Advances in Functional Hydrogel Wound Dressings: A Review
Abstract
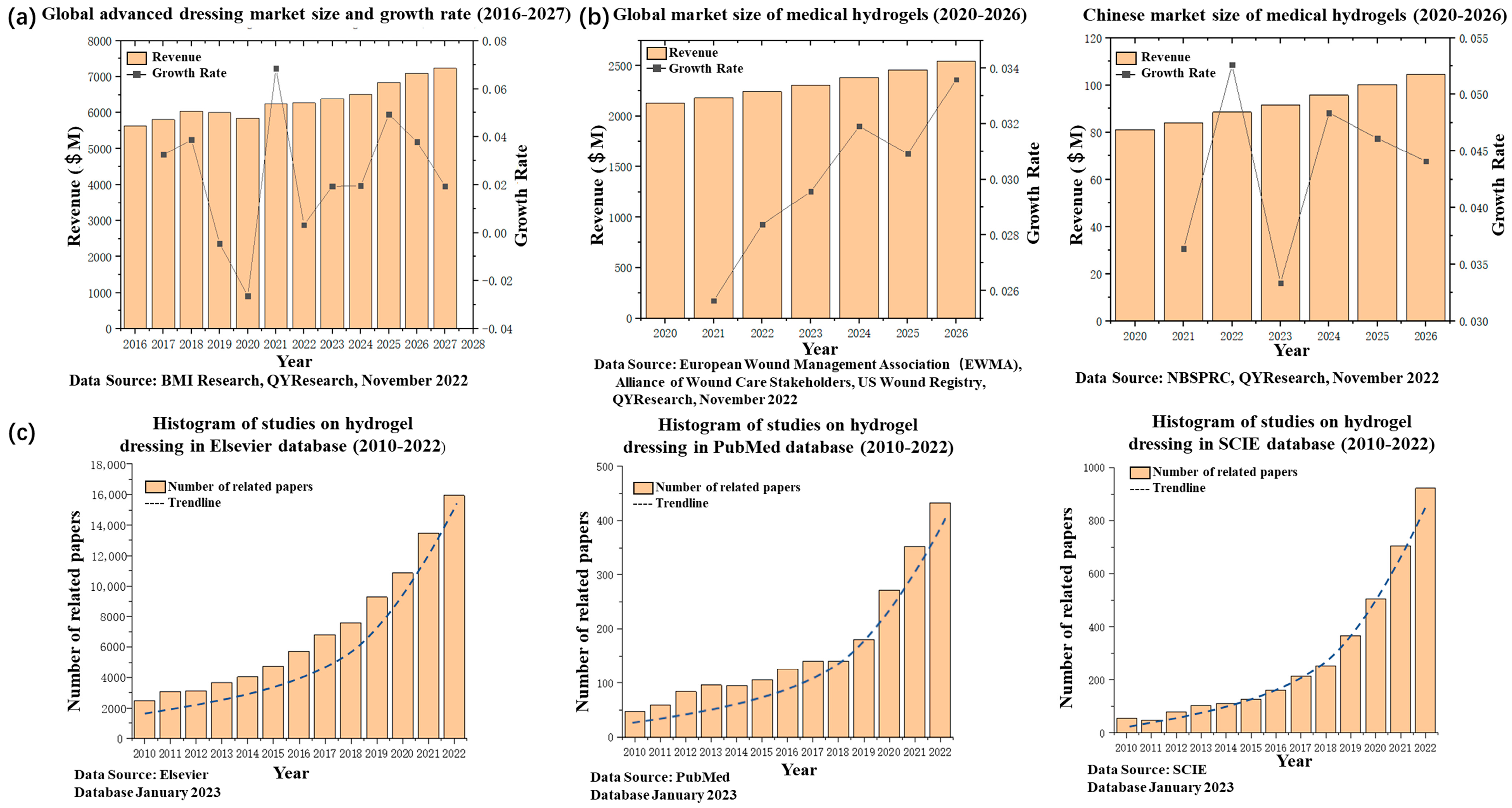
1. Introduction
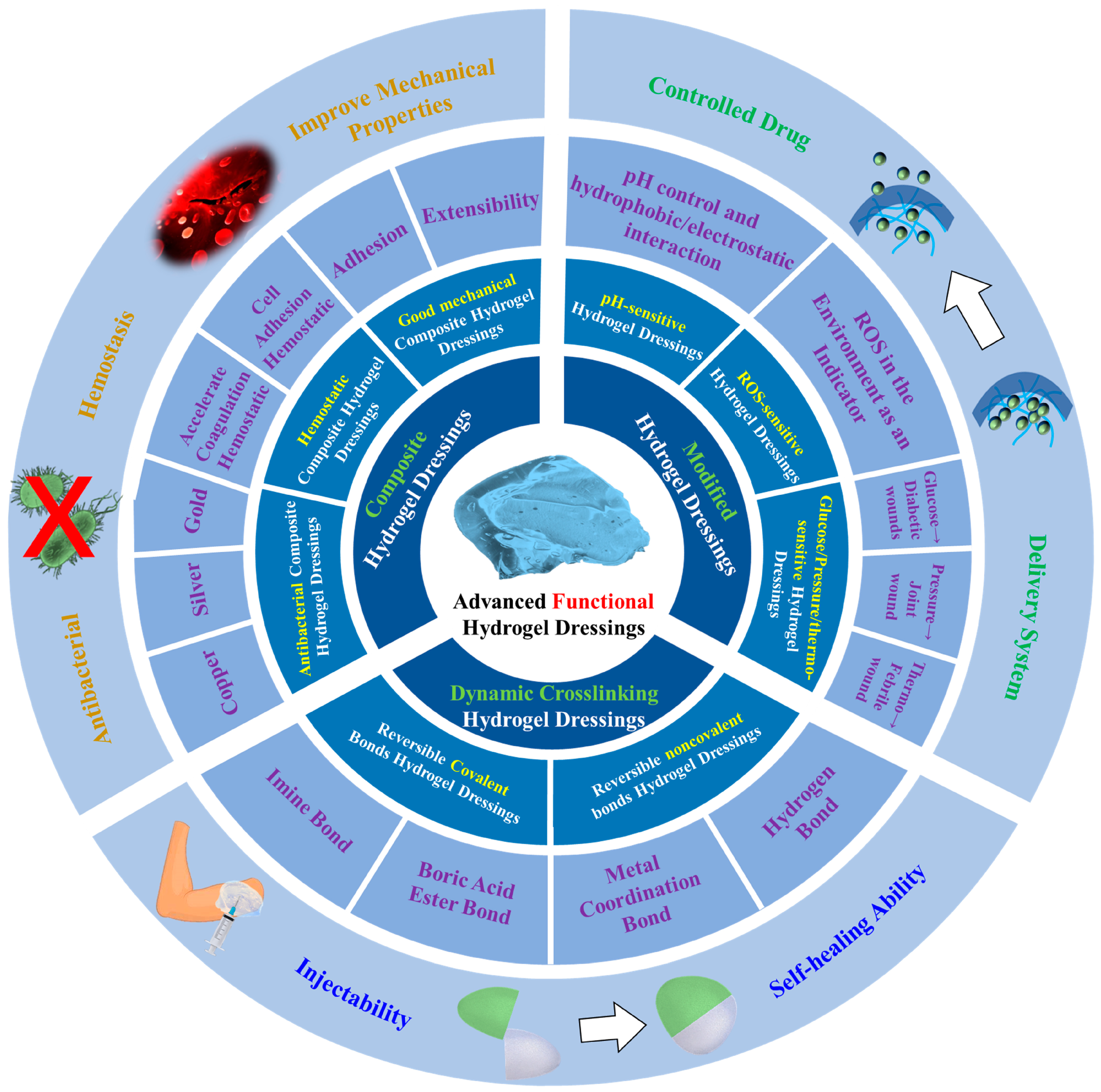
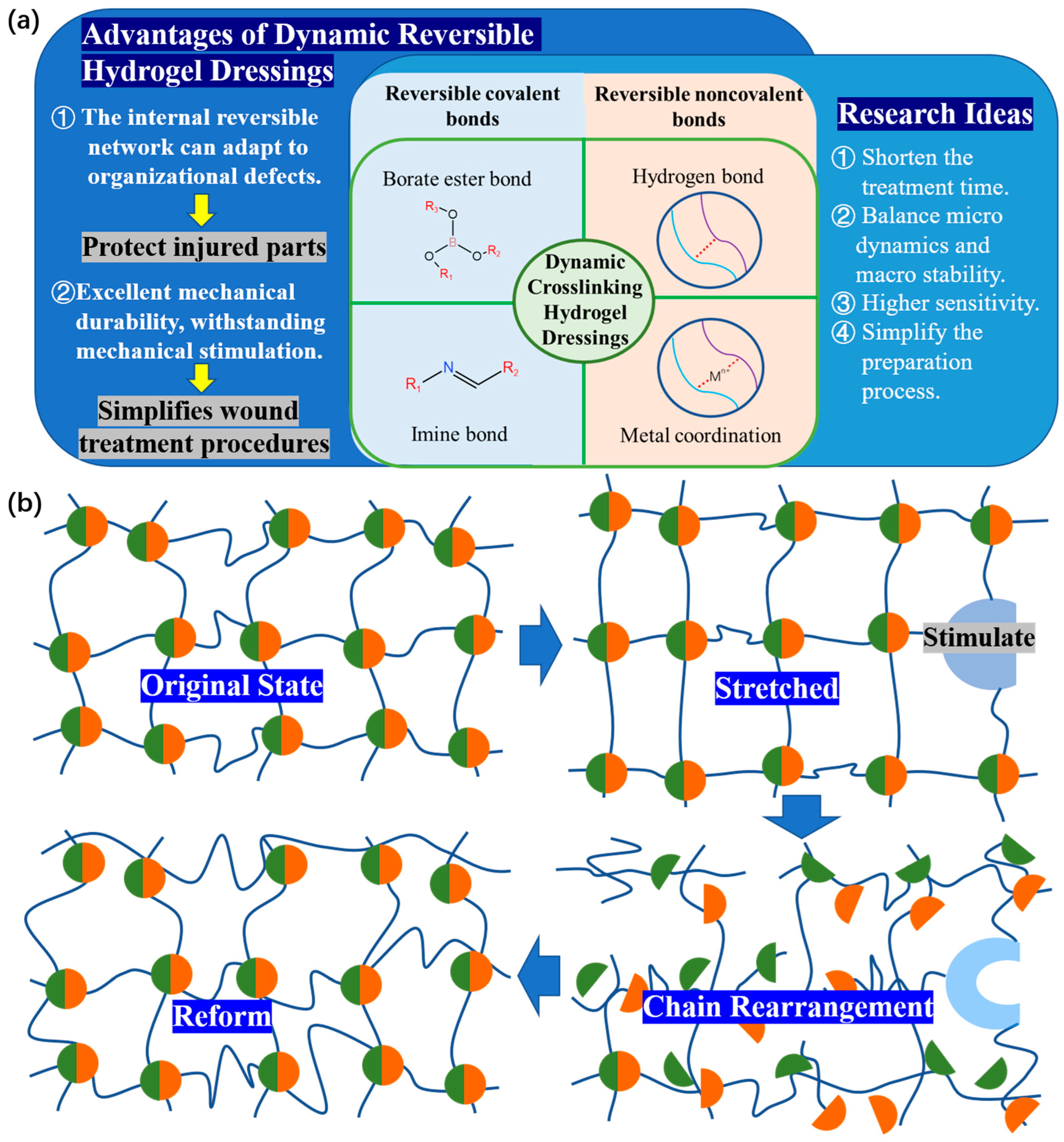
2. Dynamic Crosslinking Hydrogel Dressings
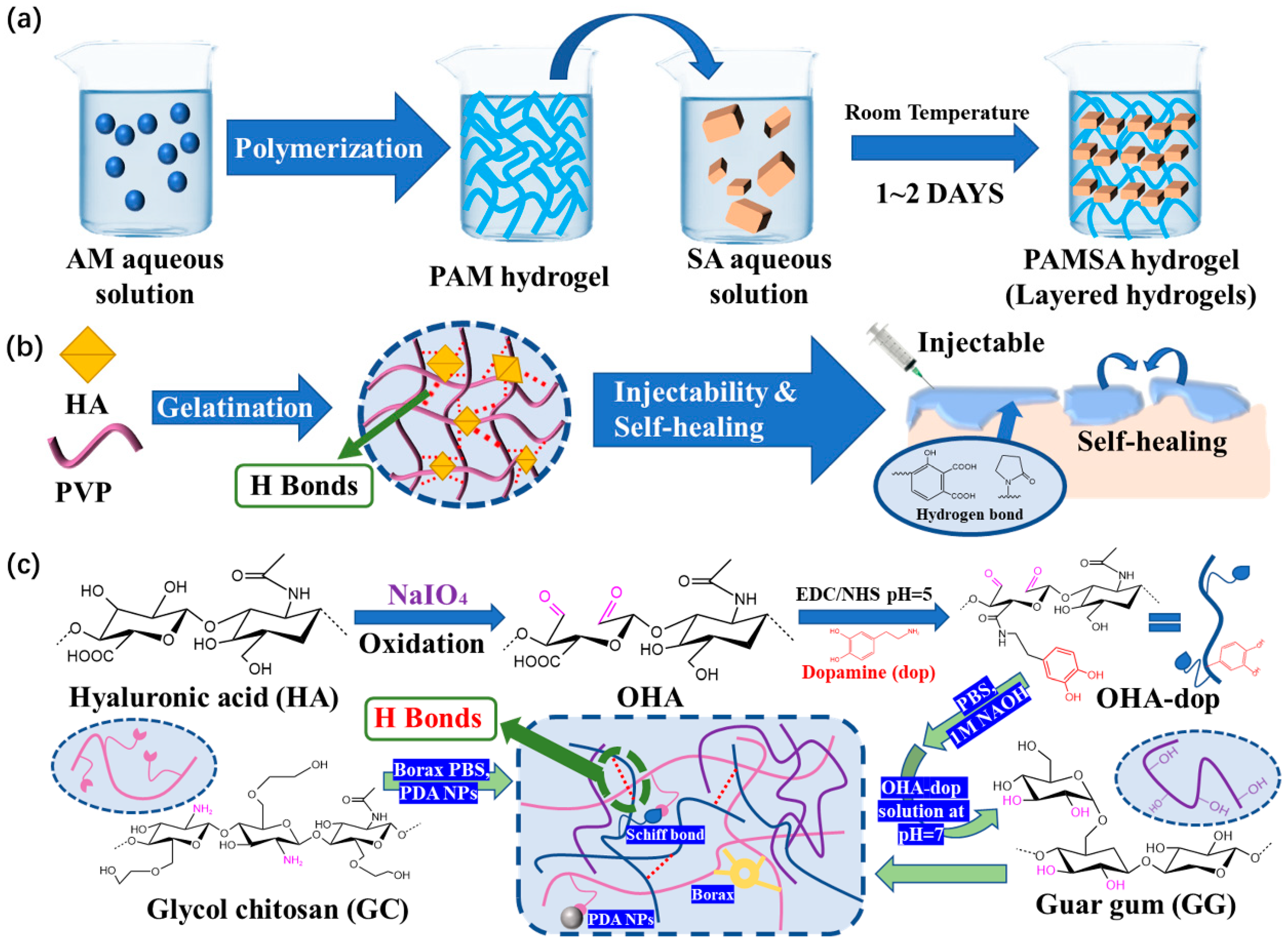
2.1. Reversible Covalent Bonds
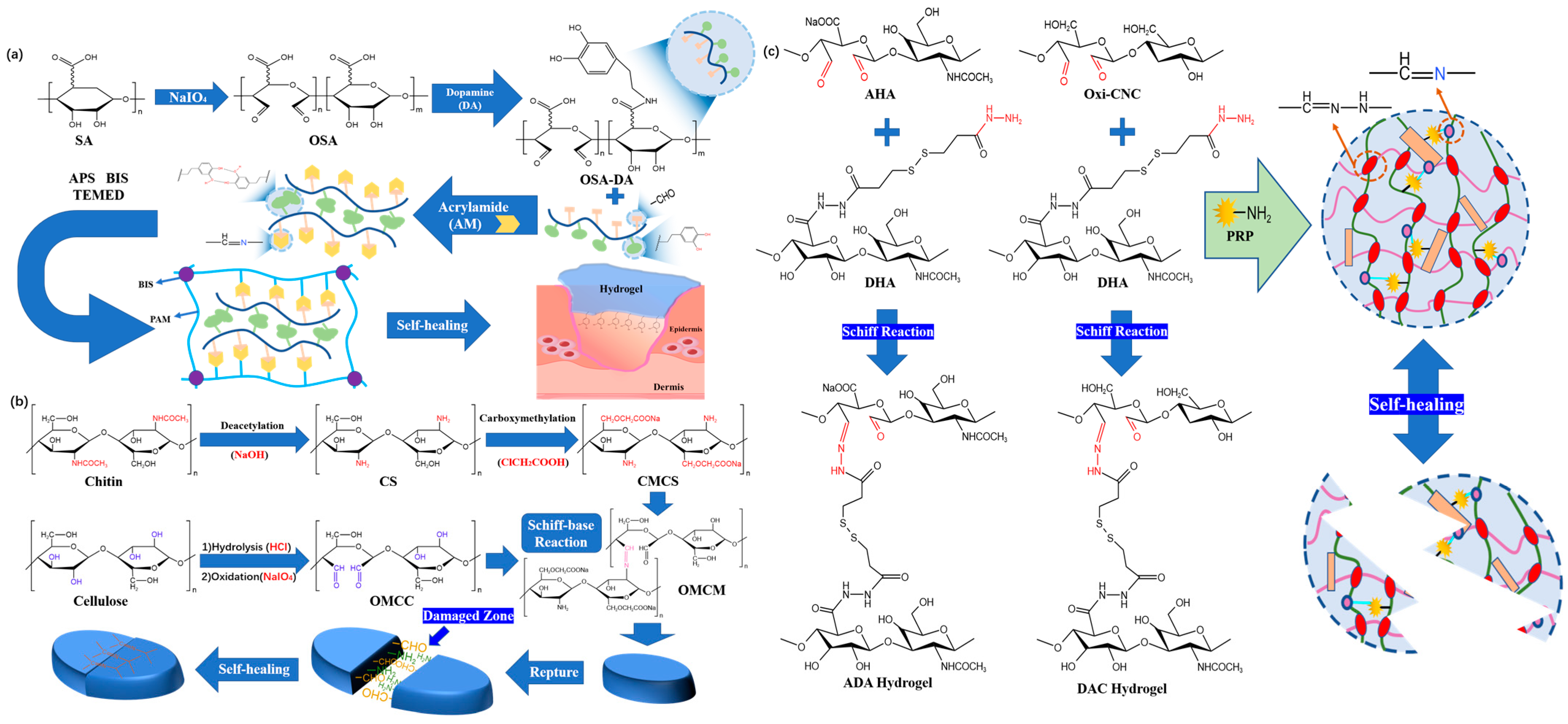
2.1.1. Imine Bonds
2.1.2. Boric Acid Ester Bonds
2.2. Reversible Noncovalent Bonds
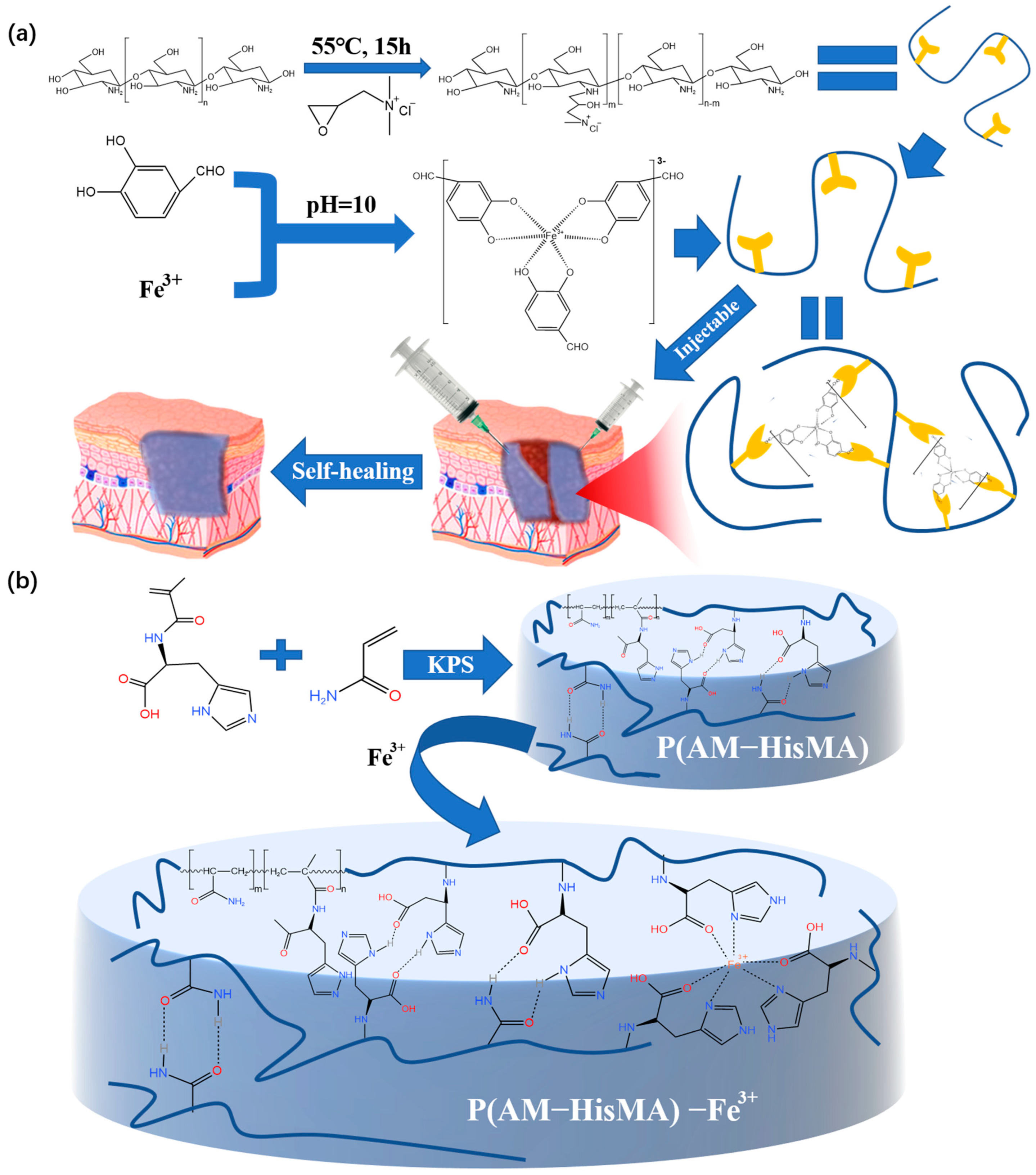
2.2.1. Metal Coordination Bond
2.2.2. Hydrogen Bond
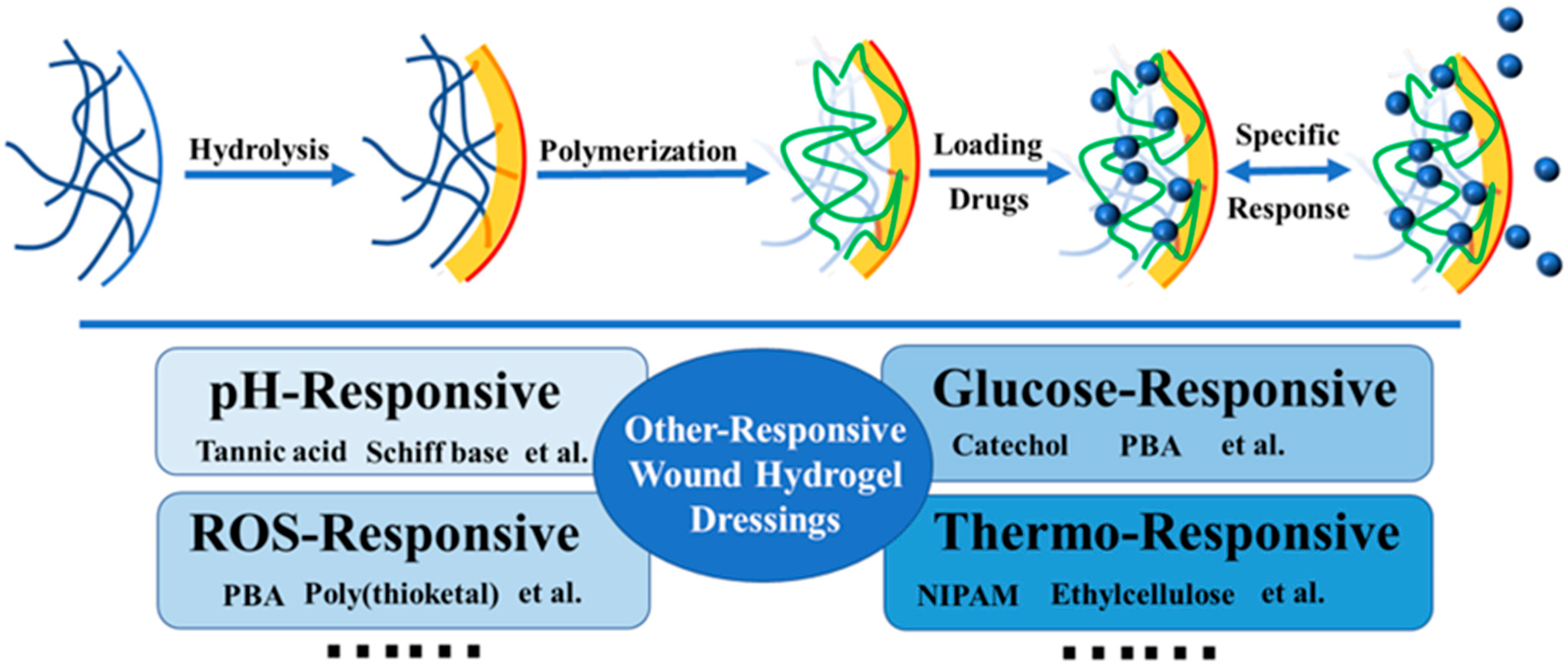
3. Modified Hydrogel Dressings
3.1. pH-Responsive Hydrogel Dressings
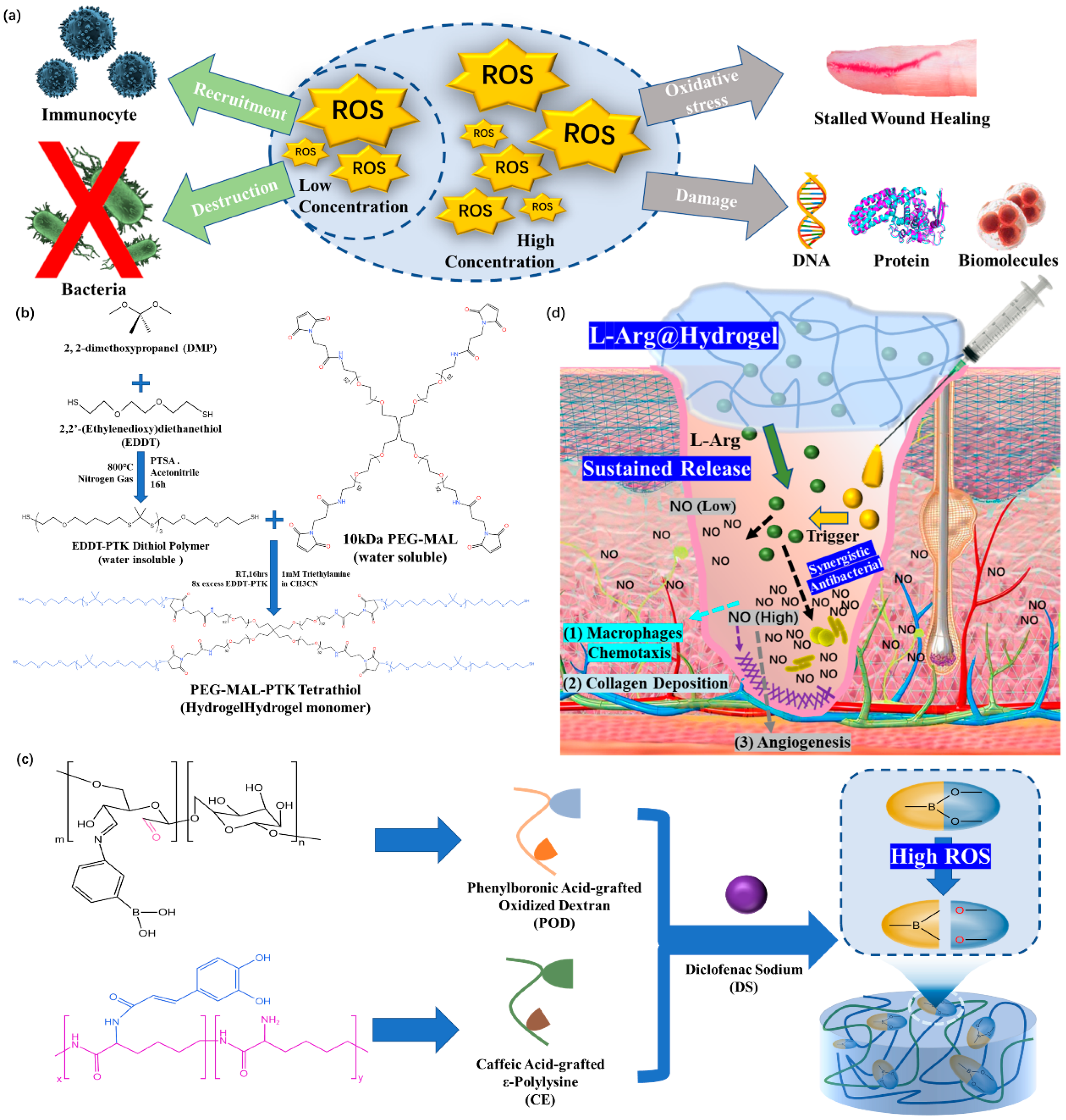
3.2. ROS-Responsive Hydrogel Dressings
3.3. Other Types of Responsive Hydrogel Dressings
3.3.1. Glucose-Responsive Hydrogel Dressings
