Optimization of Textile Waste Blends of Cotton and PET by Enzymatic Hydrolysis with Reusable Chemical Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Textile Waste Pretreatment
2.2. Enzymatic Hydrolysis
2.3. Optimization of Pretreated Textile Waste Hydrolysis
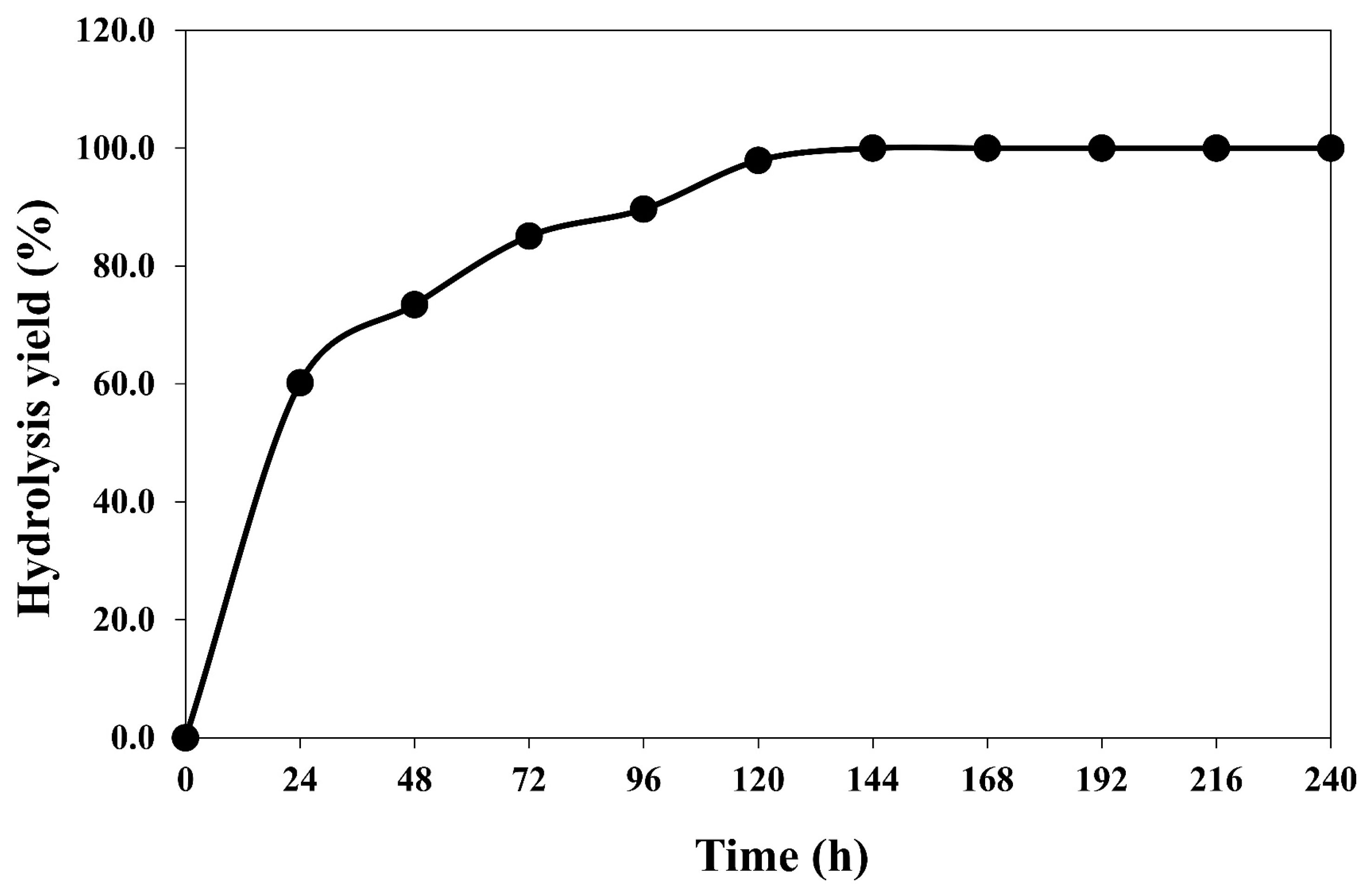
2.4. Effect of Incubation Time for Pretreated Textile Waste Hydrolysis
2.5. SEM Analysis of Textile Waste Substrate
2.6. Fourier Transform Infrared (FTIR) Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sugar Recovery from Textile Waste after Pretreatment
