Synthesis, Characterization, and Optimization Studies of Polycaprolactone/Polylactic Acid/Titanium Dioxide Nanoparticle/Orange Essential Oil Membranes for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanoparticles
Characterization of Nanoparticles of TiO2
2.3. OEO Composition
2.4. Preparation of the Membranes of PCL/PLA/TiO2/OEO
Characterization of PCL/PLA/TiO2/OEO Membranes
- Fourier Transform Infrared Spectroscopy (FTIR)
- X-ray Diffraction
- Scanning Electron Microscopy (SEM)
- Thermal Analysis
2.5. Preliminary Biocompatibility Analysis of In Vivo Membranes
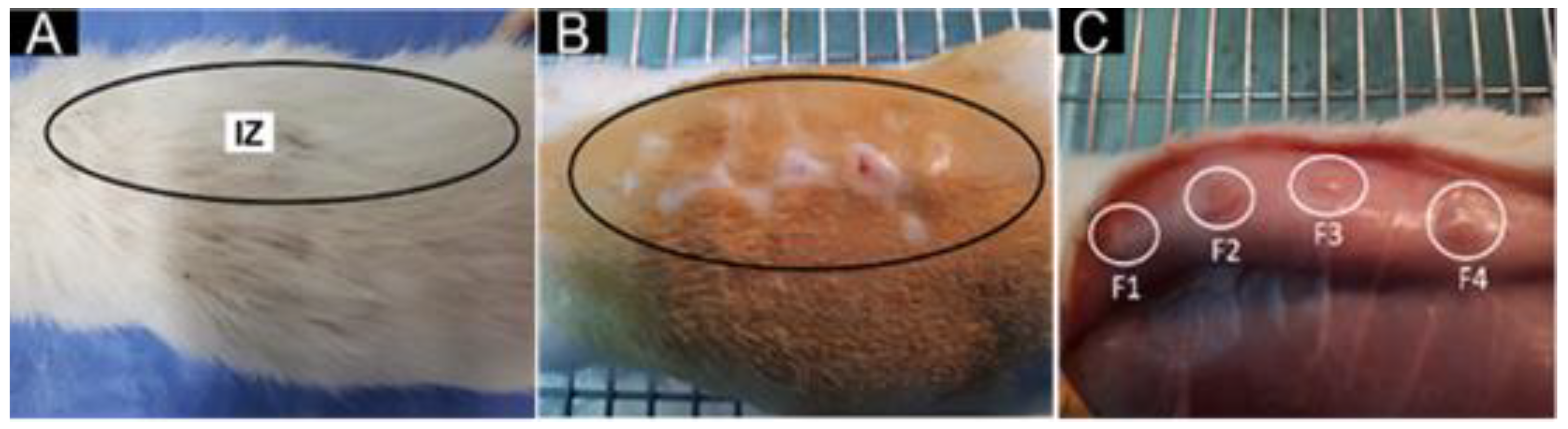
2.5.1. Surgical Preparations of Biomodels
2.5.2. Histology Tests
3. Results and Discussion
3.1. Characterization of the Nanoparticles of TiO2
3.2. Composition of OEO
3.3. Preparation of PLA/PCL/TiO2/OEO Membranes
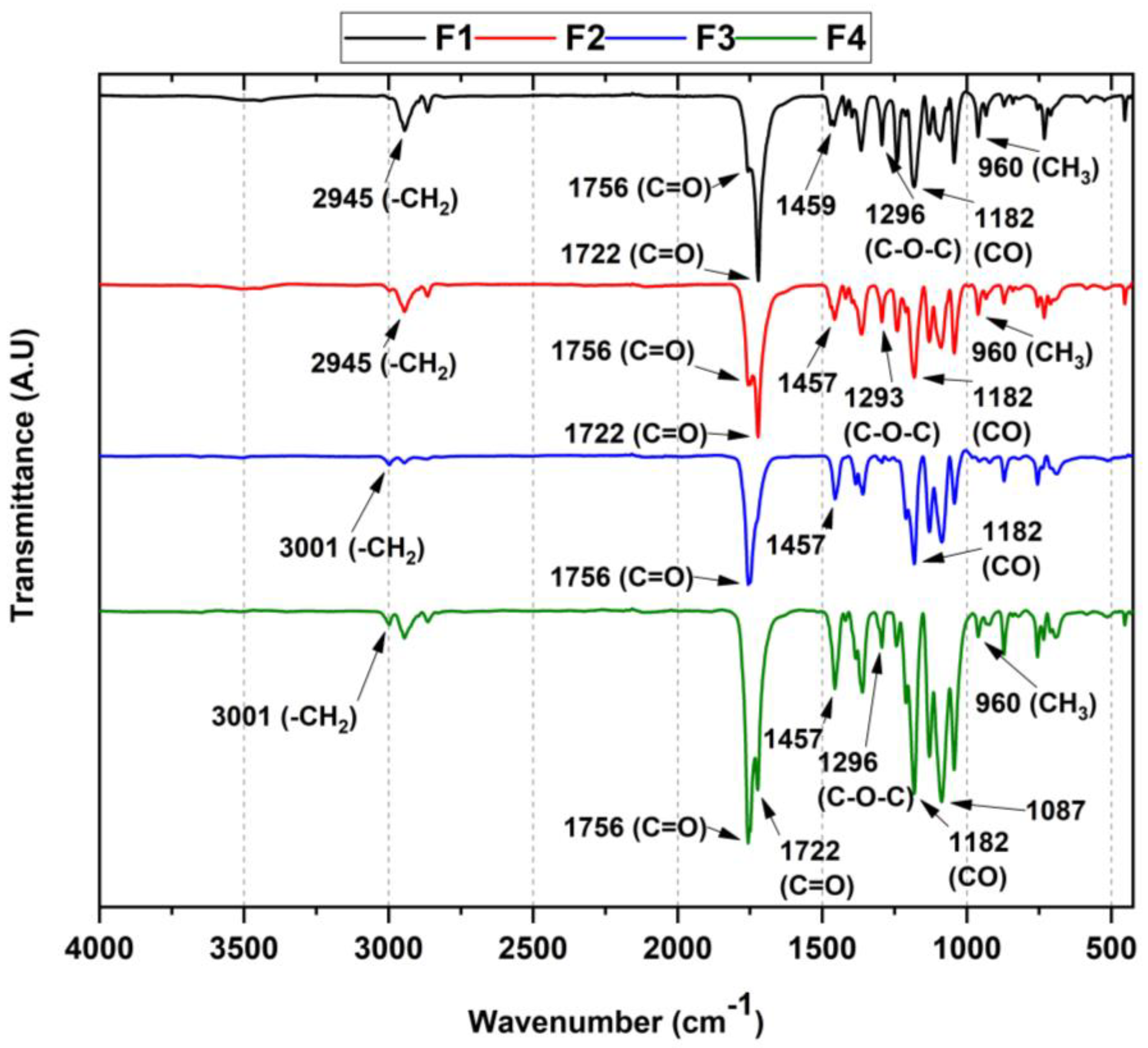
3.3.1. FT-IR for PLA/PCL/TiO2/OEO Membranes
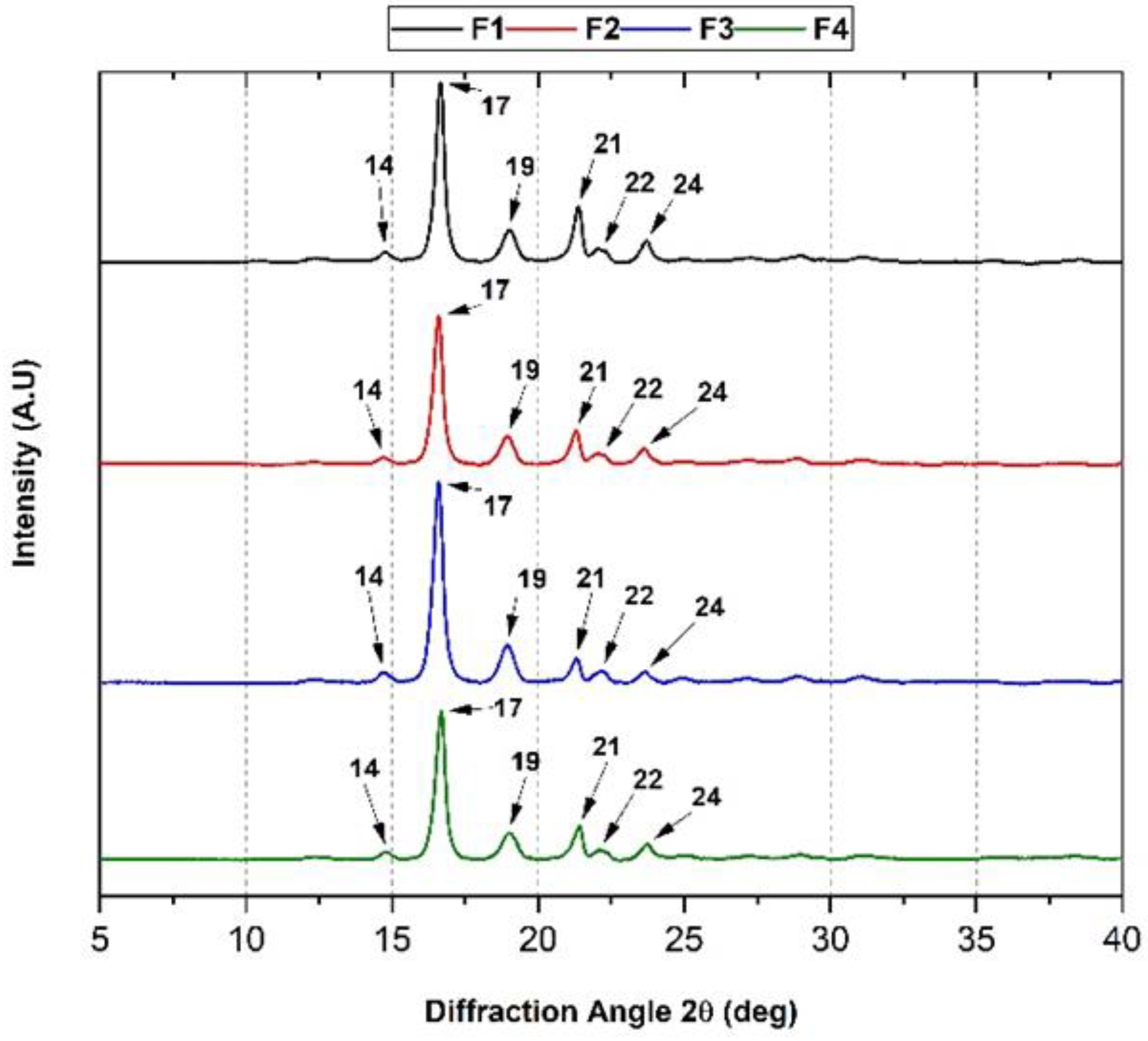
3.3.2. XRD of the PLA/PCL/TiO2/OEO Membranes
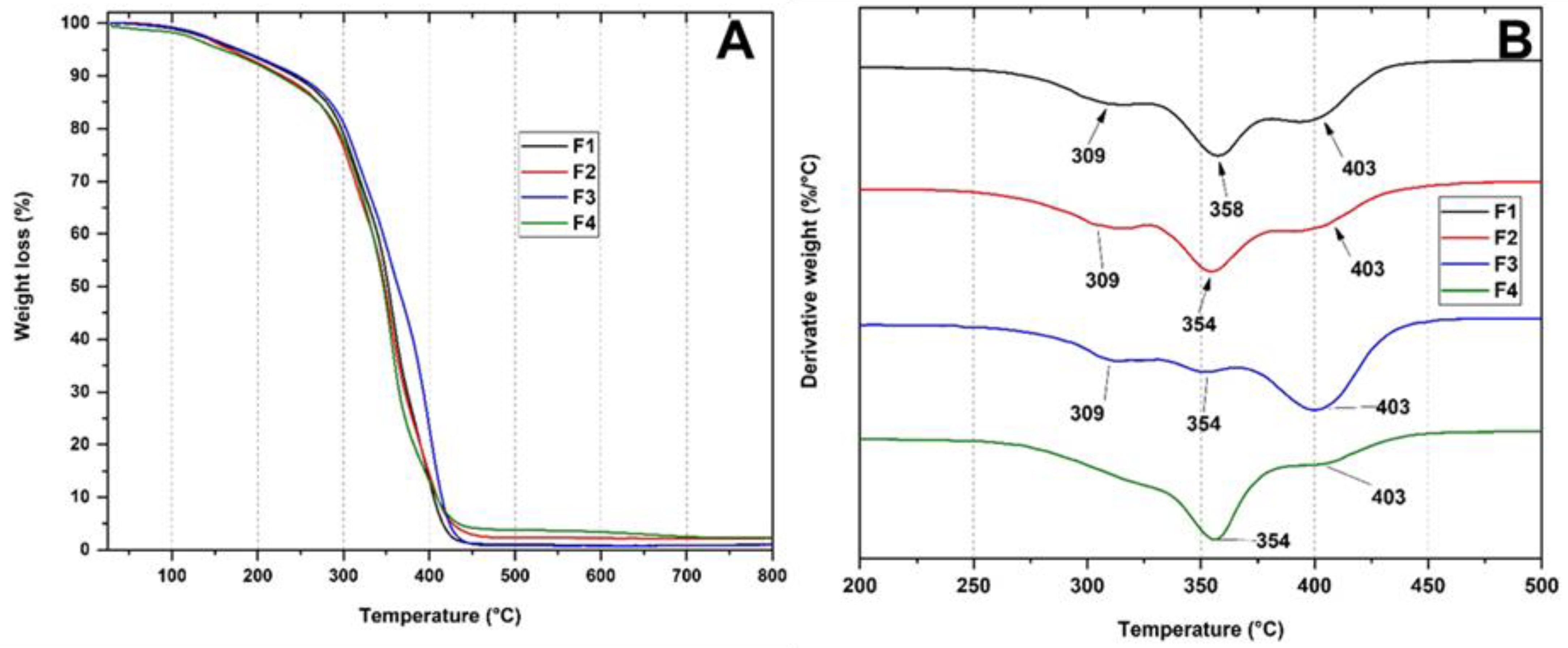
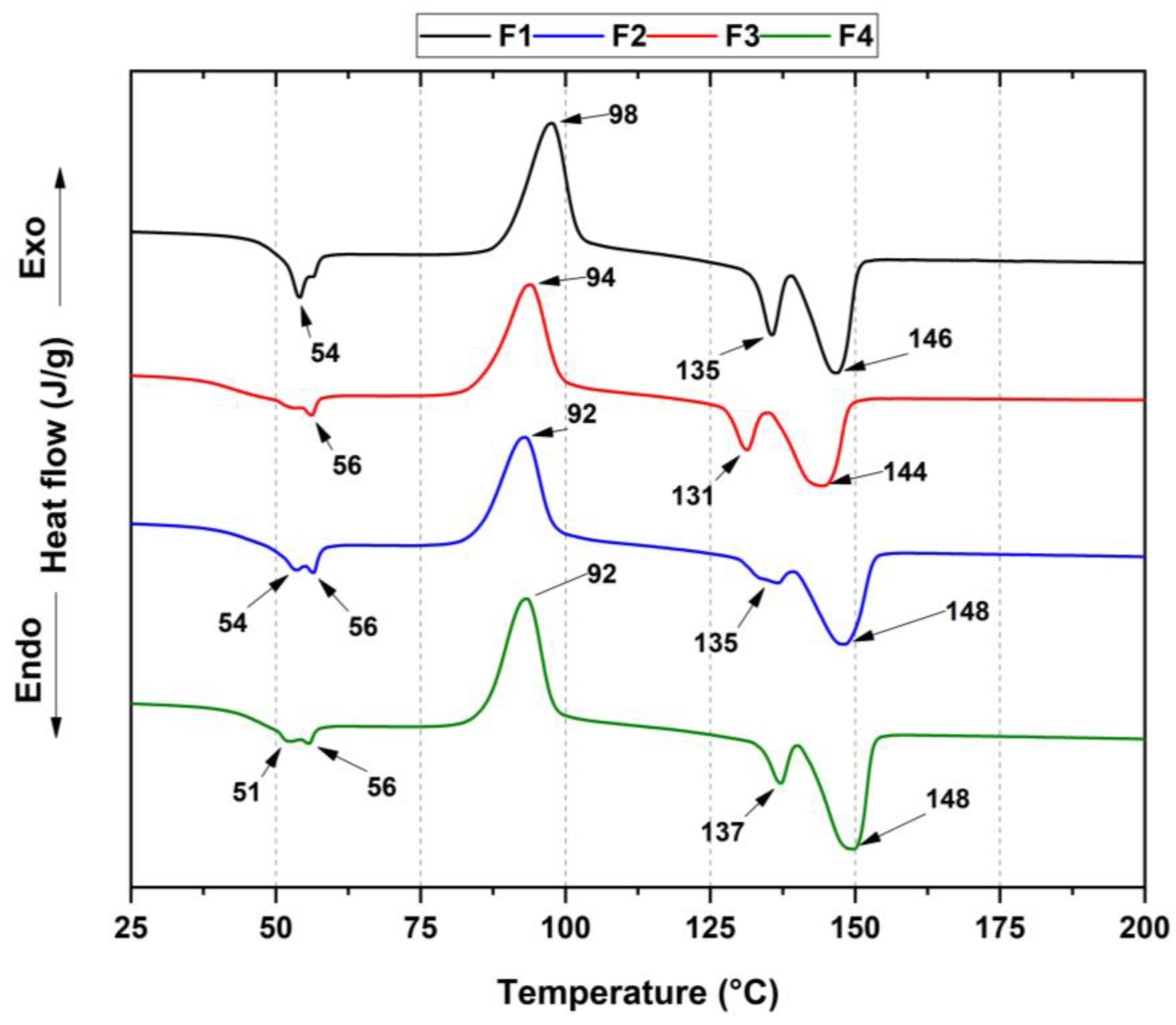
3.3.3. Thermal Analysis of PLA/PCL/TiO2/OEO Membranes
3.3.4. Scanning Electron Microscopy (SEM) of PLA/PCL/TiO2/OEO Membranes
3.3.5. In Vivo Biocompatibility Test of the PLA/PCL/TiO2/OEO Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conoscenti, G.; Carfì Pavia, F.; Ciraldo, F.E.; Liverani, L.; Brucato, V.; La Carrubba, V.; Boccaccini, A.R. In vitro degradation and bioactivity of composite poly-l-lactic (PLLA)/bioactive glass (BG) scaffolds: Comparison of 45S5 and 1393BG compositions. J. Mater. Sci. 2018, 53, 2362–2374. [Google Scholar] [CrossRef]
