Design and Application of Hybrid Polymer-Protein Systems in Cancer Therapy
Abstract
1. Introduction
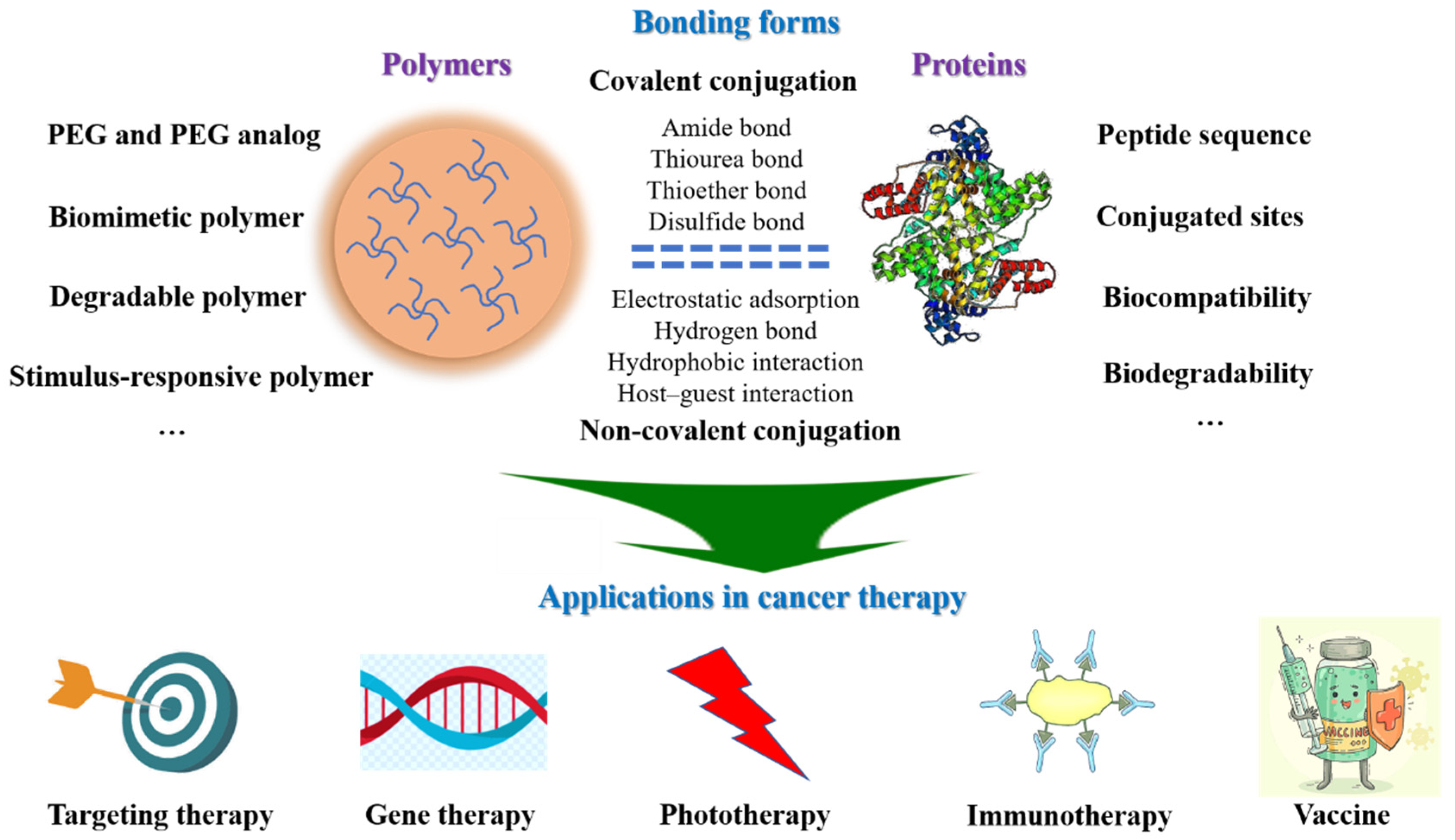
2. Design of Hybrid Polymer-Protein Systems
2.1. Selection of Polymers
2.1.1. Polyethylene Glycol (PEG) and PEG Analog
2.1.2. Biomimetic Polymer
2.1.3. Degradable Polymer
2.1.4. Stimulus-Responsive Polymer
| Category | Example | References |
|---|---|---|
| PEG and PEG analog | p(HPMA) | [32,33] |
| PCB | [34] | |
| Poly(lactic-co-glycolic acid) (PLGA) | [35] | |
| Biomimetic polymer | Trehalose-based polymer | [39] |
| Dextran, Cellulose, Pectin polymer | [41,42,43] | |
| Heparin-mimicking polymer | [45,46,47,48] | |
| Horseradish peroxidase (HRP)-catalyzed system | [49] | |
| Degradable polymer | CKA based polymer | [51] |
| Peptides | [55] | |
| HES | [56] | |
| PLGA | [61] | |
| Stimulus-responsive polymer | p(NIPAAm) | [62] |
| polyacrylic acid | [63] | |
| p(DMAEMA) | [64] | |
| Gelatin-containing supramolecular nanofibers | [67] |
2.2. Section of Proteins
2.3. Bonding Form of Polymer-Protein Systems
2.3.1. Covalent Conjugation
2.3.2. Non-Covalent Conjugation
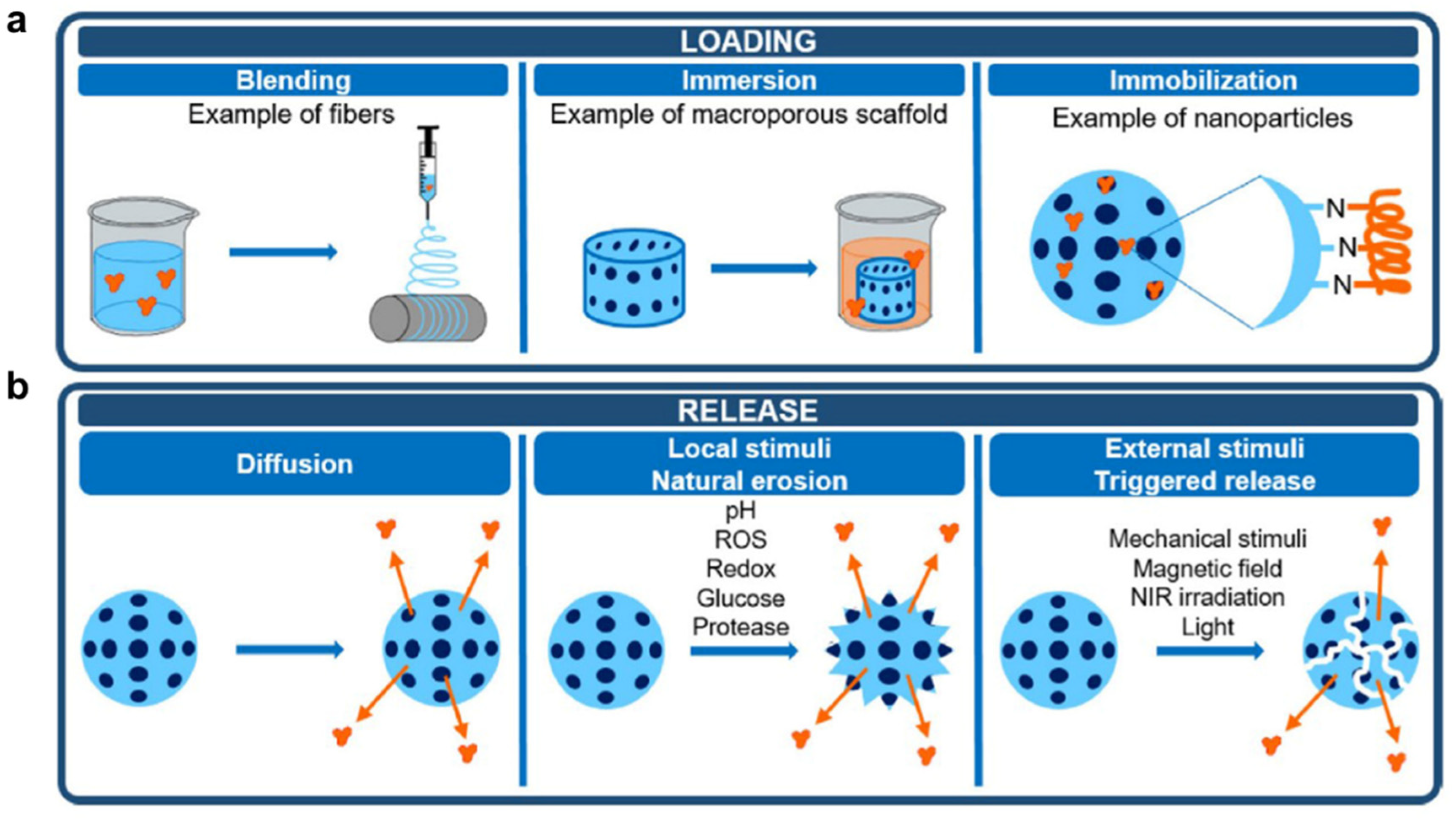
2.4. Consideration in the Design Process
3. Applications for Hybrid Polymer-Protein Systems in Cancer Therapy
3.1. Targeted Treatment
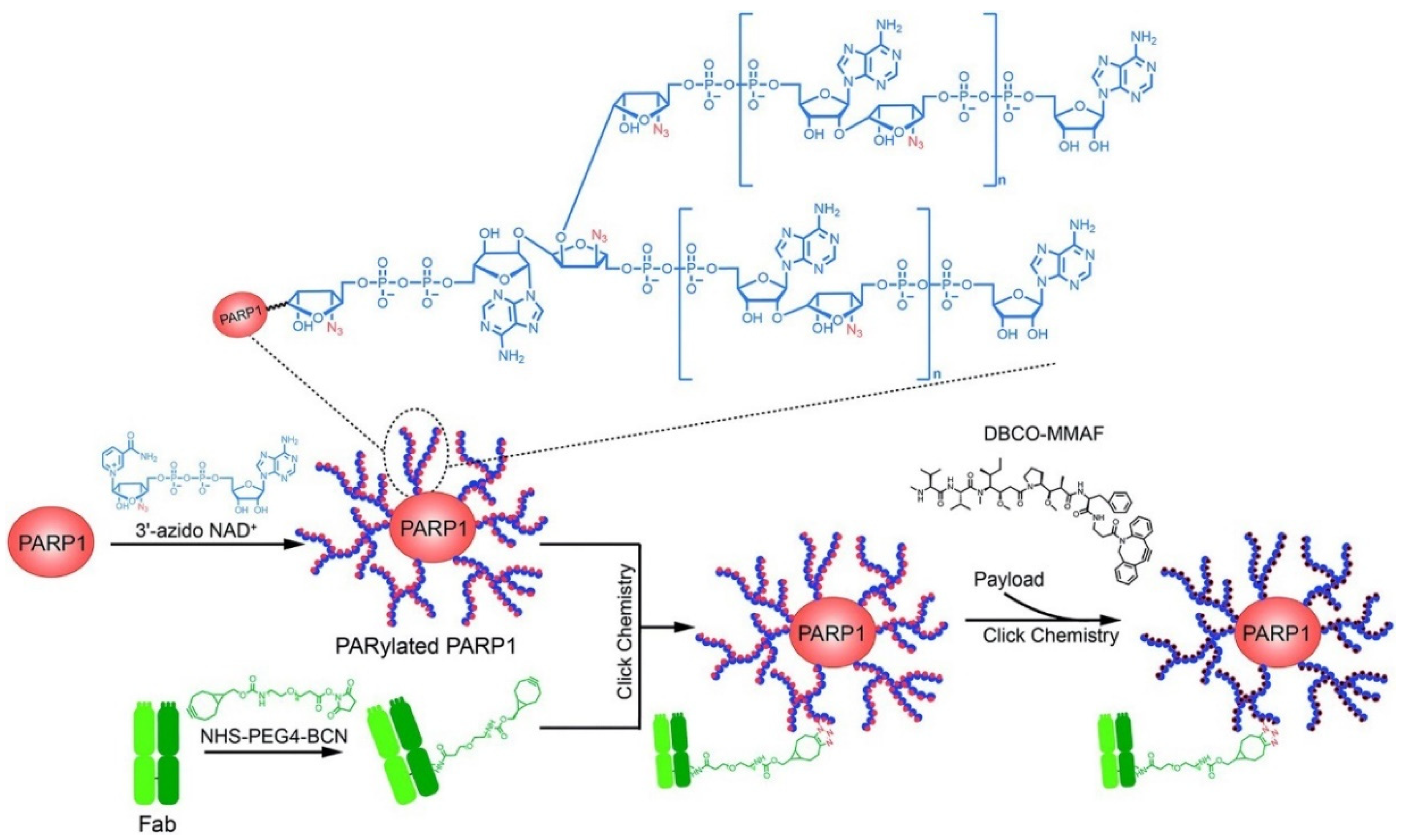
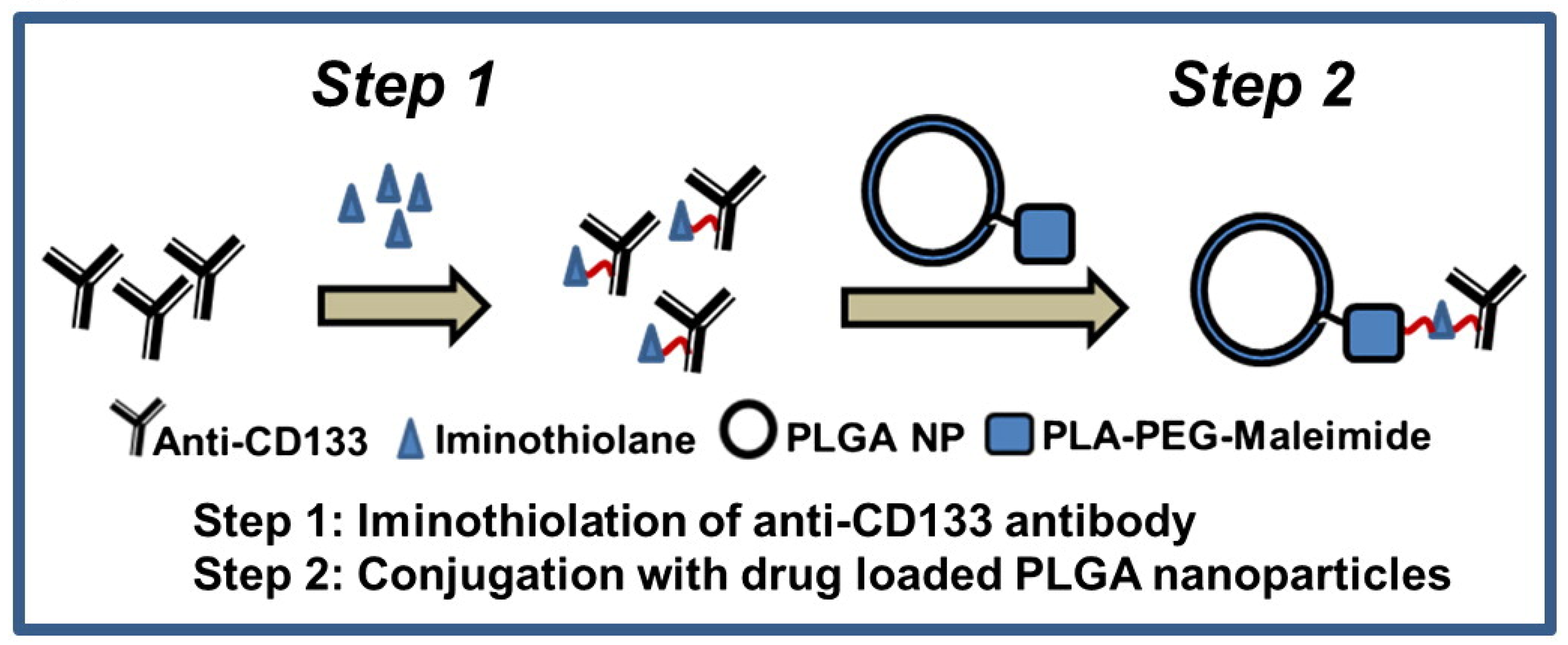
3.1.1. Tumor Cells Targeted
3.1.2. Tumor Stem Cells Targeted
3.1.3. Tumor Microenvironment (TME) Targeted
3.2. Gene Therapy
3.3. Phototherapy
3.4. Immunotherapy
3.5. Vaccines
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Stie, M.B.; Kalouta, K.; Vetri, V.; Fodera, V. Protein materials as sustainable non- and minimally invasive strategies for biomedical applications. J. Control Release 2022, 344, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gulati, N.M.; Stewart, P.L.; Steinmetz, N.F. Bioinspired shielding strategies for nanoparticle drug delivery applications. Mol. Pharm. 2018, 15, 2900–2909. [Google Scholar] [CrossRef]
- Abuchowski, A.; Vanes, T.; Palczuk, N.C.; Davis, F.F. Alteration of immunological properties of bovine serum-albumin by covalent attachment of polyethylene-glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [CrossRef]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl-methacrylate with the carbon-tetrachloride dichlorotris(triphenylphosphine)ruthenium(Ii) methylaluminum bis(2,6-Di-Tert-Butylphenoxide) initiating system–possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Willis, F.; Pettengell, R. Pegfilgrastim. Expert Opin. Biol. Ther. 2002, 2, 985–992. [Google Scholar] [CrossRef]
- Patel, J.N.; Walko, C.M. Sylatron: A pegylated interferon for use in melanoma. Ann. Pharmacother. 2012, 46, 830–838. [Google Scholar] [CrossRef]
- Reinders, M.K.; Jansen, T.L. New advances in the treatment of gout: Review of pegloticase. Ther. Clin. Risk Manag. 2010, 6, 543–550. [Google Scholar] [CrossRef][Green Version]