3.3.2. Pressure-Responsive Hydrogel Dressings
3.3.3. Thermo-Responsive Hydrogel Dressings
4. Composite Hydrogel Dressings
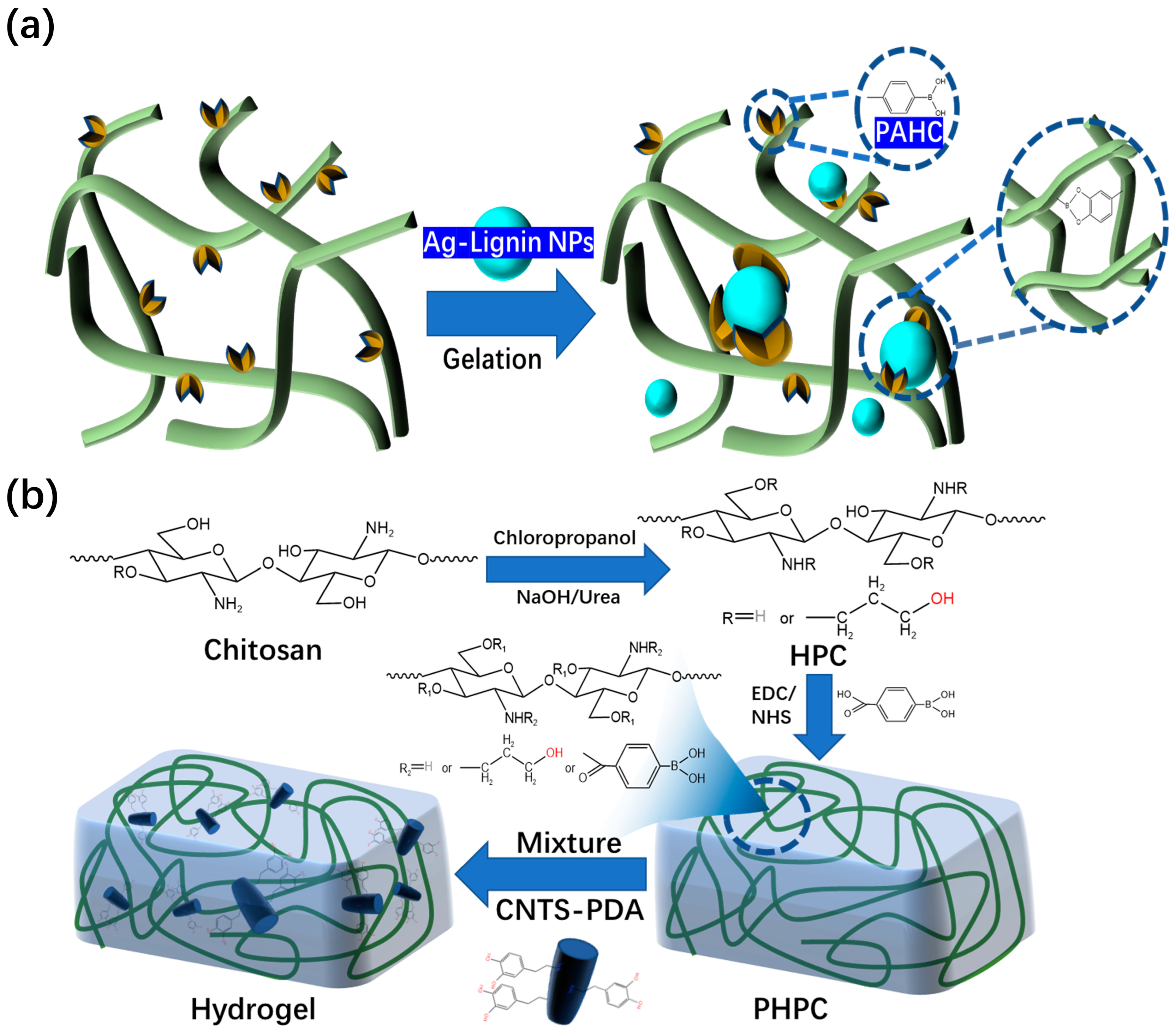
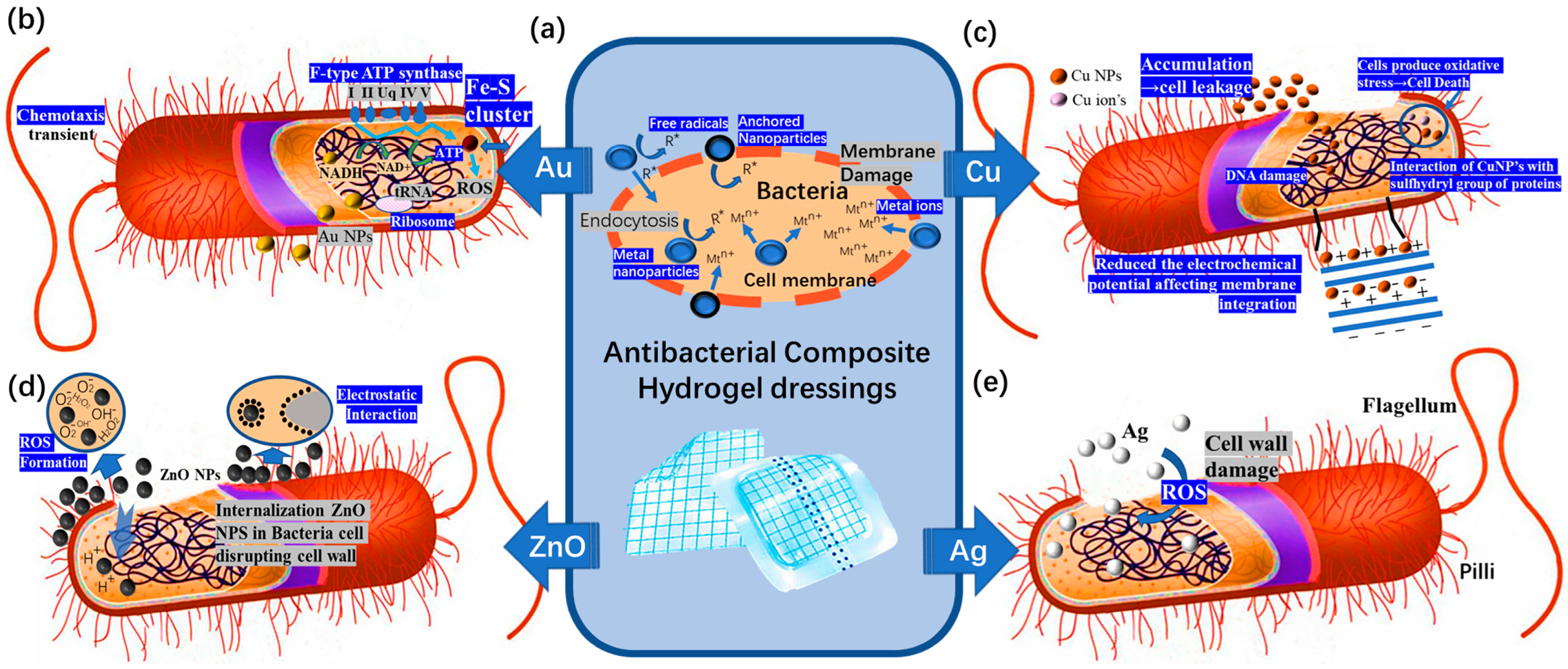
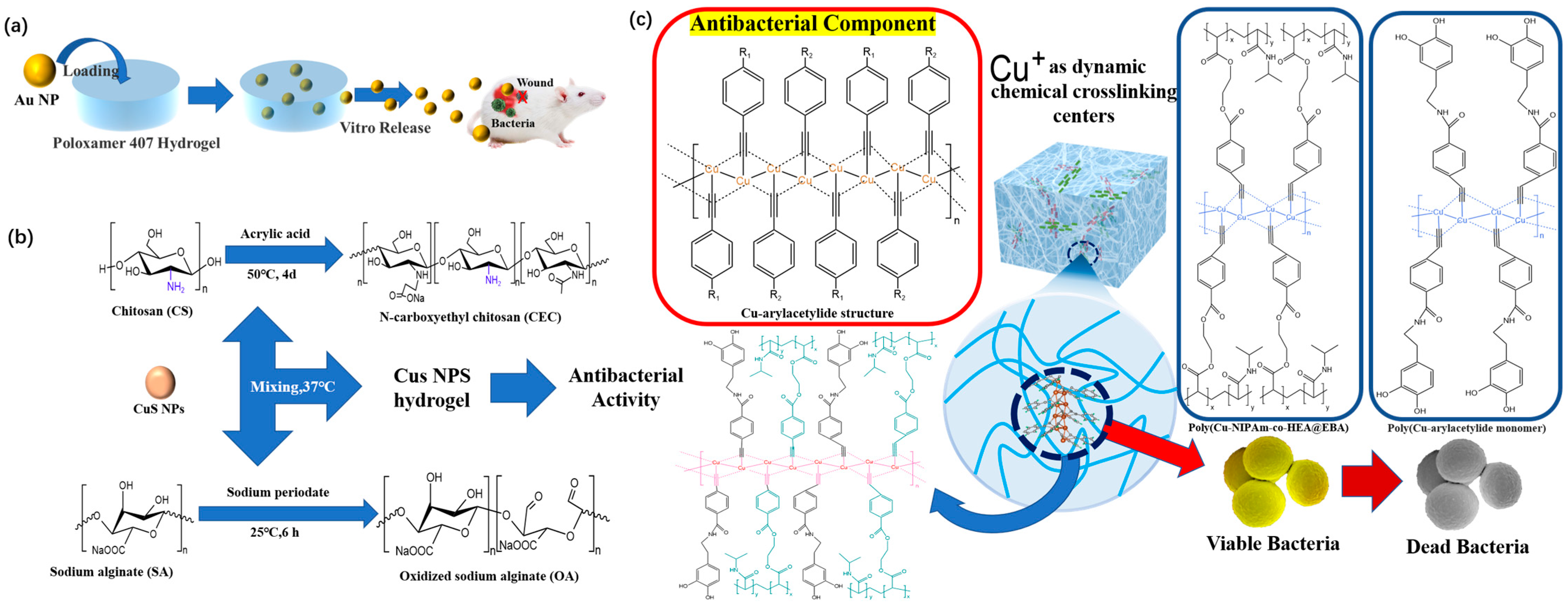
4.1. Antibacterial Composite Hydrogel Dressings
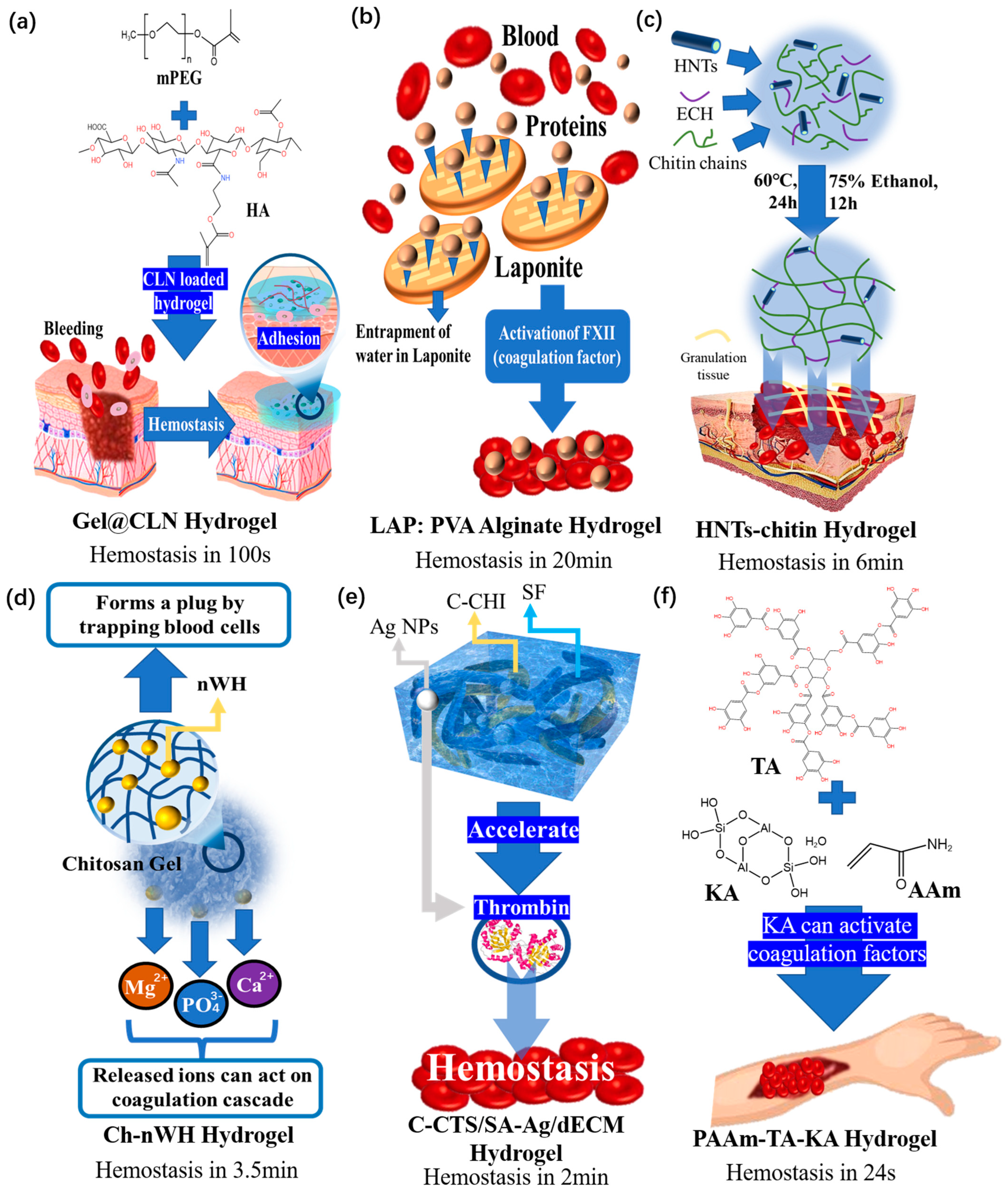
4.2. Hemostatic Composite Hydrogel Dressings
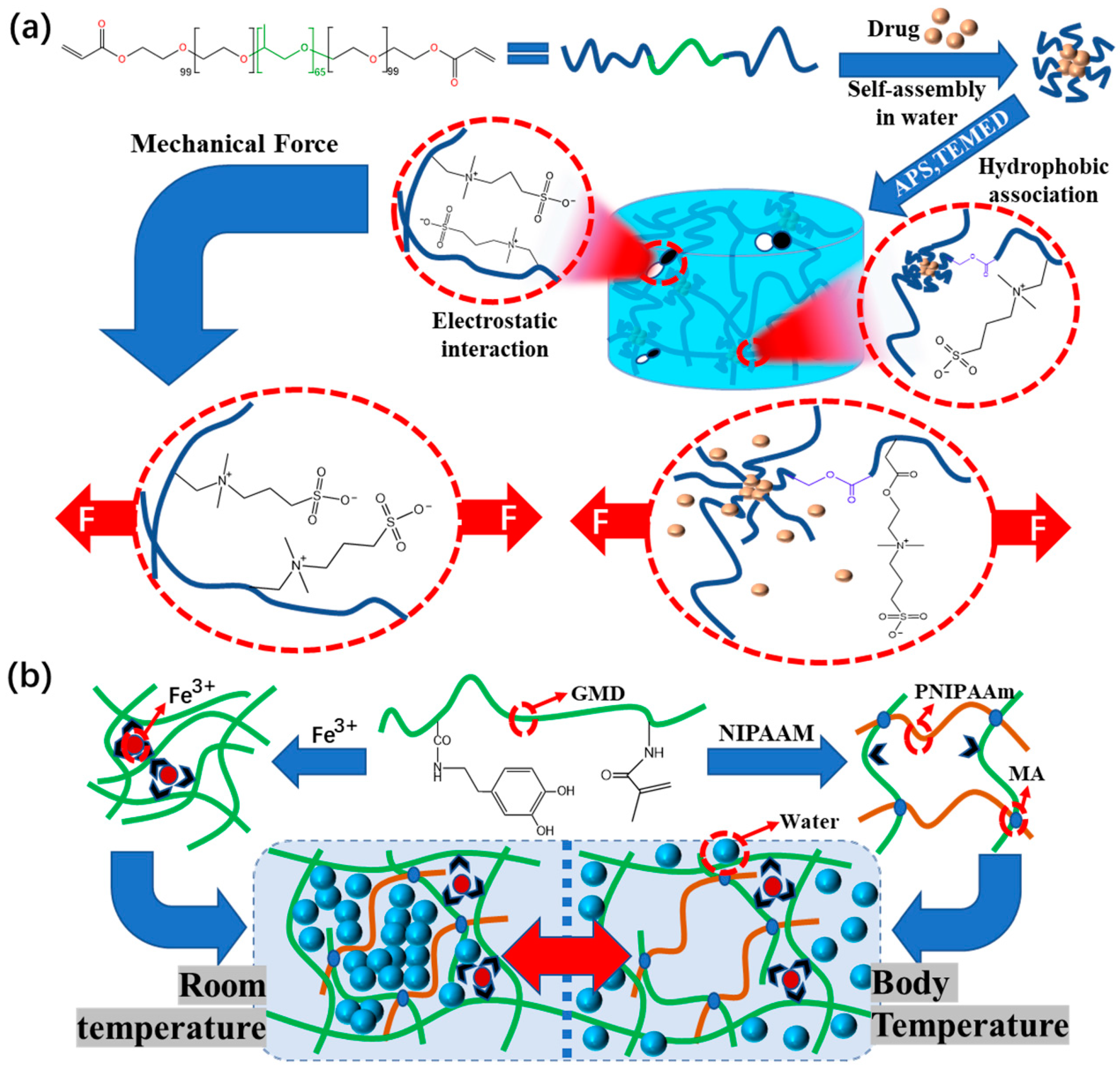
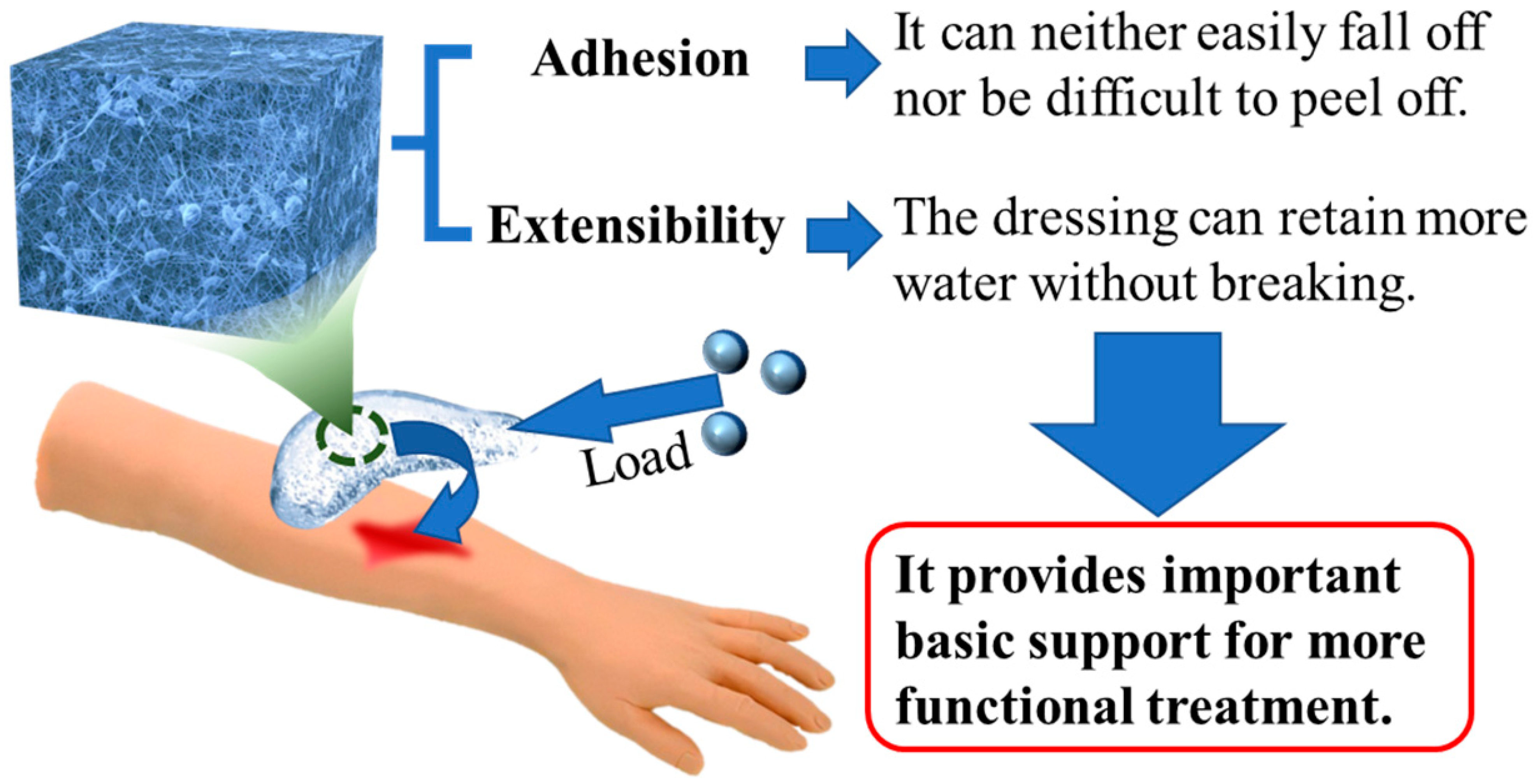
4.3. Composite Hydrogel Dressings with Good Mechanical Properties
4.3.1. Adhesion
4.3.2. Extensibility
5. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liu, S.D.; Li, D.; Wang, Y.; Zhou, G.Q.; Ge, K.; Jiang, L. Adhesive, antibacterial and double crosslinked carboxylated polyvinyl alcohol/chitosan hydrogel to enhance dynamic skin wound healing. Int. J. Biol. Macromol. 2023, 228, 744–753. [Google Scholar] [CrossRef]