- Deb, P.; Deoghare, A.B.; Borah, A.; Barua, E.; Das Lala, S. Scaffold Development Using Biomaterials: A Review. Mater. Today Proc. 2018, 5, 12909–12919. [Google Scholar] [CrossRef]
- Yang, S.L.; Wu, Z.H.; Yang, W.; Yang, M.B. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Rose, A.S.J.L.; Selvarajan, P.; Perumal, S. Growth, structural, spectral, mechanical, thermal and dielectric characterization of phosphoric acid admixtured l-alanine (PLA) single crystals. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2011, 81, 270–275. [Google Scholar] [CrossRef]
- Mina Hernandez, J.H. Effect of the incorporation of polycaprolactone (Pcl) on the retrogradation of binary blends with cassava thermoplastic starch (tps). Polymers 2021, 13, 38. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Cutright, D.E.; Hunsuck, E.E. Tissue reaction to the biodegradable polylactic acid suture. Oral Surg. Oral Med. Oral Pathol. 1971, 31, 134–139. [Google Scholar] [CrossRef]
- Haroosh, H.; Chaudhary, D.; Dong, Y.; Hawkins, B. Electrospun PLA: PCL/halloysite nanotube nanocomposites fibers for drug delivery. Process. Fabr. Adv. Mater. XIX 2011, 847–858. [Google Scholar]
- Wang, L.; Zhang, F.; Liu, Y.; Leng, J. Shape memory polymer fibers: Materials, structures, and applications. Adv. Fiber Mater. 2022, 4, 5–23. [Google Scholar] [CrossRef]
