Effects of Shellac Self-Repairing and Carbonyl Iron Powder Microcapsules on the Properties of Dulux Waterborne Coatings on Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Microcapsule Preparation and Experimental Design
2.2.1. Pretreatment of CIP Core Material
2.2.2. Preparation of Shellac Microcapsules
2.2.3. Preparation of CIP Microcapsules
2.3. Primer Coating Preparation
2.4. Testing and Characterization
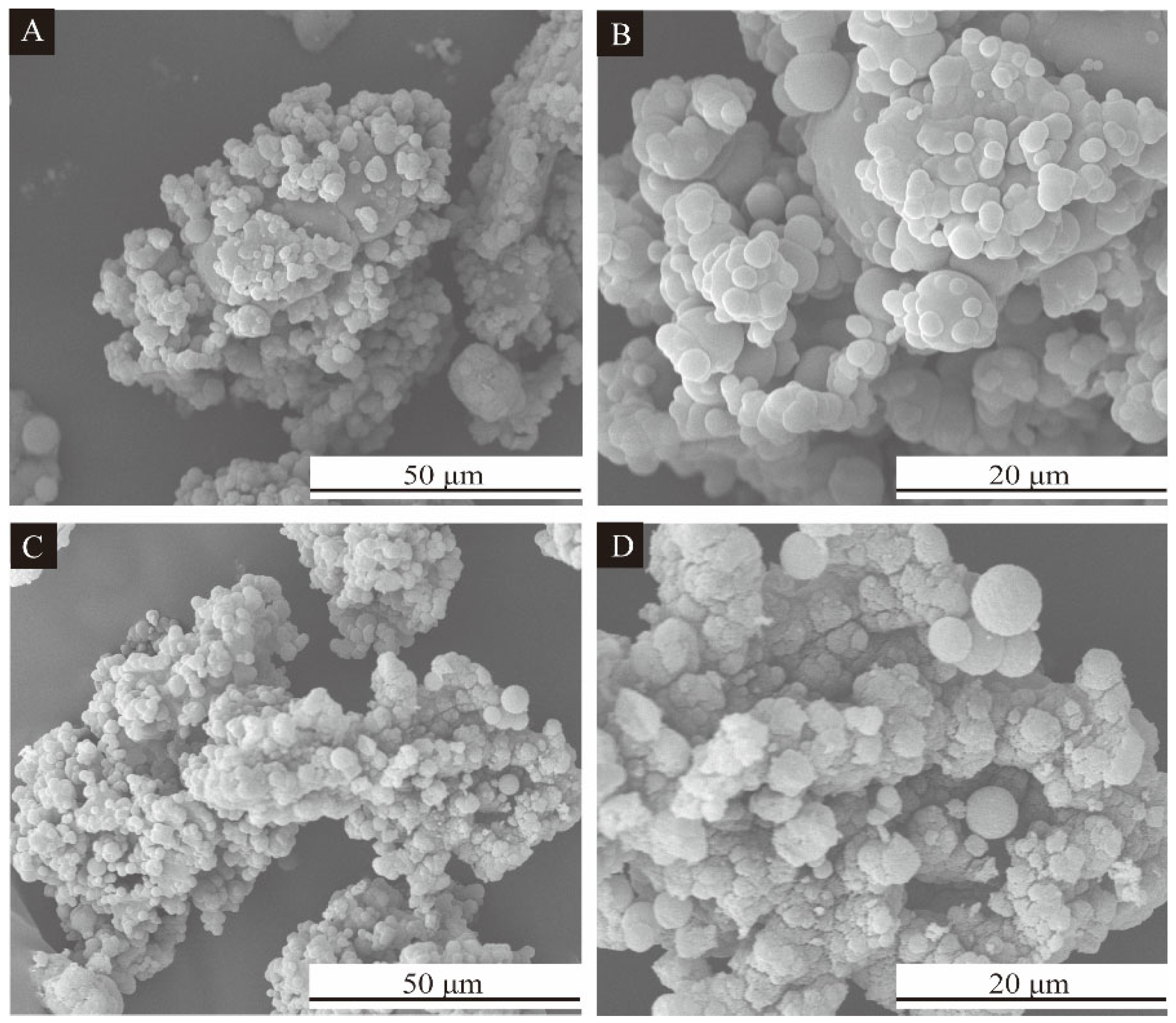
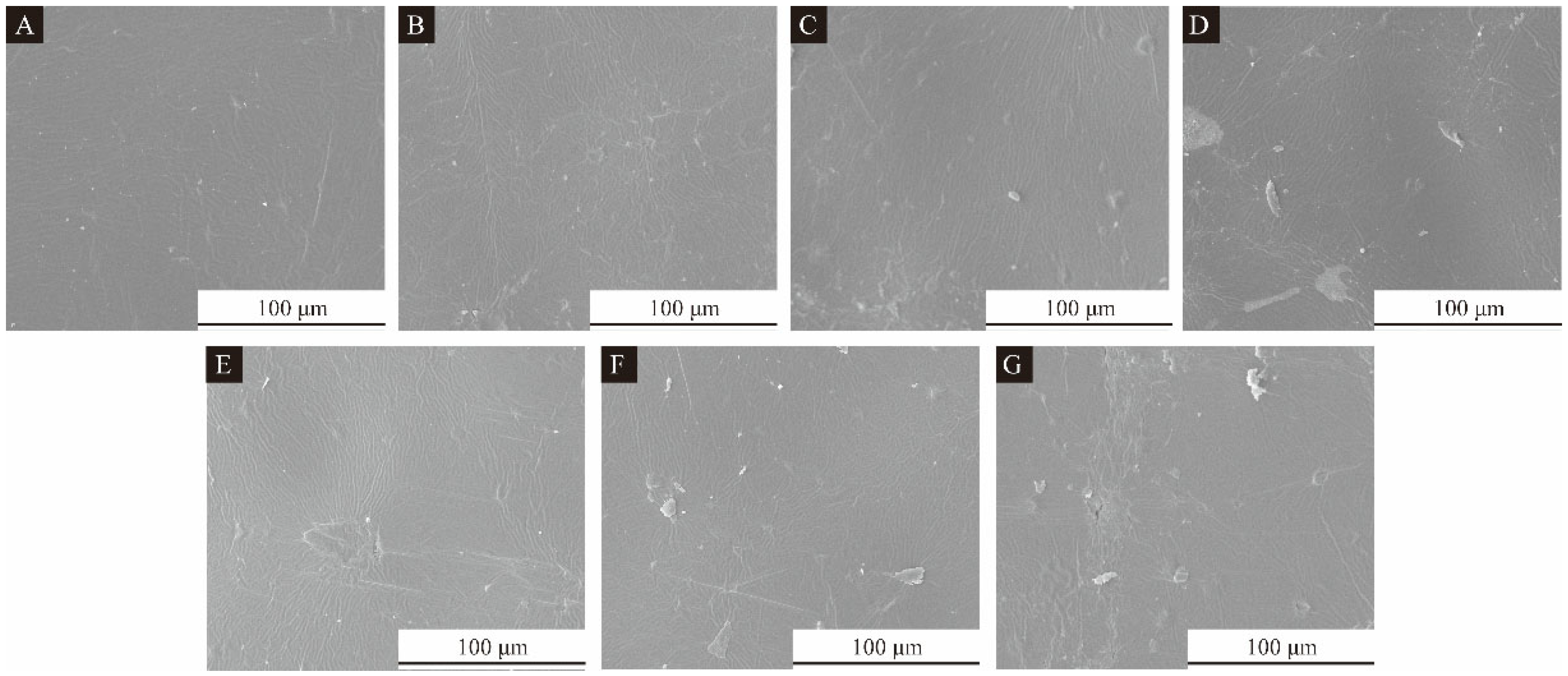
2.4.1. Microcharacterization Test of CIP Microcapsules
2.4.2. Calculation of Coating Rate of CIP Microcapsules
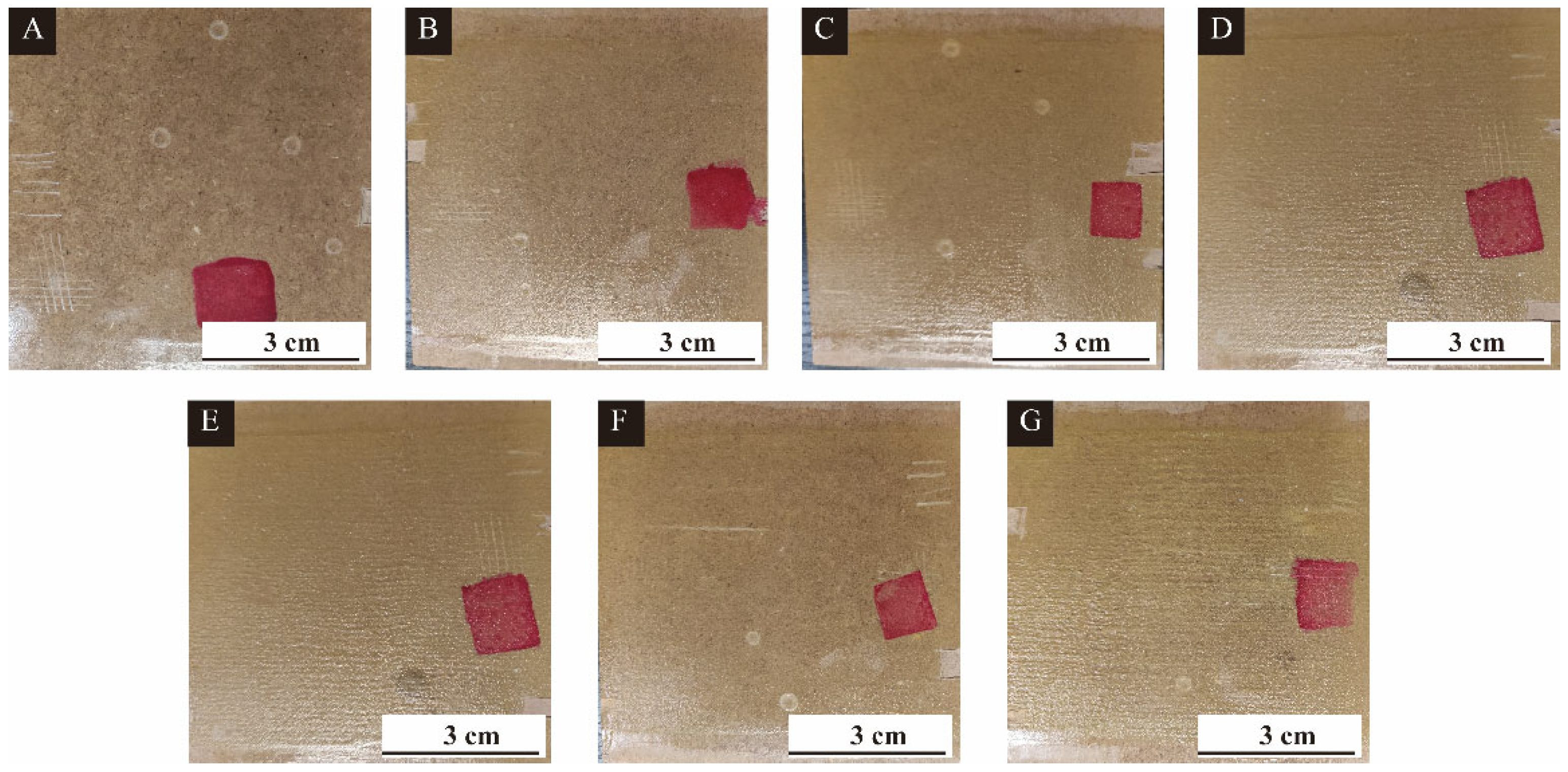
2.4.3. Test Methods for Bifunctional Coatings
2.4.4. Electromagnetic Parameter Testing of CIP Microcapsules
3. Experimental Results and Discussion
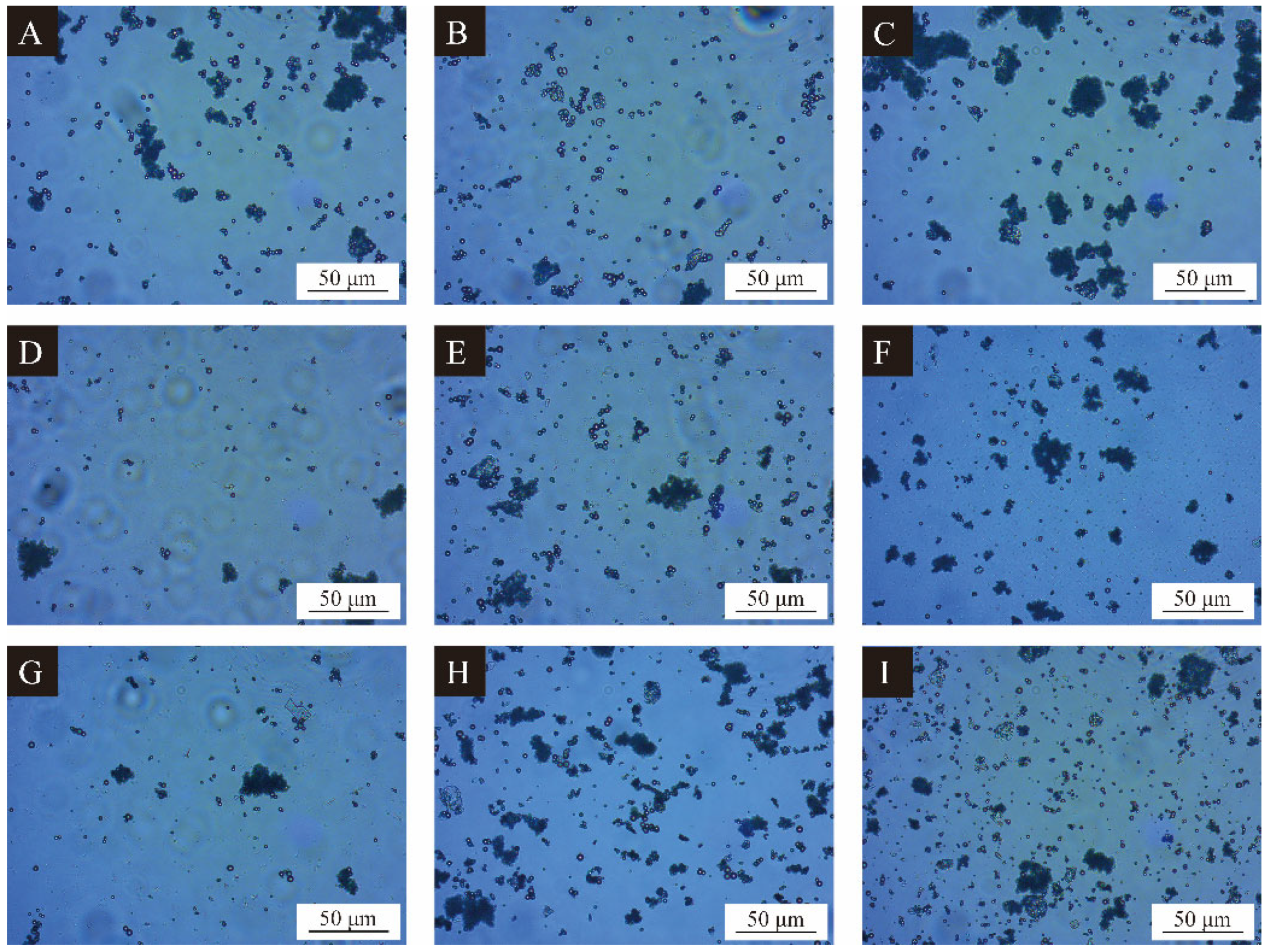
3.1. Morphological Characterization of CIP Microcapsules by Orthogonal Experiment
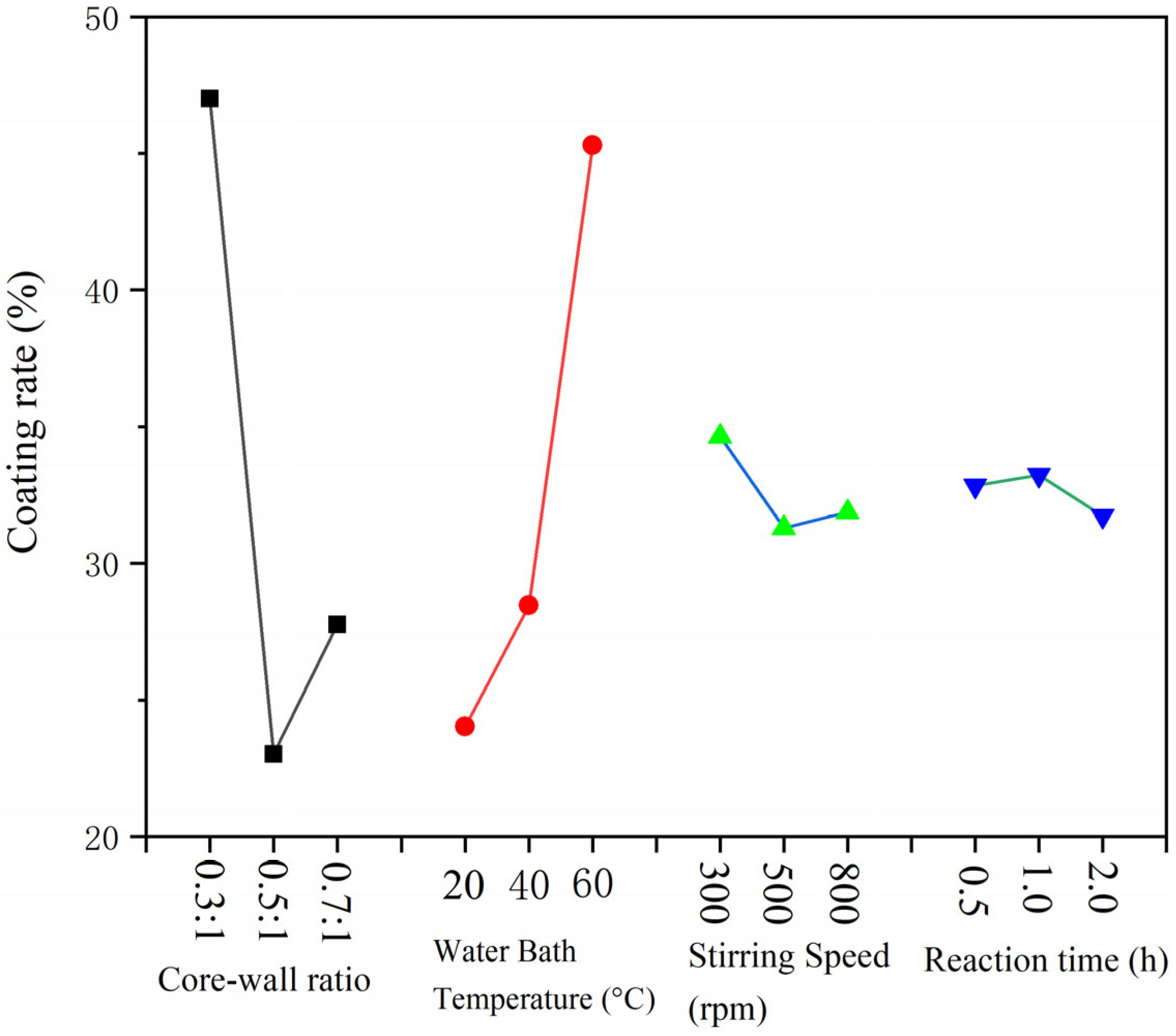
3.2. Analysis of Microcapsules Yield and Coating Rate
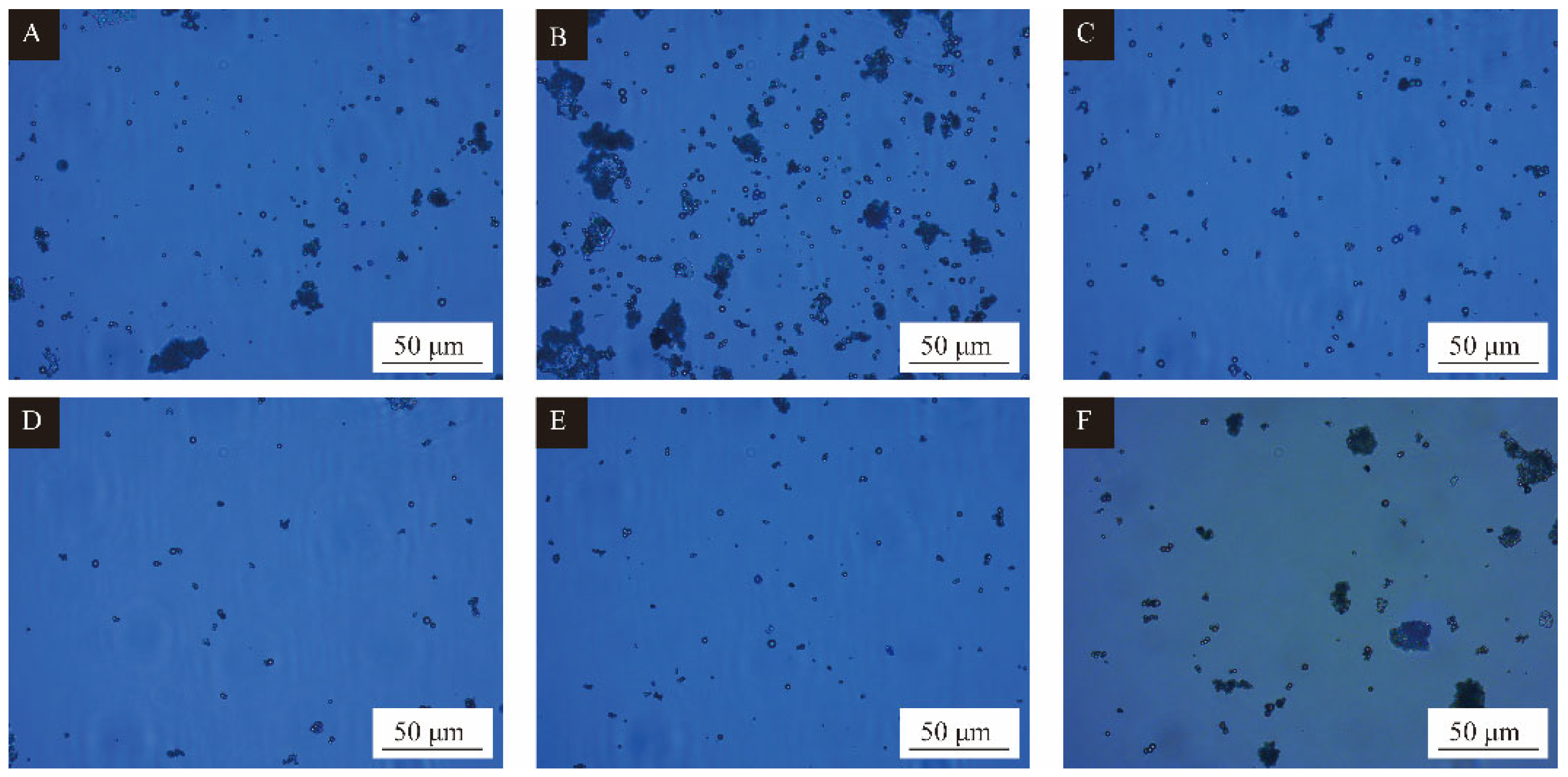
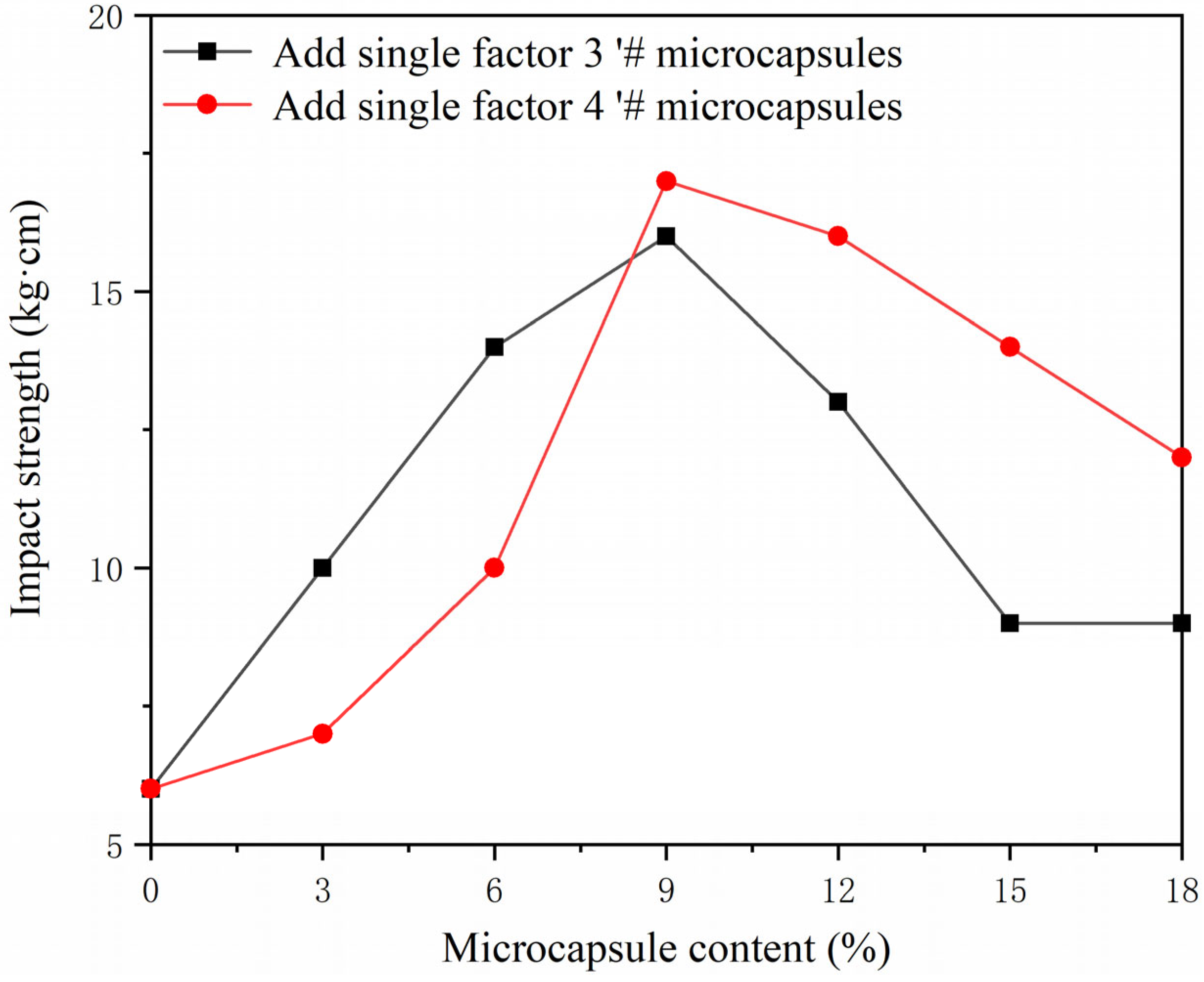
3.3. Single Factor Experimental Analysis of Microcapsules
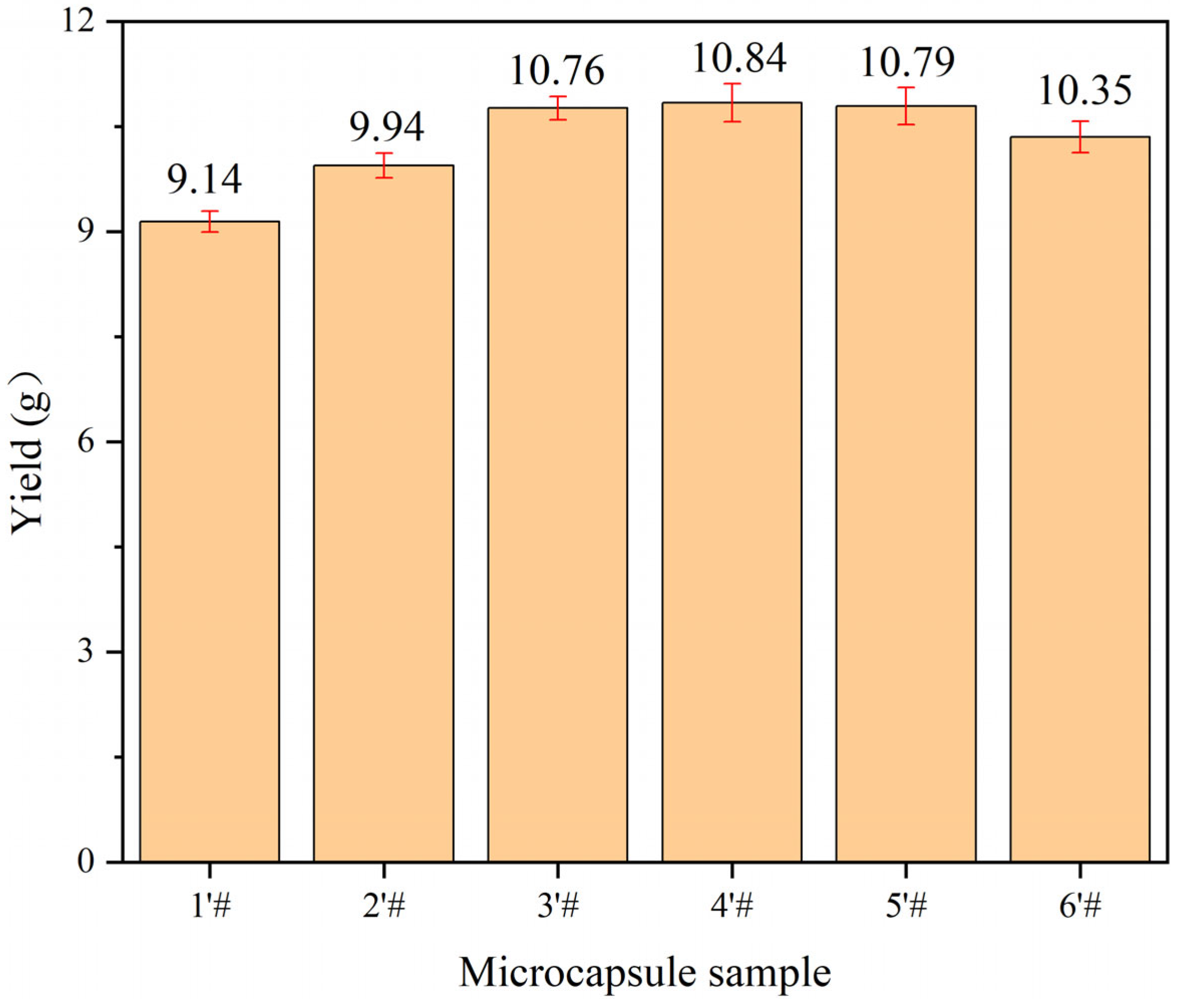
3.4. Analysis of Single-Factor Microcapsules Yield and Coating Rate
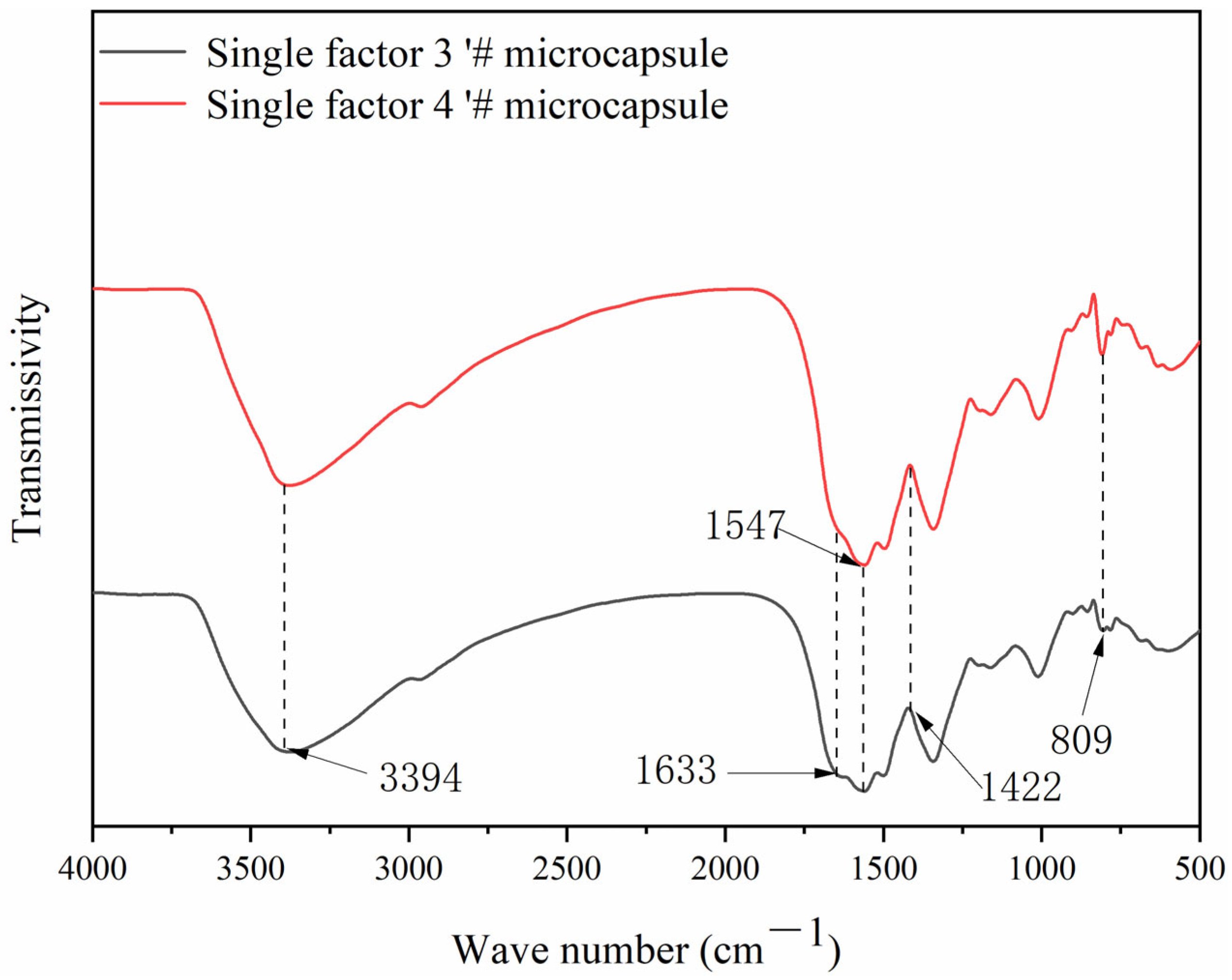
3.5. Chemical Composition Analysis of Microcapsules
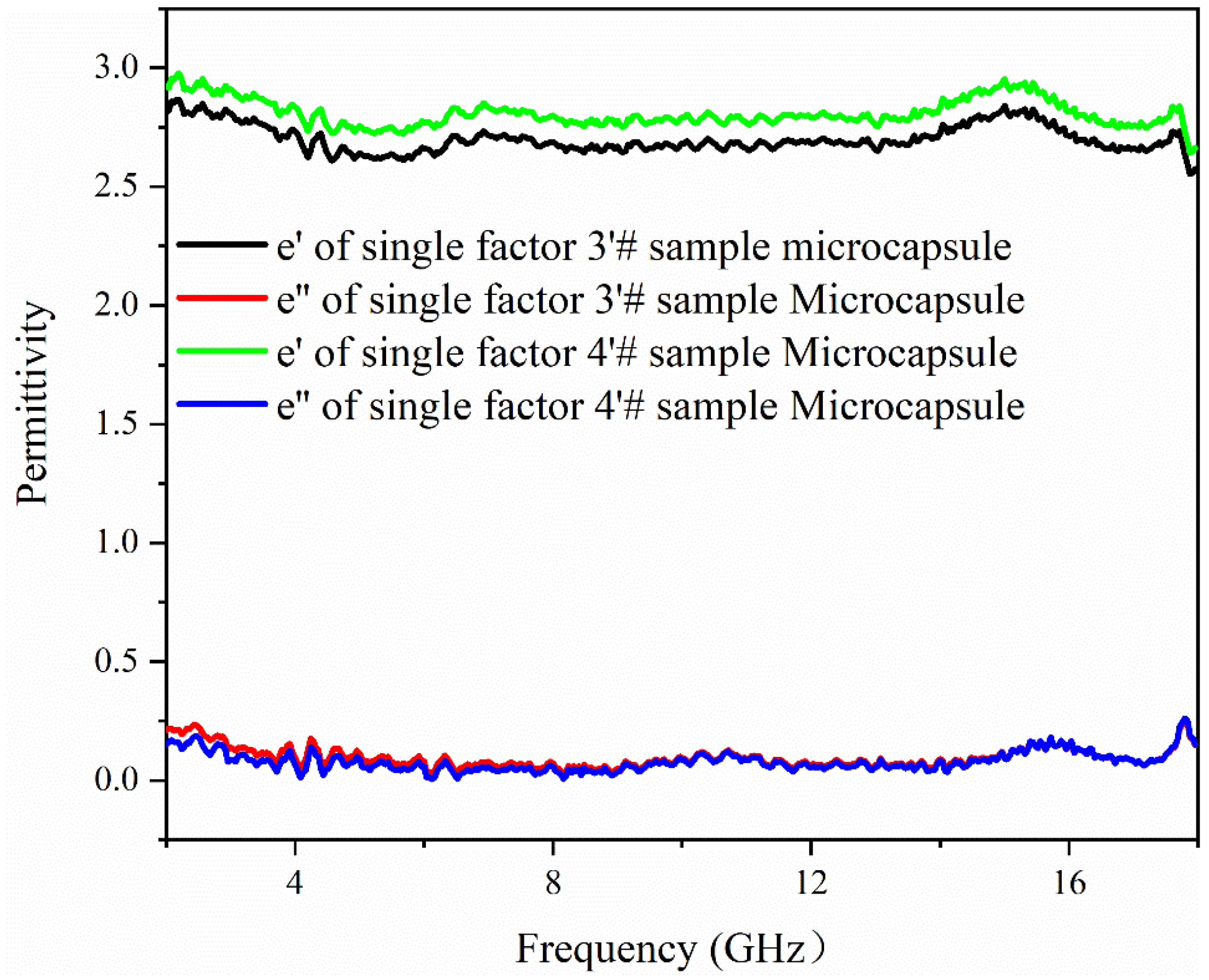
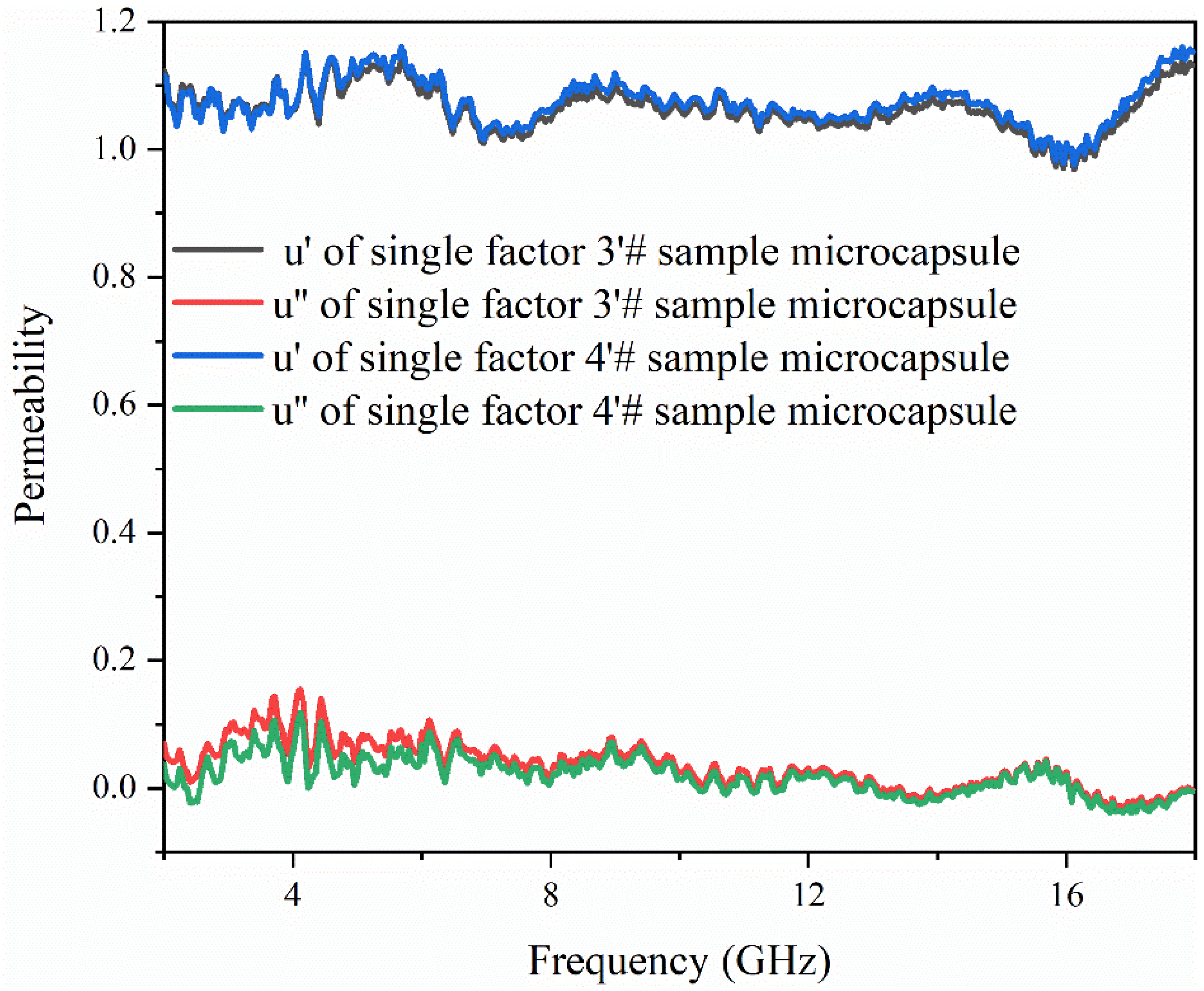
3.6. Analysis of Electromagnetic Parameters and Microwave-Absorbing Properties of the Microcapsules
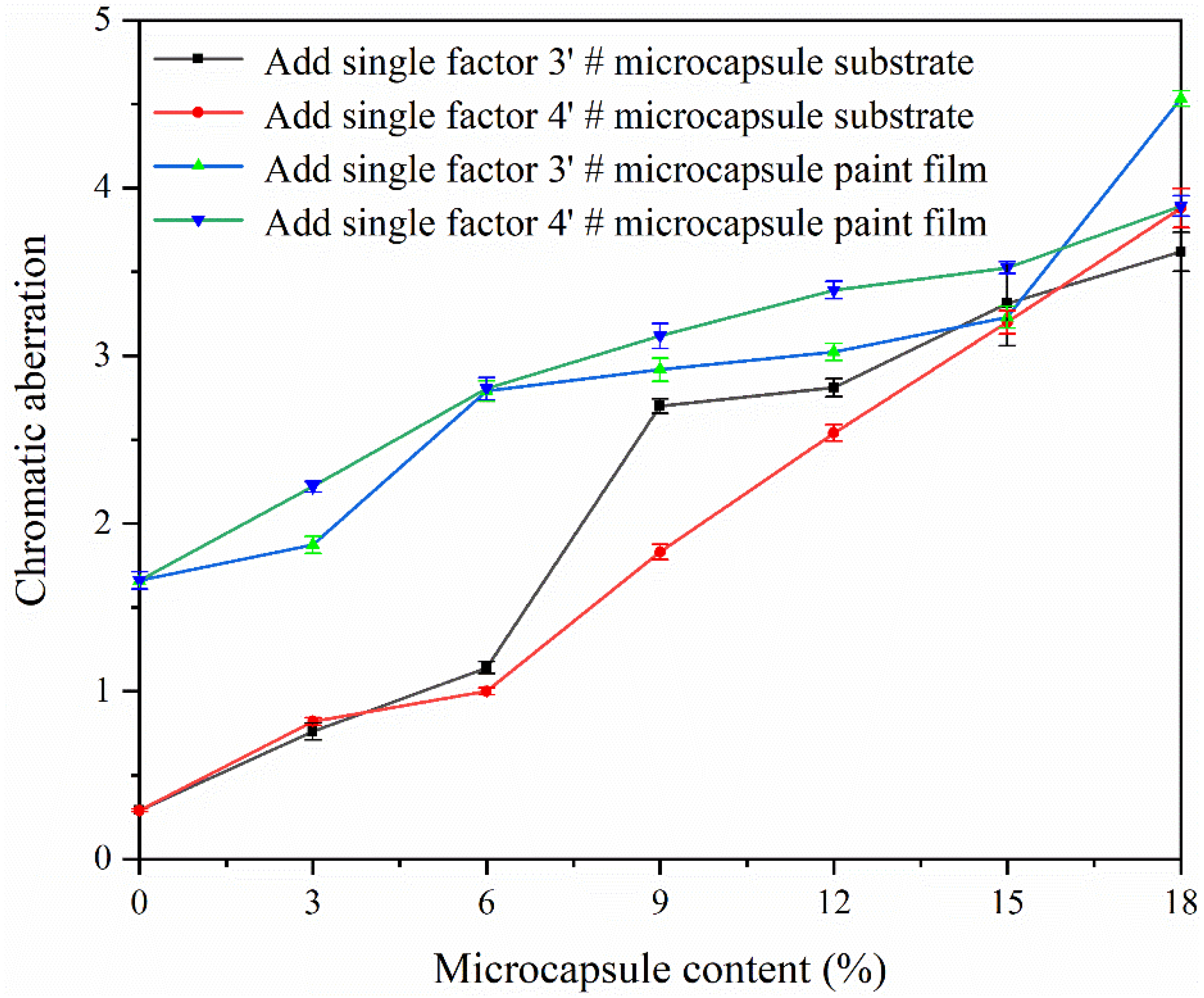
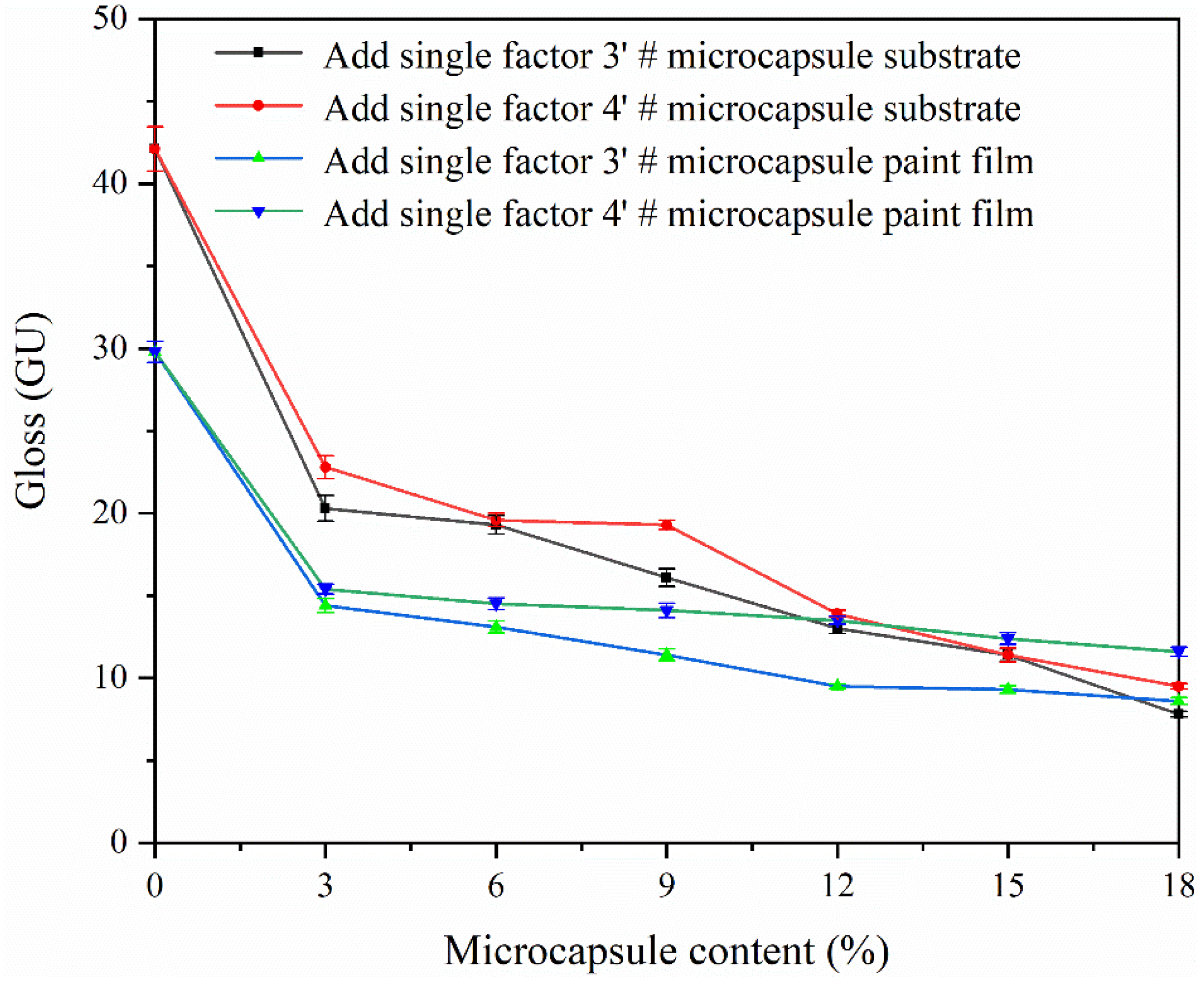
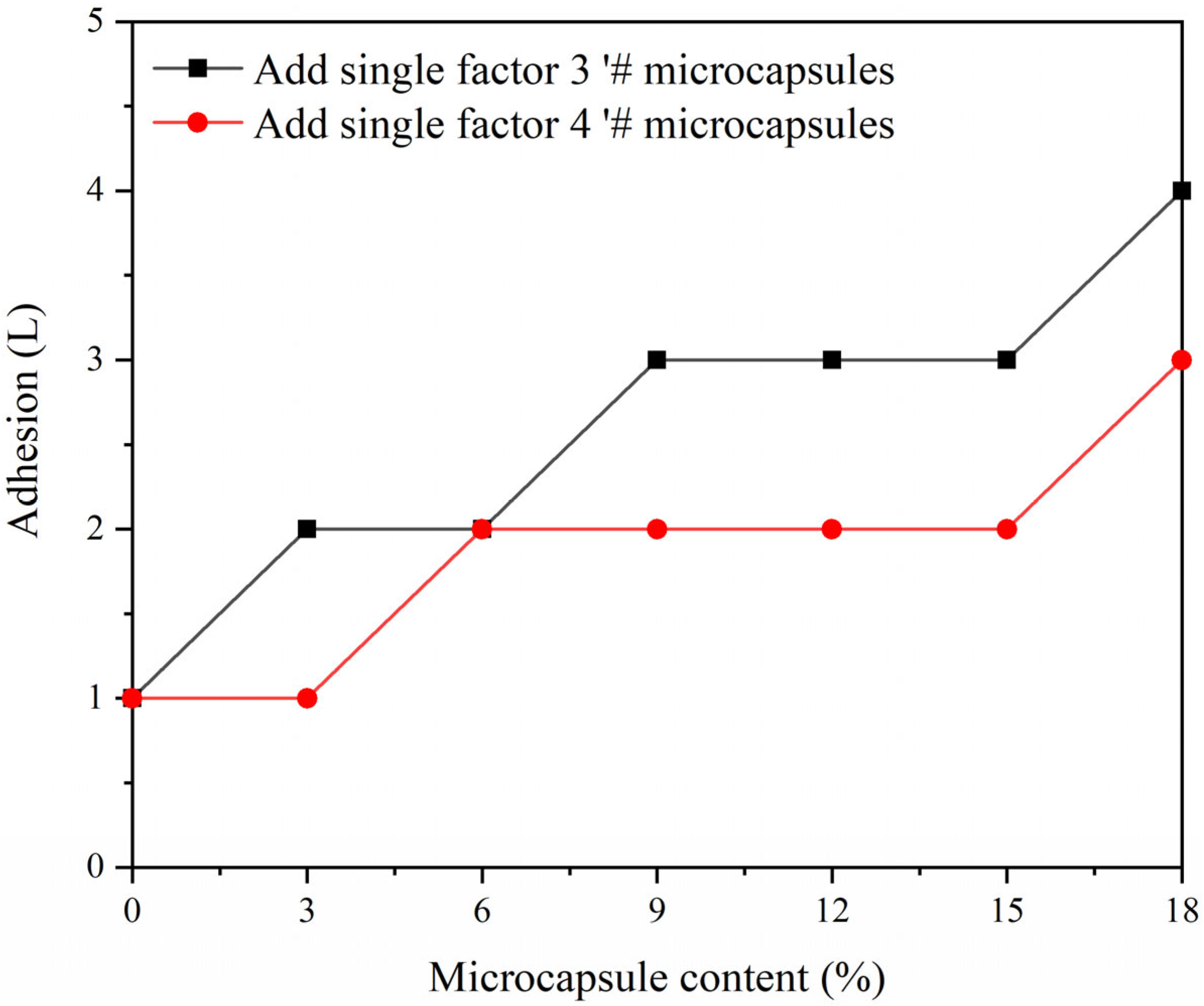
3.7. Effect of CIP Microcapsules with Different Core-Wall Ratios on the Properties of Waterborne Wood Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Gu, Y.; Xu, W.; Lu, T.; Li, W.; Fan, H. Compressive Properties of Green Velvet Material Used in Mattress Bedding. Appl. Sci. 2021, 11, 11159. [Google Scholar] [CrossRef]