- Jevsevar, S.; Kunstelj, M.; Porekar, V.G. PEGylation of therapeutic proteins. Biotechnol. J. 2010, 5, 113–128. [Google Scholar] [CrossRef][Green Version]
- Alconcel, S.N.S.; Baas, A.S.; Maynard, H.D. FDA-approved poly(ethylene glycol)-protein conjugate drugs. Polym. Chem. 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Kaushik, A.; Rubahn, H.G.; Mishra, Y.K.; Kim, H.J. Smart nanomaterials as the foundation of a combination approach for efficient cancer theranostics. Mater. Today Chem. 2022, 26, 101182. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Mistewicz, K.; Nowacki, B.; In-na, P.; Krushynska, A.; Mishra, Y.K.; Kim, H.J. A focused review on three-dimensional bioprinting technology for artificial organ fabrication. Biomater. Sci. 2022, 10, 5054–5080. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Klok, H.A. Polymer-protein conjugates: An enzymatic activity perspective. Polym. Chem. 2010, 1, 1352–1373. [Google Scholar] [CrossRef]
- Kiran, P.; Khan, A.; Neekhra, S.; Pallod, S.; Srivastava, R. Nanohybrids as protein-polymer conjugate multimodal therapeutics. Front. Med. Technol. 2021, 3, 676025. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Bailon, P.; Won, C.Y. PEG-modified biopharmaceuticals. Expert Opin. Drug. Deliv. 2009, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Dembek, C.; White, L.A.; Garrison, L.P. The cost offsets and cost-effectiveness associated with pegylated drugs: A review of the literature. Expert Rev. Pharmacoecon. Outcomes Res. 2012, 12, 775–793. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, J.; Rao, J.; Li, G.H.; Liu, Y.L.; Zhou, J. PEG modification enhances the in vivo stability of bioactive proteins immobilized on magnetic nanoparticles. Biotechnol. Lett. 2020, 42, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug. Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.U. Anaemia: The safety and efficacy of peginesatide in patients with CKD. Nat. Rev. Nephrol. 2013, 9, 192–193. [Google Scholar] [CrossRef]
- Mikhail, A. Profile of peginesatide and its potential for the treatment of anemia in adults with chronic kidney disease who are on dialysis. J. Blood Med. 2012, 3, 25–31. [Google Scholar] [CrossRef][Green Version]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug. Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, Y.; Li, C.; Tang, W.; Qian, J.; Wei, X.; Lu, W.; Ying, T.; Zhan, C. Anti-PEG scFv corona ameliorates accelerated blood clearance phenomenon of PEGylated nanomedicines. J. Control Release 2021, 330, 493–501. [Google Scholar] [CrossRef]
- Lipsky, P.E.; Calabrese, L.H.; Kavanaugh, A.; Sundy, J.S.; Wright, D.; Wolfson, M.; Becker, M.A. Pegloticase immunogenicity: The relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res. Ther. 2014, 16, R60. [Google Scholar] [CrossRef][Green Version]