- Luyt, A.S.; Gasmi, S. Influence of TiO2 Nanoparticles on the crystallization behaviour and tensile properties of biodegradable PLA and PCL nanocomposites. J. Polym. Environ. 2018, 26, 2410–2423. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, J.; Gan, Y.; Cheng, G. Titanium glycolate-derived TiO2 nanomaterials: Synthesis and applications. Adv. Powder Technol. 2018, 29, 2289–2311. [Google Scholar] [CrossRef]
- Jayakumar, R.; Ramachandran, R.; Divyarani, V.V.; Chennazhi, K.P.; Tamura, H.; Nair, S.V. International Journal of Biological Macromolecules Fabrication of chitin–chitosan/nano TiO2-composite scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Luyt, A.S.; Antunes, A.; Popelka, A.; Mahmoud, A.; Hassan, M.K.; Kasak, P. Effect of poly(ε-caprolactone) and titanium (IV) dioxide content on the UV and hydrolytic degradation of poly(lactic acid)/poly(ε-caprolactone) blends. J. Appl. Polym. Sci. 2021, 138, 1–16. [Google Scholar] [CrossRef]
- Khan, A.R.; Nadeem, M.; Aqeel Bhutto, M.; Yu, F.; Xie, X.; El-Hamshary, H.; El-Faham, A.; Ibrahim, U.A.; Mo, X. Physico-chemical and biological evaluation of PLCL/SF nanofibers loaded with oregano essential oil. Pharmaceutics 2019, 11, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Encapsulation of orange essential oil using cross-linked electrospun gelatin nanofibers. Food Bioprocess Technol. 2018, 11, 427–434. [Google Scholar] [CrossRef]
- Esthappan, S.K.; Nair, A.B.; Joseph, R. Effect of crystallite size of zinc oxide on the mechanical, thermal and flow properties of polypropylene/zinc oxide nanocomposites. Compos. Part B Eng. 2015, 69, 145–153. [Google Scholar] [CrossRef]