- QYResearch. 2021–2027 Global and China Advanced Dressing Market Status and Forecast. 2021. Available online: https://www.qyresearch.com.cn/reports/2021-2027-p911142.html (accessed on 14 February 2023).
- Tang, N.F.R.; Heryanto, H.; Armynah, B.; Tahir, D. Bibliometric analysis of the use of calcium alginate for wound dressing applications: A review. Int. J. Biol. Macromol. 2023, 228, 138–152. [Google Scholar] [CrossRef]
- Aycan, D.; Selmi, B.; Kelel, E.; Yildirim, T.; Alemdar, N. Conductive polymeric film loaded with ibuprofen as a wound dressing material. Eur. Polym. J. 2019, 121, 109308. [Google Scholar] [CrossRef]
- Ajiteru, O.; Lee, O.J.; Kim, J.H.; Lee, Y.J.; Lee, J.S.; Lee, H.; Sultan, M.T.; Park, C.H. Fabrication and characterization of a myrrh hydrocolloid dressing for dermal wound healing. Colloids Interface Sci. Commun. 2022, 48, 100617. [Google Scholar] [CrossRef]
- Tang, S.; Gong, Z.J.; Wang, Z.F.; Gao, X.; Zhang, X.N. Multifunctional hydrogels for wound dressings using xanthan gum and polyacrylamide. Int. J. Biol. Macromol. 2022, 217, 944–955. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Y.Q.; Song, W.; Yu, B.H.; Liu, H.Z. Rational design in functional hydrogels towards biotherapeutics. Mater. Des. 2022, 223, 111086. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef] [PubMed]
- QYResearch. 2022–2026 Global and China Medical Grade Hydrogel Market Status and Forecast. 2022. Available online: https://www.qyresearch.com.cn/reports/medical-grade-hydrogel-p978747.html (accessed on 14 February 2023).