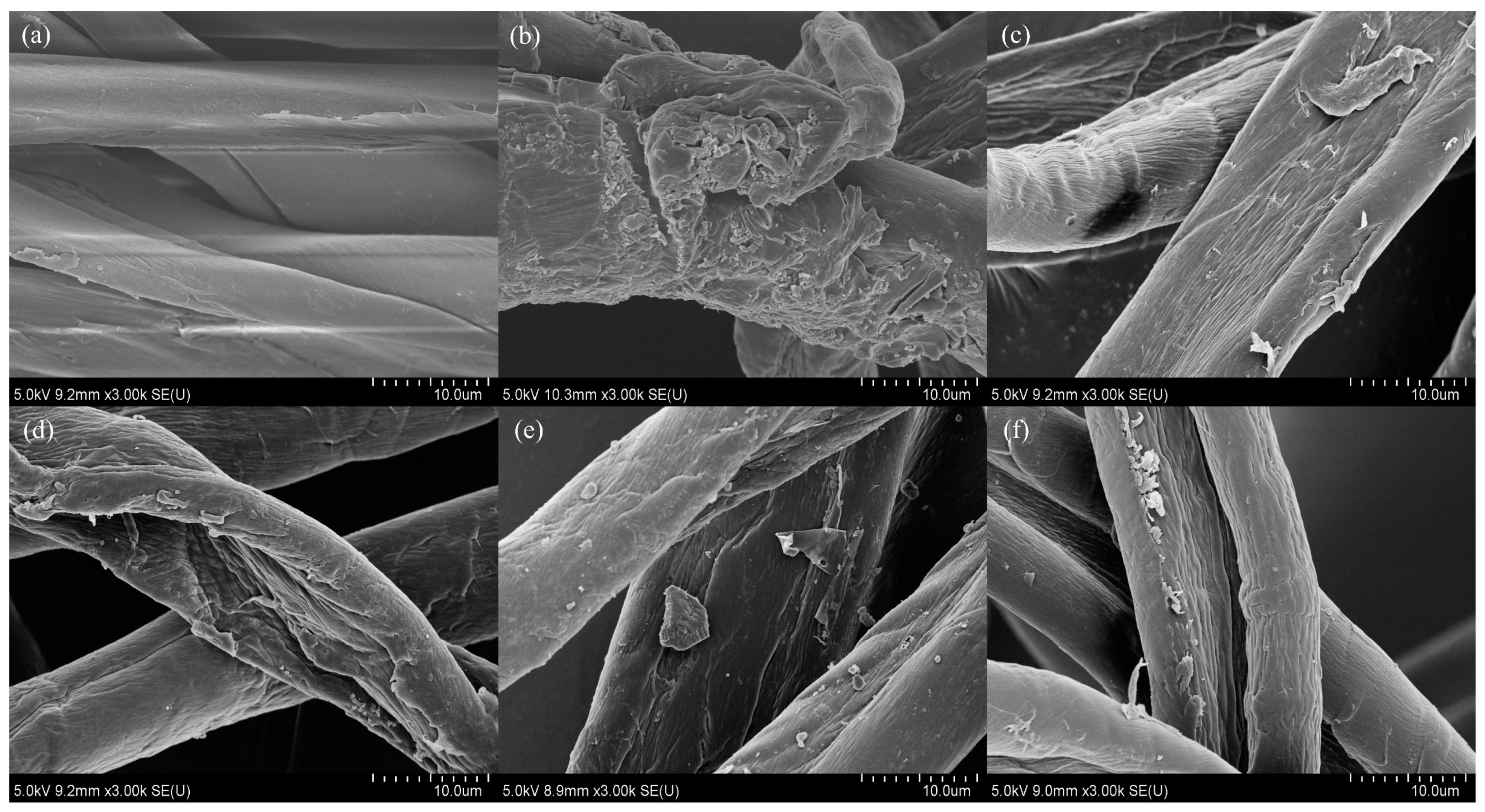
3.2. Change of Surface Morphology and FTIR Investigation
3.3. Optimization of Pretreated Textile Waste Hydrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jabbar, M.; Shaker, K. Textile raw materials. Sci. Rev. 2016, 1, 20160022. [Google Scholar]
- Fernández, L. Production of Textile Fibers Worldwide from 1975 to 2020, with a Forecast for 2025 and 2030. Available online: https://www.statista.com/statistics/1250985/global-textile-fiber-production/#statisticContainer (accessed on 29 December 2022).
