Surface Passivation for Promotes Bi-Excitonic Amplified Spontaneous Emission in CsPb(Br/Cl)3 Perovskite at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CsPb(Br/Cl)3 Perovskite and PMMA Polymer Films
2.3. Structural Characterization
2.4. Optical Characterization
2.5. ASE Measurements and Data Analysis
2.6. Time-Resolved Photoluminescence Measurements (TRPL)
3. Results and Discussion
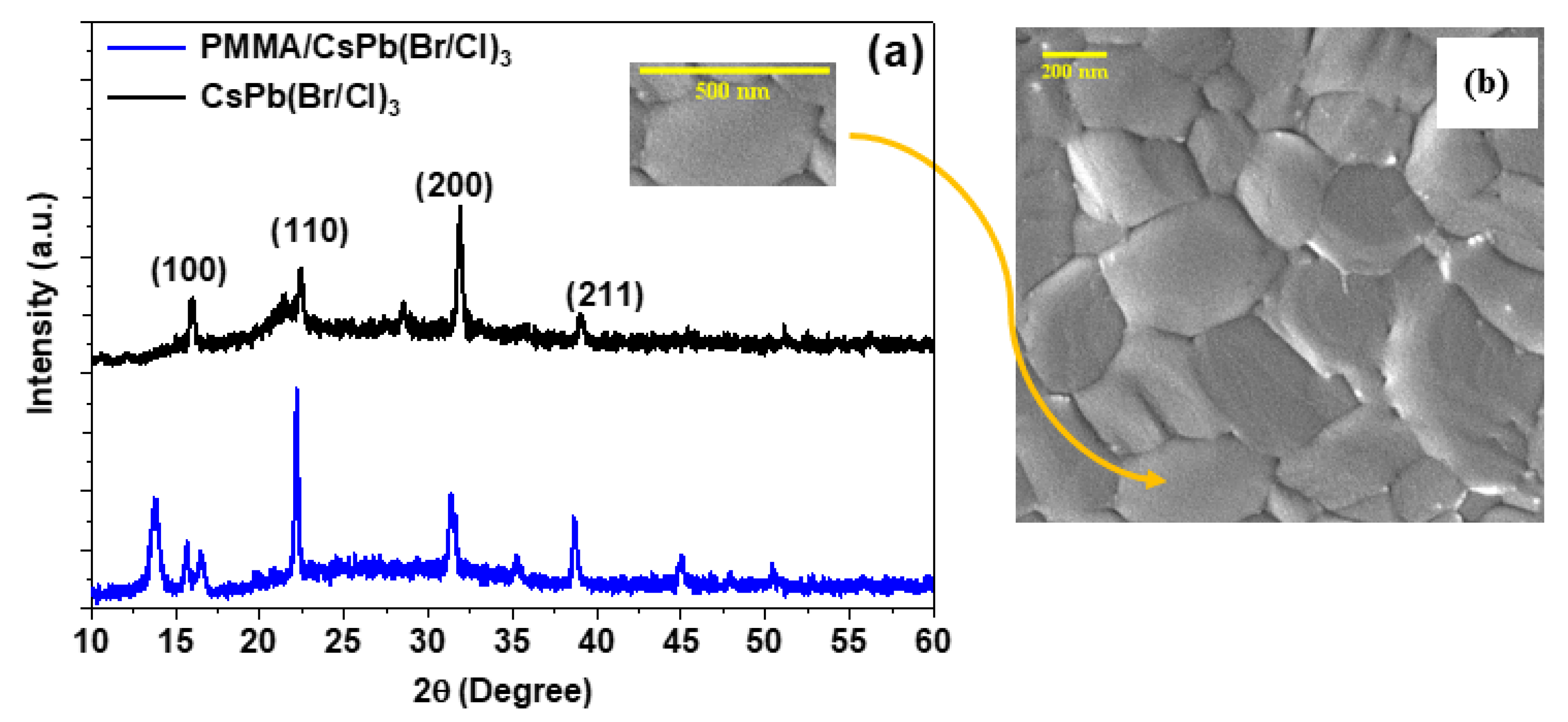
3.1. Structural and Morphological Properties
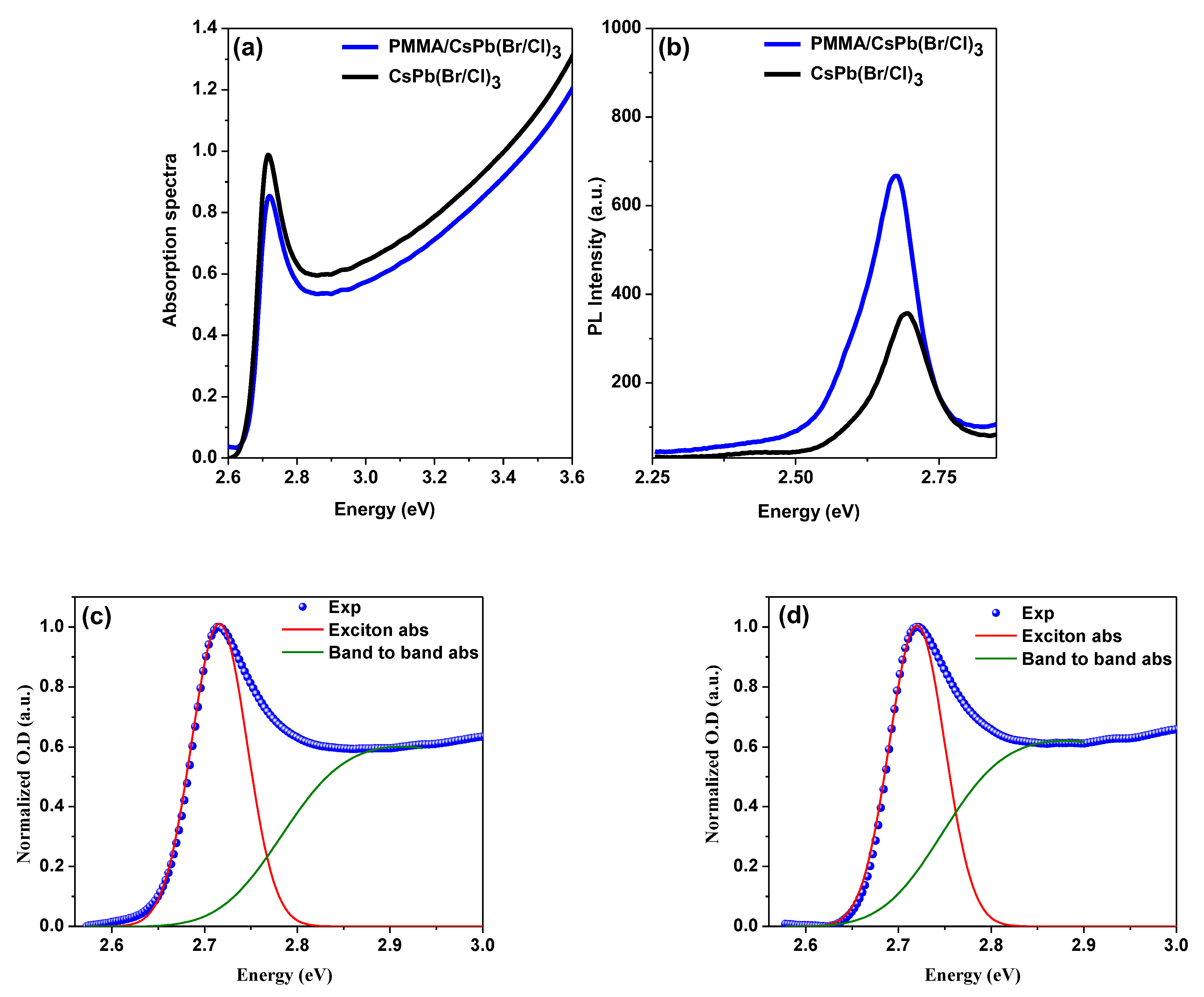
3.2. Optical Characterization
Measurements of UV-Vis Absorption & Steady-State Photoluminescence
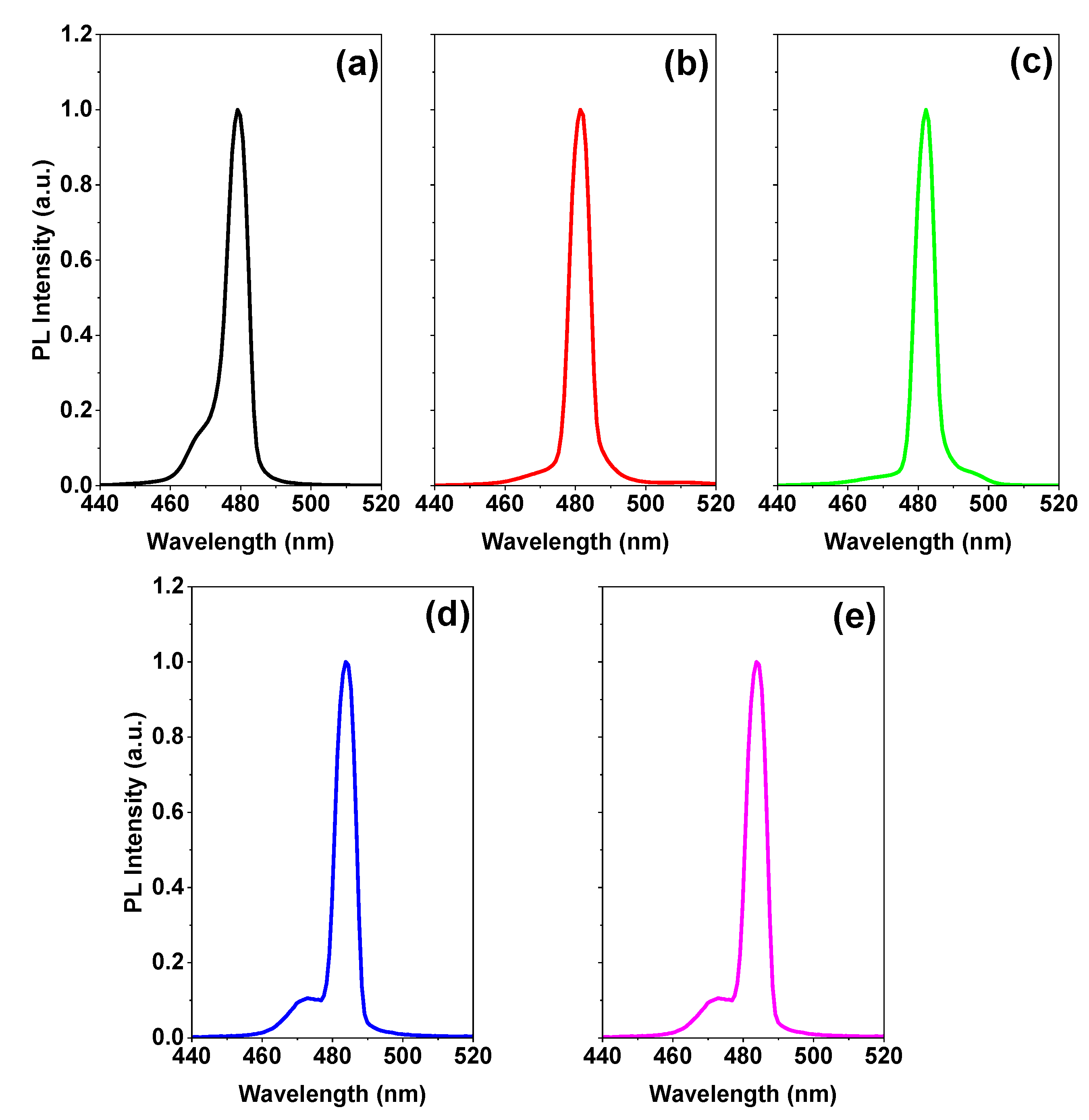
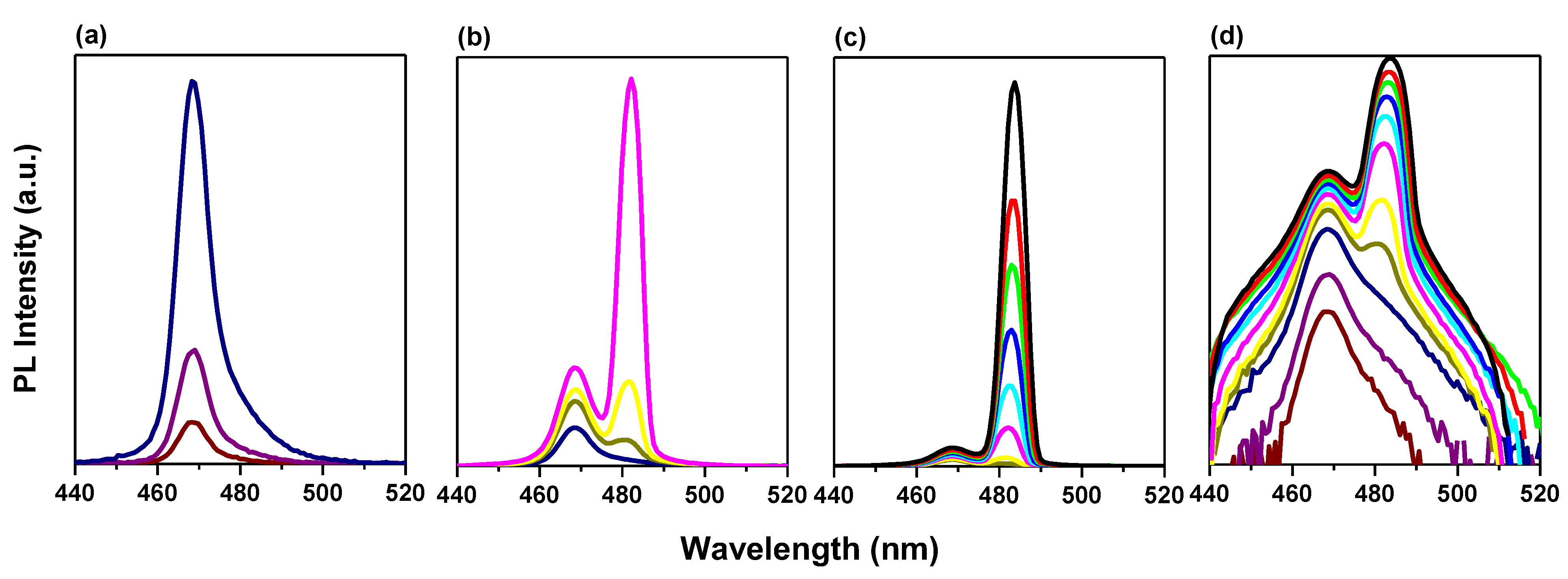
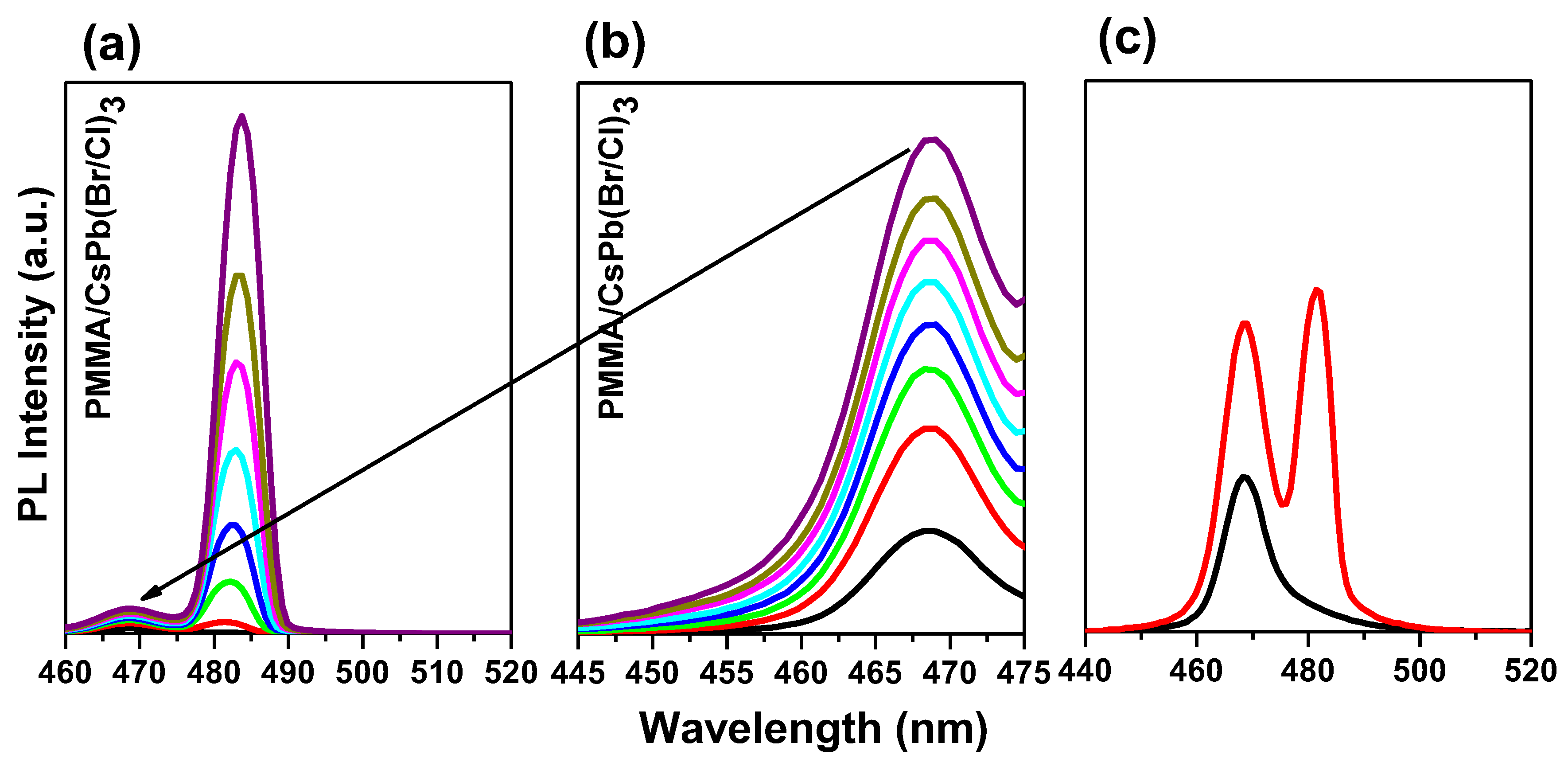
3.3. ASE Pump Fluence-Dependent Studies
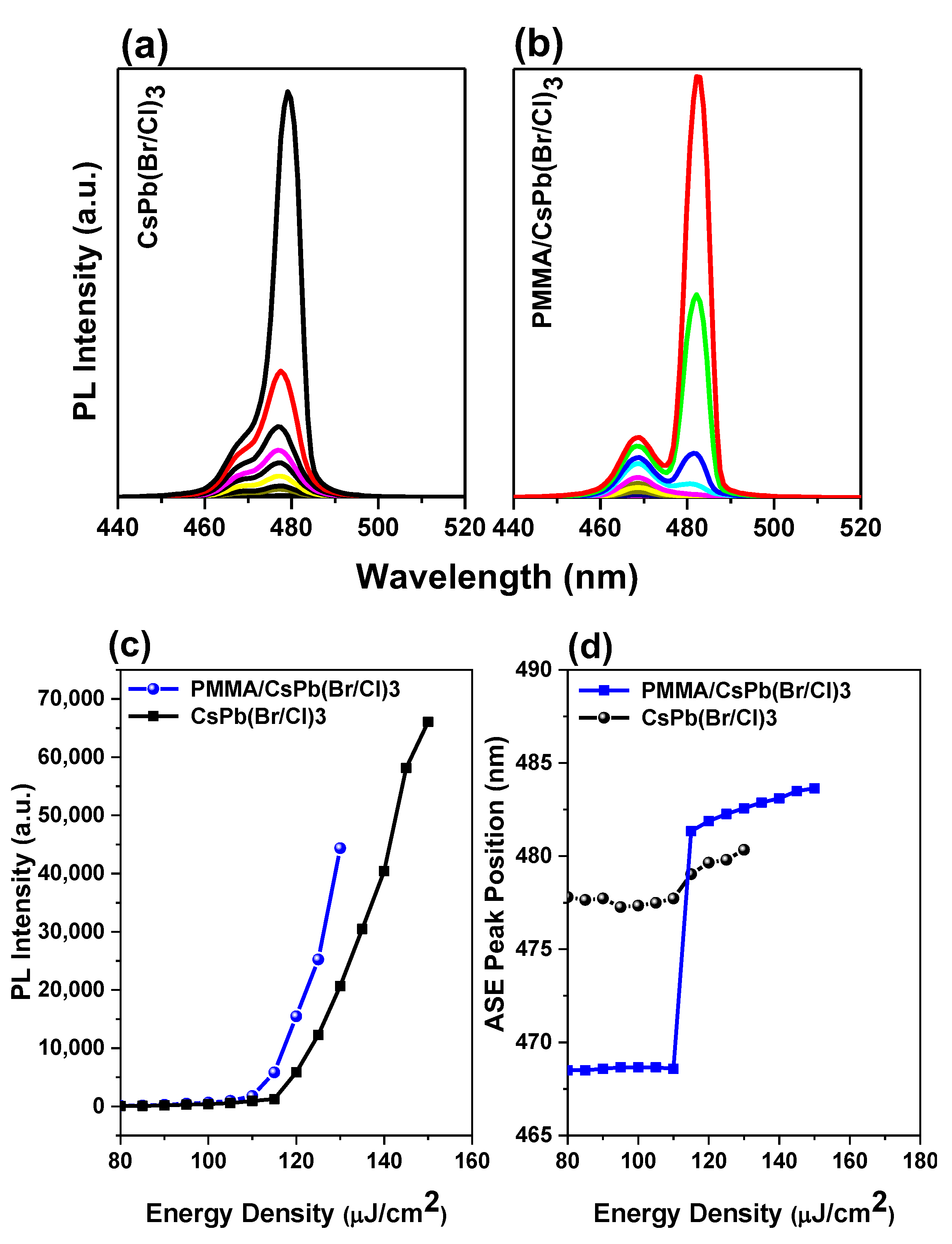
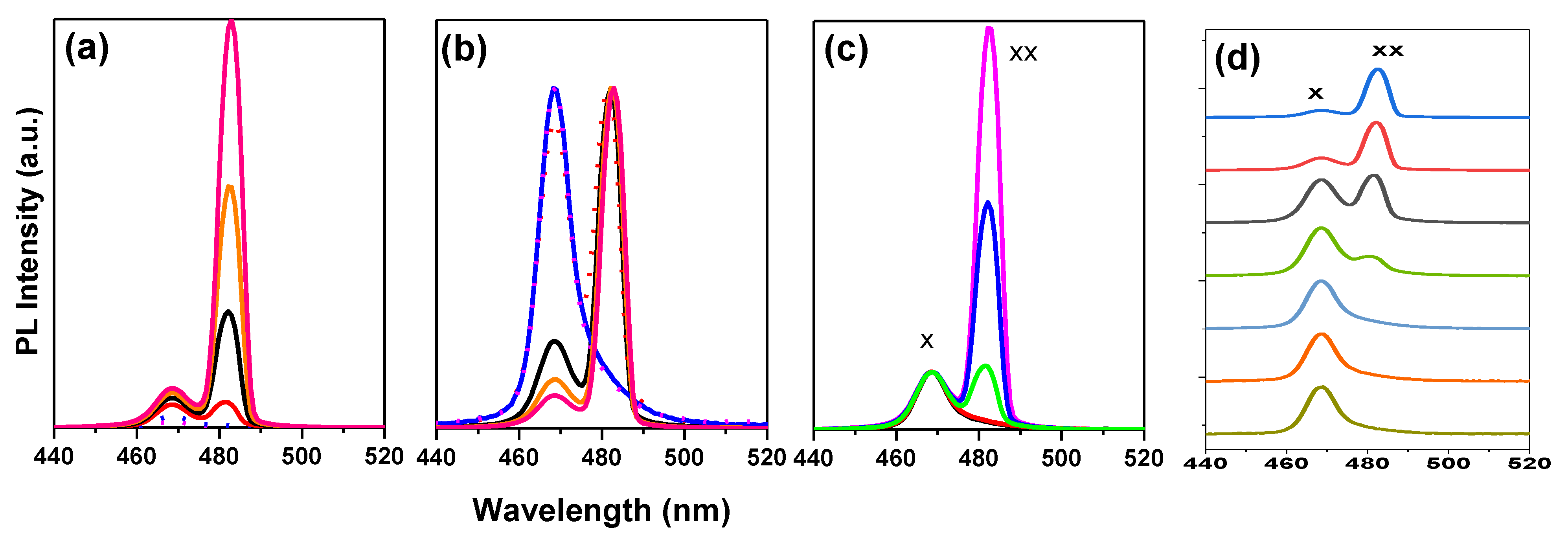
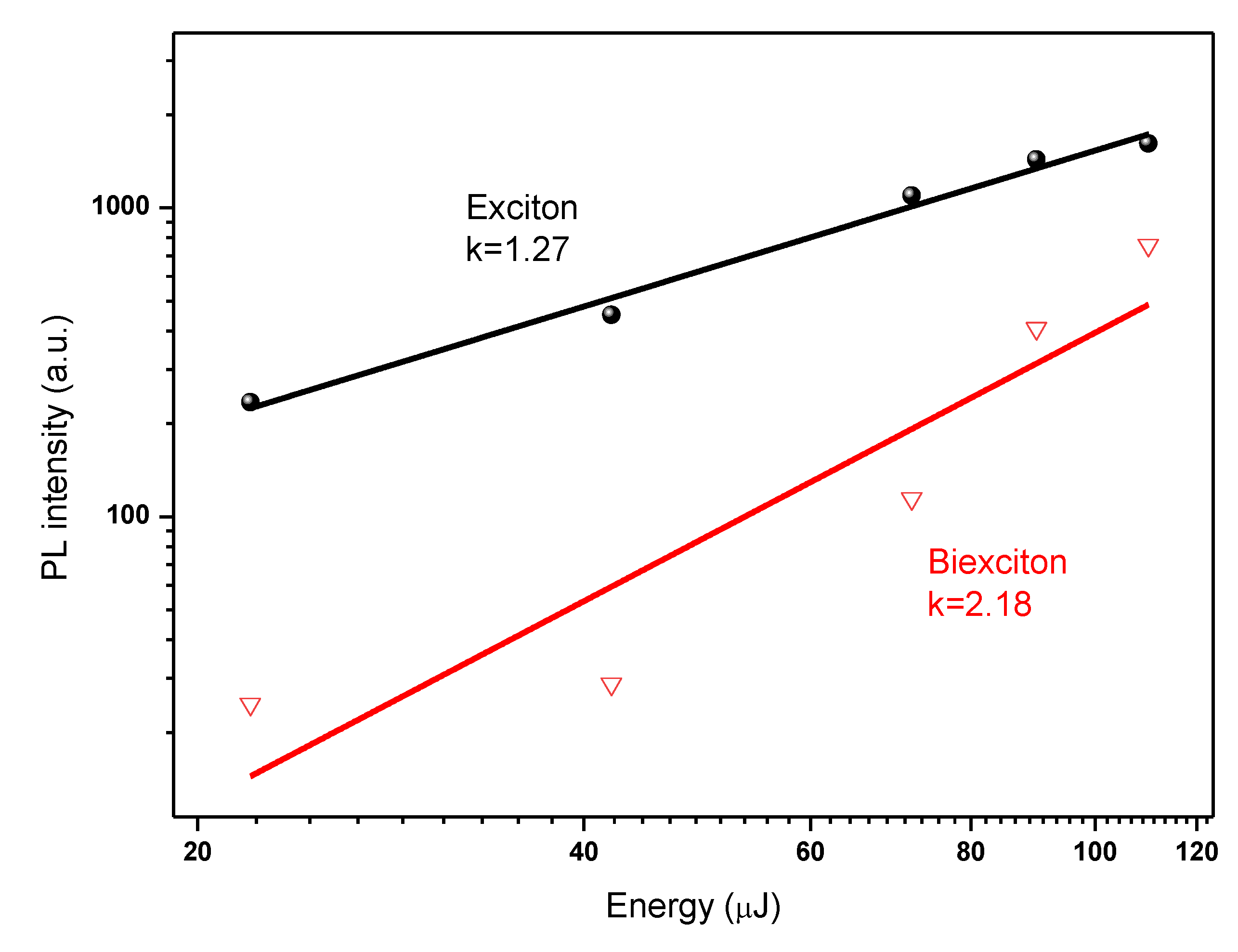
3.4. Bi-Excitonic ASE Formation and Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumawat, N.K.; Gupta, D.; Kabra, D. Recent Advances in Metal Halide-Based Perovskite Light-Emitting Diodes. Energy Technol. 2017, 5, 1734–1749. [Google Scholar] [CrossRef]
- Qaid, S.M.H.; Ghaithan, H.M.; Al-Asbahi, B.A.; Aldwayyan, A.S. Single-source thermal evaporation growth and the tuning surface passivation layer thickness effect in enhanced amplified spontaneous emission properties of CsPb(Br0.5Cl0.5)3 perovskite films. Polymers 2020, 12, 2953. [Google Scholar] [CrossRef]
- Qaid, S.M.H.; Al-Asbahi, B.A.; Ghaithan, H.M.; AlSalhi, M.S.; Al Dwayyan, A.S. Optical and structural properties of CsPbBr3 perovskite quantum dots/PFO polymer composite thin films. J. Colloid Interface Sci. 2020, 563, 426–434. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Qaid, S.M.H.; Hezam, M.; Bedja, I.; Ghaithan, H.M.; Aldwayyan, A.S. Effect of deposition method on the structural and optical properties of CH3NH3PbI3 perovskite thin films. Opt. Mater. (Amst.) 2020, 103, 109836. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Qaid, S.M.H.; Khan, M.N.; Alqasem, A.; Hezam, M.; Aldwayyan, A. Restraining effect of film thickness on the behaviour of amplified spontaneous emission from methylammonium lead iodide perovskite. IET Optoelectron. 2018, 13, 2–6. [Google Scholar] [CrossRef]
- Ghaithan, H.M.; Qaid, S.M.H.; Alahmed, Z.A.; Hezam, M.; Lyras, A.; Amer, M.; Aldwayyan, A.S. Anion Substitution Effects on the Structural, Electronic, and Optical Properties of Inorganic CsPb(I1−xBrx)3 and CsPb(Br1−xClx)3 Perovskites: Theoretical and Experimental Approaches. J. Phys. Chem. C 2021, 125, 886–897. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Ghaithan, H.M.; Qaid, S.M.H.; Aldwayyan, A.S. Structural, Electronic, and Optical Properties of CsPb (Br1−xClx)3 Perovskite: First-Principles Study with PBE–GGA and mBJ–GGA Methods. Materials 2020, 13, 4944. [Google Scholar] [CrossRef]
- Mastrikov, Y.A.; Chuklina, N.G.; Sokolov, M.N.; Popov, A.I.; Gryaznov, D.V.; Kotomin, E.A.; Maier, J. Small radius electron and hole polarons in PbX2(X = F, Cl, Br) crystals: A computational study. J. Mater. Chem. C 2021, 9, 16536–16544. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef]
