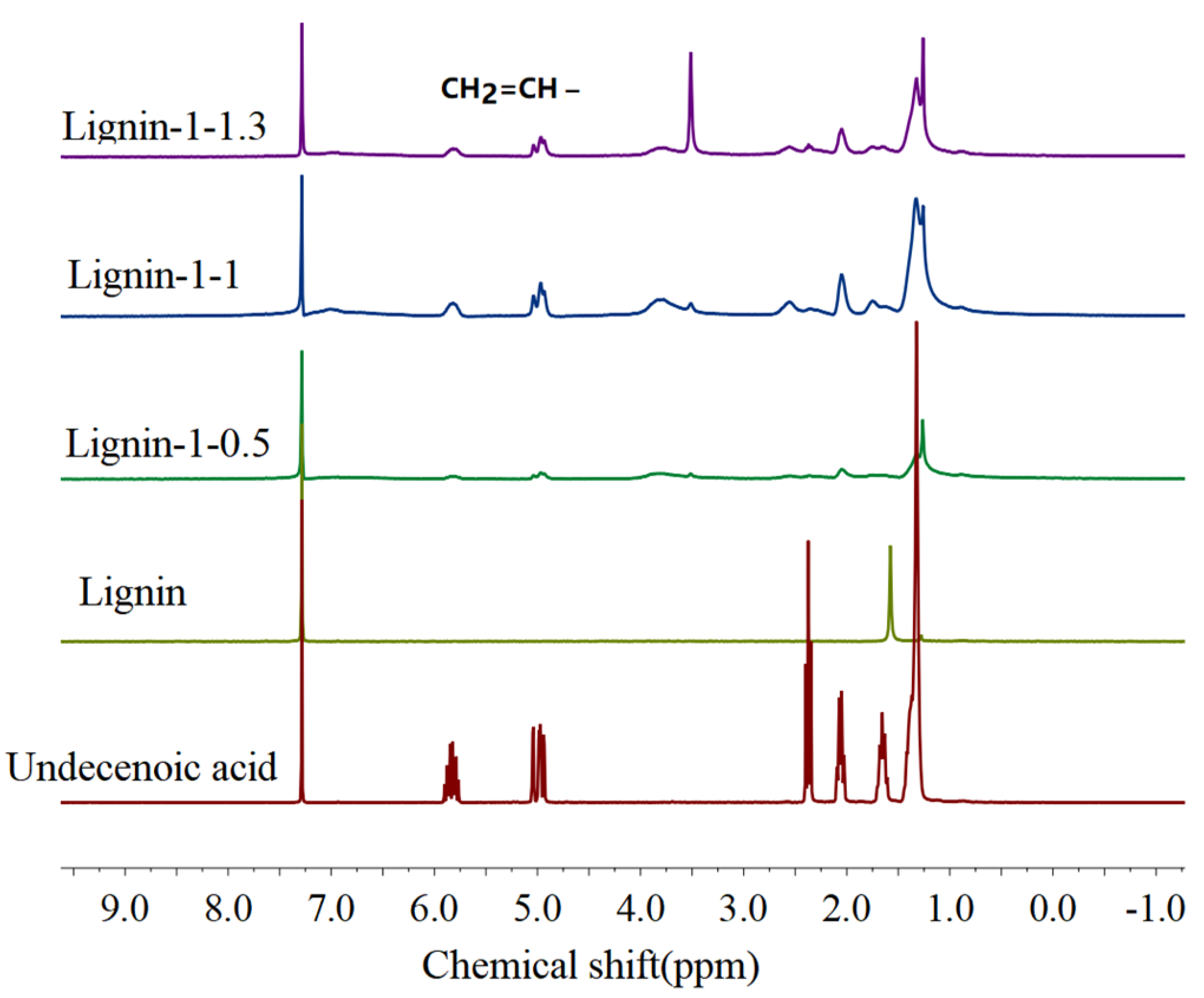
2.3. Preparation of Olefin−Functionalized Lignin (OFL)
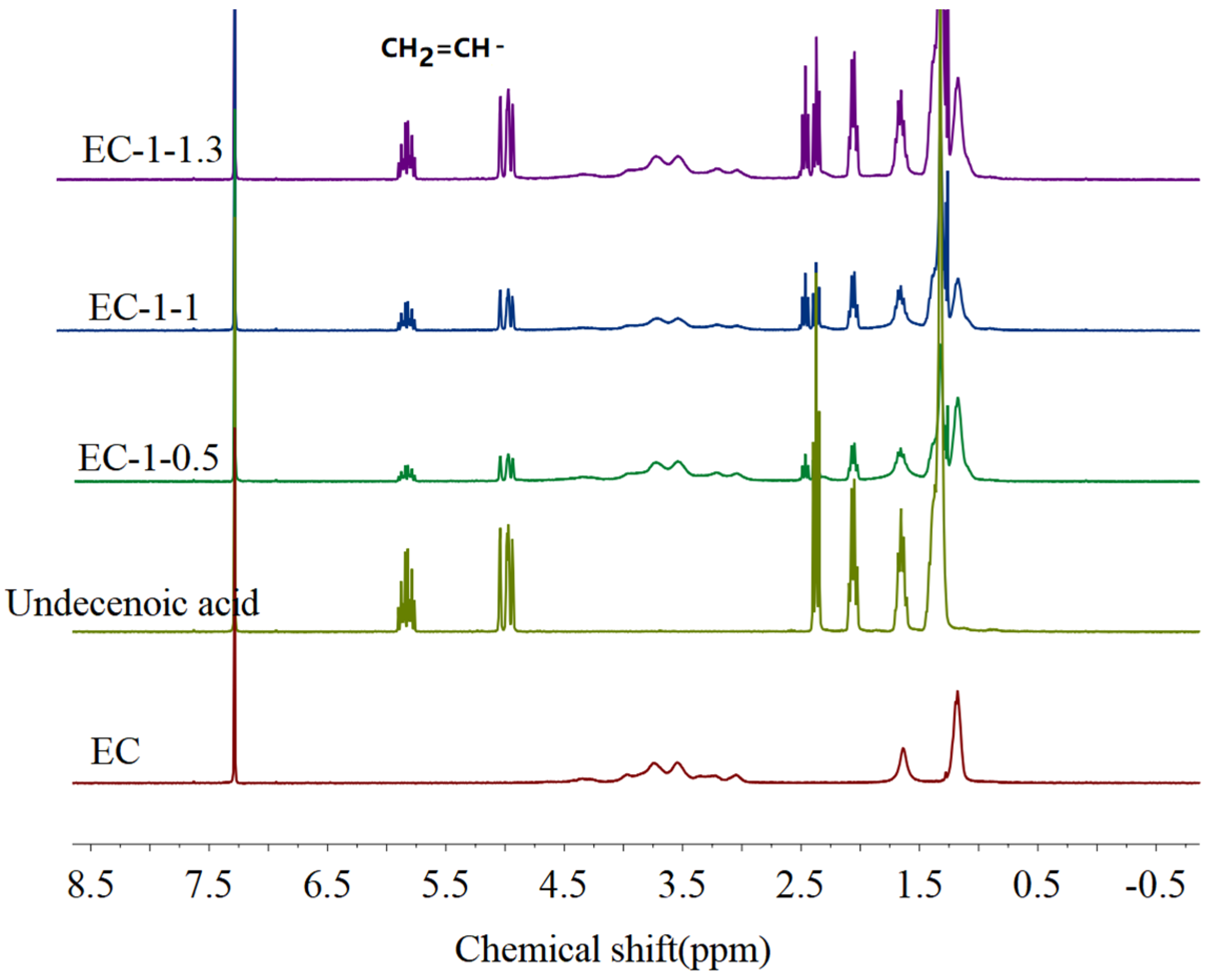
2.4. Determination of Olefin Group Concentration in OFE and OFL
2.5. Preparation of Wood−Derived Polymers through the Chemical Cross−Linking of OFE and OFL via Thiol–Ene Click Chemistry
2.6. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, M.; Chen, C.; Song, B.; Shen, M.; Cao, W.; Yang, H.; Zeng, G.; Gong, J. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Sereda, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M.; et al. Bio−Based Poly(butylene succinate)/Microcrystalline Cellulose/Nanofibrillated Cellulose−Based Sustainable Polymer Composites: Thermo−Mechanical and Biodegradation Studies. Polymers 2020, 12, 1472. [Google Scholar] [CrossRef] [PubMed]
- Calvino, C.; Macke, N.; Kato, R.; Rowan, S.J. Development, processing and applications of bio−sourced cellulose nanocrystal composites. Prog. Polym. Sci. 2020, 103, 101221. Available online: https://www.sciencedirect.com/science/article/pii/S0079670020300149 (accessed on 10 April 2023). [CrossRef]
- Li, C.; Wu, J.; Shi, H.; Xia, Z.; Sahoo, J.K.; Yeo, J.; Kaplan, D.L. Fiber-Based Biopolymer Processing as a Route toward Sustainability. Adv. Mater. 2022, 34, 2105196. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Gigante, V.; Bagherzadeh, R.; Mezzetta, A.; Milazzo, M.; Guazzelli, L.; Cinelli, P.; Lazzeri, A.; Danti, S. Cellulose−based fiber spinning processes using ionic liquids. Cellulose 2022, 29, 3079–3129. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent advances and future prospects of cellulose, starch, chitosan, polylactic acid and polyhydroxyalkanoates for sustainable food packaging applications. Int. J. Biol. Macromol. 2022, 221, 163–182. Available online: https://www.sciencedirect.com/science/article/pii/S0141813022019134 (accessed on 10 April 2023). [CrossRef]