- Ellen MacArthur Foundation. A New Textiles Economy: Redesigning Fashion’s Future. 2022. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/publications/A-New-Textiles-Economy_Full-Report.pdf (accessed on 29 December 2022).
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A review on textile recycling practices and challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Spuijbroek, M. Textile Waste in Mainland China. 2019. Available online: https://zakendoeninchina.org/wp-content/uploads/2019/08/report_Textile-Waste-in-Mainland-China_small.pdf (accessed on 29 December 2022).
- The Collective. Unspoken Crisis: Mounting Textile Waste in China. 2018. Available online: https://www.coresponsibility.com/unspoken-crisis-mounting-textile-waste-in-china (accessed on 29 December 2022).
- The State Council Information Office of the People’s Republic of China. China to up ITS Textile Recycling Capability. 2022. Available online: http://english.scio.gov.cn/chinavoices/2022-04/20/content_78175923.htm (accessed on 29 December 2022).
- CTR (Council for Textile Recycling). 2018. Available online: http://www.weardonaterecycle.org/ (accessed on 29 December 2022).
- Wicker, A. Fast Fashion Is Creating an Environmental Crisis. 2016. Available online: http://www.newsweek.com/2016/09/09/old-clothes-fashion-waste-crisis-494824.htm (accessed on 29 December 2022).
- Hong, F.; Guo, X.; Zhang, S.; Han, S.F.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Jeihanipour, A.; Karimi, K.; Niklasson, C.; Taherzadeh, M.J. A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles. Waste Manag. 2010, 30, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Cavaco-Paulo, A. Enzymatic removal of cellulose from cotton/polyester fabric blends. Cellulose 2006, 13, 611–618. [Google Scholar] [CrossRef]
- Ouchi, A.; Toida, T.; Kumaresan, S.; Ando, W.; Kato, J. A new methodology to recycle polyester from fabric blends with cellulose. Cellulose 2010, 17, 215–222. [Google Scholar] [CrossRef]
- Shen, F.; Xiao, W.; Lin, L.; Yang, G.; Zhang, Y.; Deng, S. Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Bioresour. Technol. 2013, 130, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, C.; Leu, S.Y.; Jing, H.; Li, X.; Lin, C.S.K. Valorisation of textile waste by fungal solid state fermentation: An example of circular waste-based biorefinery. Resour. Conserv. Recycl. 2018, 129, 27–35. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene terephthalate (PET). Bottle-to-bottle recycling for the beverage industry: A review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Hassan, T.; Wasim, M.; Khan, M.Q.; Salam, A.; Hassan, S.Z.U.; Abbasi, A.M.R.; Mustafa, T. Valorization of recycled PET for yarn manufacturing and knitwear fabrics used for apparel applications. Polym. Bull. 2022, 80, 2779–2799. [Google Scholar] [CrossRef]
- Thomas, S.; Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, M.G. Recycling of Polyethylene Terephthalate Bottles; William Andrew: Norwich, NY, USA, 2018. [Google Scholar]
- Gupta, V.B.; Mukherjee, A.K.; Cameotra, S.S. Poly(ethylene terephthalate) fibres. In Manufactured Fibre Technology; Gupta, V.B., Kothari, V.K., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 271–317. [Google Scholar]
- Das, J.; Halgeri, A.B.; Sahu, V.; Parikh, P.A. Alkaline hydrolysis of poly (ethylene terephthalate) in presence of a phase transfer catalyst. Indian J. Chem. Technol. 2007, 14, 173–177. [Google Scholar]