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Krasichkov, A.; Polyakova, V.O.; Uspenskaya, M.V. A Review on Chitosan and Cellulose Hydrogels for Wound Dressings. Polymers 2022, 14, 5163. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Wang, L.R.; Zhou, M.Y.; Xu, T.L.; Zhang, X.J. Multifunctional hydrogel as wound dressing for intelligent wound monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Wang, T.; Yi, W.W.; Zhang, Y.; Wu, H.; Fan, H.W.; Zhao, J.L.; Wang, S.G. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B 2023, 222, 113096. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.P.; He, J.H.; Zhang, H.L.; Guo, B.L. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Huang, X.X.; Ma, C.; Xu, Y.C.; Cao, J.F.; Li, J.C.; Li, J.Z.; Shi, S.Q.; Gao, Q. A tannin-functionalized soy protein-based adhesive hydrogel as a wound dressing. Ind. Crop. Prod. 2022, 182, 114945. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, M.; Chen, L.; Zhang, Y.; Lu, T.; Deng, Y.; Ma, W.; Xu, J.; Huang, C.; Xiong, R. Design the molecule structures to achieve functional advantages of hydrogel wound dressings: Advances and strategies. Compos. B. Eng. 2022, 247, 110313. [Google Scholar] [CrossRef]
- Nazarnezhada, S.; Abbaszadeh-Goudarzi, G.; Samadian, H.; Khaksari, M.; Ghatar, J.M.; Khastar, H.; Rezaei, N.; Mousavi, S.R.; Shirian, S.; Salehi, M. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 164, 3323–3331. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhong, K.J.; Zong, Y.; Wang, S.H.; Yang, H.Y.; Zhen, L.; Tao, S.Y.; Sun, L.Z.; Yang, J.J.; Li, J.Y. A mussel-inspired wet-adhesion hydrogel with hemostasis and local anti-inflammation for managing the development of acute wounds. Mater. Des. 2022, 213, 110347. [Google Scholar] [CrossRef]
- Zeng, H.H.; Liu, X.; Zhang, Z.Q.; Song, X.W.; Quan, J.; Zheng, J.; Shen, Z.L.; Ni, Y.Q.; Liu, C.T.; Zhang, Y.; et al. Self-healing, injectable hydrogel based on dual dynamic covalent cross-linking against postoperative abdominal cavity adhesion. Acta Biomater. 2022, 151, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.B.; Li, J.; Zhang, Z.P.; Liu, J.Z.; Wu, C.Y.; Yu, Y.Q. Incorporating self-healing capability in temperature-sensitive hydrogels by non-covalent chitosan crosslinkers. Eur. Polym. J. 2023, 182, 111728. [Google Scholar] [CrossRef]
- Erezuma, I.; Lukin, I.; Desimone, M.; Zhang, Y.S.; Dolatshahi-Pirouz, A.; Orive, G. Progress in self-healing hydrogels and their applications in bone tissue engineering. Biomater. Adv. 2023, 146, 213274. [Google Scholar] [CrossRef] [PubMed]
- Karvinen, J.; Kellomäki, M. Characterization of self-healing hydrogels for biomedical applications. Eur. Polym. J. 2022, 181, 111641. [Google Scholar] [CrossRef]
- Lv, J.H.; Fang, Y.R.; Wu, M.; Ou, X.Y.; Zhang, W.C.; Wang, S.Y.; Li, H.G.; Shang, L.; He, M.F.; Zhao, Y. Poly(acrylamide) hydrogel composites with microsized β-chitin fiber and the properties of mechanical and drug release. Mater. Today Commun. 2023, 34, 105163. [Google Scholar] [CrossRef]
- Wang, Z.G.; Chen, R.P.; Yang, S.P.; Li, S.; Gao, Z.X. Design and application of stimuli-responsive DNA hydrogels: A review. Mater. Today Bio 2022, 16, 100430. [Google Scholar] [CrossRef]
- He, W.Y.; Wang, X.C.; Gong, W.; Huang, H.B.; Hou, Y.Y.; Wang, R.; Hu, J.N. Construction of an antibacterial hydrogel based on diammonium glycyrrhizinate and gallic acid for bacterial-infected wound healing. Colloids Surf. B 2023, 222, 112975. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wu, M.; Lin, L.W.; Wu, Z.L.; Bae, M.J.; Park, S.M.; Wang, S.L.; Zhang, W.; Gao, J.F.; Wang, D.G.; et al. Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: Status and trends. Mater. Today Bio. 2022, 16, 100429. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Zhao, D.H.; Zhang, C.; Liu, K.Y.; He, Y.M.; Guan, F.X.; Yao, M.H. Nanocomposite multifunctional hyaluronic acid hydrogel with photothermal antibacterial and antioxidant properties for infected wound healing. Int. J. Biol. Macromol. 2023, 226, 870–884. [Google Scholar] [CrossRef]
- Luo, W.; Hu, B.; Zhang, H.L.; Li, C.Y.; Shi, Y.P.; Li, X.C.; Jin, L. Antibacterial photothermal and stable Ag-titanium-oxo-clusters hydrogel designed for wound healing. Mater. Des. 2023, 226, 111674. [Google Scholar] [CrossRef]
- Cao, S.J.; Bi, Z.J.; Li, Q.J.; Zhang, S.K.; Singh, M.; Chen, J.D. Shape memory and antibacterial chitosan-based cryogel with hemostasis and skin wound repair. Carbohydr. Polym. 2023, 305, 120545. [Google Scholar] [CrossRef] [PubMed]
- Takeno, H.; Kimura, Y. Molecularweight effects on tensile properties of blend hydrogels composed of clay and polymers. Polymer 2016, 85, 47–54. [Google Scholar] [CrossRef]
- Wang, W.D.; Ummartyotin, S.; Narain, R. Advances and Challenges on Hydrogels for Wound Dressing. Curr. Opin. Biomed. Eng. 2023, 26, 100443. [Google Scholar] [CrossRef]
- Ding, X.Y.; Yu, Y.R.; Zu, Y. Self-healing hydrogels based on the Knoevenagel condensation reaction for wound healing. Biomed. Technol. 2023, 2, 70–76. [Google Scholar] [CrossRef]
- Deng, Z.X.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B.L. Multifunctional stimuli-responsive hydrogels with self-Healing, high conductivity, and rapid recovery through host–guest interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Han, W.; Chen, C.; Yang, K.; Wang, H.B.; Xia, H.G.; Zhao, Y.; Teng, Y.; Feng, G.C.; Chen, Y.M. Hyaluronic acid and chitosan-based injectable and self-healing hydrogel with inherent antibacterial and antioxidant bioactivities. Int. J. Biol. Macromol. 2023, 227, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.R.; Yang, F.J.; Zhou, F.H.; He, J.; Lu, W.I.; Xiao, P.; Yan, H.Z.; Pan, C.F.; Chen, T.; Wang, Z.L. Bioinspired self-healing human-machine interactive touch pad with pressure-sensitive adhesiveness on targeted substrates. Adv. Mater. 2020, 32, 2004290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Gu, J.Y.; Zhang, D.F.; Zhang, Y.; Chen, J.H. Structurally dynamic gelatin-based hydrogels with self-healing, shape memory, and cytocompatible properties for 4D printing. Biomacromolecules 2023, 24, 109–117. [Google Scholar] [CrossRef]
- Zhang, Z.; Abidi, N.; Lucia, L.A. Dual crosslinked-network self-healing composite hydrogels exhibit enhanced water adaptivity and reinforcement. Ind. Eng. Chem. Res. 2022, 61, 17876–17884. [Google Scholar] [CrossRef]
- Feng, W.J.; Wang, Z.K. Shear-thinning and self-healing chitosan-graphene oxide hydrogel for hemostasis and wound healing. Carbohydr. Polym. 2022, 294, 119824. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Feng, Q.; Fang, Z.W.; Gu, L.; Bian, L.M. Structurally dynamic hydrogels for biomedical applications: Pursuing a fine balance between macroscopic stability and microscopic dynamics. Chem. Rev. 2021, 121, 11149–11193. [Google Scholar] [CrossRef]
- Cromwell, O.R.; Chung, J.; Guan, Z.B. Malleable and self-Healing covalent polymer networks through tunable dynamic boronic ester bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495. [Google Scholar] [CrossRef]
- Ikura, R.; Park, J.; Osaki, M.; Yamaguchi, H.; Harada, A.; Takashima, Y. Design of self-healing and self-restoring materials utilizing reversible and movable crosslinks. NPG Asia Mater. 2022, 14, 10. [Google Scholar] [CrossRef]
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Untersuchungen ü das Chinolin. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Pogostin, B.H.; Saenz, G.; Cole, C.C.; Euliano, E.M.; Hartgerink, J.D.; McHugh, K.J. Dynamic imine bonding facilitates mannan release from a nanofibrous peptide hydrogel. Bioconjug. Chem. 2023, 34, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Min, J.B.; Zhou, Z.X.; Wang, H.N.; Chen, Q.H.; Hong, M.C.; Fu, H.Q. Room temperature self-healing and recyclable conductive composites for flexible electronic devices based on imine reversible covalent bond. J. Alloys Compd. 2022, 894, 162433. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.J.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H.Z. Ultratough self-healing and tissue-adhesive hydrogel for wound dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
- Yin, H.S.; Song, P.Q.; Chen, X.Y.; Huang, Q.Y.; Huang, H.H. A self-healing hydrogel based on oxidized microcrystalline cellulose and carboxymethyl chitosan as wound dressing material. Int. J. Biol. Macromol. 2022, 221, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Wei, Q.Q.; Sun, M.; Ding, P.; Wang, L.; Sun, Y.F.; Ding, X.Y.; Okoro, O.V.; Jiang, G.H.; Shavandi, A. Injectable, self-healing, transparent, and antibacterial hydrogels based on chitosan and dextran for wound dressings. Int. J. Biol. Macromol. 2023, 233, 123494. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z.; Dong, Q.; Peng, X.T.; Chen, Y.; Yang, H.J.; Xu, W.L.; Zhao, Y.T.; Xiao, P.; Zhou, Y.S. Self-healing hyaluronic acid nanocomposite hydrogels with platelet-rich plasma impregnated for skin regeneration. ACS Nano 2022, 16, 11346–11359. [Google Scholar] [CrossRef]
- Ding, C.C.; Tian, M.D.; Feng, R.; Dang, Y.; Zhang, M. Novel self-healing hydrogel with injectable, pH-responsive, strain-sensitive, promoting wound-healing, and hemostatic properties based on collagen and chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.W.; Wu, Y.T.; Zhang, M.; Xie, X.; Liao, J.F. An injectable, self-healing carboxymethylated chitosan hydrogel with mild photothermal stimulation for wound healing. Carbohydr. Polym. 2022, 293, 119722. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, D.J.; Shao, H.R.; Hao, Y.P.; Zhang, T.; Zheng, W.P.; Ji, Y.J.; Ling, P.X.; Lu, Y.; Zhou, Q.H. Injectable and self-healing probiotics-loaded hydrogel for promoting superbacteria-infected wound healing. ACS Appl. Mater. Interfaces 2022, 14, 20538–20550. [Google Scholar] [CrossRef]
- Liu, S.X.; Jiang, N.; Chi, Y.Q.; Peng, Q.; Dai, G.R.; Qian, L.; Xu, K.M.; Zhong, W.Y.; Yue, W.Q. Injectable and self-Healing hydrogel based on chitosan-tannic acid and oxidized hyaluronic acid for wound healing. ACS Biomater. Sci. Eng. 2022, 8, 3754–3764. [Google Scholar] [CrossRef]
- Chen, M.T.; Wu, Y.; Chen, B.H.; Tucker, A.M.; Jagota, A.; Yang, S. Fast, strong, and reversible adhesives with dynamic covalent bonds for potential use in wound dressing. Proc. Natl. Acad. Sci. USA 2022, 119, e2203074119. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.E.; Larsson, H.; Galaev, I.Y.; Mattiasson, B. Synthesis of boronate-containing copolymers of N,N-dimethylacrylamide, their interaction with poly(vinyl alcohol) and rheological behaviour of the gels. Polymer 2004, 45, 2495–2505. [Google Scholar] [CrossRef]
- Chen, Y.M.; Qian, W.Q.; Chen, R.; Zhang, H.J.; Li, X.J.; Shi, D.J.; Dong, W.F.; Chen, M.Q.; Zhao, Y. One-pot preparation of autonomously self-healable elastomeric hydrogel from boricacid and random copolymer bearing hydroxyl groups. ACS Macro Lett. 2017, 6, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.J.; Seidi, F.; Li, C.C.; Wan, Z.M.; Jin, Y.C.; Song, J.L.; Xiao, H.N. Antimicrobial/biocompatible hydrogels dual-reinforced by cellulose as ultrastretchable and rapid self-Healing wound dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef]
- Zhong, Y.J.; Seidi, F.; Wang, Y.L.; Zheng, L.; Jin, Y.C.; Xiao, H.N. Injectable chitosan hydrogels tailored with antibacterial and antioxidant dual functions for regenerative wound healing. Carbohydr. Polym. 2022, 298, 120103. [Google Scholar] [CrossRef]
- Deng, P.P.; Chen, F.X.; Zhang, H.D.; Chen, Y.; Zhou, J.P. Conductive, Self-Healing, adhesive, and antibacterial hydrogels based on lignin/cellulose for rapid MRSA-infected wound repairing. ACS Appl. Mater. Interfaces 2021, 13, 52333–52345. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.P.; Liang, X.; Chen, F.X.; Chen, Y.; Zhou, J.P. Novel multifunctional dual-dynamic-bonds crosslinked hydrogels for multi-strategy therapy of MRSA-infected wounds. Appl. Mater. Today 2022, 26, 101362. [Google Scholar] [CrossRef]
- Xue, K.; Liow, S.S.; Karim, A.A.; Li, Z.B.; Loh, X.J. A recent perspective on noncovalently formed polymeric hydrogels. Chem. Rec. 2018, 18, 1517–1529. [Google Scholar] [CrossRef]
- Hou, B.N.; Shen, H.L.; Li, J.; Xie, W.Q.; Li, Z.Z. Self-healing polymer hydrogel based on dynamic chemical bonds. J. Mater. Eng. 2020, 48, 73–82. [Google Scholar]
- Qian, J.M.; Ji, L.J.; Xu, W.J.; Hou, G.H.; Wang, J.L.; Wang, Y.P.; Wang, T.B. Copper-hydrazide coordinated multifunctional hyaluronan hydrogels for infected wound healing. ACS Appl. Mater. Interfaces 2022, 14, 16018–16031. [Google Scholar] [CrossRef] [PubMed]
- Rather, R.A.; Sarwara, R.K.; Das, N.; Pal, B. Impact of reducing and capping agents on carbohydrates for the growth of Ag and Cu nanostructures and their antibacterial activities. Particuology 2019, 43, 219–226. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.Y.; Zhao, X.; Zhang, Y.H.; Tam, A.; Yan, Y.F.; Shen, H.K.; Zhang, Y.S.; Qi, J.; Feng, Y.H.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Hu, J.L.; Feng, K.K.; Cong, Y.Y.; Li, X.Y.; Jiang, Y.J.; Jiao, X.D.; Li, Y.R.; Zhang, Y.Q.; Dong, X.Y.; Lu, W.F.; et al. Nanosized shikonin-Fe(III) coordination material for synergistic wound treatment: An initial explorative study. ACS Appl. Mater. Interfaces 2022, 14, 56510–56524. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Li, Z.L.; Huang, Y.; Yu, R.; Guo, B.L. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Zhang, H.; He, J.D.; Qu, J.Q. Metal-coordinated amino acid hydrogels with ultra-stretchability, adhesion, and self-healing properties for wound healing. Eur. Polym. J. 2022, 179, 111548. [Google Scholar] [CrossRef]