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective Esterification and Etherification of Cellulose: A Review. Biomacromolecules 2011, 12, 1956–1972. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional nanomaterials through esterification of cellulose: A review of chemistry and application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef][Green Version]
- Shokri, Z.; Seidi, F.; Saeb, M.R.; Jin, Y.; Li, C.; Xiao, H. Elucidating the impact of enzymatic modifications on the structure, properties, and applications of cellulose, chitosan, starch and their derivatives: A review. Mater. Today Chem. 2022, 24, 100780. Available online: https://www.sciencedirect.com/science/article/pii/S246851942200009X (accessed on 10 April 2023). [CrossRef]
- Svinterikos, E.; Zuburtikudis, I.; Al−Marzouqi, M.H. Electrospun Lignin−Derived Carbon Micro− and Nanofibers: A Review on Precursors, Properties, and Applications. ACS Sustain. Chem. Eng. 2020, 8, 13868–13893. [Google Scholar] [CrossRef]
- Lee, D.-W.; Jin, M.-H.; Park, J.-H.; Lee, Y.-J.; Choi, Y.-C. Flexible Synthetic Strategies for Lignin−Derived Hierarchically Porous Carbon Materials. ACS Sustain. Chem. Eng. 2018, 6, 10454–10462. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, D.; Qiu, X.; Qian, Y.; Yan, M.; Li, Q. Influences of aggregation behavior of lignin on the microstructure and adsorptive properties of lignin−derived porous carbons by potassium compound activation. J. Ind. Eng. Chem. 2020, 82, 220–227. Available online: https://www.sciencedirect.com/science/article/pii/S1226086X19305532 (accessed on 10 April 2023). [CrossRef]
- Tian, Y.; Zhou, H. A novel nitrogen−doped porous carbon derived from black liquor for efficient removal of Cr(VI) and tetracycline: Comparison with lignin porous carbon. J. Clean. Prod. 2022, 333, 130106. Available online: https://www.sciencedirect.com/science/article/pii/S0959652621042724 (accessed on 10 April 2023). [CrossRef]