- Ozer, I.; Kelly, G.; Gu, R.; Li, X.; Zakharov, N.; Sirohi, P.; Nair, S.K.; Collier, J.H.; Hershfield, M.S.; Hucknall, A.M.; et al. Polyethylene glycol-like brush polymer conjugate of a protein drug does not induce an antipolymer immune response and has enhanced pharmacokinetics than its polyethylene glycol counterpart. Adv. Sci. 2022, 9, e2103672. [Google Scholar] [CrossRef]
- Ozer, I.; Pitoc, G.A.; Layzer, J.M.; Moreno, A.; Olson, L.B.; Layzer, K.D.; Hucknall, A.M.; Sullenger, B.A.; Chilkoti, A. PEG-like brush polymer conjugate of RNA aptamer that shows reversible anticoagulant activity and minimal immune response. Adv. Mater. 2022, 34, e2107852. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Sahoo, R.K.; Nakhate, K.T.; Ajazuddin; Gupta, U. Biotinylated HPMA centered polymeric nanoparticles for Bortezomib delivery. Int. J. Pharm. 2020, 579, 119173. [Google Scholar] [CrossRef] [PubMed]
- Subasic, C.N.; Ardana, A.; Chan, L.J.; Huang, F.; Scoble, J.A.; Butcher, N.J.; Meagher, L.; Chiefari, J.; Kaminskas, L.M.; Williams, C.C. Poly(HPMA-co-NIPAM) copolymer as an alternative to polyethylene glycol-based pharmacokinetic modulation of therapeutic proteins. Int. J. Pharm. 2021, 608, 121075. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yuan, Z.; Hung, H.C.; Ma, J.; Jain, P.; Tsao, C.; Xie, J.; Zhang, P.; Lin, X.; Wu, K.; et al. Revealing the Immunogenic Risk of Polymers. Angew. Chem. Int. Ed. Engl. 2018, 57, 13873–13876. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, M.T.; Dragulska, S.A.; Chen, Y.; Poursharifi, M.; Santiago, M.A.; Martignetti, J.A.; Mieszawska, A.J. Pt(II)-PLGA Hybrid in a pH-Responsive Nanoparticle System Targeting Ovarian Cancer. Pharmaceutics 2023, 15, 607. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Wood, J.; Tran, B.; Patapoff, T.W.; Nivaggioli, T. Trehalose limits BSA aggregation in spray-dried formulations at high temperatures: Implications in preparing polymer implants for long-term protein delivery. J. Pharm. Sci. 2013, 102, 2655–2666. [Google Scholar] [CrossRef]
- Sharp, D.M.; Picken, A.; Morris, T.J.; Hewitt, C.J.; Coopman, K.; Slater, N.K. Amphipathic polymer-mediated uptake of trehalose for dimethyl sulfoxide-free human cell cryopreservation. Cryobiology 2013, 67, 305–311. [Google Scholar] [CrossRef][Green Version]
- Olsson, C.; Jansson, H.; Swenson, J. The role of trehalose for the stabilization of proteins. J. Phys. Chem. B 2016, 120, 4723–4731. [Google Scholar] [CrossRef]
- Diaz-Dussan, D.; Peng, Y.Y.; Sengupta, J.; Zabludowski, R.; Adam, M.K.; Acker, J.P.; Ben, R.N.; Kumar, P.; Narain, R. Trehalose-Based polyethers for cryopreservation and three-dimensional cell scaffolds. Biomacromolecules 2020, 21, 1264–1273. [Google Scholar] [CrossRef]
- Krywko-Cendrowska, A.; di Leone, S.; Bina, M.; Yorulmaz-Avsar, S.; Palivan, C.G.; Meier, W. Recent Advances in Hybrid Biomimetic Polymer-Based Films: From Assembly to Applications. Polymers 2020, 12, 1003. [Google Scholar] [CrossRef]