- Yang, W.; Gao, F.; Qiu, Y.; Liu, W.; Xu, H.; Yang, L.; Liu, Y. CsPbBr3-Quantum-Dots/Polystyrene@Silica Hybrid Microsphere Structures with Significantly Improved Stability for White LEDs. Adv. Opt. Mater. 2019, 7, 1900546. [Google Scholar] [CrossRef]
- Jin, X.; Ma, K.; Chakkamalayath, J.; Morsby, J.; Gao, H. In Situ Photocatalyzed Polymerization to Stabilize Perovskite Nanocrystals in Protic Solvents. ACS Energy Lett. 2022, 7, 610–616. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead Halide Perovskite Nanocrystals as Photocatalysts for PET-RAFT Polymerization under Visible and Near-Infrared Irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef]
- Qaid, S.M.H.; Ghaithan, H.M.; Al-Asbahi, B.A.; Aldwayyan, A.S. Investigation of the Surface Passivation Effect on the Optical Properties of CsPbBr3 Perovskite Quantum Dots. Surf. Interfaces 2021, 23, 100948. [Google Scholar] [CrossRef]
- Nishida, M. Energy Distribution of Dangling-Orbital Surface States on the (110) Surface of III-V Compounds. Phys. Status Solidi 1980, 99, K39–K43. [Google Scholar] [CrossRef]
- Wang, L.; Wu, K.; Dong, Q.M.; Li, X.Y.; Xiong, S.Y.; Xu, L.T.; Liang, P. Effect of surface passivation on optical and electronic properties of ultrathin silicon nanosheets. Sci. China Inf. Sci. 2012, 55, 1469–1474. [Google Scholar] [CrossRef]
- Nguyen, V.; Si, J.; Yan, L.; Hou, X. Electron-hole recombination dynamics in carbon nanodots. Carbon N. Y. 2015, 95, 659–663. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Chen, H.; Chen, J.; Zhu, R.; Ma, P.; Towers, A.; Lin, Y.; Gesquiere, A.J.; Wu, S.T.; et al. Ultrastable, Highly Luminescent Organic–Inorganic Perovskite–Polymer Composite Films. Adv. Mater. 2016, 28, 10710–10717. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Guo, S.; Cao, S.; Wang, L.; Gao, F.; Yang, Z.; Zheng, J.; Yang, W. A General Strategy for In Situ Growth of All-Inorganic CsPbX3 (X = Br, I, and Cl) Perovskite Nanocrystals in Polymer Fibers toward Significantly Enhanced Water/Thermal Stabilities. Adv. Opt. Mater. 2018, 6, 1800346. [Google Scholar] [CrossRef]
- Yassitepe, E.; Yang, Z.; Voznyy, O.; Kim, Y.; Walters, G.; Castañeda, J.A.; Kanjanaboos, P.; Yuan, M.; Gong, X.; Fan, F.; et al. Amine-Free Synthesis of Cesium Lead Halide Perovskite Quantum Dots for Efficient Light-Emitting Diodes. Adv. Funct. Mater 2016, 26, 8757–8763. [Google Scholar] [CrossRef]
- Shen, X.; Sun, C.; Bai, X.; Zhang, X.; Wang, Y.; Wang, Y.; Song, H.; Yu, W.W. Efficient and Stable CsPb(Br/I)3@Anthracene Composites for White Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 16768–16775. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Fan, Y.; Chen, Z.; Tang, Z.; Zhao, J.; Lv, Y.; Lin, J.; Guo, X.; Zhang, J.; et al. Toward Highly Luminescent and Stabilized Silica-Coated Perovskite Quantum Dots through Simply Mixing and Stirring under Room Temperature in Air. ACS Appl. Mater. Interfaces 2018, 10, 13053–13061. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, L.; Zhou, T.; Zheng, P.; Li, Y.; Xie, R.J. Improved stability of CsPbBr3 perovskite quantum dots achieved by suppressing interligand proton transfer and applying a polystyrene coating. Nanoscale 2018, 10, 21441–21450. [Google Scholar] [CrossRef]