- Kosowska, K.; Szatkowski, P. Influence of ZnO, SiO2 and TiO2 on the aging process of PLA fibers produced by electrospinning method. J. Therm. Anal. Calorim. 2020, 140, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Zapata, P.A.; Palza, H.; Delgado, K.; Rabagliati, F.M. Novel antimicrobial polyethylene composites prepared by metallocenic in situ polymerization with TiO2-based nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4055–4062. [Google Scholar] [CrossRef]
- Castro, J.I.; Valencia-Llano, C.H.; Valencia Zapata, M.E.; Restrepo, Y.J.; Mina Hernandez, J.H.; Navia-Porras, D.P.; Valencia, Y.; Valencia, C.; Grande-Tovar, C.D. Chitosan/Polyvinyl Alcohol/Tea Tree Essential Oil Composite Films for Biomedical Applications. Polymers 2021, 13, 3753. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly (lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Carmona, V.B.; Correa, A.C.; Marconcini, J.M.; Capparel-Li Mattoso, L.H. Properties of a Biodegradable Ternary Blend of Thermoplastic Starch (TPS), Poly(e-Caprolactone) (PCL) and Poly(Lactic Acid) (PLA). J. Polym. Environ. 2015, 23, 83–89. [Google Scholar] [CrossRef]
- Vargas Urbano, M.A.; Ochoa Muñoz, Y.H.; Ortegón Fernández, Y.; Mosquera, P.; Rodríguez Páez, J.E.; Camargo Amado, R.J. Nanopartículas de TiO2, fase anatasa, sintetizadas por métodos químicos. Ing. Y Desarro. 2011, 29, 186–201. [Google Scholar]
- Galvão, J.G.; Silva, V.F.; Ferreira, S.G.; França, F.R.M.; Santos, D.A.; Freitas, L.S.; Alves, P.B.; Araújo, A.A.S.; Cavalcanti, S.C.H.; Nunes, R.S. β-cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: An alternative to control Aedes aegypti larvae. Thermochim. Acta 2015, 608, 14–19. [Google Scholar] [CrossRef]