- Staat, R.H.; Gawronski, T.H.; Schachtele, C.F. Detection and preliminary studies on dextranase-producing microorganisms from human dental plaque. Infect. Immun. 1973, 8, 1009–1016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, L.; Liu, M.; Wang, J.; Zhai, G. Chondroitin sulfate-based nanocarriers for drug/gene delivery. Carbohydr. Polym. 2015, 133, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.B.V.; Yoshida, C.M.P.; Pereira, S.; Goycoolea, F.M.; Franco, T.T. Electrostatic Self-Assembled Chitosan-Pectin Nano- and Microparticles for Insulin Delivery. Molecules 2017, 22, 1707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turecek, P.L.; Siekmann, J. PEG–Protein Conjugates; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–101. [Google Scholar] [CrossRef]
- Mauzac, M.; Aubert, N.; Jozefonvicz, J. Antithrombic activity of some polysaccharide resins. Biomaterials 1982, 3, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Paluck, S.J.; McGahran, A.J.; Maynard, H.D. Poly(vinyl sulfonate) Facilitates bFGF-Induced Cell Proliferation. Biomacromolecules 2015, 16, 2684–2692. [Google Scholar] [CrossRef][Green Version]
- Paluck, S.J.; Nguyen, T.H.; Lee, J.P.; Maynard, H.D. A Heparin-mimicking block copolymer both stabilizes and increases the activity of fibroblast growth factor 2 (FGF2). Biomacromolecules 2016, 17, 3386–3395. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shudo, H.; Seto, H.; Hoshino, Y.; Miura, Y. Syntheses of sulfated glycopolymers and analyses of their BACE-1 inhibitory activity. Bioorg. Med. Chem. Lett. 2013, 23, 6390–6395. [Google Scholar] [CrossRef]
- Liu, S.; Yan, T.; Sun, J.; Li, F.; Xu, J.; Sun, H.; Yu, S.; Liu, J. Biomimetic cascade polymer nanoreactors for starvation and photodynamic cancer therapy. Molecules 2021, 26, 5609. [Google Scholar] [CrossRef]
- Shastri, V.P. Non-degradable biocompatible polymers in medicine: Past, present and future. Curr. Pharm. Biotechnol. 2003, 4, 331–337. [Google Scholar] [CrossRef][Green Version]
- Hill, M.R.; Guegain, E.; Tran, J.; Figg, C.A.; Turner, A.C.; Nicolas, J.; Sumerlin, B.S. Radical ring-opening copolymerization of cyclic ketene acetals and maleimides affords homogeneous incorporation of degradable units. ACS Macro Lett. 2017, 6, 1071–1077. [Google Scholar] [CrossRef]
- Gigmes, D.; Van Steenberge, P.H.M.; Siri, D.; D’Hooge, D.R.; Guillaneuf, Y.; Lefay, C. Simulation of the degradation of cyclic ketene acetal and vinyl-based copolymers synthesized via a radical process: Influence of the reactivity ratios on the degradability properties. Macromol. Rapid Commun. 2018, 39, e1800193. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.B.; Davidson, M.G.; Edler, K.J.; Brown, S. Synthesis, properties, and applications of bio-based cyclic aliphatic polyesters. Biomacromolecules 2021, 22, 3649–3667. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, R.P.; Dove, A.P. Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 2016, 5, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Rubin, G.M.; Dhakal, D.; Chen, M.; Ding, Y. Biocatalytic synthesis of peptidic natural products and related analogues. iScience 2021, 24, 102512. [Google Scholar] [CrossRef]