- Kosmidis, V.A.; Achilias, D.S.; Karayannidis, G.P. Poly(ethylene terephthalate) recycling and recovery of pure terephthalic acid. Kinetics of a phase transfer catalyzed alkaline hydrolysis. Macromol. Mater. Eng. 2001, 286, 640–647. [Google Scholar] [CrossRef]
- López-Fonseca, R.; González-Marcos, M.P.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I. A kinetic study of the depolymerisation of poly(ethylene terephthalate) by phase transfer catalysed alkaline hydrolysis. J. Chem. Technol. Biotechnol. 2009, 84, 92–99. [Google Scholar] [CrossRef]
- Polk, M.B.; Leboeuf, L.L.; Shah, M.; Won, C.-Y.; Hu, X.; Ding, W. Nylon 66, nylon 46, and PET phase-transfer-catalyzed alkaline depolymerization at atmospheric pressure. Polym. Plast. Technol. Eng. 1999, 38, 459–470. [Google Scholar] [CrossRef]
- Palme, A.; Peterson, A.; de la Motte, H.; Theliander, H.; Brelid, H. Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text. Cloth. Sustain. 2017, 3, 4. [Google Scholar] [CrossRef]
- Bayer, E.A.; Shoham, Y.; Lamed, R. Cellulose-decomposing bacteria and their enzyme systems. Prokaryotes 2006, 2, 578–617. [Google Scholar]
- Eriksson, K.E.L.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Bhat, M.K. Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 2000, 18, 355–383. [Google Scholar] [CrossRef]
- Cherry, J.R.; Fidantsef, A.L. Directed evolution of industrial enzymes: An update. Curr. Opin. Biotechnol. 2003, 14, 438–443. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Laohakunjit, N.; Kerdchoechuen, O.; Kyu, K.L.; Ratanakhanokchai, K. Present and potential applications of cellulases in agriculture, biotechnology, and bioenergy. Folia Microbiol. 2013, 58, 163–176. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Hossain, M.S.; Mohamad Kassim, M.H.; Ahmad, M.I.; Omar, F.M.; Balakrishnan, V.; Zulkifli, M.; Yahaya, A.N.A. Recycling waste cotton cloths for the isolation of cellulose nanocrystals: A sustainable approach. Polymers 2021, 13, 626. [Google Scholar] [CrossRef]
- Liu, X.; Ai, N.; Zhang, H.; Lu, M.; Ji, D.; Yu, F.; Ji, J. Quantification of glucose, xylose, arabinose, furfural, and HMF in corncob hydrolysate by HPLC-PDA–ELSD. Carbohydr. Res. 2012, 353, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.; Masek, A. Cellulose fibers hydrophobization via a hybrid chemical modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Yoon, L.W.; Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenergy 2014, 67, 319–338. [Google Scholar] [CrossRef]
- Gao, L.; Shi, S.; Hou, W.; Wang, S.; Yan, Z.; Ge, C. NaOH/urea swelling treatment and hydrothermal degradation of waste cotton fiber. J. Renew. Mater. 2020, 8, 703–713. [Google Scholar] [CrossRef]
- Qiang, D.; Zhang, M.; Li, J.; Xiu, H.; Liu, Q. Selective hydrolysis of cellulose for the preparation of microcrystalline cellulose by phosphotungstic acid. Cellulose 2016, 23, 1199–1207. [Google Scholar] [CrossRef]
- Isogai, A.; Atalla, R. Dissolution of cellulose in aqueous NaOH solutions. Cellulose 1998, 5, 309–319. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Deng, Y. Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr. Polym. 2008, 72, 178–184. [Google Scholar] [CrossRef]
- Dos Santos Pereira, A.P.; da Silva, M.H.P.; Lima Júnior, É.P.; dos Santos Paula, A.; Tommasini, F.J. Processing and characterization of PET composites reinforced with geopolymer concrete waste. Mater. Res. 2017, 20, 411–420. [Google Scholar] [CrossRef]