- Xu, W.; Chen, P.; Yang, Y.; Wang, X.; Liu, X. Effects of freezing and steam treatments on the permeability of Populus tomentosa. Mater. Werkst. 2021, 52, 907–915. [Google Scholar] [CrossRef]
- Liu, X.; Lei, Y.Z.; Zhu, X.; Liu, G.G.; Wang, C.Q.; Chang, S.S.; Zhang, X.; Hu, J.B. Electrostatic deposition of TiO2 nanoparticles on porous wood veneer for improved membrane filtration performance and antifouling properties. Environ. Res. 2023, 220, 115170. [Google Scholar] [CrossRef]
- Mai, C.; Schmitt, U.; Niemz, P. A brief overview on the development of wood research. Holzforschung 2022, 76, 102–119. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, H.; Zhang, L.; Zhao, X.; Hu, Y. Lightweight, High-Strength Wood Prepared by Deep Eutectic Solvent Treatment as a Green Structural Material. ACS Sustain. Chem. Eng. 2022, 10, 9600–9611. [Google Scholar] [CrossRef]
- Ali, R.; Abdullah, U.H.; Ashaari, Z.; Hamid, N.H.; Hua, L.S. Hydrothermal Modification of Wood: A Review. Polymers 2021, 13, 2612. [Google Scholar] [CrossRef]
- Yan, X.; Chang, Y.; Qian, X. Effect of the Concentration of Pigment Slurry on the Film Performances of Waterborne Wood Coatings. Coatings 2019, 9, 635. [Google Scholar] [CrossRef]
- Liu, C.; Xu, W. Effect of Coating Process on Properties of Two-Component Waterborne Polyurethane Coatings for Wood. Coatings 2022, 12, 1857. [Google Scholar] [CrossRef]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. Recent progress in preparation and agricultural application of microcapsules. J. Biomed. Mater. Res. Part A 2019, 107, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Cui, J.; Qiu, H.; Yang, J.; Gao, S.; Li, J. Microcapsule/silica dual-fillers for self-healing, self-reporting and corrosion protection properties of waterborne epoxy coatings. Prog. Org. Coat. 2021, 159, 106394. [Google Scholar] [CrossRef]