- Zhang, J.; Boyd, I.; Boyd, I.; Boyd, I. Nanocrystalline TiO2 films studied by optical, XRD and FTIR spectroscopy Related papers. J. Non. Cryst. Solids 2002, 303, 134–138. [Google Scholar] [CrossRef]
- Kringel, D.H.; Antunes, M.D.; Klein, B.; Crizel, R.L.; Wagner, R.; de Oliveira, R.P.; Dias, A.R.G.; Zavareze, E.d.R. Production, Characterization, and Stability of Orange or Eucalyptus Essential Oil/β-Cyclodextrin Inclusion Complex. J. Food Sci. 2017, 82, 2598–2605. [Google Scholar] [CrossRef]
- Pardini, F.; Iregui, Á.; Faccia, P.; Amalvy, J.; González, A.; Irusta, L. Development and characterization of electrosprayed microcaspules of poly ε-caprolactone with citronella oil for mosquito-repellent application. Int. J. Polym. Anal. Charact. 2021, 26, 497–516. [Google Scholar] [CrossRef]
- Ocelić, V.; Vilko, B.; Dajana, M.; Grgić, K.; Ivančić, A. Biodegradable Polymer Blends Based on Thermoplastic Starch. J. Polym. Environ. 2020, 29, 492–508. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed poly (lactic acid)(PLA)/poly (ε-caprolactone)(PCL) biodegradable polymer blend nanocomposites with TiO2 as filler. Polym. Test. 2015, 45, 93–100. [Google Scholar] [CrossRef]
- Wongkanya, R.; Teeranachaideekul, V.; Makarasen, A.; Chuysinuan, P. Electrospun poly(lactic acid) nanofiber mats for controlled transdermal delivery of essential oil from Zingiber cassumunar Roxb. Mater. Res. 2020, 7, 055305. [Google Scholar] [CrossRef]