- Li, Z.; Kong, L.; Huang, S.; Li, L. Highly Luminescent and Ultrastable CsPbBr3 Perovskite Quantum Dots Incorporated into a Silica/Alumina Monolith. Angew. Chem. 2017, 129, 8246–8250. [Google Scholar] [CrossRef]
- Yoon, H.C.; Lee, S.; Song, J.K.; Yang, H.; Do, Y.R. Efficient and Stable CsPbBr3 Quantum-Dot Powders Passivated and Encapsulated with a Mixed Silicon Nitride and Silicon Oxide Inorganic Polymer Matrix. ACS Appl. Mater. Interfaces 2018, 10, 11756–11767. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Lin, S.-Y.; Tang, A.-C.; Singh, B.P.; Tong, H.-C.; Chen, C.-Y.; Lee, Y.-C.; Tsai, T.-L.; Liu, R.-S. Mesoporous Silica Particles Integrated with All-Inorganic CsPbBr3 Perovskite Quantum-Dot Nanocomposites (MP-PQDs) with High Stability and Wide Color Gamut Used for Backlight Display. Angew. Chem. 2016, 128, 8056–8061. [Google Scholar] [CrossRef]
- Li, Z.J.; Hofman, E.; Li, J.; Davis, A.H.; Tung, C.H.; Wu, L.Z.; Zheng, W. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2018, 28, 1704288. [Google Scholar] [CrossRef]
- Leijtens, T.; Lauber, B.; Eperon, G.E.; Stranks, S.D.; Snaith, H.J. The importance of perovskite pore filling in organometal mixed halide sensitized TiO2-based solar cells. J. Phys. Chem. Lett. 2014, 5, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, D.; Pan, G.; Zhou, D.; Xu, W.; Zhu, J.; Wang, H.; Chen, C.; Song, H. All-inorganic perovskite quantum dot/TiO2 inverse opal electrode platform: Stable and efficient photoelectrochemical sensing of dopamine under visible irradiation. Nanoscale 2018, 10, 10505–10513. [Google Scholar] [CrossRef] [PubMed]
- Qaid, S.M.H.; Alharbi, F.H.; Bedja, I.; Nazeeruddin, M.K.; Aldwayyan, A.S. Reducing amplified spontaneous emission threshold in CsPbBr3 quantum dot films by controlling TiO2 compact layer. Nanomaterials 2020, 10, 1605. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, N.; Gauvreau, B.; Rajabian, M.; Skorobogata, O.; Pone, E.; Zabeida, O.; Martinu, L.; Dubois, C.; Skorobogatiy, M. Consecutive solvent evaporation and co-rolling techniques for polymer multilayer hollow fiber preform fabrication. J. Mater. Res. 2006, 21, 2246–2254. [Google Scholar] [CrossRef]
- Skorobogatiy, M. Efficient antiguiding of TE and TM polarizations in low-index core waveguides without the need for an omnidirectional reflector. Opt. Lett. 2005, 30, 2991. [Google Scholar] [CrossRef]
- Ramnath, L.; DaCosta, S.; Akong, S.; Hassanali, H.; Omonze, D.; Powers, J.T. Fiber optics profiles real-time temperature across horizontal lateral. Oil Gas J. 2003, 101, 33–40. [Google Scholar]
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Henry, A.C.; Tutt, T.J.; Galloway, M.; Davidson, Y.Y.; McWhorter, C.S.; Soper, S.A.; McCarley, R.L. Surface modification of poly(methyl methacrylate) used in the fabrication of microanalytical devices. Anal. Chem. 2000, 72, 5331–5337. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, F.; Cui, Z.C.; Zheng, J.; Zhang, H.M.; Zhang, D.M.; Liu, S.Y.; Yi, M.B. Study of reactive ion etching process to fabricate the PMMA-based polymer waveguide. Microelectron. J. 2004, 35, 605–608. [Google Scholar] [CrossRef]