- Fathabadi, H.F.; Javidi, M. Self-healing and corrosion performance of polyurethane coating containing polyurethane microcapsules. J. Coat. Technol. Res. 2021, 18, 1365–1378. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, W.; Qian, X. Effect of Water-Based Emulsion Core Microcapsules on Aging Resistance and Self-Repairing Properties of Water-Based Coatings on Linden. Appl. Sci. 2021, 11, 4662. [Google Scholar] [CrossRef]
- Yan, X.; Qian, X.; Chang, Y. Preparation and Characterization of Urea Formaldehyde @ Epoxy Resin Microcapsule on Waterborne Wood Coatings. Coatings 2019, 9, 475. [Google Scholar] [CrossRef]
- Cheng, Z.; Wei, Y.; Liu, C.; Chen, Y.; Ma, Y.; Chen, H.; Liang, X.; Sun, N.X.; Zhu, H. Lightweight and Construable Magnetic Wood for Electromagnetic Interference Shielding. Adv. Eng. Mater. 2020, 22, 2000257. [Google Scholar] [CrossRef]
- Gan, W.; Liu, Y.; Gao, L.; Zhan, X.; Li, J. Magnetic property, thermal stability, UV-resistance, and moisture absorption behavior of magnetic wood composites. Polym. Compos. 2017, 38, 1646–1654. [Google Scholar] [CrossRef]
- Yan, H.; Song, X.; Wang, Y. Study on wave absorption properties of carbonyl iron and SiO2 coated carbonyl iron particles. AIP Adv. 2018, 8, 065322. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wu, J. Preparation and Characterization of Graphene Oxide/Polyaniline/Carbonyl Iron Nanocomposites. Materials 2022, 15, 484. [Google Scholar] [CrossRef]
- Gan, W.T.; Gao, L.K.; Liu, Y.; Zhan, X.X.; Li, J. The magnetic, mechanical, thermal properties and UV resistance of COFe2O4/SiO2-coated film on wood. J. Wood Chem. Technol. 2015, 36, 94–104. [Google Scholar] [CrossRef]
- Tang, T.; Fu, Y. Formation of Chitosan/Sodium Phytate/Nano-Fe3O4 Magnetic Coatings on Wood Surfaces via Layer-by-Layer Self-Assembly. Coatings 2020, 10, 51. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kumar, V.; Lee, D.-J. Compressive properties of magnetorheological elastomer with different magnetic fields and types of filler. Polym. Adv. Technol. 2019, 30, 1106–1115. [Google Scholar] [CrossRef]