- Thiyagu, T.T.; Uvaraja, G.G.V.C.; Arun, T.M.V.R. Effect of SiO2/TiO2 and ZnO Nanoparticle on Cardanol Oil Compatibilized PLA/PBAT Biocomposite Packaging Film. Silicon 2022, 14, 3795–3808. [Google Scholar] [CrossRef]
- Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Yahyaoui, M.; Gordobil, O.; Herrera Díaz, R.; Abderrabba, M.; Labidi, J. Development of novel antimicrobial films based on poly(lactic acid) and essential oils. React. Funct. Polym. 2016, 109, 1–8. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Tsubakihara, S.; Iura, K.; Ono, Y.; Dan, Y.; Takahashi, K. Crystallization behavior of poly(l-lactic acid). Polymer 2006, 47, 7554–7563. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Camino, G. Nanocomposites of PLA and PCL based on montmorillonite and sepiolite. Mater. Sci. Eng. C 2009, 29, 1433–1441. [Google Scholar] [CrossRef]
- Loyo, C.; Moreno-Serna, V.; Fuentes, J.; Amigo, N.; Sepúlveda, F.A.; Ortiz, J.A.; Rivas, L.M.; Ulloa, M.T.; Benavente, R.; Zapata, P.A. PLA/CaO nanocomposites with antimicrobial and photodegradation properties. Polym. Degrad. Stab. 2022, 197, 109865. [Google Scholar] [CrossRef]
- Lu, W.; Cui, R.; Zhu, B.; Qin, Y.; Cheng, G.; Li, L.; Yuan, M. Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly (lactic acid) biocomposite food packaging film. J. Mater. Res. Technol. 2021, 11, 1152–1161. [Google Scholar] [CrossRef]
- Hemmrich, K.; von Heimburg, D.; Rendchen, R.; Di Bartolo, C.; Milella, E.; Pallua, N. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials 2005, 26, 7025–7037. [Google Scholar] [CrossRef]