- Xi, Y.; Yang, D.; Lou, H.; Gong, Y.; Yi, C.; Lyu, G.; Han, W.; Kong, F.; Qiu, X. Designing the effective microstructure of lignin−based porous carbon substrate to inhibit the capacity decline for SnO2 anode. Ind. Crop. Prod. 2021, 161, 113179. Available online: https://www.sciencedirect.com/science/article/pii/S0926669020310967 (accessed on 10 April 2023). [CrossRef]
- Gong, X.; Meng, Y.; Lu, J.; Tao, Y.; Cheng, Y.; Wang, H. A Review on Lignin-Based Phenolic Resin Adhesive. Macromol. Chem. Phys. 2022, 223, 2100434. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, X.; Yang, G.; Li, Q.; Zhou, N. Preparation and characterization of a nanolignin phenol formaldehyde resin by replacing phenol partially with lignin nanoparticles. RSC Adv. 2019, 9, 29255–29262. [Google Scholar] [CrossRef]
- Huang, C.; Peng, Z.; Li, J.; Li, X.; Jiang, X.; Dong, Y. Unlocking the role of lignin for preparing the lignin−based wood adhesive: A review. Ind. Crop. Prod. 2022, 187, 115388. Available online: https://www.sciencedirect.com/science/article/pii/S0926669022008718 (accessed on 10 April 2023). [CrossRef]
- Weng, S.; Li, Z.; Bo, C.; Song, F.; Xu, Y.; Hu, L.; Zhou, Y.; Jia, P. Design lignin doped with nitrogen and phosphorus for flame retardant phenolic foam materials. React. Funct. Polym. 2023, 185, 105535. Available online: https://www.sciencedirect.com/science/article/pii/S138151482300038X (accessed on 10 April 2023). [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio−Based Alternatives to Phenol and Formaldehyde for the Production of Resins. Polymers 2020, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio−based active food packaging materials: Sustainable alternative to conventional petrochemical−based packaging materials. Food Res. Int. 2020, 137, 109625. Available online: https://www.sciencedirect.com/science/article/pii/S0963996920306505 (accessed on 10 April 2023). [CrossRef] [PubMed]