- Jang, D.; Yoon, H.N.; Nam, I.W.; Lee, H.K. Effect of CIP incorporation on the piezoresistive sensing characteristics of CNT-based polymeric sensor. Compos. Struct. 2020, 244, 112260. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.; Tao, Y. Effect of Transparent, Purple, and Yellow Shellac Microcapsules on the Optical Properties and Self-Healing Performance of Waterborne Coatings. Coatings 2022, 12, 1056. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.X.; Tao, Y. Effect of transparent, purple, and yellow shellac microcapsules on properties of the coating on paraberlinia bifoliolata surface. Polymers 2022, 14, 3304. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, W.; Han, Y.; Yin, T. Preparation of Melamine/Rice Husk Powder Coated Shellac Microcapsules and Effect of Different Rice Husk Powder Content in Wall Material on Properties of Wood Waterborne Primer. Polymers 2022, 14, 72. [Google Scholar] [CrossRef]
- Hazir, E.; Koc, K.H. Evaluation of wood-based coating performance for ultraviolet roller and conventional air-atomization processes. Maderas-Cienc. Technol. 2021, 23, 12. [Google Scholar] [CrossRef]
- Gao, J.; Wang, R.; Zhang, Z.; Song, D.; Li, X. Effect of membrane structure of waterborne coatings on the transport process of corrosive medium. Prog. Org. Coat. 2018, 124, 8–15. [Google Scholar] [CrossRef]
- GB/T 11186.3-1989; Methods for Measuring the Colour of Paint Films. Part III: Calculation of Colour Differences. Standardization Administration of the People’s Republic of China: Beijing, China, 1990.
- GB/T 4893.6-2013; Test of Surface Coatings of Furniture. Part VI: Determination of Gloss Value. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 6739-2006; Paint and Varnishes-Determination of Film Hardness by Pencil Test. Standardization Administration of the People’s Republic of China: Beijing, China, 1998.