- Marsi, T.C.O.; Ricci, R.; Toniato, T.V.; Vasconcellos, L.M.R.; Elias, C.d.M.V.; Silva, A.D.R.; Furtado, A.S.A.; Magalhães, L.S.S.M.; Silva-Filho, E.C.; Marciano, F.R.; et al. Electrospun Nanofibrous Poly (Lactic Acid)/Titanium Dioxide Nanocomposite Membranes for Cutaneous Scar Minimization. Front. Bioeng. Biotechnol. 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Valencia, C.H. Hydrolytic degradation and in vivo resorption of poly-l-lactic acid-chitosan biomedical devices in the parietal bones of Wistar rats. J. Int. Med. Res. 2019, 47, 1705–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Pan, Y.; Liu, R.; Luo, X.; Zeng, X.; Zhi, D.; Li, J.; Cheng, Q.; Huang, Z.; Zhang, H.; et al. Biocompatibility and biodegradation properties of polycaprolactone/polydioxanone composite scaffolds prepared by blend or co-electrospinning. J. Bioact. Compat. Polym. 2019, 34, 115–130. [Google Scholar] [CrossRef]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Adib, Y.; Bensussan, A.; Michel, L. Cutaneous Wound Healing: A Review about Innate Immune Response and Current Therapeutic Applications. Mediat. Inflamm. 2022, 2022, 5344085. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Ruiz, A.M.; Requena, L. Foreign Body Granulomas. Dermatol. Clin. 2015, 33, 497–523. [Google Scholar] [CrossRef]
- Adams, P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2004. [Google Scholar]
- NIST Mass Spectrometry Data Center. Available online: http://webbook.nist.gov/chemistry/ (accessed on 4 December 2022).

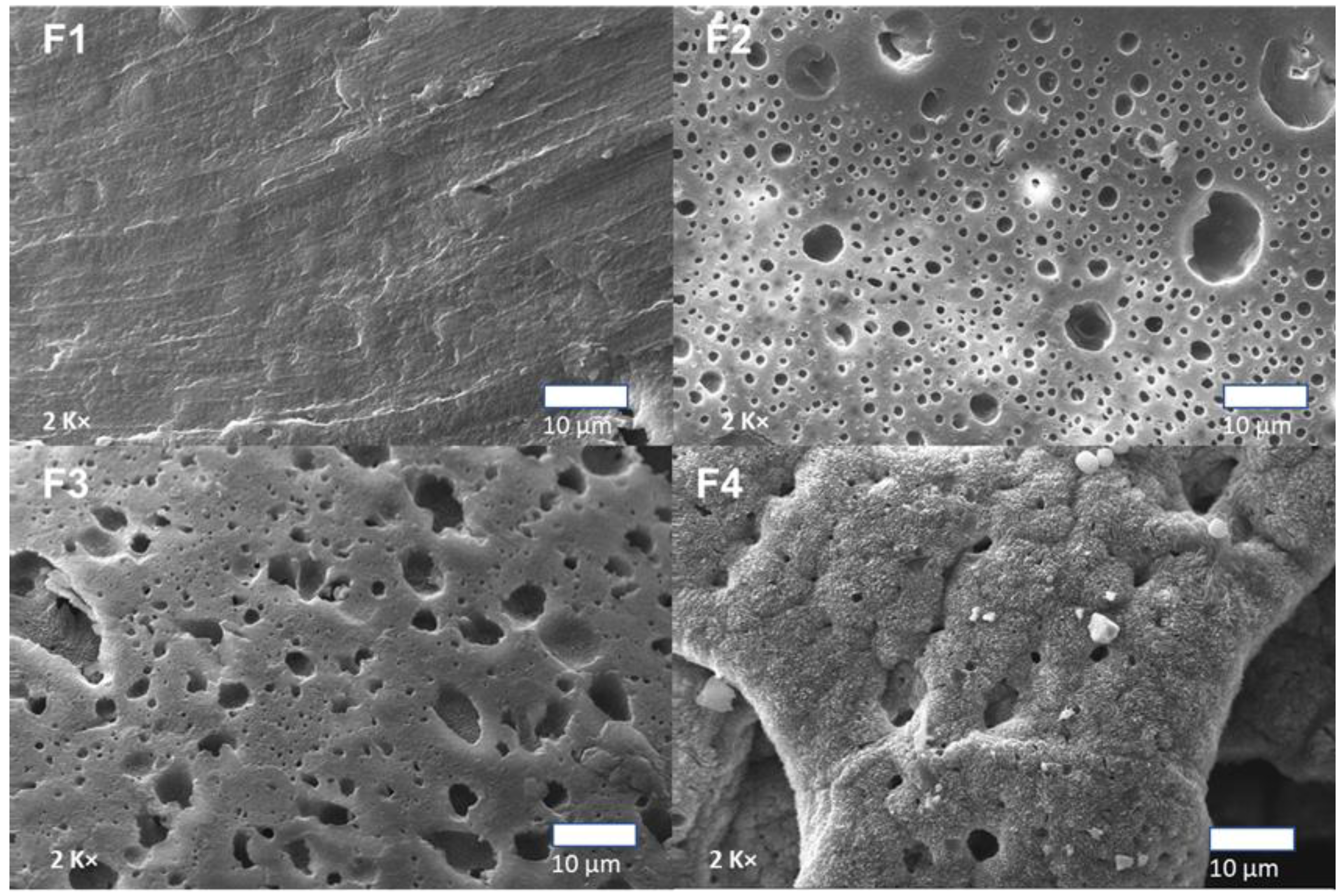

| Components | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| PCL (%) | 30 | 27 | 20 | 17 |