- Yang, W.; Rallini, M.; Natali, M.; Kenny, J.; Ma, P.; Dong, W.; Torre, L.; Puglia, D. Preparation and properties of adhesives based on phenolic resin containing lignin micro and nanoparticles: A comparative study. Mater. Des. 2019, 161, 55–63. Available online: https://www.sciencedirect.com/science/article/pii/S0264127518308396 (accessed on 10 April 2023). [CrossRef]
- Xu, Y.; Guo, L.; Zhang, H.; Zhai, H.; Ren, H. Research status, industrial application demand and prospects of phenolic resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef][Green Version]
- Firdaus, M. Thiol−Ene (Click) Reactions as Efficient Tools for Terpene Modification. Asian J. Org. Chem. 2017, 6, 1702–1714. [Google Scholar] [CrossRef]
- Firdaus, M.; Montero de Espinosa, L.; Meier, M.A.R. Terpene−Based Renewable Monomers and Polymers via Thiol–Ene Additions. Macromolecules 2011, 44, 7253–7262. [Google Scholar] [CrossRef]
- Ahangarpour, M.; Kavianinia, I.; Harris, P.W.R.; Brimble, M.A. Photo−induced radical thiol–ene chemistry: A versatile toolbox for peptide−based drug design. Chem. Soc. Rev. 2021, 50, 898–944. [Google Scholar] [CrossRef] [PubMed]
- Kazybayeva, D.S.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Thiol−Ene “Click Reactions” as a Promising Approach to Polymer Materials. Polym. Sci. Ser. B 2022, 64, 1–16. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol−Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Jawerth, M.; Johansson, M.; Lundmark, S.; Gioia, C.; Lawoko, M. Renewable Thiol–Ene Thermosets Based on Refined and Selectively Allylated Industrial Lignin. ACS Sustain. Chem. Eng. 2017, 5, 10918–10925. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Zheng, B.; Ou, R.; Fan, Q.; Li, L.; Guo, C.; Liu, T.; Wang, Q. Synthesis of lignin−based polyols via thiol−ene chemistry for high−performance polyurethane anticorrosive coating. Compos. Part B Eng. 2020, 200, 108295. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, P.; Li, X.; Yin, Y.; Chen, K.; Wang, C. Facile fabrication of durable superhydrophobic and oleophobic surface on cellulose substrate via thiol−ene click modification. Appl. Surf. Sci. 2019, 493, 1004–1012. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Y.; Wang, Z.; Han, Y.; Tang, C. Plant Oil and Lignin−Derived Elastomers via Thermal Azide–Alkyne Cycloaddition Click Chemistry. ACS Sustain. Chem. Eng. 2018, 7, 2593–2601. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Z.; Trenor, N.M.; Tang, C. Preparation and Applications of Polymers with Pendant Fatty Chains from Plant Oils. In Sustainable Polymers from Biomass; Wiley: Hoboken, NJ, USA, 2017; pp. 181–207. [Google Scholar] [CrossRef]
- Yan, K.; Wang, J.; Wang, Z.; Yuan, L. Bio−based monomers for amide−containing sustainable polymers. Chem. Commun. 2023, 59, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Yasa, S.R.; Cheguru, S.; Krishnasamy, S.; Korlipara, P.V.; Rajak, A.K.; Penumarthy, V. Synthesis of 10−undecenoic acid based C22−dimer acid esters and their evaluation as potential lubricant basestocks. Ind. Crop. Prod. 2017, 103, 141–151. Available online: https://www.sciencedirect.com/science/article/pii/S092666901730225X (accessed on 10 April 2023). [CrossRef]
- Kontham, V.; Ansari, K.R.; Padmaja, K.V. Tribological Properties of 10−Undecenoic Acid−Derived Schiff Base Lubricant Additives. Arab. J. Sci. Eng. 2021, 46, 5593–5603. [Google Scholar] [CrossRef]
- Lluch, C.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cadiz, V. “Click” Synthesis of Fatty Acid Derivatives as Fast−Degrading Polyanhydride Precursors. Macromol. Rapid Commun. 2011, 32, 1343–1351. [Google Scholar] [CrossRef]