- Devi VK, A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Zhao, D.W.; Feng, M.; Zhang, L.; He, B.; Chen, X.Y.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, Q.H.; Qi, G.L.; Chen, F.X.; Tu, B.Y.; Zhang, S.; Li, Y.P.; Chen, Y.; Yu, H.; Duan, P. A hydrogen bonds-crosslinked hydrogels with self-healing and adhesive properties for hemostatic. Front. Bioeng. Biotechnol. 2022, 10, 855013. [Google Scholar] [CrossRef]
- Guo, H.L.; Huang, S.; Xu, A.; Xue, W. Injectable adhesive self-healing multiple-dynamic-bond crosslinked hydrogel with photothermal antibacterial activity for infected wound healing. Chem. Mater. 2022, 34, 2655–2671. [Google Scholar] [CrossRef]
- Ying, R.; Huang, W.C.; Mao, X.Z. Synthesis of agarose-based multistimuli-responsive hydrogel dressing for accelerated wound healing. ACS Biomater. Sci. Eng. 2022, 8, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.M.; Wang, L.L.; Shao, P.; Sun, P.L.; Yang, C.S. A review on chemical and physical modifications of phytosterols and their influence on bioavailability and safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 5638–5657. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.K.; Yao, H.; Fan, D.; Zhou, L.; Wei, S.H. Hyaluronic acid thiol modified injectable hydrogel: Synthesis, characterization, drug release, cellular drug uptake and anticancer activity. Carbohydr. Polym. 2021, 254, 117286. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. [Google Scholar]
- Komatsu, S.; Tago, M.; Ando, Y.; Asoh, T.A.; Kikuchi, A. Facile preparation of multi-stimuli-responsive degradable hydrogels for protein loading and release. J. Control. Release 2021, 331, 1–6. [Google Scholar] [CrossRef]
- Hu, Y.W.; Gao, S.J.; Lu, H.F.; Ying, J.Y. Acid-resistant and physiological pH-responsive DNA hydrogel composed of A-motif and i-motif toward oral insulin delivery. J. Am. Chem. Soc. 2022, 144, 5461–5470. [Google Scholar] [CrossRef]
- Martin, J.R.; Patil, P.; Yu, F.; Gupta, M.K.; Duvall, C.L. Enhanced stem cell retention and antioxidative protection with injectable, ROS-degradable PEG hydrogels. Biomaterials 2020, 263, 120377. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Long, L.Y.; Hu, C.; Kong, Q.Q.; Wang, Y.B. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, R.L.; Chen, B.H.; Liu, X.L.; Jia, Q.; Wang, X.F.; Yang, Z.; Ning, P.B.; Wang, Z.L.; Yang, Y. Injectable reactive oxygen species-responsive hydrogel dressing with sustained nitric oxide release for bacterial ablation and wound healing. Adv. Funct. Mater. 2022, 32, 2202857. [Google Scholar] [CrossRef]
- Azadbakht, A.; Alizadeh, S.; Ahovan, Z.A.; Khosrowpour, Z.; Majidi, M.; Pakzad, S.; Shojaei, S.; Chauhan, N.P.S.; Jafari, M.; Gholipourmalekabadi, M. Chitosan-placental ECM composite thermos-responsive hydrogel as a biomimetic wound dressing with angiogenic property. Macromol. Biosci. 2022, 23, 2200386. [Google Scholar] [CrossRef]
- Liu, X.H.; Hou, M.D.; Luo, X.M.; Zheng, M.H.; Wang, X.C.; Zhang, H.J.; Guo, J.L. Thermoresponsive hemostatic hydrogel with a biomimetic nanostructure constructed from aggregated collagen nanofibers. Biomacromolecules 2021, 22, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Duan, Z.G.; Fu, J.Z.R.Z.; Zhu, C.H.; Fan, D.D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Zeng, W.N.; Xu, P.; Fu, X.X.; Yu, X.J.; Chen, L.; Leng, F.; Yu, C.; Yang, Z.Y. Glucose-responsive multifunctional metal-organic drug-loaded hydrogel for diabetic wound healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Li, D.R.; Fei, X.; Xu, L.Q.; Wang, Y.; Tian, J.; Li, Y. Pressure-sensitive antibacterial hydrogel dressing for wound monitoring in bed ridden patients. J. Colloid Interface Sci. 2022, 627, 942–955. [Google Scholar] [CrossRef]
- Fang, K.; Wang, R.; Zhang, H.; Zhou, L.J.; Xu, T.; Xiao, Y.; Zhou, Y.; Gao, G.R.; Chen, J.; Liu, D.L.; et al. Mechano-responsive tough and antibacterial zwitterionic hydrogels with controllable drug release for wound healing applications. ACS Appl. Mater. Interfaces 2020, 12, 52307–52318. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in nature humans skin. Dermatol. J. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Siegel, R.A. Stimuli sensitive polymers and self regulated drug delivery systems: A very partial review. J. Control. Release 2014, 190, 337–351. [Google Scholar] [CrossRef]
- Cui, T.T.; Yu, J.F.; Wang, C.F.; Chen, S.; Li, Q.; Guo, K.; Qing, R.K.; Wang, G.F.; Ren, J.N. Micro-gel ensembles for accelerated healing of chronic wound via pH regulation. Adv. Sci. 2022, 9, 2201254. [Google Scholar] [CrossRef]
- Fan, X.C.; Yang, L.; Wang, T.; Sun, T.D.; Lu, S.T. pH-responsive cellulose-based dual drug-loaded hydrogel for wound dressing. Eur. Polym. J. 2019, 121, 109290. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, L.L.; Wang, T.; Wang, H.N. A pH-responsive hydrogel system based on cellulose and dopamine with controlled hydrophobic drug delivery ability and long-term bacteriostatic property. Colloid Polym. Sci. 2019, 297, 705–717. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.J.; Zhou, H.T.; Wang, H.R.; Xu, Y.Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.Z.; Duan, Z.G.; Zhu, C.H.; Fan, D.D. Injectable hydrogel based on defect-rich multi-nanozymes for diabetic wound healing via an oxygen self-supplying cascade reaction. Small 2022, 18, 2200165. [Google Scholar] [CrossRef] [PubMed]
- Pachua, L. Chapter 5—Nanocellulose and nanohydrogel-mediated sustained drug delivery: Smart medical technology. In Micro and Nano Technologies, Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Mohammad, F., Al-Lohedan, H.A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–130. [Google Scholar]
- Wu, M.; Lu, Z.H.; Wu, K.K.; Nam, C.; Zhang, L.; Guo, J.S. Recent advances in the development of nitric oxide-releasing biomaterials and their application potentials in chronic wound healing. J. Mater. Chem. B 2021, 9, 7063–7075. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.P.; Zuo, H.Q.; Chen, B.; Wang, R.J.; Ahmed, A.; Hu, Y.; Ouyang, J. Doxorubicin-loaded platelets as a smart drug delivery system: An improved therapy for lymphoma. Sci. Rep. 2017, 7, 42632. [Google Scholar] [CrossRef]
- Liang, Y.P.; Li, M.; Yang, Y.T.; Qiao, L.P.; Xu, H.R.; Guo, B.L. pH/glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic dabetic foot wound healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Liu, G.T.; Huang, J.; Wu, J. Novel Glucose-Responsive Antioxidant hybrid hydrogel for enhanced diabetic wound repair. ACS Appl. Mater. Interfaces 2022, 14, 7680–7689. [Google Scholar] [CrossRef]
- Hou, M.D.; Wang, X.C.; Yue, O.Y.; Zheng, M.H.; Zhang, H.J.; Liu, X.H. Development of a multifunctional injectable temperature-sensitive gelatin-based adhesive double-network hydrogel. Biomater. Adv. 2022, 134, 112556. [Google Scholar] [CrossRef]
- FDA. SOLARAZE® GEL. 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021005s013lbl.pdf (accessed on 16 April 2023).
- Horrocks, A. Prontosan wound irrigation and gel: Management of chronic wounds. Br. J. Nurs. 2006, 15, 1222–1228. [Google Scholar] [CrossRef]
- Rajati, H.; Alvandi, H.; Rahmatabadi, S.S.; Hosseinzadeh, L.; Arkan, E. A nanofiber-hydrogel composite from green synthesized AgNPs embedded to PEBAX/PVA hydrogel and PA/Pistacia atlantica gum nanofiber for wound dressing. Int. J. Biol. Macromol. 2023, 226, 1426–1443. [Google Scholar] [CrossRef]
- Zhang, X.M.; Liu, Q.; Zhu, S.M.; Yu, M. Green and facile fabrication of nano-ZnO coated cellulose/starch/activated carbon hydrogel for enhanced dyes adsorption and antibacterial activity. Mater. Today Commun. 2022, 33, 104355. [Google Scholar] [CrossRef]
- Sundaram, M.N.; Amirthalingam, S.; Mony, U.; Varma, P.K.; Jayakumar, R. Injectable chitosan-nano bioglass composite hemostatic hydrogel for effective bleeding control. Int. J. Biol. Macromol. 2019, 129, 936–943. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.Y.; Wang, F.Y.; Li, Y.L.; Ou, Z.W.; Jiang, J.Y. A novel strategy to reinforce double network hydrogels with enhanced mechanical strength and swelling ratio by nano cement hydrates. Polymer 2023, 269, 125725. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: Concept; design, and applications of these promising, multi-functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Wang, A.H.; Fan, G.S.; Qi, H.L.; Li, H.Y.; Pang, C.C.; Zhu, Z.K.; Ji, S.C.; Liang, H.; Jiang, B.P.; Shen, X.C. H2O2-activated in situ polymerization of aniline derivative in hydrogel for real-time monitoring and inhibition of wound bacterial infection. Biomaterials 2022, 289, 121798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, Q.X.; Ling, G.X.; Zhang, P. A cross-linked hydrogel of bismuth sulfide nanoparticles with excellent photothermal antibacterial and mechanical properties to combat bacterial infection and prompt wound healing. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130832. [Google Scholar] [CrossRef]
- Xiang, J.X.; Shen, L.; Hong, Y.L. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Shen, J.F.; Dai, Y.; Xia, F.; Zhang, X.J. Role of divalent metal ions in the function and application of hydrogels. Prog. Polym. Sci. 2022, 135, 101622. [Google Scholar] [CrossRef]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.R.; Fan, Z.J. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef]
- Liu, Y.L.; Mao, J.; Guo, Z.Y.; Hu, Y.F.; Wang, S. Polyvinyl alcohol/carboxymethyl chitosan hydrogel loaded with silver nanoparticles exhibited antibacterial and self-healing properties. Int. J. Biol. Macromol. 2022, 220, 211–222. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Ghith, D.A.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Wang, S.Y.; Liu, R.Q.; Bi, S.W.; Zhao, X.S.; Zeng, G.X.; Li, X.Y.; Wang, H.B.; Gu, J. Mussel-inspired adhesive zwitterionic composite hydrogel with antioxidant and antibacterial properties for wound healing. Colloids Surf. B Biointerfaces 2022, 220, 112914. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Paul, P.; Behera, S.K.; Jena, B.; Tripathy, S.K.; Lundborg, C.S.; Mishra, A. To decipher the antibacterial mechanism and promotion of wound healing activity by hydrogels embedded with biogenic Ag@ZnO core-shell nanocomposites. Chem. Eng. J. 2021, 417, 128025. [Google Scholar] [CrossRef]
- McMahon, S.; Kennedy, R.; Duffy, P.; Vasquez, J.M.; Wall, J.G.; Tai, H.Y.; Wang, W.X. Poly(ethylene glycol)-based hyperbranched polymer from RAFT and its application as a silver-sulfadiazine-loaded antibacterial hydrogel in wound care. ACS Appl. Mater. Interfaces 2016, 8, 26648–26656. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Z.Y.; Yang, J.; Zhang, J.J.; Zheng, W.F.; Jiang, X.Y. Activating the antibacterial effect of 4,6-diamino-2-pyrimidinethiol-modified gold nanoparticles by reducing their sizes. Angew. Chem. Int. Ed. 2020, 59, 23471–23475. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries 2022: U.S. Geological Survey; USGS: Sunrise Valley Drive Reston, VA, USA, 2022; 202p. [Google Scholar]
- Kong, Y.; Hou, Z.S.; Zhou, L.Q.; Zhang, P.F.; Ouyang, Y.W.; Wang, P.W.; Chen, Y.W.; Luo, X.L. Injectable self-healing hydrogels containing CuS nanoparticles with abilities of hemostasis, antibacterial activity, and promoting wound healing. ACS Biomater. Sci. Eng. 2021, 7, 335–349. [Google Scholar] [CrossRef]
- Xia, X.J.; Liang, Q.D.; Sun, X.G.; Yu, D.H.; Huang, X.R.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q.; Conductive, I.E. Antibacterial, and Anti-swelling Hydrogels as Implantable Sensors for Bioelectronics. Adv. Funct. Mater. 2022, 32, 2208024. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Lek. Listy 2019, 120, 397–409. [Google Scholar] [CrossRef]
- Kircheva, N.; Dobrev, S.; Nikolova, V.; Angelova, S.; Dudev, T. Theoretical insight into the phosphate-targeted silver’s antibacterial action: Differentiation between gram(+) and gram(−) bacteria. Inorg. Chem. 2022, 61, 10089–10100. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Xiao, D.D.; Yu, H.N.; Ke, R.Y.; Shi, S.L.; Tang, Y.; Zhong, Y.; Zhang, L.P.; Sui, X.F.; Wang, B.J.; et al. Asymmetric composite wound dressing with hydrophobic flexible bandage and tissue-adhesive hydrogel for joints skin wound healing. Compos. B Eng. 2022, 235, 109762. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Wahid, F.; Zhao, X.J.; Wang, F.P.; Wang, T.F.; Xie, Y.Y.; Jia, S.R.; Zhong, C. Fabrication of amino acid-based supramolecular hydrogel with silver ions for improved antibacterial properties. Mater. Lett. 2021, 300, 130161. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef]
- Lohmann & Rauscher. Suprasorb® A + Ag in the Treatment of Wounds at Risk of Infection and Infected Wounds. 2022. Available online: https://beta.clinicaltrials.gov/study/NCT05646121 (accessed on 16 April 2023).