| PLA (%) | 70 | 70 | 70 | 70 |
| TiO2 (%) | 0 | 3 | 0 | 3 |
| OEO (%) | 0 | 0 | 10 | 10 |
| Tg (°C) | Tcc (°C) | Tm1 (°C) | Tm2 (°C) | Tm3 (°C) | Xc PLA (%) | |
|---|---|---|---|---|---|---|
| F1 | 54 | 98 | 135 | 146 | 54 | 5.2 |
| F2 | 56 | 94 | 131 | 144 | 56 | 1.8 |
| F3 | 54 | 92 | 135 | 148 | 56 | 4.4 |
| F4 | 51 | 92 | 137 | 148 | 56 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, J.I.; Astudillo, S.; Mina Hernandez, J.H.; Saavedra, M.; Zapata, P.A.; Valencia-Llano, C.H.; Chaur, M.N.; Grande-Tovar, C.D. Synthesis, Characterization, and Optimization Studies of Polycaprolactone/Polylactic Acid/Titanium Dioxide Nanoparticle/Orange Essential Oil Membranes for Biomedical Applications. Polymers 2023, 15, 135. https://doi.org/10.3390/polym15010135
Castro JI, Astudillo S, Mina Hernandez JH, Saavedra M, Zapata PA, Valencia-Llano CH, Chaur MN, Grande-Tovar CD. Synthesis, Characterization, and Optimization Studies of Polycaprolactone/Polylactic Acid/Titanium Dioxide Nanoparticle/Orange Essential Oil Membranes for Biomedical Applications. Polymers. 2023; 15(1):135. https://doi.org/10.3390/polym15010135
Chicago/Turabian StyleCastro, Jorge Ivan, Stiven Astudillo, Jose Herminsul Mina Hernandez, Marcela Saavedra, Paula A. Zapata, Carlos Humberto Valencia-Llano, Manuel N. Chaur, and Carlos David Grande-Tovar. 2023. "Synthesis, Characterization, and Optimization Studies of Polycaprolactone/Polylactic Acid/Titanium Dioxide Nanoparticle/Orange Essential Oil Membranes for Biomedical Applications" Polymers 15, no. 1: 135. https://doi.org/10.3390/polym15010135