- Noga, M.; Edinger, D.; Wagner, E.; Winter, G.; Besheer, A. Characterization and compatibility of hydroxyethyl starch-polyethylenimine copolymers for DNA delivery. J. Biomater. Sci. Polym. Ed. 2014, 25, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control Release 2014, 190, 465–476. [Google Scholar] [CrossRef][Green Version]
- Paleos, C.M.; Sideratou, Z.; Tsiourvas, D. Drug delivery systems based on hydroxyethyl starch. Bioconjug. Chem. 2017, 28, 1611–1624. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Tang, Y.; Zhang, Y.; Zhang, Y.; Yin, T.; Xu, H.; Cai, C.; Tang, X. Hydroxyethyl starch conjugates for improving the stability, pharmacokinetic behavior and antitumor activity of 10-hydroxy camptothecin. Int. J. Pharm. 2014, 471, 234–244. [Google Scholar] [CrossRef]
- Wang, H.; Hu, H.; Yang, H.; Li, Z. Hydroxyethyl starch based smart nanomedicine. RSC Adv. 2021, 11, 3226–3240. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Dharmasiri, M.B.; Mudiyanselage, T.K. Thermo-responsive poly(N-isopropyl acrylamide) hydrogel with increased response rate. Polym. Bull 2021, 78, 3183–3198. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter. 2016, 12, 2542–2549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyaci, T.; Orakdogen, N. pH-responsive poly(N,N-dimethylaminoethyl methacrylate-co-2-acrylamido-2-methyl-propanosulfonic acid) cryogels: Swelling, elasticity and diffusive properties. RSC Adv. 2015, 5, 77235–77247. [Google Scholar] [CrossRef]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Hayashi, K.; Matsuda, M.; Nakahata, M.; Takashima, Y.; Tanaka, M. Stimulus-Responsive, Gelatin-Containing Supramolecular Nanofibers as Switchable 3D Microenvironments for Cells. Polymers 2022, 14, 4407. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.H.; Arboleda, C.; Stubelius, A.; Li, L.W.; Olejniczak, J.; Almutairi, A. Highly responsive and rapid hydrogen peroxide-triggered degradation of polycaprolactone nanoparticles. Biomater. Sci. 2020, 8, 2394–2397. [Google Scholar] [CrossRef]
- Chen, A.; Chen, J.; Wang, D.; Xu, J.; Zeng, H. CO2/N2-responsive oil-in-water emulsions using a novel switchable surfactant. J. Colloid Interface Sci. 2020, 571, 134–141. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, G.; Liu, S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012, 41, 5933–5949. [Google Scholar] [CrossRef]
- Chen, F.; Ren, Y.; Guo, J.; Yan, F. Thermo- and electro-dual responsive poly(ionic liquid) electrolyte based smart windows. Chem. Commun. 2017, 53, 1595–1598. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertrand, O.; Gohy, J.F. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2017, 8, 52–73. [Google Scholar] [CrossRef]