- Ziaie, B.; Baldi, A.; Lei, M.; Gu, Y.; Siegel, R.A. Hard and soft micromachining for BioMEMS: Review of techniques and examples of applications in microfluidics and drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Simmons, B.A.; McGraw, G.J.; Davalos, R.V.; Fiechtner, G.J.; Fintschenko, Y.; Cummings, E.B. The development of polymeric devices as dielectrophoretic separators and concentrators. MRS Bull. 2006, 31, 120–124. [Google Scholar] [CrossRef]
- Ravagnan, L.; Divitini, G.; Rebasti, S.; Marelli, M.; Piseri, P.; Milani, P. Poly(methyl methacrylate)-palladium clusters nanocomposite formation by supersonic cluster beam deposition: A method for microstructured metallization of polymer surfaces. J. Phys. D Appl. Phys. 2009, 42, 10031080314. [Google Scholar] [CrossRef]
- Kondo, T.; Azuma, T.; Yuasa, T.; Ito, R. Biexciton lasing in the layered perovskite-type material (C6H13NH3)2PbI4. Solid State Commun. 1998, 105, 253–255. [Google Scholar] [CrossRef]
- Arora, N.; Dar, M.I.; Hezam, M.; Tress, W.; Jacopin, G.; Moehl, T.; Gao, P.; Aldwayyan, A.S.; Deveaud, B.; Grätzel, M.; et al. Photovoltaic and Amplified Spontaneous Emission Studies of High-Quality Formamidinium Lead Bromide Perovskite Films. Adv. Funct. Mater. 2016, 26, 2846–2854. [Google Scholar] [CrossRef]
- Priante, D.; Dursun, I.; Alias, M.S.; Shi, D.; Melnikov, V.A.; Ng, T.K.; Mohammed, O.F.; Bakr, O.M.; Ooi, B.S. The recombination mechanisms leading to amplified spontaneous emission at the true-green wavelength in CH3NH3PbBr3 perovskites. Appl. Phys. Lett. 2015, 106, 081902. [Google Scholar] [CrossRef]
- Chen, K.; Barker, A.J.; Morgan, F.L.C.; Halpert, J.E.; Hodgkiss, J.M. Effect of carrier thermalization dynamics on light emission and amplification in organometal halide perovskites. J. Phys. Chem. Lett. 2015, 6, 153–158. [Google Scholar] [CrossRef]
- Valenta, J.; Luterová, K.; Tomasiunas, R.; Dohnalová, K.; Hönerlage, B.; Pelant, I. Optical Gain Measurements with Variable Stripe Length Technique. Towards First Silicon Laser 2003, 93, 223–242. [Google Scholar]
- Malko, A.V.; Mikhailovsky, A.A.; Petruska, M.A.; Hollingsworth, J.A.; Htoon, H.; Bawendi, M.G.; Klimov, V.I. From amplified spontaneous emission to microring lasing using nanocrystal quantum dot solids. Appl. Phys. Lett. 2002, 81, 1303–1305. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Shi, J.; Zhang, S.; Du, W.; Mi, Y.; Shang, Q.; Liu, P.; Sui, X.; Wu, X.; et al. Room temperature continuous-wave excited biexciton emission in perovskite nanoplatelets via plasmonic nonlinear fano resonance. Commun. Phys. 2019, 2, 80. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Lim, S.S.; Yantara, N.; Liu, X.; Sabba, D.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef]