- GB/T 4893.4-2013; Test of Surface Coatings of Furniture. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 1732-1993; Determination of Impact Resistance of Film. Standardization Administration of the People’s Republic of China: Beijing, China, 1993.
- Yang, X.; Liu, Y.; Lv, Z.; Hua, Q.; Liu, L.; Wang, B.; Tang, J. Synthesis of high latent heat lauric acid/silica microcapsules by interfacial polymerization method for thermal energy storage. J. Energy Storage 2021, 33, 102059. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Xin, B.; Liu, Y.; Zhang, F. Preparation and characterization of wormwood-oil-contained microcapsules. J. Microencapsul. 2020, 37, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Tao, Y.; Qian, X.Y. Preparation of microcapsules for core materials and their effects on properties of waterborne coatings on basswood. J. For. Eng. 2022, 7, 186–192. [Google Scholar]
- Han, Y.; Yan, X.X.; Yin, T.Y. Effects of different proportions of mixed microcapsules on aging resistance and self-healing properties of waterborne coatings. J. For. Eng. 2022, 7, 183–189. [Google Scholar]
- DaAloia, A.G.; Bidsorkhi, H.C.; De Bellis, G.; Sarto, M.S. Graphene Based Wideband Electromagnetic Absorbing Textiles at Microwave Bands. IEEE Trans. Electromagn. Compat. 2022, 64, 710–719. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, D.; Xu, W. Influence of the Bottom Color Modification and Material Color Modification Process on the Performance of Modified Poplar. Coatings 2021, 11, 660. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, D.; Xu, W. Effect of Polyurethane Non-Transparent Coating Process on Paint Film Performance Applied on Modified Poplar. Coatings 2022, 12, 39. [Google Scholar] [CrossRef]