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.X.; Wang, X.L.; Yu, J.Y.; Wu, D.Q. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef]
- Golafshan, N.; Rezahasani, R.; Esfahani, M.T.; Kharaziha, M.; Khorasani, S.N. Nanohybrid hydrogels of laponite: PVA-Alginate as a potential wound healing material. Carbohydr. Polym. 2017, 176, 392–401. [Google Scholar] [CrossRef]
- Zhao, P.X.; Feng, Y.; Zhou, Y.Q.; Tan, C.Y.; Liu, M.X. Gold@Halloysite nanotubes-chitin composite hydrogel with antibacterial and hemostatic activity for wound healing. Bioact. Mater. 2023, 20, 355–367. [Google Scholar] [CrossRef]
- Pillai, N.S.M.; Eswar, K.; Amirthalingam, S.; Mony, U.; Varma, P.K.; Jayakumar, R. Injectable nano whitlockite incorporated chitosan hydrogel for effective hemostasis. ACS Appl. Bio Mater. 2019, 2, 865–873. [Google Scholar] [CrossRef]
- Pan, G.X.; Li, F.H.; He, S.H.; Li, W.D.; Wu, Q.M.; He, J.J.; Ruan, R.J.; Xiao, Z.X.; Zhang, J.; Yang, H.H. Mussel- and barnacle cement proteins-inspired dual-bionic bioadhesive with repeatable wet-tissue adhesion, multimodal self-gealing, and antibacterial capability for nonpressing hemostasis and promoted wound healing. Adv. Funct. Mater. 2022, 32, 2200908. [Google Scholar] [CrossRef]
- Fan, X.M.; Wang, S.B.; Fang, Y.; Li, P.Y.; Zhou, W.K.; Wang, Z.C.; Chen, M.F.; Liu, H.Q. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater. Sci. Eng. C 2020, 109, 110649. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Avery, R.K.; Assmann, A.; Paul, A.; McKinley, G.H.; Khademhosseini, A.; Olsen, B.D. Shear-Thinning Nanocomposite Hydrogels for the Treatment of Hemorrhage. ACS Nano 2014, 8, 9833–9842. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Ding, L.P.; Chen, Y.; Chen, G.Q.; Zhao, T.B.; Yu, Y.L. Nano-silver functionalized polysaccharides as a platform for wound dressings: A review. Int. J. Biol. Macromol. 2022, 194, 644–653. [Google Scholar] [CrossRef]
- Zhao, B.N.; Zhang, Y.Z.; Li, D.D.; Mo, X.M.; Pan, J.F. Hofmeister effect-enhanced gelatin/oxidized dextran hydrogels with improved mechanical properties and biocompatibility for wound healing. Acta Biomater. 2022, 151, 235–253. [Google Scholar] [CrossRef]
- Arno, M.C.; Inam, M.; Weems, A.C.; Li, Z.H.; Binch, A.L.A.; Platt, C.I.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P.; O'Reilly, R.K. Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties. Nat. Commun. 2020, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Lin, P.L.; Song, X.F.; Chen, K.; Yang, Y.X.; Xu, Y.L.; Zhang, Q.; Wu, Y.S.; Zhang, Y.F.; Cheng, Y.L. Injectable and self-healing hydrogels with tissue adhesiveness and antibacterial activity as wound dressings for infected wound healing. J. Polym. Sci. 2022, 60, 1511–1520. [Google Scholar] [CrossRef]
- Lu, J.W.; Fan, X.K.; Hu, J.W.; Li, J.; Rong, J.J.; Wang, W.J.; Chen, Y.; Liu, W.Y.; Chen, J.; Chen, Y. Construction and function of robust and moist bilayer chitosan-based hydrogel wound dressing. Mater. Des. 2023, 226, 111604. [Google Scholar] [CrossRef]
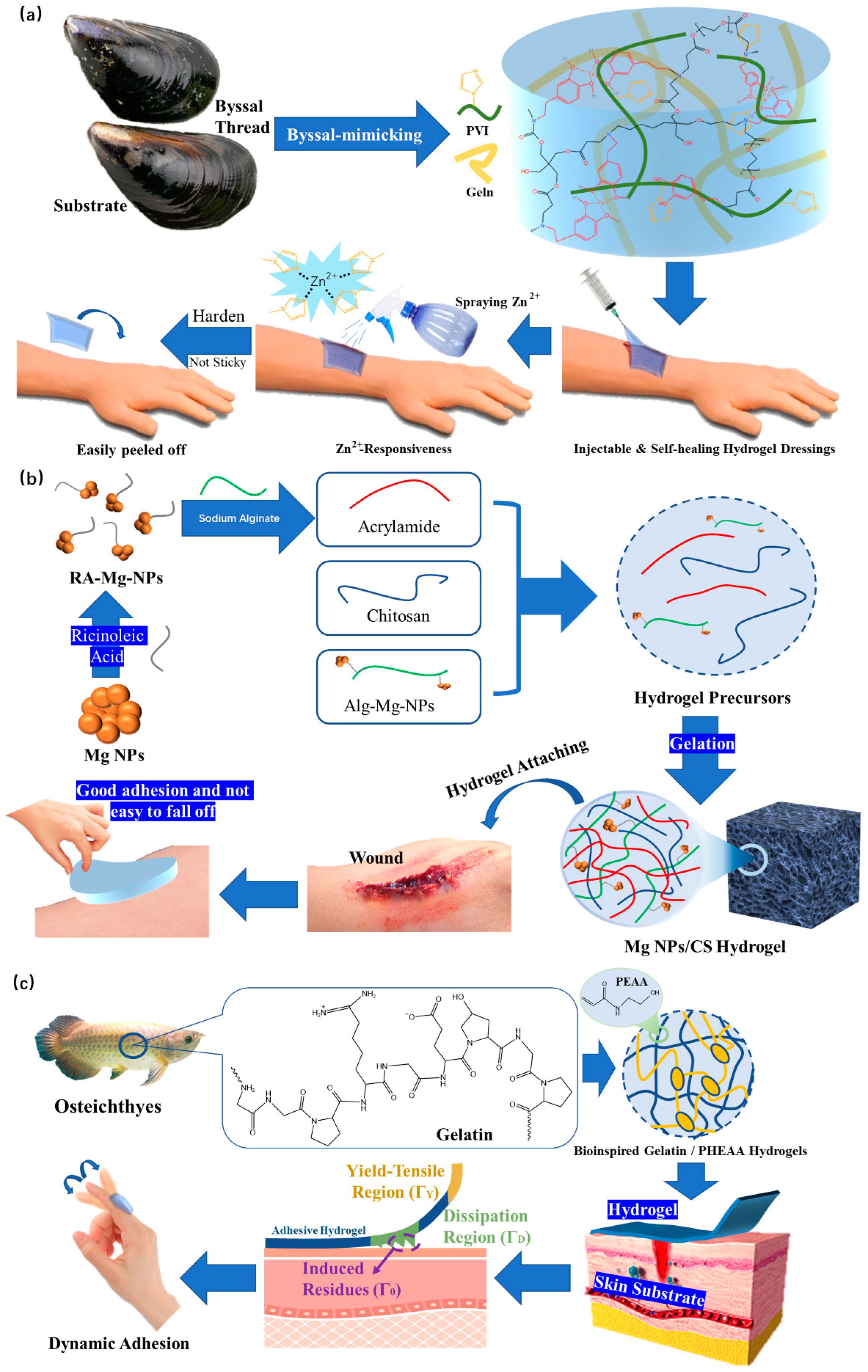
- Xie, T.; Ding, J.; Han, X.X.; Jia, H.Z.; Yang, Y.; Liang, S.; Wang, W.X.; Liu, W.G.; Wang, W. Wound dressing change facilitated by spraying zinc ions. Mater. Horizons 2020, 7, 605–614. [Google Scholar] [CrossRef]
- Qu, J.H.; Li, J.; Zhu, W.P.; Xu, Y.F.; Zhou, S.M.; Yang, Y.Y.; Qian, X.H. Hybrid nanocomposite multinetwork hydrogel containing magnesium hydroxide nanoparticles with enhanced antibacterial activity for wound dressing applications. Polymer 2022, 251, 124902. [Google Scholar] [CrossRef]
- Wang, S.G.; Shi, F.W.K.; Yuan, J.F.; Sun, W.L.; Yang, J.T.; Chen, Y.X.; Zhang, D.; Che, L.B. Osteichthyes skin-inspired tough and sticky composite hydrogels for dynamic adhesive dressings. Compos. B Eng. 2022, 241, 110010. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Huang, F.; Wang, Y.J.; Zou, T.; Zheng, J.S.; Lin, Z. Recycling Mg(OH)2 nanoadsorbent during treating the low concentration of CrVI. Environ. Sci. Technol. 2011, 45, 1955–1961. [Google Scholar] [CrossRef]
- Qiao, Z.; Parks, J.; Choi, P.; Ji, H.F. Applications of Highly Stretchable and Tough Hydrogels. Polymers 2019, 11, 1773. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Zhang, K.Y.; Yao, Y.M.; Li, X.R.; Yu, J.Y.; Ding, B. Bioinspired sequentially crosslinked nanofibrous hydrogels with robust adhesive and stretchable capability for joint wound dressing. Compos. Commun. 2021, 26, 100785. [Google Scholar] [CrossRef]
- Hu, T.; Wu, G.P.; Bu, H.T.; Zhang, H.Y.; Li, W.X.; Song, K.; Jiang, G.B. An injectable; adhesive, and self-healable composite hydrogel wound dressing with excellent antibacterial activity. Chem. Eng. J. 2022, 450, 138201. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.K.; Yan, L.L.; Huo, D.M.; Wang, F.C. Dynamic compressive properties and failure mechanism of glass fiber reinforced silica hydrogel. Mater. Sci. Eng. A 2010, 527, 824–827. [Google Scholar] [CrossRef]
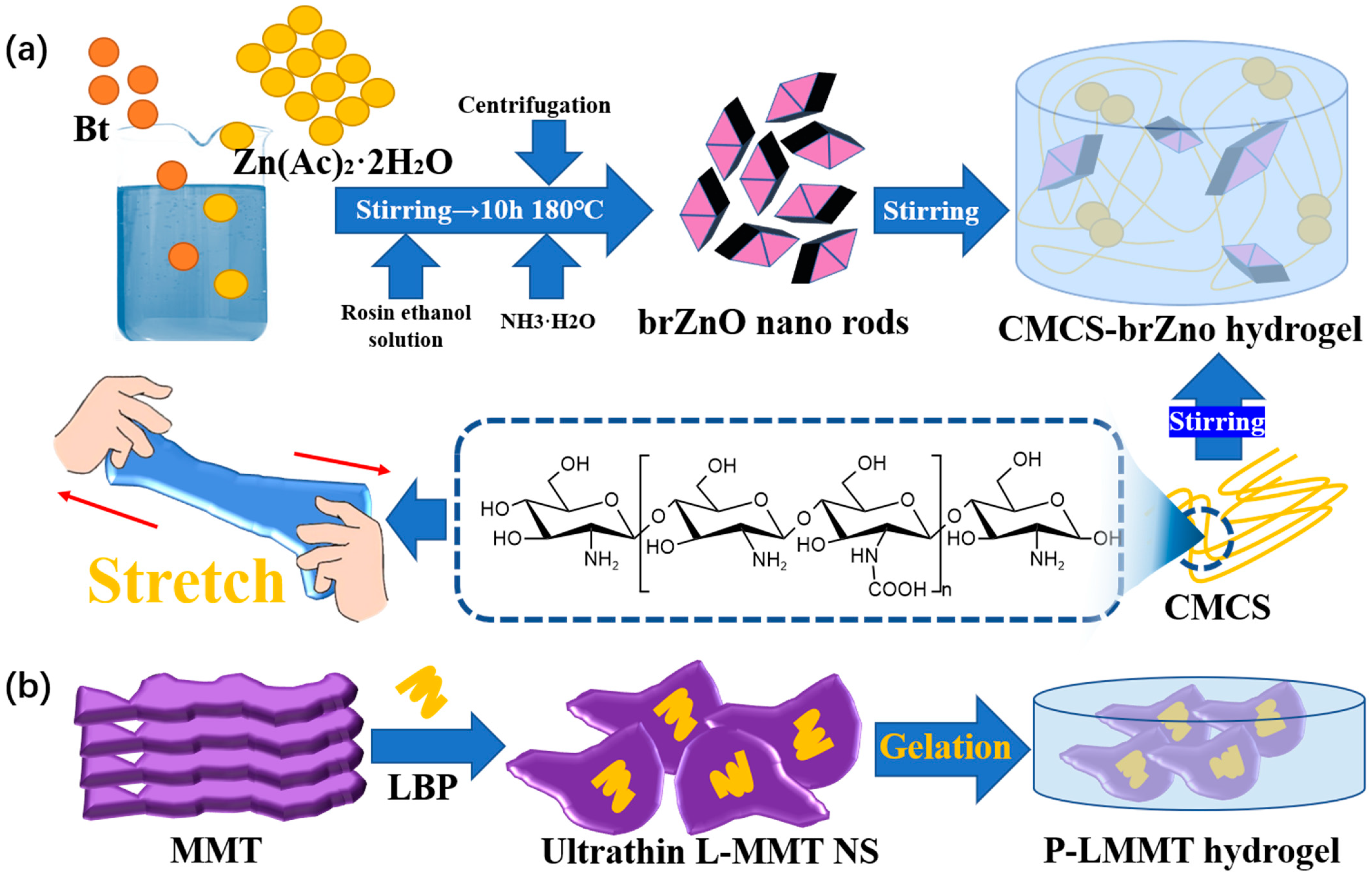
- Shi, S.; Lan, X.; Feng, J.X.; Ni, Y.S.; Zhu, M.Q.; Sun, J.; Wang, J.L. Hydrogel loading 2D montmorillonite exfoliated by anti-inflammatory Lycium barbarum L. polysaccharides for advanced wound dressing. Int. J. Biol. Macromol. 2022, 209, 50–58. [Google Scholar] [CrossRef]
- Tao, S.W.; Zhu, L.; Xu, N. Exosomes from Stem Cells Loaded by Hydrogel in Skin Wound Repair. Chin. J. Biochem. Mol. Biol. 2021, 37, 1024–1031. [Google Scholar]
- Wang, L.L.; Janes, M.E.; Kumbhojkar, N.; Kapate, N.; Clegg, J.R.; Prakash, S.; Heavey, M.K.; Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Cell therapies in the clinic. Bioeng. Transl. Med. 2021, 6, e10214. [Google Scholar] [CrossRef] [PubMed]
- KBV Research, Global Hydrogel Dressing Market Size, Share & Industry Trends Analysis Report By Application, By End-use, By Product, By Regional Outlook and Forecast, 2022–2028. 2022. Available online: https://www.reportlinker.com/p06364533/Global-Hydrogel-Dressing-Market-Size-Share-Industry-Trends-Analysis-Report-By-Application-By-End-use-By-Product-By-Regional-Outlook-and-Forecast.html?utm_source=GNW (accessed on 17 April 2023).