- Vale, B.R.C.; Socie, E.; Burgos-Caminal, A.; Bettini, J.; Schiavon, M.A.; Moser, J.E. Exciton, Biexciton, and Hot Exciton Dynamics in CsPbBr3 Colloidal Nanoplatelets. J. Phys. Chem. Lett. 2020, 11, 387–394. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, L.; Liu, W.; Wu, C.; Liu, C.; Wu, Z.; Xiao, L.; Chen, Z.; Wang, S. Exciton and bi-exciton mechanisms in amplified spontaneous emission from CsPbBr3 perovskite thin films. Opt. Express 2019, 27, 29124. [Google Scholar] [CrossRef]
- Navarro-Arenas, J.; Suárez, I.; Chirvony, V.S.; Gualdrón-Reyes, A.F.; Mora-Seró, I.; Martínez-Pastor, J. Single-Exciton Amplified Spontaneous Emission in Thin Films of CsPbX3 (X = Br, I) Perovskite Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 6389–6398. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Li, X.; Liang, C.; Zhang, Z.; Guo, J.; Hong, G.; Xing, G.; Huang, W. Recent Progress in Metal Halide Perovskite Micro- and Nanolasers. Adv. Opt. Mater. 2019, 7, 1900080. [Google Scholar] [CrossRef]
- Ding, J.; Hagerott, M.; Ishihara, T.; Jeon, H.; Nurmikko, A.V. (Zn,Cd)Se/ZnSe quantum-well lasers: Excitonic gain in an inhomogeneously broadened quasi-two-dimensional system. Phys. Rev. B 1993, 47, 10528–10542. [Google Scholar] [CrossRef]
- Popov, A.I.; Kotomin, E.A.; Maier, J. Analysis of self-trapped hole mobility in alkali halides and metal halides. Solid State Ionics 2017, 302, 3–6. [Google Scholar] [CrossRef]
- D’Innocenzo, V.; Grancini, G.; Alcocer, M.J.P.; Kandada, A.R.S.; Stranks, S.D.; Lee, M.M.; Lanzani, G.; Snaith, H.J.; Petrozza, A. Excitons versus free charges in organo-lead tri-halide perovskites. Nat. Commun. 2014, 5, 045214. [Google Scholar] [CrossRef] [PubMed]
- Wehrenfennig, C.; Liu, M.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Homogeneous emission line broadening in the organo lead halide perovskite CH3NH3PbI3−xClx. J. Phys. Chem. Lett. 2014, 5, 1300–1306. [Google Scholar] [CrossRef]
- Wu, K.; Bera, A.; Ma, C.; Du, Y.; Yang, Y.; Li, L.; Wu, T. Temperature-dependent excitonic photoluminescence of hybrid organometal halide perovskite films. Phys. Chem. Chem. Phys. 2014, 16, 22476–22481. [Google Scholar] [CrossRef] [PubMed]
- Kunugita, H.; Hashimoto, T.; Kiyota, Y.; Udagawa, Y.; Takeoka, Y.; Nakamura, Y.; Sano, J.; Matsushita, T.; Kondo, T.; Miyasaka, T.; et al. Excitonic Feature in Hybrid Perovskite CH3NH3PbBr3 single crystals. Chem. Lett. 2015, 44, 852–854. [Google Scholar] [CrossRef]
- Wang, Y.; Zhi, M.; Chang, Y.Q.; Zhang, J.P.; Chan, Y. Stable, Ultralow Threshold Amplified Spontaneous Emission from CsPbBr3 Nanoparticles Exhibiting Trion Gain. Nano Lett. 2018, 18, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, J.; Wang, H.; Fang, C.; Luo, H.; Li, D. Biexcitons in 2D (iso-BA)2PbI4perovskite crystals. Nanophotonics 2020, 9, 2001–2006. [Google Scholar] [CrossRef]
- Thouin, F.; Neutzner, S.; Cortecchia, D.; Dragomir, V.A.; Soci, C.; Salim, T.; Lam, Y.M.; Leonelli, R.; Petrozza, A.; Kandada, A.R.S.; et al. Stable biexcitons in two-dimensional metal-halide perovskites with strong dynamic lattice disorder. Phys. Rev. Mater. 2018, 2, 034001. [Google Scholar] [CrossRef]
- Wei, X.; Liu, H.; Zhang, Z.; Xu, W.; Huang, W.; Luo, L.B.; Liu, J. Low-temperature architecture of a cubic-phase CsPbBr3 single crystal for ultrasensitive weak-light photodetectors. Chem. Commun. 2021, 57, 7798–7801. [Google Scholar] [CrossRef]