- Çiçek, S.S.; Girreser, U.; Zidorn, C. Quantification of the total amount of black cohosh cycloartanoids by integration of one specific 1 H NMR signal. J. Pharm. Biomed. Anal. 2018, 155, 109–115. Available online: https://www.sciencedirect.com/science/article/pii/S0731708518303376 (accessed on 10 April 2023). [CrossRef]
- Crenshaw, M.D.; Tefft, M.E.; Buehler, S.S.; Brinkman, M.C.; Clark, P.I.; Gordon, S.M. Determination of nicotine, glycerol, propylene glycol and water in electronic cigarette fluids using quantitative 1 H NMR. Magn. Reson. Chem. 2016, 54, 901–904. [Google Scholar] [CrossRef][Green Version]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate−Based Materials: A Review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef][Green Version]
- Lucena, M.d.C.C.; de Alencar, A.E.V.; Mazzeto, S.E.; Soares, S.d.A. The effect of additives on the thermal degradation of cellulose acetate. Polym. Degrad. Stab. 2003, 80, 149–155. Available online: https://www.sciencedirect.com/science/article/pii/S0141391002003968 (accessed on 10 April 2023). [CrossRef]
- Mülhaupt, R. Green Polymer Chemistry and Bio−based Plastics: Dreams and Reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Williams, P.T.; Onwudili, J. Subcritical and Supercritical Water Gasification of Cellulose, Starch, Glucose, and Biomass Waste. Energy Fuels 2006, 20, 1259–1265. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Liu, C.; Hu, L.; Feng, G.; Bo, C.; Zhou, Y. Effect of chlorinated phosphate ester based on castor oil on thermal degradation of poly (vinyl chloride) blends and its flame retardant mechanism as secondary plasticizer. RSC Adv. 2015, 5, 41169–41178. [Google Scholar] [CrossRef]
- Jia, P.; Hu, L.; Zhang, M.; Zhou, Y.-H. TG−FTIR and TG−MS analysis applied to study the flame retardancy of PVC–castor oil−based chlorinated phosphate ester blends. J. Therm. Anal. Calorim. 2016, 124, 1331–1339. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Hu, L.; Liu, C.; Feng, G.; Yang, X.; Bo, C.; Zhou, Y. Development of a vegetable oil based plasticizer for preparing flame retardant poly(vinyl chloride) materials. RSC Adv. 2015, 5, 76392–76400. [Google Scholar] [CrossRef]