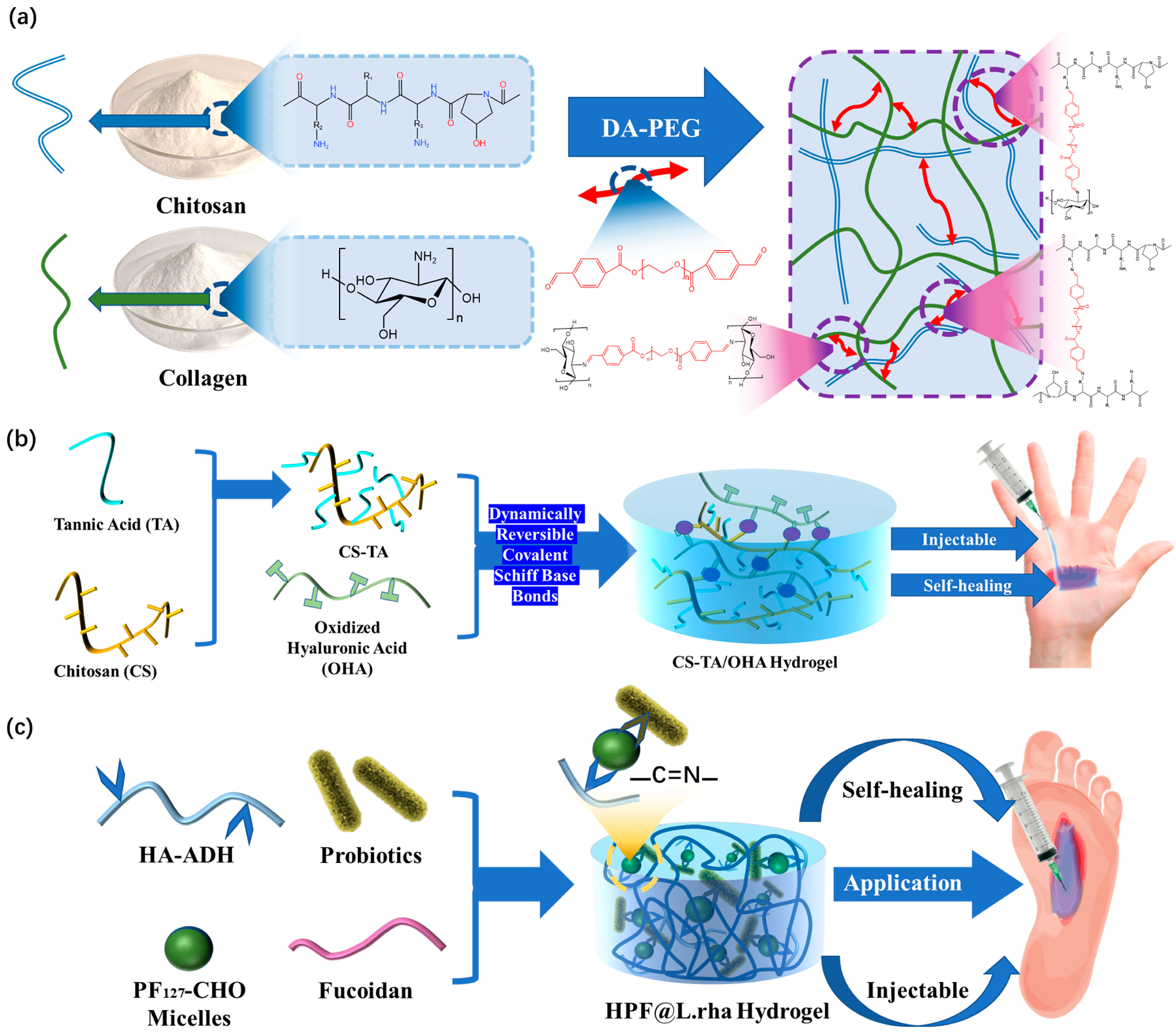
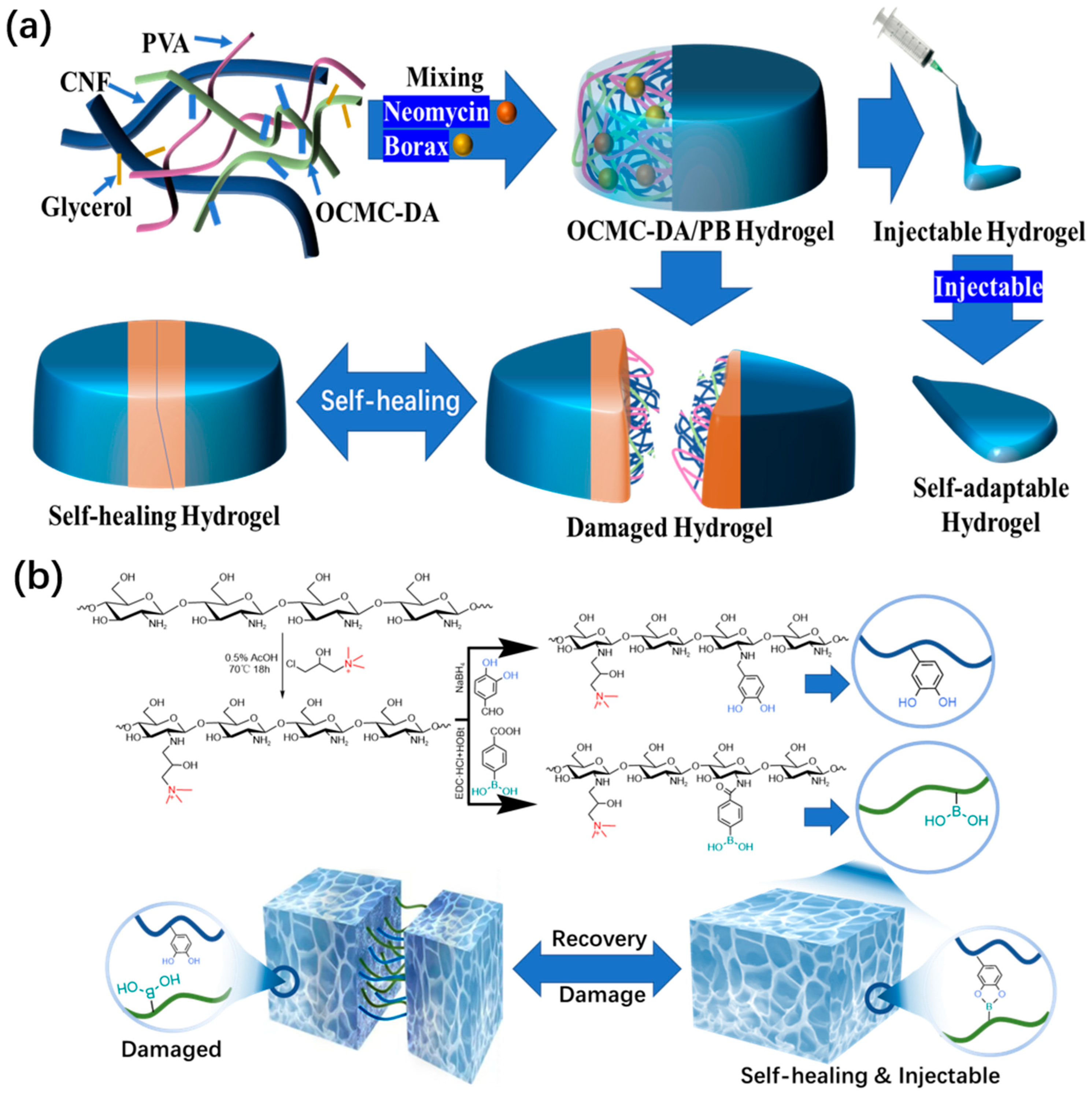





| Hydrogel Components | Dynamic Bond | Self-Healing Time | Injectable | Advantages | Ref. |
|---|---|---|---|---|---|
| OMCC from PP+ CMCS from HER | Imine bond | 5 h | YES | Non-toxicity and biosecurity | [47] |
| AHA + ADA + oxi-CNC | Imine bond | 4 h | YES | Improves hydrogels’ strength without sacrificing their self-healing property | [49] |
| CS + COL | Imine bond | 45 min | YES | DA-PEG allows the chain between the two crosslinking points to remain flexible | [50] |
| OSA-DA + PAM | Imine bond | 15 min | NO | Ultratough and self-healing | [46] |
| HA-ADH + PF127-CHO | Imine bond | 10 min | YES | The use of biological materials greatly improves the performance of the dressing | [52] |
| HPC + Phenylboronic Acid | Borate ester bond | 10 min | YES | Blood compatibility, anti-oxidation, good tissue adhesion | [59] |
| boronic acid + catechol groups | Borate ester bond | 5 min | YES | Treats wounds with high microbial bioburdens | [58] |
| CS + OHA | Imine bond | 2 min | YES | Self-heals quickly and its materials have anti-inflammatory effects | [53] |
| OCMC-DA + PVA + CNF | Borate ester bond | 12 s | YES | Ultrastretchable and simple preparation method | [57] |
| Catechin of PDA + Phenylboric Acid | Borate ester bond | Immediately | YES | Has the fastest self-healing speed | [60] |
| Stimuli | Stimuli Responsive Component | Advantage | Ref. |
|---|---|---|---|
| pH | Cystamine | Drug delivery carrier, can recognize acidic or reducing conditions | [78] |
| Oligonucleotides rich in adenine and cytosine | Biocompatibility and biodegradability | [79] | |
| ROS | Thiol-maleimide | Protected encapsulated mesenchymal stem cells from cytotoxic doses of ROS | [80] |
| Diclofenac sodium and mangiferin | Eliminate oxygen free radicals, inhibit inflammatory reaction and generate new skin | [81] | |
| L-Arginine | It has the ability of absorbing wound exudates and controlling drug release | [82] | |
| Temperature | Chitosan and solubilized placental extracellular matrix | Rapid response to temperature changes | [83] |
| Muco-mimetic poloxamer 407 (F-107) and TS-Gel-Ag-col | Prevent gel from being diluted in contact with blood | [84] | |
| Glucose | Polyvinyl alcohol and chitosan grafted with phenylboric acid | The drug is released as needed according to the glucose concentration | [85] |
| Glucose oxidase and DG@Gel | Breaks down excess glucose | [86] | |
| Pressure | Conductive fillers | Secondary injury caused by unconscious physical movement | [87] |
| F127DA micelles | Wounds on frequently moving body parts can be effectively treated | [88] |
| Name | Classification | Component | Advantages | Refs |
|---|---|---|---|---|
| Nano-silver hydrogel | Nanoparticles | Ag-NPs | Maintains antibacterial efficacy | [114] |
| PVA/CMCS/AgNPs/Borax hydrogel | Nanoparticles | Ag-NPs | 1. Extended antibacterial activity 2. Increased mechanical strength | [115] |
| PAA-AuNPs hydrogel | Nanoparticles | Au-NPs | 1. Improved antibacterial activity of drugs 2. Activates drug precursors | [116] |
| PSNZn composite hydrogel | Metal ions | PDA/PSBMA/NFC/Zn2+ | 1. Antibacterial property 2. Hemostasis | [117] |
| Ag@ZnO NCs impregnated hydrogels | Nanoparticles | PVP/PVA/Ag@ZnO | 1. Antibacterial 2. Anti-biofilm activity | [118] |
| Silver-sulfadiazine-loaded antibacterial hydrogel | Sulfa antibiotics with metal | Silver sulfadiazine | 1. Antiresistant 2. Antibacterial | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Zhang, C.; Wang, T.; Xu, J. Advances in Functional Hydrogel Wound Dressings: A Review. Polymers 2023, 15, 2000. https://doi.org/10.3390/polym15092000
Shen Z, Zhang C, Wang T, Xu J. Advances in Functional Hydrogel Wound Dressings: A Review. Polymers. 2023; 15(9):2000. https://doi.org/10.3390/polym15092000
Chicago/Turabian StyleShen, Zihao, Chenrui Zhang, Ting Wang, and Juan Xu. 2023. "Advances in Functional Hydrogel Wound Dressings: A Review" Polymers 15, no. 9: 2000. https://doi.org/10.3390/polym15092000
APA StyleShen, Z., Zhang, C., Wang, T., & Xu, J. (2023). Advances in Functional Hydrogel Wound Dressings: A Review. Polymers, 15(9), 2000. https://doi.org/10.3390/polym15092000