- Liu, L.; Shan, H.R.; Jia, X.K.; Duan, S.J.; Gao, Y.J.; Xu, C.Y.; Wei, S.Y. Study on UV curable tung oil based waterborne polyurethane wood coatings. J. For. Eng. 2022, 7, 115–121. [Google Scholar]
- Liu, Q.; Gao, D.; Xu, W. Effect of Paint Process on the Performance of Modified Poplar Wood Antique. Coatings 2021, 11, 1174. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, Y.; Xu, W.; Lu, T.; Li, W.; Fan, H. Compressive Properties of Polyurethane Fiber Mattress Filling Material. Appl. Sci. 2022, 12, 6139. [Google Scholar] [CrossRef]
- Fallah, F.; Khorasani, M.; Ebrahimi, M. Improving the mechanical properties of waterborne nitrocellulose coating using nano-silica particles. Prog. Org. Coat. 2017, 109, 110–116. [Google Scholar] [CrossRef]
- Haase, J.G.; Leung, L.H.; Evans, P.D. Plasma Pre-treatments to Improve the Weather Resistance of Polyurethane Coatings on Black Spruce Wood. Coatings 2019, 9, 8. [Google Scholar] [CrossRef]
- Manabe, K.; Nakano, M.; Miyake, K.; Norikane, Y. Bioinspired extremely rapid self-repairing coatings for long-life repeated features. Chem. Eng. J. 2021, 424, 130568. [Google Scholar] [CrossRef]





| Experimental Materials | Molecular Mass (g/mol) | CAS | Manufacturer |
|---|---|---|---|
| 99.9% melamine | 126.12 | 108-78-1 | Nanjing Houxin Biotechnology Co., Ltd., Nanjing, China |
| 37% formaldehyde | 30.03 | 50-00-0 | Jinan Chuangtong Chemical Co., Ltd., Jinan, China |
| triethanolamine | 149.1882 | 102-71-6 | Jinan Chuangtong Chemical Co., Ltd., Jinan, China |
| 12.5% yellow shellac solution | - | - | Shanghai Yuhe Industrial Co., Ltd., Shanghai, China |
| rosin solution | - | - | Shenzhen Maner Technology Co., Ltd., Suzhou, China |
| Span-20 | 346.459 | 133-39-2 | Nanjing Houxin Biotechnology Co., Ltd., Nanjing, China |
| Tween-20 | 604.813 | 9005-64-5 | Nanjing Houxin Biotechnology Co., Ltd., Nanjing, China |
| CIP | 195.897 | 13463-40-6 | Nangong Xindun Alloy Welding Material Spraying Co., Ltd., Nangong, China |