- Qi, Y.; Amiram, M.; Gao, W.; McCafferty, D.G.; Chilkoti, A. Sortase-catalyzed initiator attachment enables high yield growth of a stealth polymer from the C terminus of a protein. Macromol. Rapid. Commun. 2013, 34, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Carmali, S.; Murata, H.; Matyjaszewski, K.; Russell, A.J. Tailoring Site Specificity of Bioconjugation Using Step-Wise Atom-Transfer Radical Polymerization on Proteins. Biomacromolecules 2018, 19, 4044–4051. [Google Scholar] [CrossRef] [PubMed]
- Kuan, S.L.; Wang, T.; Weil, T. Site-Selective Disulfide Modification of Proteins: Expanding Diversity beyond the Proteome. Chemistry 2016, 22, 17112–17129. [Google Scholar] [CrossRef] [PubMed]
- Pelegri-O’Day, E.M.; Lin, E.W.; Maynard, H.D. Therapeutic protein-polymer conjugates: Advancing beyond PEGylation. J. Am. Chem. Soc. 2014, 136, 14323–14332. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Ng, D.Y.W.; Weil, T. Polymer bioconjugates: Modern design concepts toward precision hybrid materials. Prog. Polym. Sci. 2020, 105, 101241. [Google Scholar] [CrossRef]
- Sereda, T.J.; Mant, C.T.; Quinn, A.M.; Hodges, R.S. Effect of Alpha-Amino Group on Peptide Retention Behavior in Reversed-Phase Chromatography–Determination of the Pk(a) Values of the Alpha-Amino Group of 19 Different N-Terminal Amino-Acid-Residues. J. Chromatogr. 1993, 646, 17–30. [Google Scholar] [CrossRef]
- Ward, C.M.; Seymour, L.W. Conjugation of NHS-folate to preformed poly(L-lysine)/DNA complexes for specific receptor mediated uptake into KB cells. Hum. Gene Ther. 1999, 10, 853. [Google Scholar]
- Usha, R.; Sreeram, K.J.; Rajaram, A. Stabilization of collagen with EDC/NHS in the presence of L-lysine: A comprehensive study. Colloid Surface B 2012, 90, 83–90. [Google Scholar] [CrossRef]
- Kikuchi, S.; Kanoh, D.; Sato, S.; Sakurai, Y.; Suzuki, M.; Nakamura, H. Maleimide-functionalized closo-dodecaborate albumin conjugates (MID-AC): Unique ligation at cysteine and lysine residues enables efficient boron delivery to tumor for neutron capture therapy. J. Control Release 2016, 237, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Struck, R.F.; Roychowdhury, A.; Maddry, J.A.; Waud, W.R. Development and anti-cancer testing of halogenated analogues of isophosphoramide mustard-lysine (IPM-L; ZIO-201), ZIO-202 and ZIO-203. Cancer Res. 2006, 66, 130. [Google Scholar]
- Mitsiogianni, M.; Mantso, T.; Trafalis, D.T.; Rupasinghe, H.P.V.; Zoumpourlis, V.; Franco, R.; Botaitis, S.; Pappa, A.; Panayiotidis, M.I. Allyl isothiocyanate regulates lysine acetylation and methylation marks in an experimental model of malignant melanoma. Eur. J. Nutr. 2020, 59, 557–569. [Google Scholar] [CrossRef][Green Version]
- Aly, A.M.; Hoyer, L.W. The Substitution of Cysteine for Arginine-1689 in Factor-Viii-East-Hartford Reduces Its Procoagulant Activity through Formation of a Disulfide Bond. Clin. Res. 1990, 38, A427. [Google Scholar]