- Mykhaylyk, V.B.; Rudko, M.; Kraus, H.; Kapustianyk, V.; Kolomiets, V.; Vitoratou, N.; Chornodolskyy, Y.; Voloshinovskii, A.S.; Vasylechko, L. Ultra-fast low temperature scintillation and X-ray luminescence of CsPbCl3 crystals. J. Mater. Chem. C 2022, 11, 656–665. [Google Scholar] [CrossRef]
- Sandor, E.; Wooster, W.A. Crystal Structure and Photoconductivity of Cæsium Plumbohalides. Nature 1958, 182, 1436. [Google Scholar]
- Rodova, M.; Brozek, J.; Knizek, K.; Nitsch, K. Phase transitions in ternary caesium lead bromide. J. Therm. Anal. Calorim. 2003, 71, 667–673. [Google Scholar] [CrossRef]
- Kirschner, M.S.; Diroll, B.T.; Guo, P.; Harvey, S.M.; Helweh, W.; Flanders, N.C.; Brumberg, A.; Watkins, N.E.; Leonard, A.A.; Evans, A.M.; et al. Photoinduced, reversible phase transitions in all-inorganic perovskite nanocrystals. Nat. Commun. 2019, 10, 504. [Google Scholar] [CrossRef]
- Dos Reis, R.; Yang, H.; Ophus, C.; Ercius, P.; Bizarri, G.; Perrodin, D.; Shalapska, T.; Bourret, E.; Ciston, J.; Dahmen, U. Determination of the structural phase and octahedral rotation angle in halide perovskites. Appl. Phys. Lett. 2018, 112, 071901. [Google Scholar] [CrossRef]
- Møller, C.K. The structure of perovskite-like cesium plumbo trihalides. Mat. Fys. Medd. Dan. Vid. Selsk. 1959, 32, 1–27. [Google Scholar]
- Stoumpos, C.C.; Malliakas, C.D.; Peters, J.A.; Liu, Z.; Sebastian, M.; Im, J.; Chasapis, T.C.; Wibowo, A.C.; Chung, D.Y.; Freeman, A.J.; et al. Crystal growth of the perovskite semiconductor CsPbBr3: A new material for high-energy radiation detection. Cryst. Growth Des. 2013, 13, 2722–2727. [Google Scholar] [CrossRef]
- Moore, D.T.; Kamino, B.A.; Patel, J.B.; Miranda, L.; Johnston, M.B.; Parrott, E.S.; Haghighirad, A.A.; Hörantner, M.T.; Sutton, R.J.; Snaith, H.J.; et al. Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar]
- Zhang, M.; Zheng, Z.; Fu, Q.; Chen, Z.; He, J.; Zhang, S.; Chen, C.; Luo, W. Synthesis and single crystal growth of perovskite semiconductor CsPbBr3. J. Cryst. Growth 2018, 484, 37–42. [Google Scholar] [CrossRef]
- Bertolotti, F.; Protesescu, L.; Kovalenko, M.V.; Yakunin, S.; Cervellino, A.; Billinge, S.J.L.; Terban, M.W.; Pedersen, J.S.; Masciocchi, N.; Guagliardi, A. Coherent Nanotwins and Dynamic Disorder in Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2017, 11, 3819–3831. [Google Scholar] [CrossRef] [PubMed]
- Shobhana, E. X-ray Diffraction and UV-Visible Studies of PMMA Thin Films. Int. J. Mod. Eng. Res. 2012, 2, 1092–1095. [Google Scholar]
- Hu, W.; Orlova, T.; Bernstein, G.H. Technique for preparation of precise wafer cross sections and applications to electron beam lithography of poly(methylmethacrylate) resist. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2002, 20, 3085–3088. [Google Scholar] [CrossRef]