| absolute ethanol | 46.07 | 64-17-5 | Wuxi Jingke Chemical Co., Ltd., Wuxi, China |
| citric acid monohydrate | 210.14 | 5949-29-1 | Nanjing Quanlong Biotechnology Co., Ltd., Nanjing, China |
| 0.05 mol/L dilute hydrochloric acid | 36.46 | 7647-01-0 | Nanjing Kehua Test Reagent Consumables, Nanjing, China |
| Dulux waterborne primer | - | - | Akzo Nobel Paint (Shanghai) Co., Ltd., Shanghai, China |
| Sample | Core-Wall Ratio | Water Bath Temperature (°C) | Stirring Speed (rpm) | Reaction Time (h) |
|---|---|---|---|---|
| 1 # | 0.3:1 | 20 | 300 | 0.5 |
| 2 # | 0.3:1 | 40 | 500 | 1.0 |
| 3 # | 0.3:1 | 60 | 800 | 2.0 |
| 4 # | 0.5:1 | 20 | 500 | 2.0 |
| 5 # | 0.5:1 | 40 | 800 | 0.5 |
| 6 # | 0.5:1 | 60 | 300 | 1.0 |
| 7 # | 0.7:1 | 20 | 800 | 1.0 |
| 8 # | 0.7:1 | 40 | 300 | 2.0 |
| 9 # | 0.7:1 | 60 | 500 | 0.5 |
| Sample | CIP (g) | Deionized Water for Core (g) | Melamine (g) | 37% Formaldehyde (g) | Deionized Water for Wall (g) |
|---|---|---|---|---|---|
| 1 # | 2.58 | 23.22 | 5.00 | 10.00 | 40.00 |
| 2 # | 2.58 | 23.22 | 5.00 | 10.00 | 40.00 |
| 3 # | 2.58 | 23.22 | 5.00 | 10.00 | 40.00 |
| 4 # | 4.30 | 38.70 | 5.00 | 10.00 | 40.00 |
| 5 # | 4.30 | 38.70 | 5.00 | 10.00 | 40.00 |
| 6 # | 4.30 | 38.70 | 5.00 | 10.00 | 40.00 |
| 7 # | 6.02 | 54.18 | 5.00 | 10.00 | 40.00 |
| 8 # | 6.02 | 54.18 | 5.00 | 10.00 | 40.00 |
| 9 # | 6.02 | 54.18 | 5.00 | 10.00 | 40.00 |
| Sample | Core-Wall Ratio | CIP (g) | Deionized Water for Core (g) | Melamine (g) | 37% Formaldehyde (g) | Deionized Water for Wall (g) |
|---|---|---|---|---|---|---|
| 1′ # | 0.55:1 | 4.73 | 42.57 | 5.00 | 10.00 | 40.00 |
| 2′ # | 0.60:1 | 5.16 | 46.44 | 5.00 | 10.00 | 40.00 |
| 3′ # | 0.65:1 | 5.59 | 50.31 | 5.00 | 10.00 | 40.00 |
| 4′ # | 0.70:1 | 6.02 | 54.18 | 5.00 | 10.00 | 40.00 |
| 5′ # | 0.75:1 | 6.45 | 58.05 | 5.00 | 10.00 | 40.00 |
| 6′ # | 0.80:1 | 6.88 | 61.92 | 5.00 | 10.00 | 40.00 |
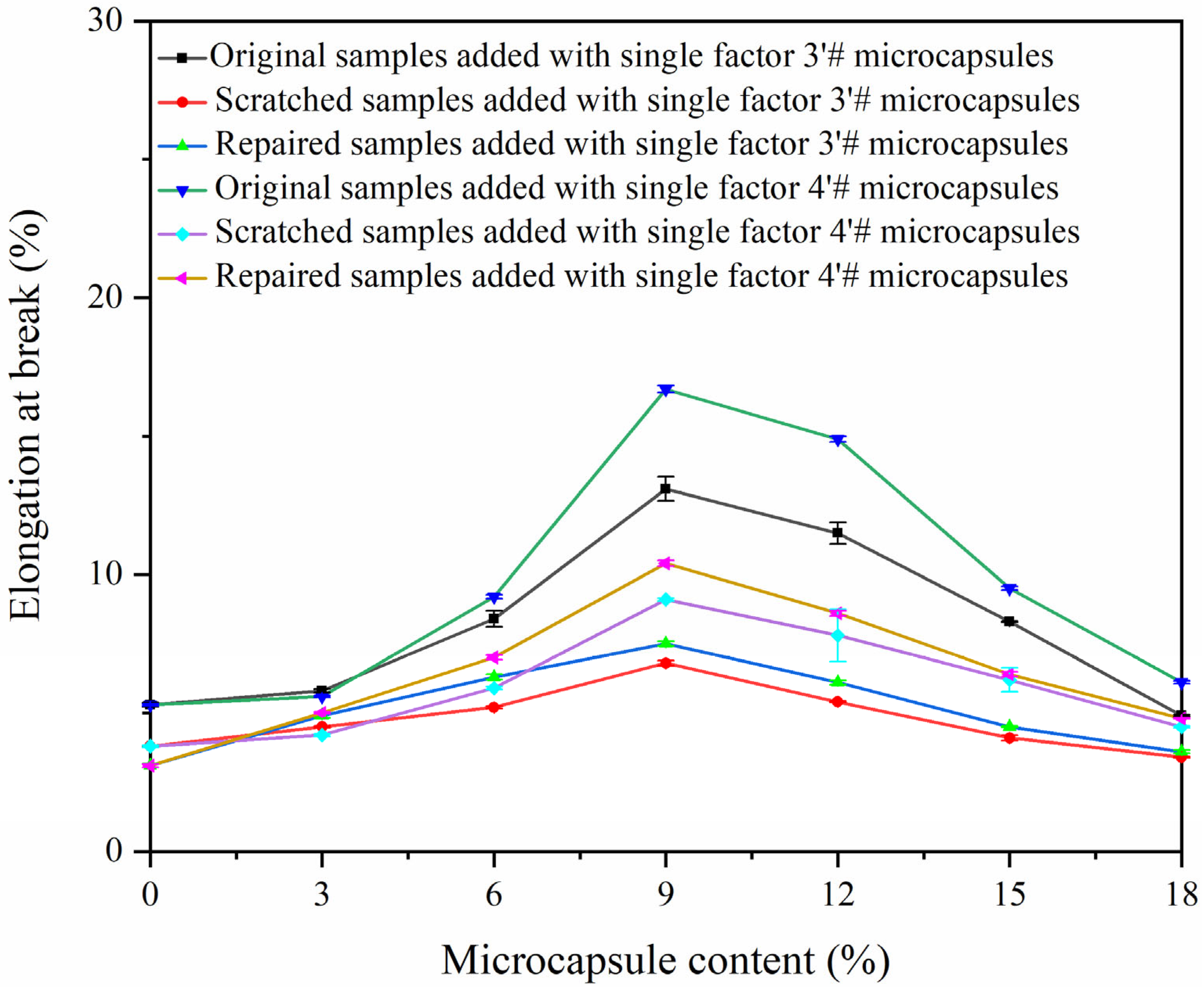