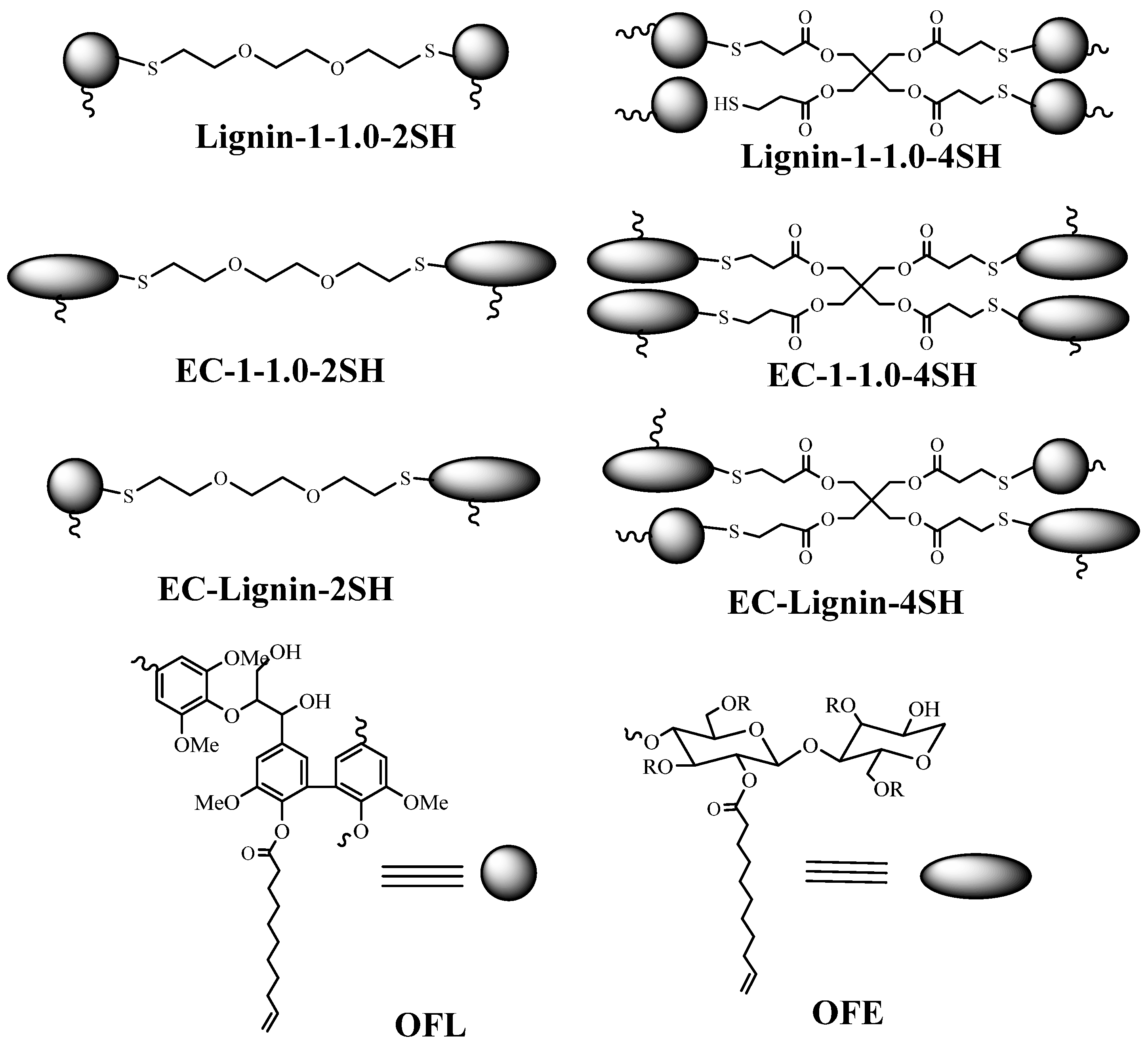

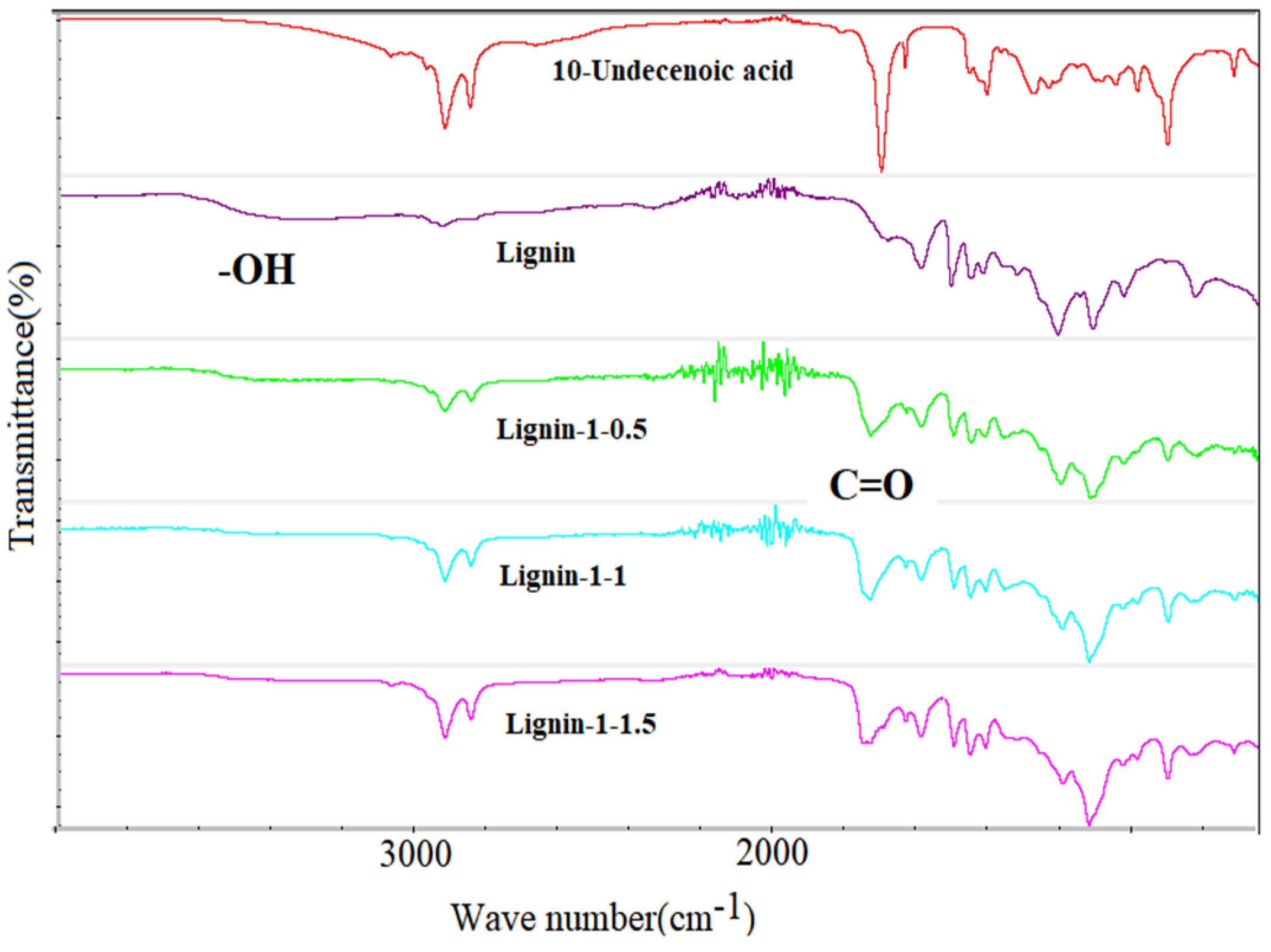
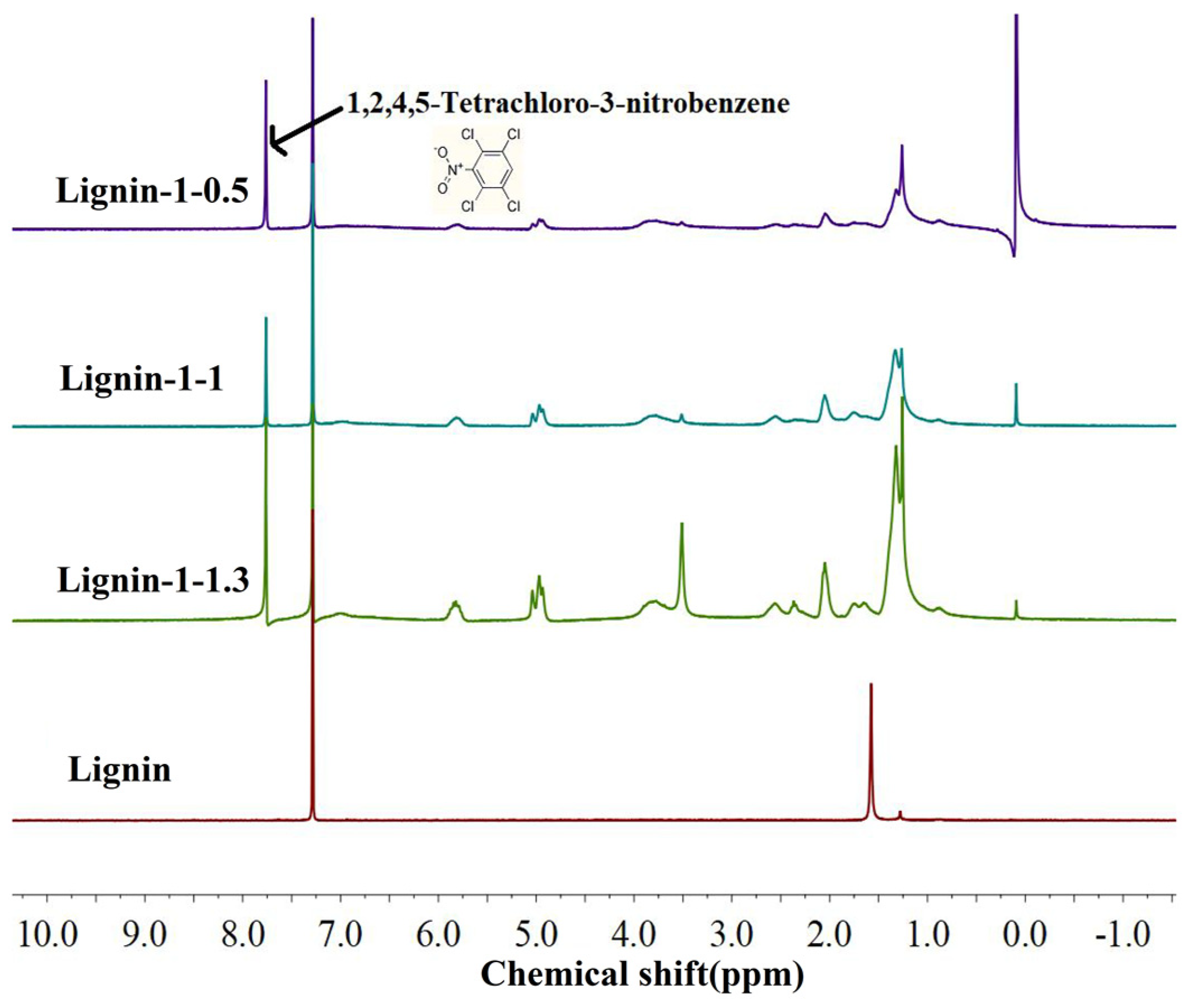

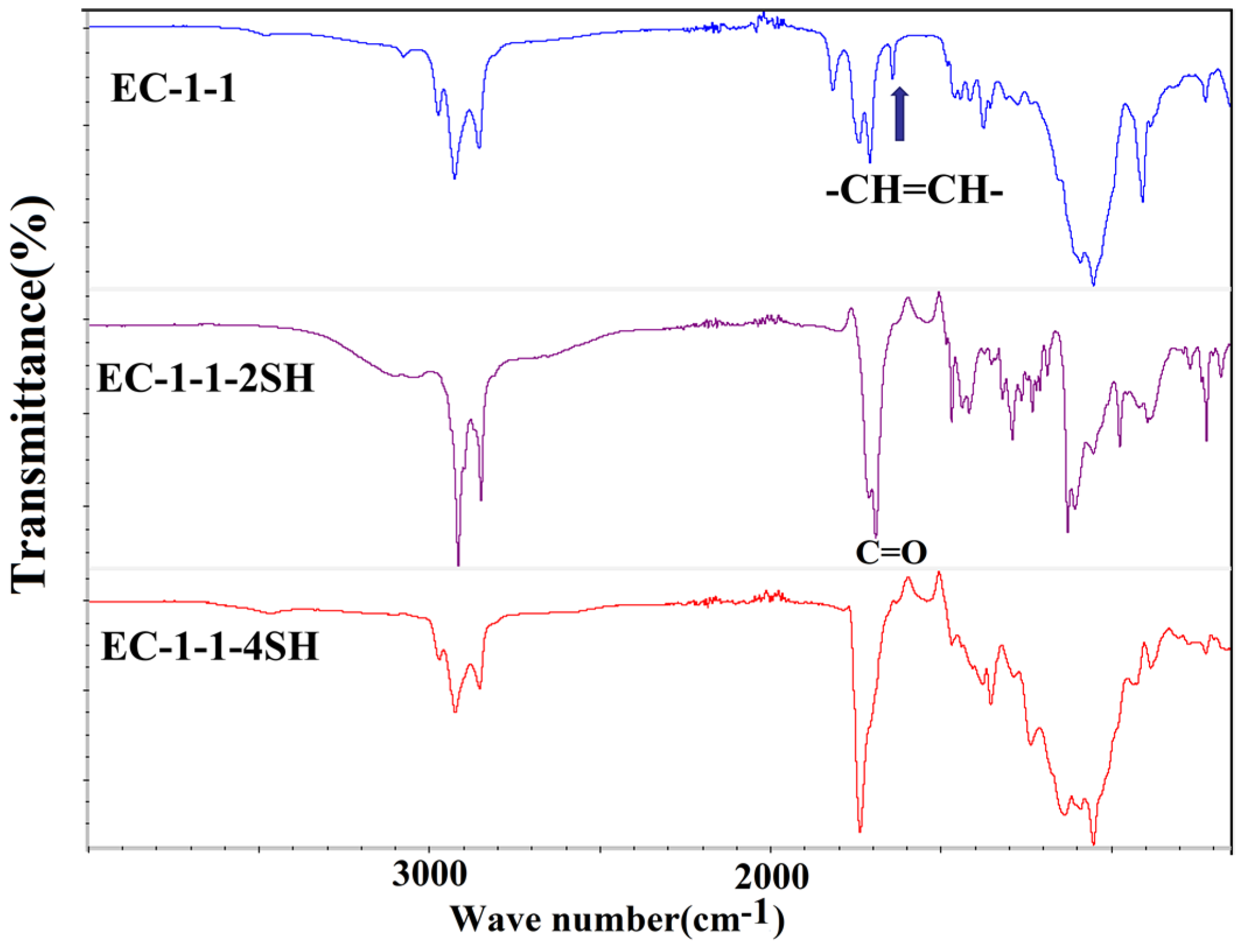


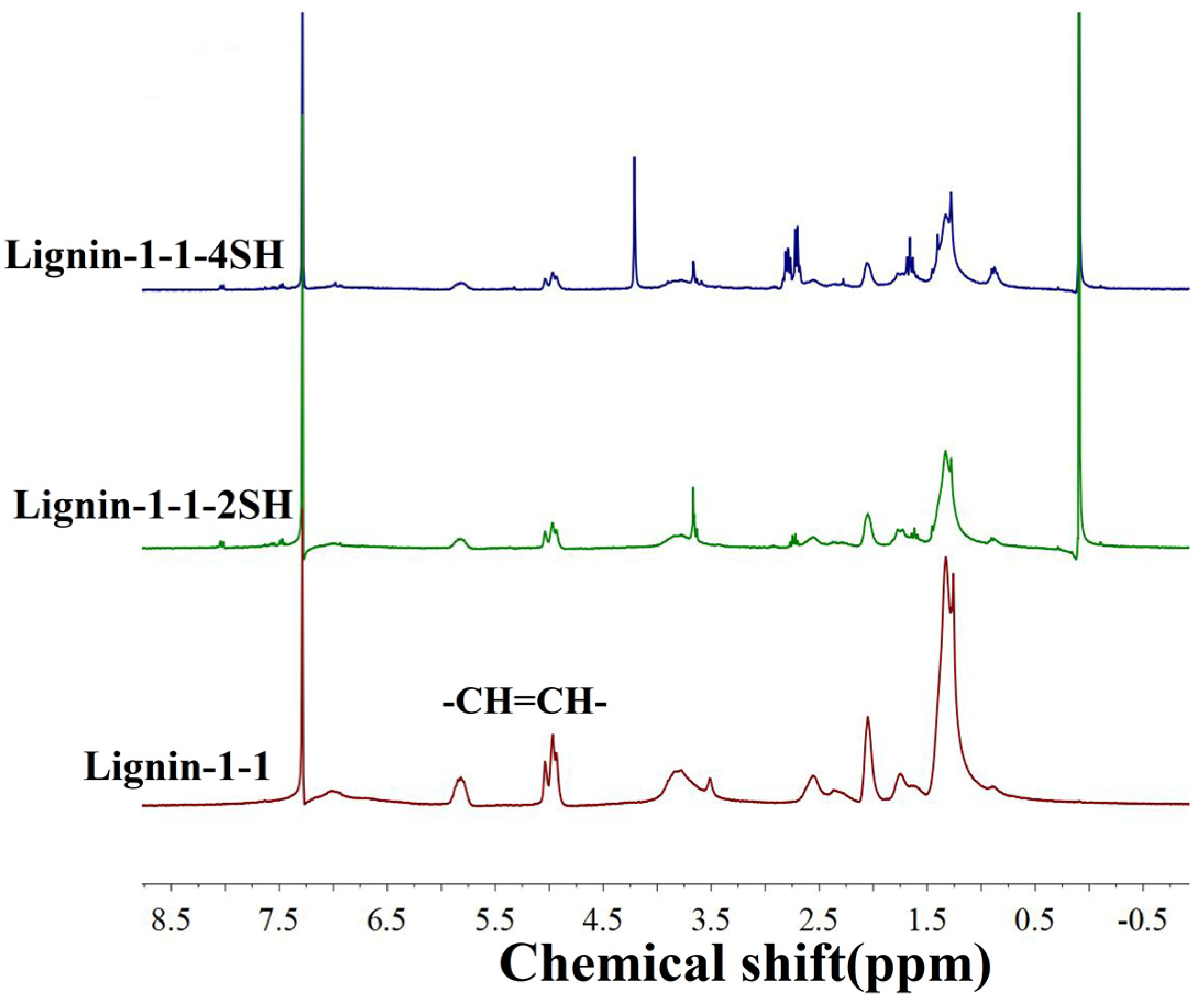
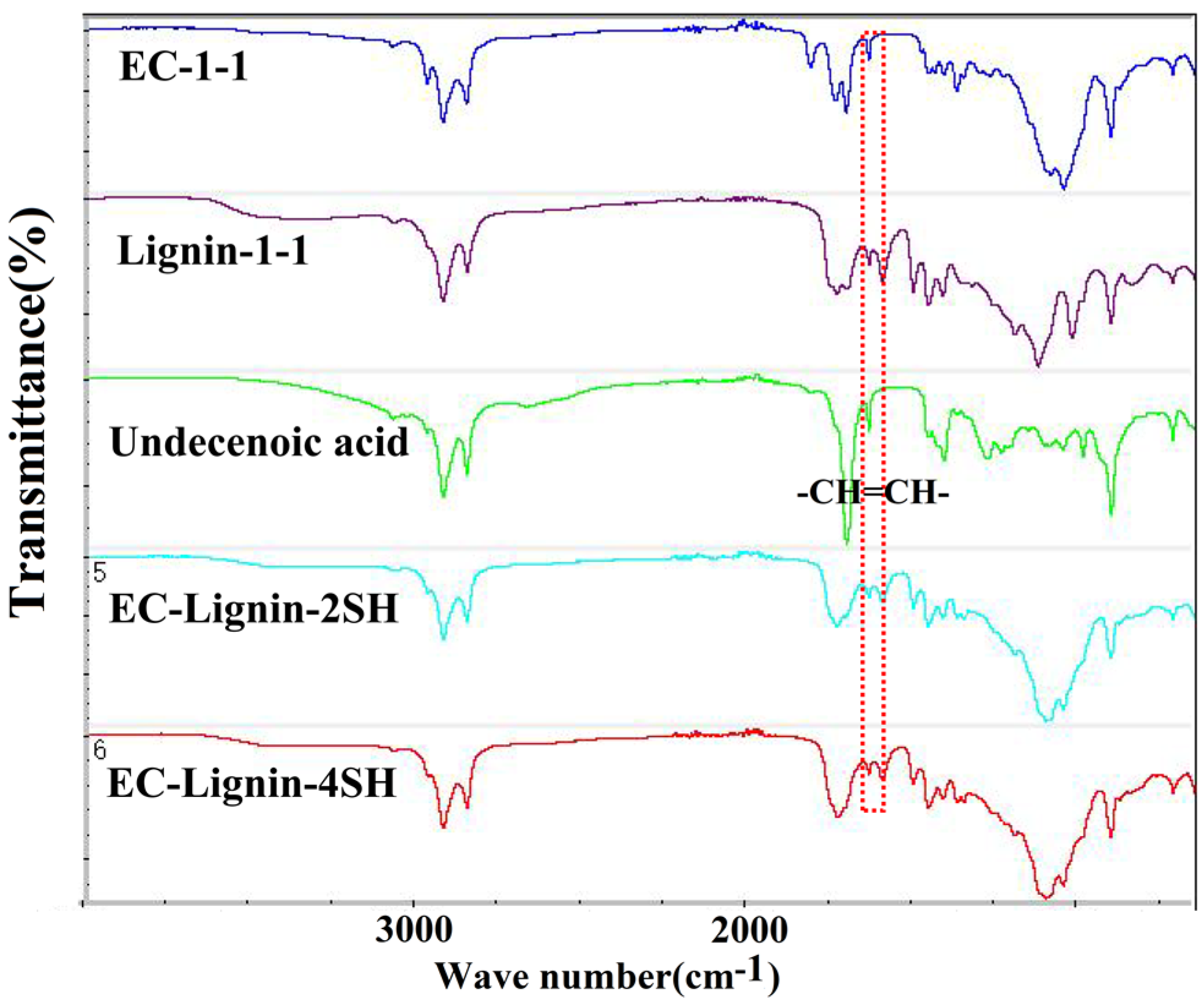



| Cross−linked Polymer | Reactants |
|---|---|
| EC−1−0.5−2SH | EC−1−0.5 and 3,6−Dioxa−1,8−octanedithiol, n(−CH=CH−) = n(−SH) |
| EC−1−1.0−2SH | EC−1−1.0 and 3,6−Dioxa−1,8−octanedithiol, n(−CH=CH−) = n(−SH) |
| EC−1−1.3−2SH | EC−1−1.3 and 3,6−Dioxa−1,8−octanedithiol, n(−CH=CH−) = n(−SH) |
| EC−1−0.5−4SH | EC−1−0.5 and pentaerythritol tetra(3−mercaptopropionate), n(−CH=CH−) = n(−SH) |
| EC−1−1.0−4SH | EC−1−1.0 and pentaerythritol tetra(3−mercaptopropionate), n(−CH=CH−) = n(−SH) |
| EC−1−1.3−4SH | EC−1−1.3 and pentaerythritol tetra(3−mercaptopropionate), n(−CH=CH−) = n(−SH) |
| EC−Lignin−2SH | EC−1−1, Lignin−1−1, and 3,6−Dioxa−1,8−octanedithiol, n(−CH=CH−)/n(−CH=CH−)/n(−SH) = 0.5:0.5:1 |
| EC−Lignin−4SH | EC−1−1, lignin−1−1, and pentaerythritol tetra(3−mercaptopropionate), n(−CH=CH−)/n(−CH=CH−)/n(−SH) = 0.5:0.5:1 |
| Lignin−1−1−2SH | Lignin−1−1 and 3,6−Dioxa−1,8−octanedithiol, n(−CH=CH−) = n(−SH) |
| Lignin−1−1−4SH | Lignin−1−1 and pentaerythritol tetra(3−mercaptopropionate), n(−CH=CH−) = n(−SH) |
| OFL and OFE Samples | Olefin Group Concentration in OFL and OFE (mmol/g) |
|---|---|
| Lignin−1−0.5 | 1.8060 |
| Lignin−1−1 | 2.3818 |
| Lignin−1−1.3 | 2.8096 |
| EC−1−0.5 | 2.9200 |
| EC−1−1 | 3.5873 |
| EC−1−1.3 | 3.7000 |
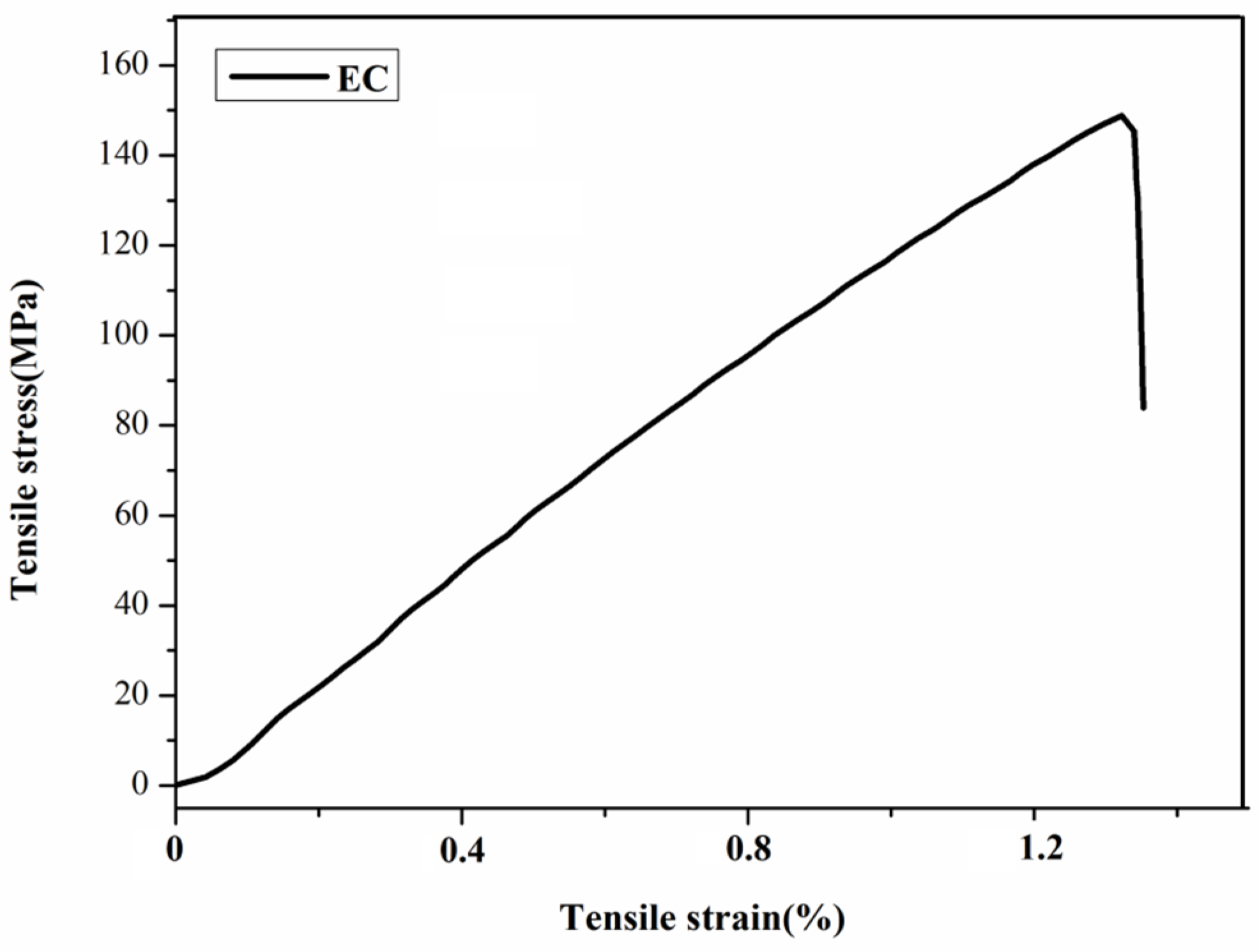
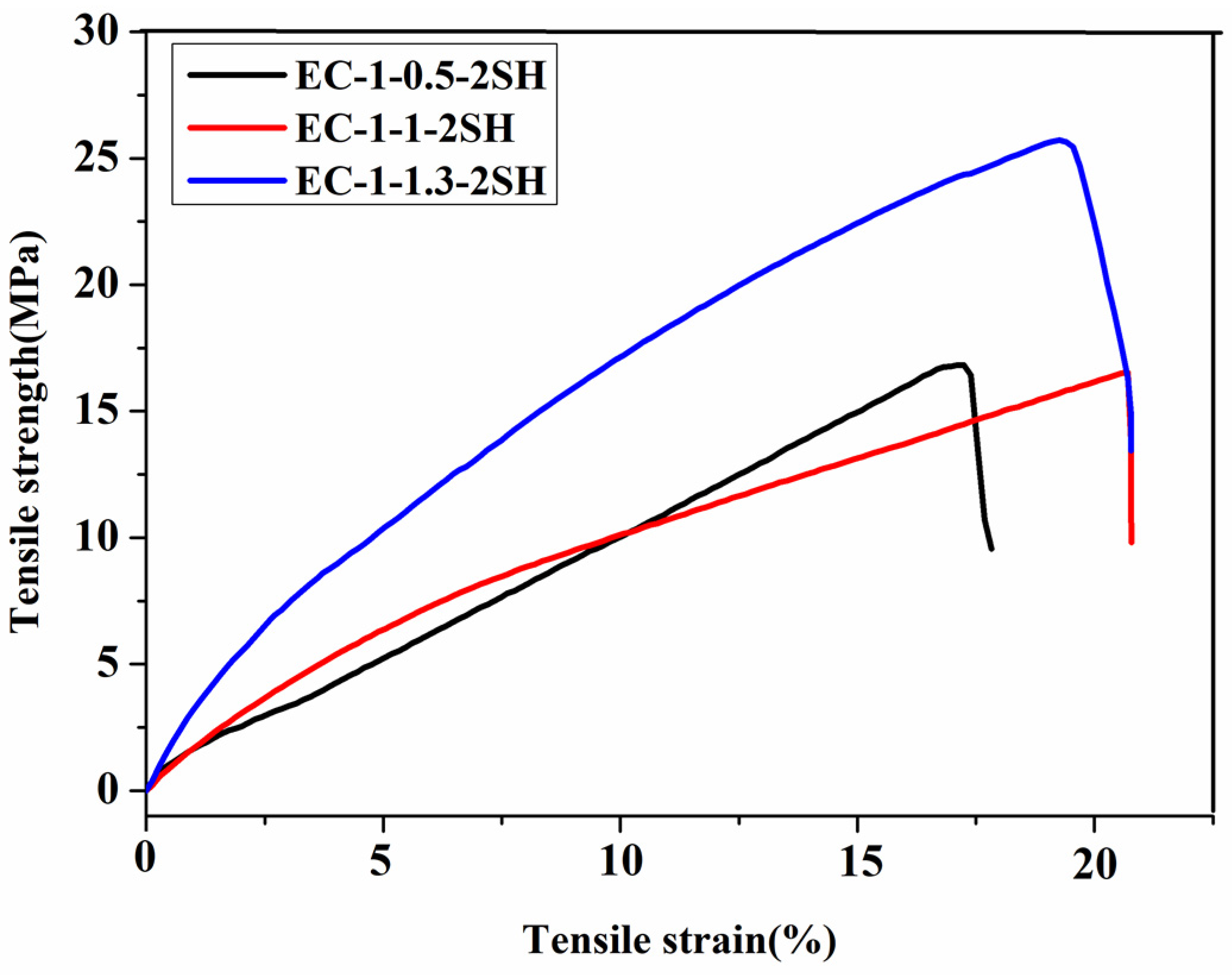
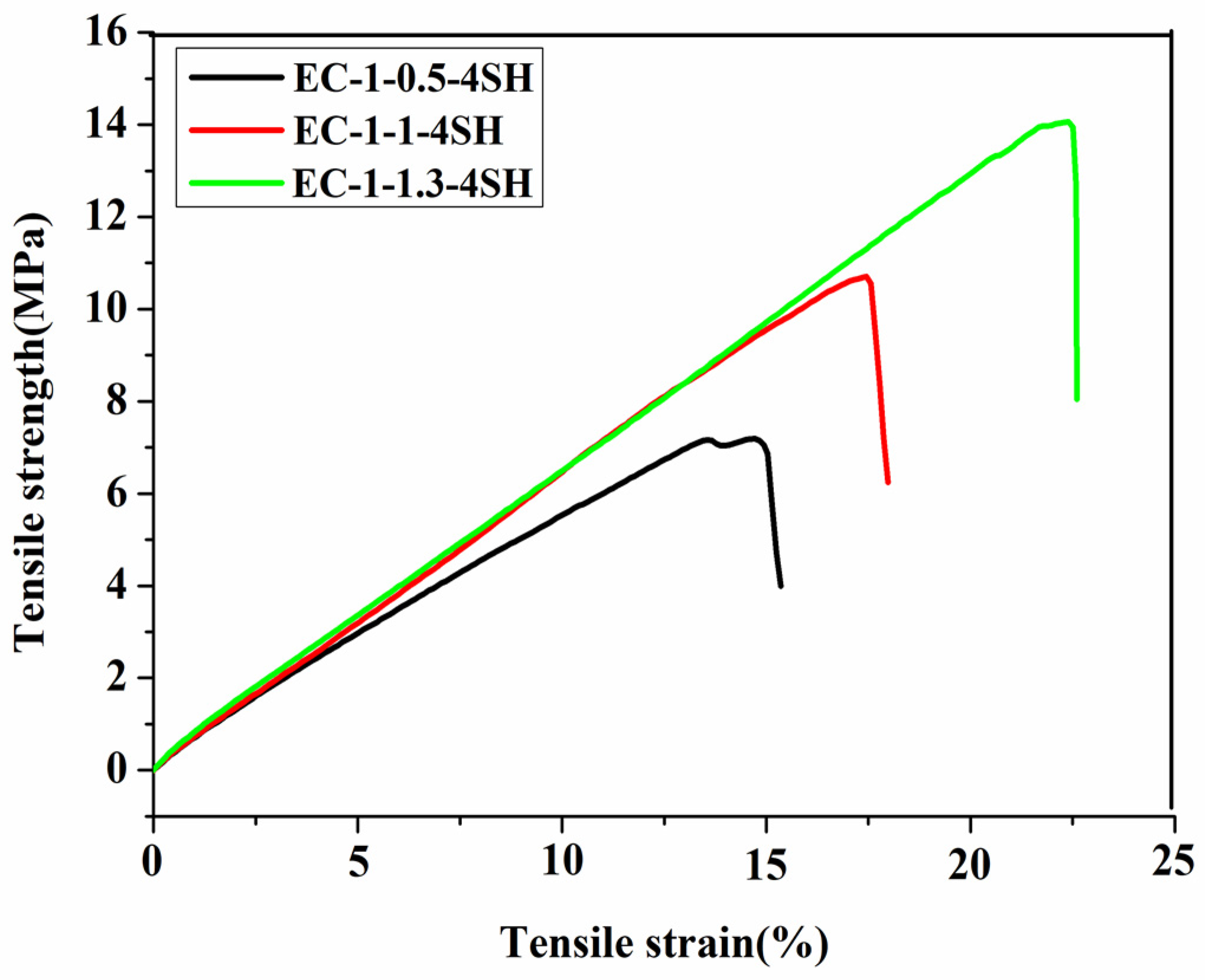
| Polymer Films | Tensile Stress (MPa) | Tensile Strain (%) | Cgel (%) |