- Takeo, T.; Horikoshi, Y.; Nakao, S.; Sakoh, K.; Ishizuka, Y.; Tsutsumi, A.; Fukumoto, K.; Kondo, T.; Haruguchi, Y.; Takeshita, Y.; et al. Cysteine Analogs with a Free Thiol Group Promote Fertilization by Reducing Disulfide Bonds in the Zona Pellucida of Mice. Biol. Reprod. 2015, 92, 90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, M.; Lau, T.Y.; Carr, S.A.; Krieger, M. Contributions of a Disulfide Bond and a Reduced Cysteine Side Chain to the Intrinsic Activity of the High-Density Lipoprotein Receptor SR-BI. Biochemistry 2012, 51, 10044–10055. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calce, E.; De Luca, S. The Cysteine S-Alkylation Reaction as a Synthetic Method to Covalently Modify Peptide Sequences. Chem. -Eur. J. 2017, 23, 224–233. [Google Scholar] [CrossRef]
- Mason, A.F.; Thordarson, P. Synthesis of Protein Bioconjugates via Cysteine-maleimide Chemistry. JoVE-J. Vis. Exp. 2016, 113, e54157. [Google Scholar]
- Zhou, J.R.; Chen, P.P.; Deng, C.; Meng, F.H.; Cheng, R.; Zhong, Z.Y. A Simple and Versatile Synthetic Strategy to Functional Polypeptides via Vinyl Sulfone-Substituted L-Cysteine N-Carboxyanhydride. Macromolecules 2013, 46, 6723–6730. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, R.R.; Jiao, Y.F.; Sun, Y.F.; Shen, S.; Wang, Y.J.; Lu, D.R.; Jiang, X.G.; Yang, W.L. Redox stimuli-responsive hollow mesoporous silica nanocarriers for targeted drug delivery in cancer therapy. Nanoscale Horiz. 2016, 1, 480–487. [Google Scholar] [CrossRef]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.D.; Lu, D.C.; You, R.Y.; Liu, J.L.; Huang, L.Q.; Su, J.Q.; Feng, S.Y. Diazotization-Coupling Reaction-Based Determination of Tyrosine in Urine Using Ag Nanocubes by Surface-Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ban, H.; Nagano, M.; Gavrilyuk, J.; Hakamata, W.; Inokuma, T.; Barbas, C.F. Facile and Stabile Linkages through Tyrosine: Bioconjugation Strategies with the Tyrosine-Click Reaction. Bioconjug. Chem. 2013, 24, 520–532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fancy, D.A.; Melcher, K.; Johnston, S.A.; Kodadek, T. New chemistry for the study of multiprotein complexes: The six-histidine tag as a receptor for a protein crosslinking reagent. Chem. Biol. 1996, 3, 551–559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, H.; Hayashi, E.; Yamada, N.; Yatagai, M.; Takahara, Y. Further studies on the site-specific protein modification by microbial transglutaminase. Bioconjug. Chem. 2001, 12, 701–710. [Google Scholar] [CrossRef]
- Carrico, I.S.; Carlson, B.L.; Bertozzi, C.R. Introducing genetically encoded aldehydes into proteins. Nat. Chem. Biol. 2007, 3, 321–322. [Google Scholar] [CrossRef]
- Broyer, R.M.; Grover, G.N.; Maynard, H.D. Emerging synthetic approaches for protein-polymer conjugations. Chem. Commun. 2011, 47, 2212–2226. [Google Scholar] [CrossRef][Green Version]
- Nicolas, J.; Mantovani, G.; Haddleton, D.M. Living radical polymerization as a tool for the synthesis of polymer-protein/peptide bioconjugates. Macromol. Rapid. Commun. 2007, 28, 1083–1111. [Google Scholar] [CrossRef]