| Sample | Addition Amount of Microcapsule (%) | Elongation at Break (%) | Repair Rate (%) | ||
|---|---|---|---|---|---|
| Original Sample | Scratched Sample | Sample After Repair | |||
| Blank sample | 0 | 5.3 ± 0.2 | 3.8 ± 0.1 | 3.1±0.1 | 0 |
| 3–1 | 3 | 5.8 ± 0.2 | 4.5 ± 0.2 | 4.9 ± 0.2 | 30.8 ± 1.5 |
| 3–2 | 6 | 8.4 ± 0.4 | 5.2 ± 0.2 | 6.3 ± 0.3 | 34.4 ± 1.7 |
| 3–3 | 9 | 13.1 ± 0.6 | 6.8 ± 0.3 | 7.5 ± 0.3 | 11.1 ± 0.5 |
| 3–4 | 12 | 11.5 ± 0.5 | 5.4 ± 0.2 | 6.1 ± 0.3 | 11.5 ± 0.5 |
| 3–5 | 15 | 8.3 ± 0.4 | 4.1 ± 0.2 | 4.5 ± 0.2 | 9.5 ± 0.4 |
| 3–6 | 18 | 4.9 ± 0.2 | 3.4 ± 0.1 | 3.6 ± 0.1 | 1.3 |
| 4–1 | 3 | 5.6 ± 0.2 | 4.2 ± 0.2 | 4.6 ± 0.2 | 28.6 ± 1.4 |
| 4–2 | 6 | 9.2 ± 0.4 | 5.9 ± 0.2 | 7.0 ± 0.3 | 33.3 ± 1.6 |
| 4–3 | 9 | 16.7 ± 0.8 | 9.1 ± 0.4 | 10.4 ± 0.5 | 17.1 ± 0.8 |
| 4–4 | 12 | 14.9 ± 0.7 | 7.8 ± 0.3 | 8.6 ± 0.4 | 16.9 ± 0.8 |
| 4–5 | 15 | 9.5 ± 0.4 | 6.2 ± 0.3 | 6.4 ± 0.3 | 6.1 ± 0.3 |
| 4–6 | 18 | 6.1 ± 0.3 | 4.5 ± 0.2 | 4.6 ± 0.2 | 6.2 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Yan, X. Effects of Shellac Self-Repairing and Carbonyl Iron Powder Microcapsules on the Properties of Dulux Waterborne Coatings on Wood. Polymers 2023, 15, 2016. https://doi.org/10.3390/polym15092016
Li W, Yan X. Effects of Shellac Self-Repairing and Carbonyl Iron Powder Microcapsules on the Properties of Dulux Waterborne Coatings on Wood. Polymers. 2023; 15(9):2016. https://doi.org/10.3390/polym15092016
Chicago/Turabian StyleLi, Wenbo, and Xiaoxing Yan. 2023. "Effects of Shellac Self-Repairing and Carbonyl Iron Powder Microcapsules on the Properties of Dulux Waterborne Coatings on Wood" Polymers 15, no. 9: 2016. https://doi.org/10.3390/polym15092016
APA StyleLi, W., & Yan, X. (2023). Effects of Shellac Self-Repairing and Carbonyl Iron Powder Microcapsules on the Properties of Dulux Waterborne Coatings on Wood. Polymers, 15(9), 2016. https://doi.org/10.3390/polym15092016