|---|---|---|---|
| EC | 146.89 ± 10.24 | 1.32 ± 0.02 | − |
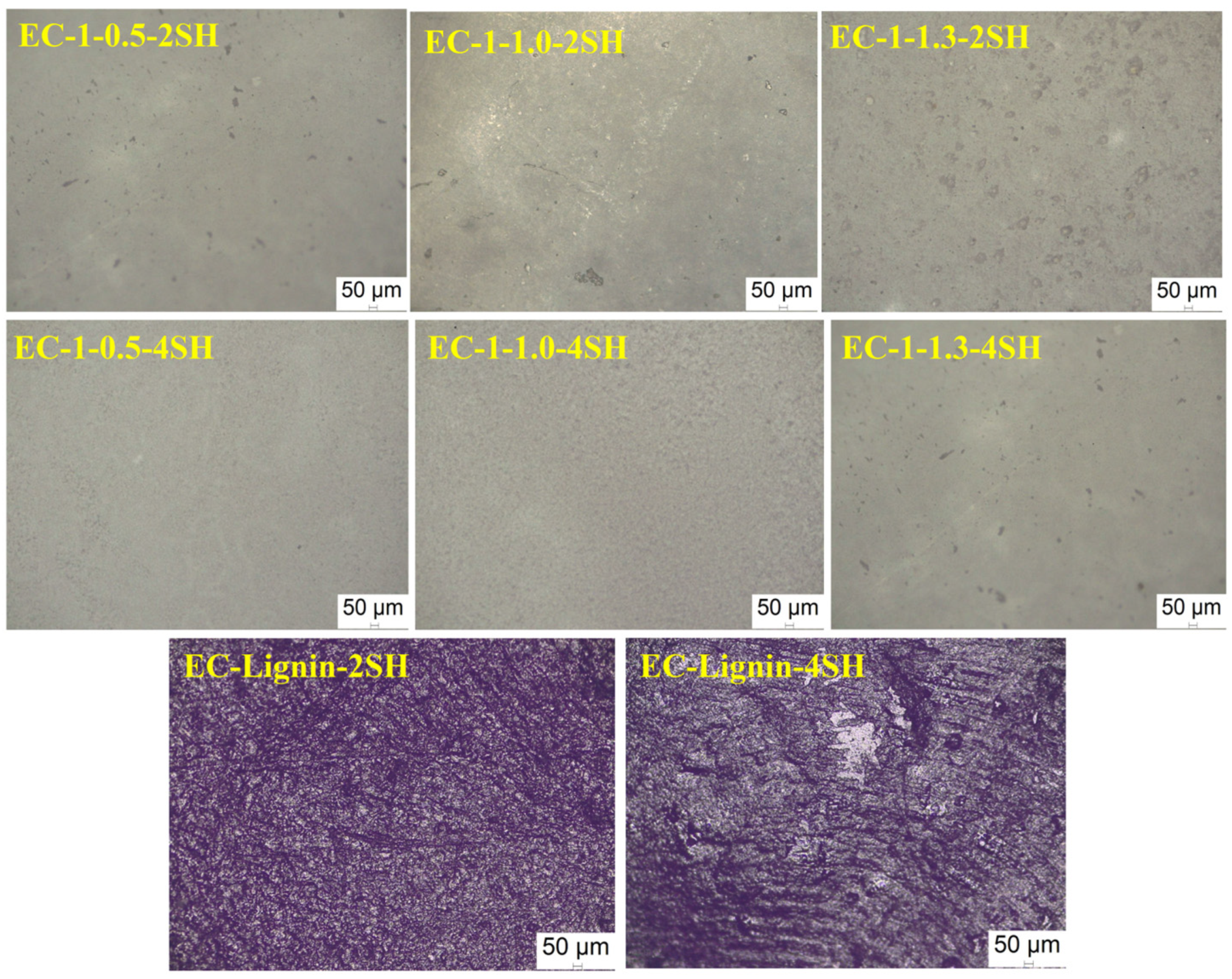
| EC−1−0.5−2SH | 16.41 ± 1.22 | 17.25 ± 0.19 | 94.9 ± 0.8 |
| EC−1−1.0−2SH | 16.53 ± 1.10 | 19.41 ± 2.18 | 96.4 ± 0.4 |
| EC−1−1.3−2SH | 23.59 ± 3.01 | 18.12 ± 0.13 | 98.0 ± 0.4 |
| EC−1−0.5−4SH | 6.86 ± 0.21 | 14.85 ± 1.11 | 92.7 ± 0.6 |
| EC−1−1.0−4SH | 10.55 ± 0.89 | 16.92 ± 1.76 | 94.3 ± 0.7 |
| EC−1−1.3−4SH | 13.94 ± 1.00 | 22.38 ± 3.20 | 95.2 ± 0.9 |
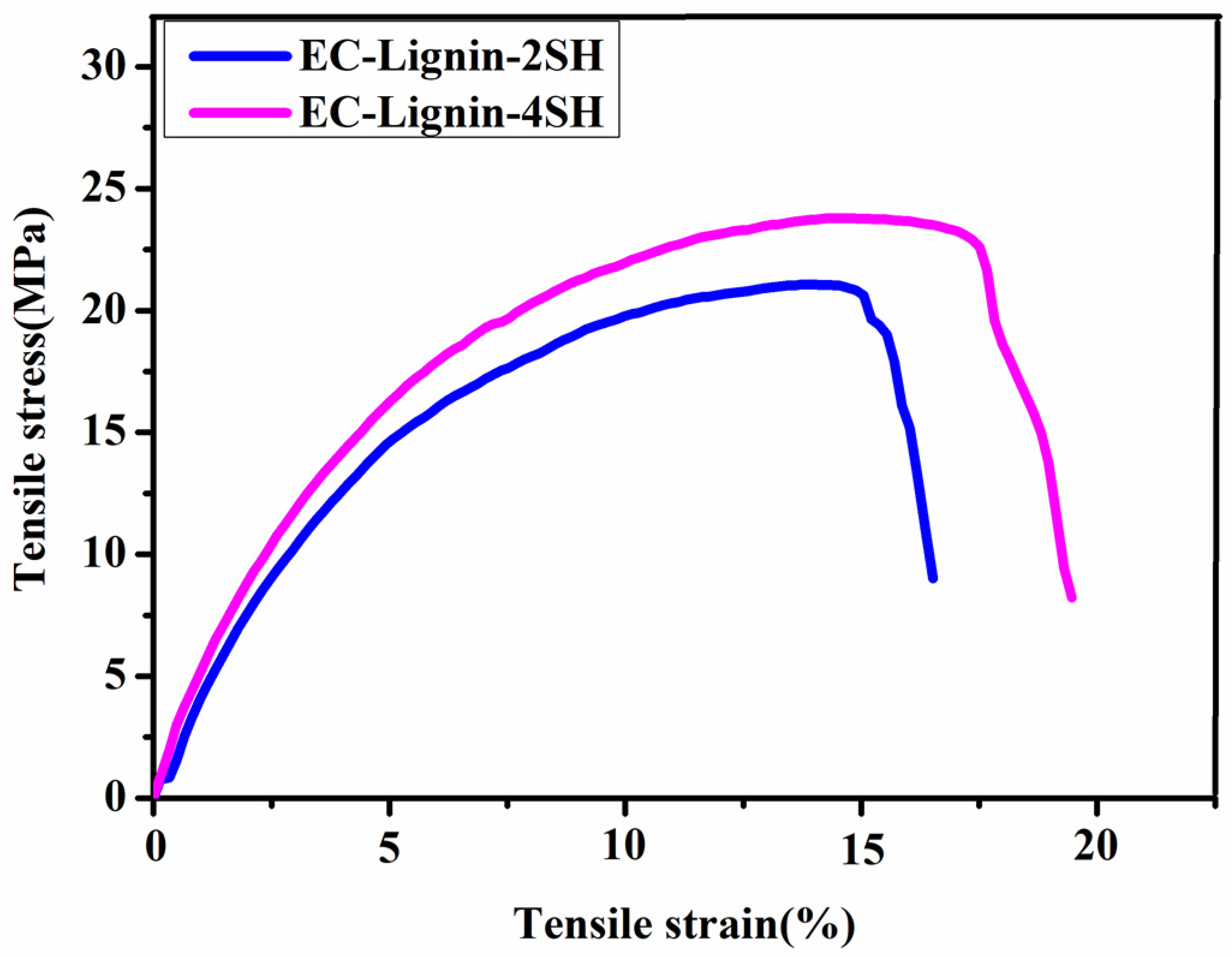
| EC−Lignin−2SH | 13.72 ± 0.02 | 15.12 ± 1.19 | 94.8 ± 0.2 |
| EC−Lignin−4SH | 15.19 ± 1.03 | 17.25 ± 2.10 | 97.2 ± 0.5 |
| Lignin−1−1−2SH | − | − | − |
| Lignin−1−1−4SH | − | − | − |
| Cross−linked Polymers | Td (°C) | TP (°C) | Char Residue (%) |
|---|---|---|---|
| EC | 339.8 | 364.1 | 0.9 |
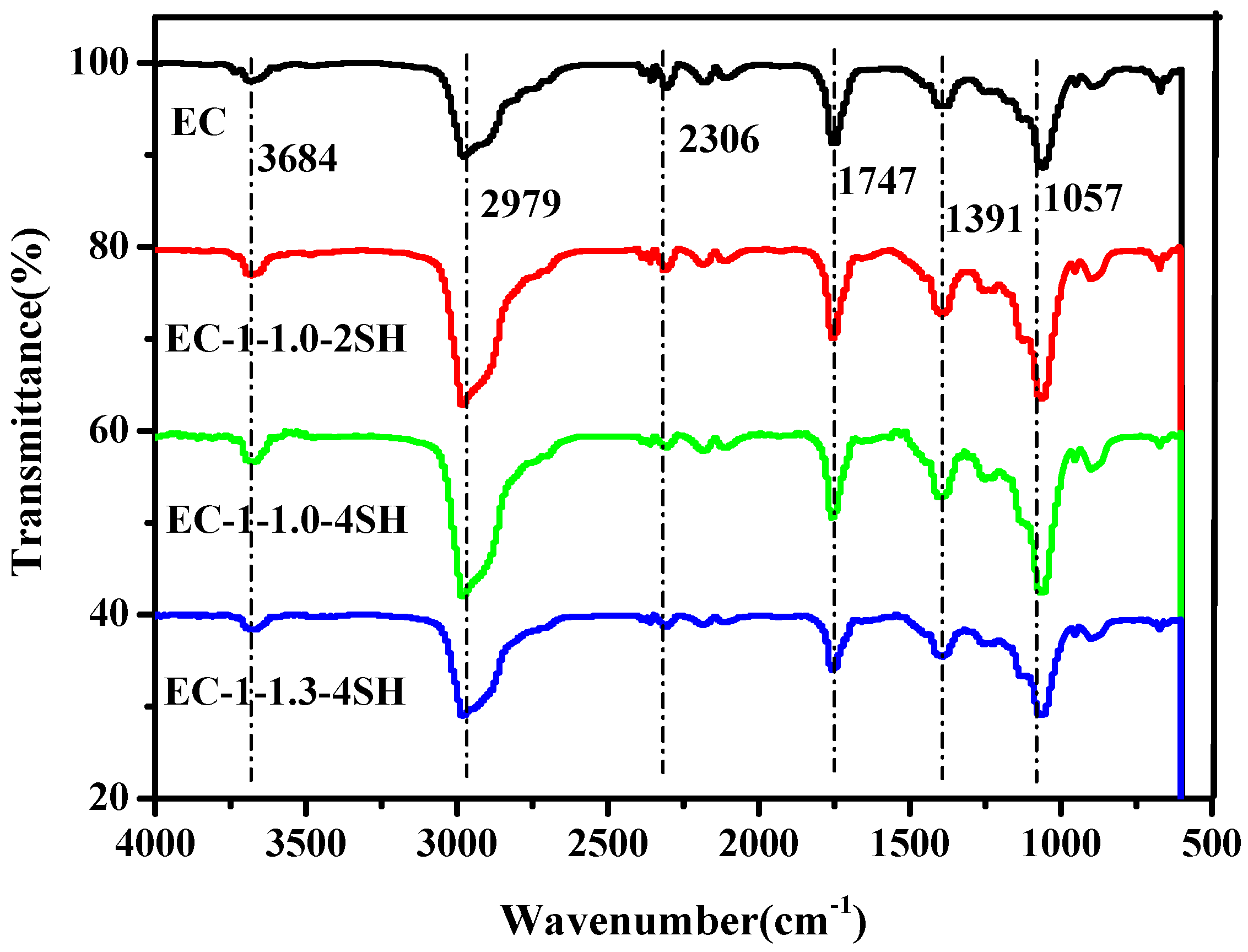
| EC−1−1.0−2SH | 348.2 | 370.2 | 6.6 |
| EC−1−1.0−4SH | 346.8 | 371.2 | 7.2 |
| EC−1−1.3−4SH | 344.0 | 374.1 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, R.; Liu, C.; Wang, J.; Jia, P. Wood−Derived Polymers from Olefin−Functionalized Lignin and Ethyl Cellulose via Thiol–Ene Click Chemistry. Polymers 2023, 15, 1923. https://doi.org/10.3390/polym15081923
An R, Liu C, Wang J, Jia P. Wood−Derived Polymers from Olefin−Functionalized Lignin and Ethyl Cellulose via Thiol–Ene Click Chemistry. Polymers. 2023; 15(8):1923. https://doi.org/10.3390/polym15081923
Chicago/Turabian StyleAn, Rongrong, Chengguo Liu, Jun Wang, and Puyou Jia. 2023. "Wood−Derived Polymers from Olefin−Functionalized Lignin and Ethyl Cellulose via Thiol–Ene Click Chemistry" Polymers 15, no. 8: 1923. https://doi.org/10.3390/polym15081923
APA StyleAn, R., Liu, C., Wang, J., & Jia, P. (2023). Wood−Derived Polymers from Olefin−Functionalized Lignin and Ethyl Cellulose via Thiol–Ene Click Chemistry. Polymers, 15(8), 1923. https://doi.org/10.3390/polym15081923