- Margariti, C. The application of FTIR microspectroscopy in a non-invasive and nondestructive way to the study and conservation of mineralised excavated textiles. Herit. Sci. 2019, 7, 63. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Du, C.; Lin, C.S.K. Recovery of glucose and polyester from textile waste by enzymatic hydrolysis. Waste Biomass Valorization 2019, 10, 3763–3772. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Jeihanipour, A.; Karimi, K.; Taherzadeh, M.J. Alkali pretreatment of softwood spruce and hardwood birch by NaOH/thiourea, NaOH/urea, NaOH/urea/thiourea, and NaOH/PEG to improve ethanol and biogas production. J. Chem. Technol. Biotechnol. 2012, 87, 1209–1214. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Taherzadeh, M.J. Ethanol production from cotton-based waste textiles. Bioresour. Technol. 2009, 100, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P.; Himmel, M.E.; Mielenz, J.R. Outlook for cellulase improvement: Screening and selection strategies. Biotechnol. Adv. 2006, 24, 452–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Navone, L.; Moffitt, K.; Hansen, K.-A.; Blinco, J.; Payne, A.; Speight, R. Closing the textile loop: Enzymatic fibre separation and recycling of wool/polyester fabric blends. J. Waste Manag. 2020, 102, 149–160. [Google Scholar] [CrossRef]
- Akpinar, O.; Ak, O.; Kavas, A.; Bakir, U.; Yilmaz, L. Enzymatic production of xylooligosaccharides from cottonstalks. J. Agric. Food Chem. 2007, 55, 5544–5551. [Google Scholar] [CrossRef]



| Run No. | Level | Actual Level | Hydrolysis Yield (%) | |||
|---|---|---|---|---|---|---|
| Observed | Predicted | |||||
| 1 | −1 | −1 | 20 | 3 | 39.21 | 37.85 |
| 2 | 1 | −1 | 30 | 3 | 62.54 | 63.61 |
| 3 | −1 | 1 | 20 | 7 | 66.34 | 61.37 |
| 4 | 1 | 1 | 30 | 7 | 90.36 | 87.81 |
| 5 | −1.41 | 0 | 17.93 | 5 | 46.27 | 49.91 |
| 6 | 1.41 | 0 | 32.07 | 5 | 86.61 | 86.71 |
| 7 | 0 | −1.41 | 25 | 2.17 | 40.68 | 40.12 |
| 8 | 0 | 1.41 | 25 | 7.83 | 69.31 | 73.76 |
| 9 | 0 | 0 | 25 | 5 | 57.59 | 56.78 |
| 10 | 0 | 0 | 25 | 5 | 53.17 | 56.78 |
| 11 | 0 | 0 | 25 | 5 | 59.59 | 56.78 |
| Source | Sum of Squares | DF | Mean Square | F | p-Value |
|---|---|---|---|---|---|
| Model | 2707.56 | 5 | 541.51 | 30.14 | 0.001 * |
| 1362.31 | 1 | 1362.31 | 75.82 | 0.0003 * | |
| 1138.8 | 1 | 1138.8 | 63.38 | 0.0005 * | |
| 0.12 | 1 | 0.12 | 0.00641 | 0.9393 | |
| 190 | 1 | 190 | 10.57 | 0.0227 * | |
| 0.037 | 1 | 0.037 | 0.00204 | 0.9658 | |
| Residual | 89.84 | 5 | 17.97 | ||
| Lack of fit | 68.26 | 3 | 22.75 | 2.11 | 0.3378 |
| Total | 2797.41 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boondaeng, A.; Keabpimai, J.; Srichola, P.; Vaithanomsat, P.; Trakunjae, C.; Niyomvong, N. Optimization of Textile Waste Blends of Cotton and PET by Enzymatic Hydrolysis with Reusable Chemical Pretreatment. Polymers 2023, 15, 1964. https://doi.org/10.3390/polym15081964
Boondaeng A, Keabpimai J, Srichola P, Vaithanomsat P, Trakunjae C, Niyomvong N. Optimization of Textile Waste Blends of Cotton and PET by Enzymatic Hydrolysis with Reusable Chemical Pretreatment. Polymers. 2023; 15(8):1964. https://doi.org/10.3390/polym15081964
Chicago/Turabian StyleBoondaeng, Antika, Jureeporn Keabpimai, Preeyanuch Srichola, Pilanee Vaithanomsat, Chanaporn Trakunjae, and Nanthavut Niyomvong. 2023. "Optimization of Textile Waste Blends of Cotton and PET by Enzymatic Hydrolysis with Reusable Chemical Pretreatment" Polymers 15, no. 8: 1964. https://doi.org/10.3390/polym15081964
APA StyleBoondaeng, A., Keabpimai, J., Srichola, P., Vaithanomsat, P., Trakunjae, C., & Niyomvong, N. (2023). Optimization of Textile Waste Blends of Cotton and PET by Enzymatic Hydrolysis with Reusable Chemical Pretreatment. Polymers, 15(8), 1964. https://doi.org/10.3390/polym15081964