- Grancini, G.; Srimath Kandada, A.R.; Frost, J.M.; Barker, A.J.; De Bastiani, M.; Gandini, M.; Marras, S.; Lanzani, G.; Walsh, A.; Petrozza, A. Role of microstructure in the electron–hole interaction of hybrid lead halide perovskites. Nat. Photonics 2015, 9, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Qaid, S.M.H.; Ghaithan, H.M.; Alharbi, K.K.; Bin Ajaj, A.F.; Al-Asbahi, B.A.; Aldwayyan, A.S. Investigation of threshold carrier densities in the optically pumped amplified spontaneous emission of formamidinium lead bromide perovskite using different excitation wavelengths. Photonics 2022, 9, 4. [Google Scholar] [CrossRef]
- Kumawat, N.K.; Dey, A.; Kumar, A.; Gopinathan, S.P.; Narasimhan, K.L.; Kabra, D. Band Gap Tuning of CH3NH3Pb(Br1−xClx)3 Hybrid Perovskite for Blue Electroluminescence. ACS Appl. Mater. Interfaces 2015, 7, 13119–13124. [Google Scholar] [CrossRef]
- Kumawat, N.K.; Tripathi, M.N.; Waghmare, U.; Kabra, D. Structural, optical, and electronic properties of wide bandgap perovskites: Experimental and theoretical investigations. J. Phys. Chem. A 2016, 120, 3917–3923. [Google Scholar] [CrossRef]
- Kitazawa, N.; Watanabe, Y.; Nakamura, Y. Optical properties of CH3NH3PbX3 (X = halogen) and their mixed-halide crystals. J. Mater. Sci. 2002, 37, 3585–3587. [Google Scholar] [CrossRef]
- Qaid, S.M.H.; Ghaithan, H.M.; Al-Asbahi, B.A.; Aldwayyan, A.S. Achieving Optical Gain of the CsPbBr3 Perovskite Quantum Dots and Influence of the Variable Stripe Length Method. ACS Omega 2021, 6, 5297–5309. [Google Scholar] [CrossRef]
- Kumar, P.; Lynch, J.; Song, B.; Ling, H.; Barrera, F.; Kisslinger, K.; Zhang, H.; Anantharaman, S.B.; Digani, J.; Zhu, H.; et al. Light–matter coupling in large-area van der Waals superlattices. Nat. Nanotechnol. 2022, 17, 182–189. [Google Scholar] [CrossRef]
- Huang, C.Y.; Zou, C.; Mao, C.; Corp, K.L.; Yao, Y.C.; Lee, Y.J.; Schlenker, C.W.; Jen, A.K.Y.; Lin, L.Y. CsPbBr3 Perovskite Quantum Dot Vertical Cavity Lasers with Low Threshold and High Stability. ACS Photonics 2017, 4, 2281–2289. [Google Scholar] [CrossRef]
- Dang, C.; Nurmikko, A. Beyond quantum dot LEDs: Optical gain and laser action in red, green, and blue colors. MRS Bull. 2013, 38, 737–742. [Google Scholar] [CrossRef]
- Dang, C.; Lee, J.; Breen, C.; Steckel, J.S.; Coe-Sullivan, S.; Nurmikko, A. Red, green and blue lasing enabled by single-exciton gain in colloidal quantum dot films. Nat. Nanotechnol. 2012, 7, 335–339. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef] [PubMed]
- Grim, J.Q.; Christodoulou, S.; Di Stasio, F.; Krahne, R.; Cingolani, R.; Manna, L.; Moreels, I. Continuous-wave biexciton lasing at room temperature using solution-processed quantum wells. Nat. Nanotechnol. 2014, 9, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Klimov, V.I.; Mikhailovsky, A.A.; Xu, S.; Malko, A.; Hollingsworth, J.A.; Leatherdale, C.A.; Eisler, H.J.; Bawendi, M.G. Optical gain and stimulated emission in nanocrystal quantum dots. Science 2000, 290, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Aneesh, J.; Kumar Ravi, V.; Sharma, R.; Mir, W.J.; Beard, M.C.; Nag, A.; Adarsh, K.V. Ultrafast exciton many-body interactions and hot-phonon bottleneck in colloidal cesium lead halide perovskite nanocrystals. Phys. Rev. B 2018, 98, 115418. [Google Scholar] [CrossRef]
- Robel, I.; Gresback, R.; Kortshagen, U.; Schaller, R.D.; Klimov, V.I. Universal size-dependent trend in auger recombination in direct-gap and indirect-gap semiconductor nanocrystals. Phys. Rev. Lett. 2009, 102, 177404. [Google Scholar] [CrossRef]
- Yang, Y.; Ostrowski, D.P.; France, R.M.; Zhu, K.; Van De Lagemaat, J.; Luther, J.M.; Beard, M.C. Observation of a hot-phonon bottleneck in lead-iodide perovskites. Nat. Photonics 2016, 10, 53–59. [Google Scholar] [CrossRef]
- Olbright, G.R.; Klem, J.; Owyoung, A.; Brennan, T.M.; State, S.; Directorate, S.; Laboratories, S.N.; Binder, R.; Koch, S.W. Many-body effects in the luminescence of highly excited indirect superlattices. J. Opt. Soc. Am. B 1990, 7, 1473–1480. [Google Scholar] [CrossRef]
- Veldhuis, S.A.; Boix, P.P.; Yantara, N.; Li, M.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. Perovskite Materials for Light-Emitting Diodes and Lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Puls, J.; Wünsche, H.J.; Henneberger, F. Bi-excitons in wide-gap II-VI quantum wells. Localization by alloy disorder. Chem. Phys. 1996, 210, 235–247. [Google Scholar] [CrossRef]
- Kunneman, L.T.; Tessier, M.D.; Heuclin, H.; Dubertret, B.; Aulin, Y.V.; Grozema, F.C.; Schins, J.M.; Siebbeles, L.D.A. Bimolecular auger recombination of electron-hole pairs in two-dimensional CdSe and CdSe/CdZnS core/shell nanoplatelets. J. Phys. Chem. Lett. 2013, 4, 3574–3578. [Google Scholar] [CrossRef]
- Qaid, S.M.H.; Ghaithan, H.M.; Al-Asbahi, B.A.; Alqasem, A.; Aldwayyan, A.S. Fabrication of thin films from powdered cesium lead bromide (CsPbBr3) perovskite quantum dots for coherent green light emission. ACS Omega 2020, 5, 30111–30122. [Google Scholar] [CrossRef]
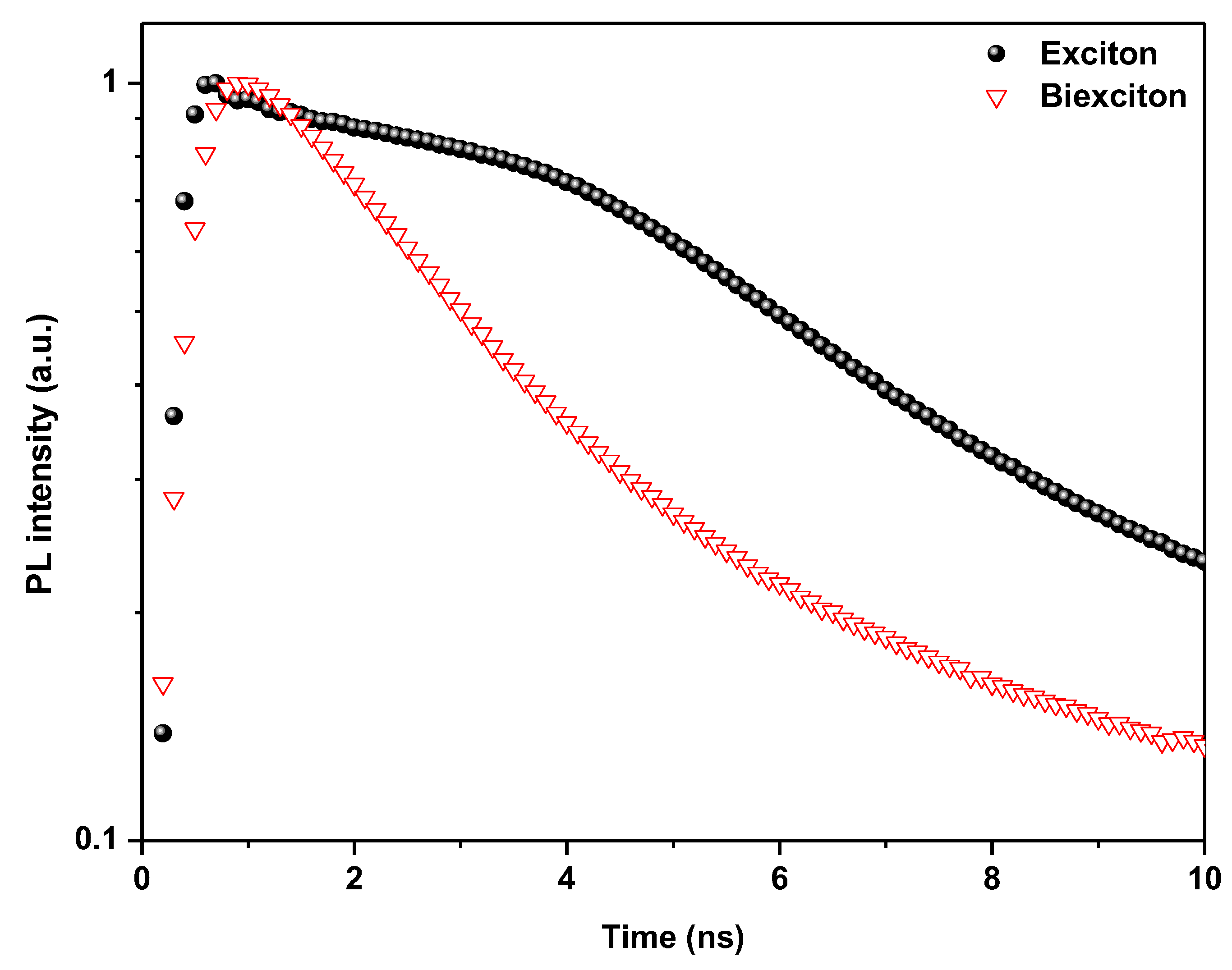

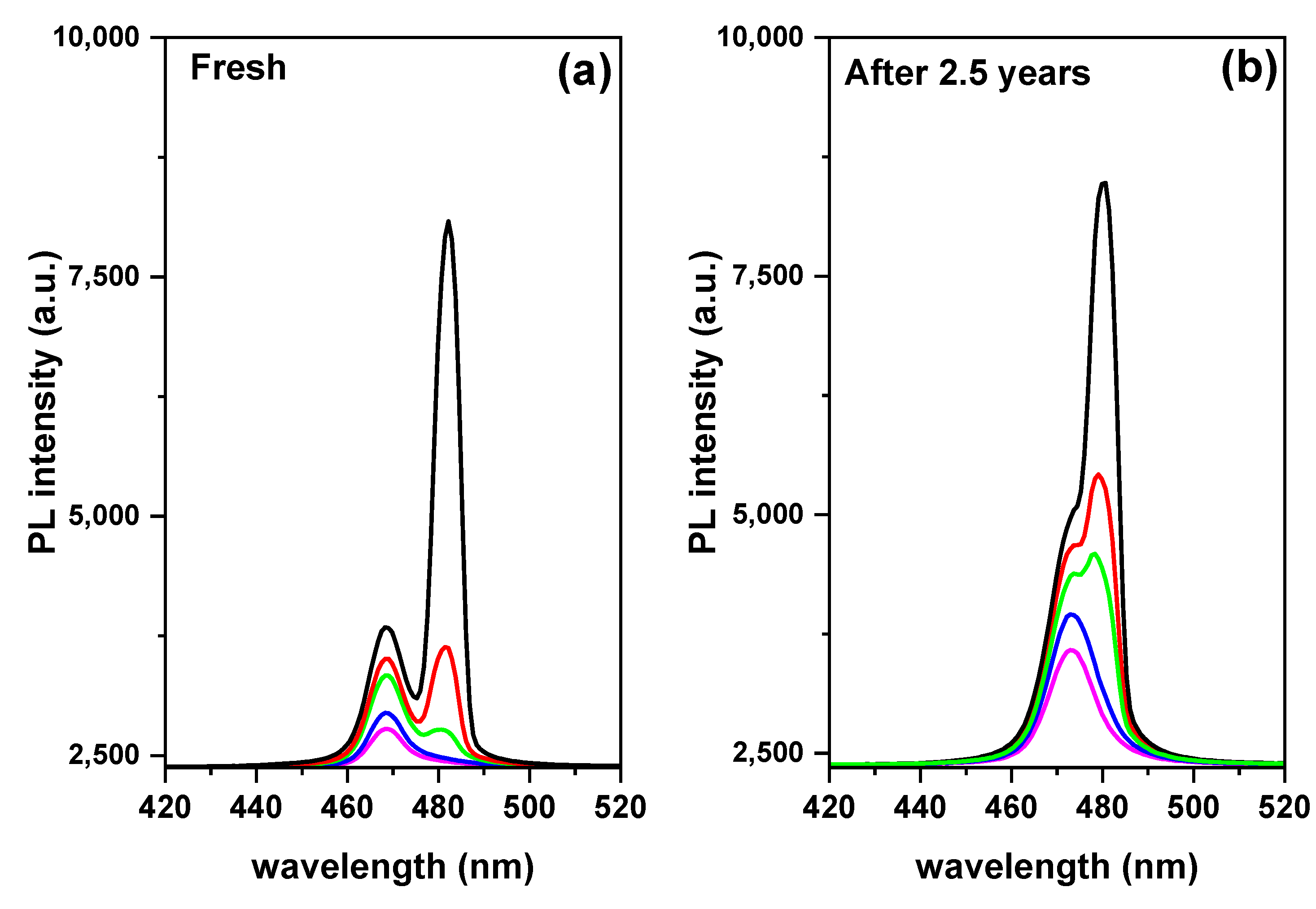
| Compound | Eg (eV) | Eb (meV) | Exciton | PL | Stock Shift (meV) | ||
|---|---|---|---|---|---|---|---|
| Peak (eV) | FWHM (meV) | Peak (eV) | FWHM (nm) | ||||
| CsPb(Br/Cl)3 | 2.787 | 67.0 | 2.719 | 70.67 | 2.675 | 97.00 | 41 |
| PMMA/[CsPb(Br/Cl)3] | 2.786 | 70.2 | 2.716 | 70.67 | 2.693 | 93.72 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qaid, S.M.H.; Ghaithan, H.M.; Bawazir, H.S.; Aldwayyan, A.S. Surface Passivation for Promotes Bi-Excitonic Amplified Spontaneous Emission in CsPb(Br/Cl)3 Perovskite at Room Temperature. Polymers 2023, 15, 1978. https://doi.org/10.3390/polym15091978
Qaid SMH, Ghaithan HM, Bawazir HS, Aldwayyan AS. Surface Passivation for Promotes Bi-Excitonic Amplified Spontaneous Emission in CsPb(Br/Cl)3 Perovskite at Room Temperature. Polymers. 2023; 15(9):1978. https://doi.org/10.3390/polym15091978
Chicago/Turabian StyleQaid, Saif M. H., Hamid M. Ghaithan, Huda S. Bawazir, and Abdullah S. Aldwayyan. 2023. "Surface Passivation for Promotes Bi-Excitonic Amplified Spontaneous Emission in CsPb(Br/Cl)3 Perovskite at Room Temperature" Polymers 15, no. 9: 1978. https://doi.org/10.3390/polym15091978
APA StyleQaid, S. M. H., Ghaithan, H. M., Bawazir, H. S., & Aldwayyan, A. S. (2023). Surface Passivation for Promotes Bi-Excitonic Amplified Spontaneous Emission in CsPb(Br/Cl)3 Perovskite at Room Temperature. Polymers, 15(9), 1978. https://doi.org/10.3390/polym15091978