- Li, M.; Li, H.M.; De, P.; Sumerlin, B.S. Thermoresponsive Block Copolymer-Protein Conjugates Prepared by Grafting-from via RAFT Polymerization. Macromol. Rapid. Commun. 2011, 32, 354–359. [Google Scholar] [CrossRef]
- Foster, J.C.; Radzinski, S.C.; Matson, J.B. Graft Polymer Synthesis by RAFT Transfer-To. J. Polym. Sci. Pol. Chem. 2017, 55, 2865–2876. [Google Scholar] [CrossRef][Green Version]
- Boyer, C.; Bulmus, V.; Liu, J.Q.; Davis, T.P.; Stenzel, M.H.; Barner-Kowollik, C. Well-defined protein-polymer conjugates via in situ RAFT polymerization. J. Am. Chem. Soc. 2007, 129, 7145–7154. [Google Scholar] [CrossRef] [PubMed]
- Vanparijs, N.; Maji, S.; Louage, B.; Voorhaar, L.; Laplace, D.; Zhang, Q.; Shi, Y.; Hennink, W.E.; Hoogenboom, R.; De Geest, B.G. Polymer-protein conjugation via a ’grafting to’ approach–a comparative study of the performance of protein-reactive RAFT chain transfer agents. Polym. Chem. 2015, 6, 5798. [Google Scholar] [CrossRef][Green Version]
- Gieseler, D.; Jordan, R. Poly(2-oxazoline) molecular brushes by grafting through of poly(2-oxazoline)methacrylates with aqueous ATRP. Polym. Chem. 2015, 6, 4678–4689. [Google Scholar] [CrossRef][Green Version]
- Horbett, T.A. Proteins at interfaces––An overview. ACS Sym. Ser. 1995, 602, 1–23. [Google Scholar]
- Cao, L.M.; Shi, X.J.; Cui, Y.C.; Yang, W.K.; Chen, G.J.; Yuan, L.; Chen, H. Protein-polymer conjugates prepared via host-guest interactions: Effects of the conjugation site, polymer type and molecular weight on protein activity. Polym. Chem. 2016, 7, 5139–5146. [Google Scholar] [CrossRef]
- Kim, J. Systematic approach to characterize the dynamics of protein adsorption on the surface of biomaterials using proteomics. Colloid Surface B 2020, 188, 110756. [Google Scholar] [CrossRef]
- Tan, S.N.; Saito, K.; Hearn, M.T.W. Adsorption of a Humanized Monoclonal Antibody onto Thermoresponsive Copolymer-Grafted Sepharose Fast Flow Sorbents. Langmuir 2021, 37, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.S.; Bae, Y.H.; Cremers, H.; Feijen, J.; Kim, S.W. Release of Proteins Via Ion-Exchange from Albumin-Heparin Microspheres. J. Control Release 1992, 22, 83–93. [Google Scholar] [CrossRef][Green Version]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Froehlich, E.; Mandeville, J.S.; Tajmir-Riahi, H.A. Protein conjugation with PAMAM nanoparticles: Microscopic and thermodynamic analysis. Colloid Surface B 2017, 150, 168–174. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Liu, F.; Ma, X.T.; Yang, X.L.; Zhao, H.Y. Hydrophobic Interaction-Induced Coassembly of Homopolymers and Proteins. Langmuir 2019, 35, 10958–10964. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Lin, C.; Hu, X.Y.; Wang, L.Y. GSH- and pH-responsive drug delivery system constructed by water-soluble pillar [5] arene and lysine derivative for controllable drug release. Chem. Commun. 2015, 51, 6832–6835. [Google Scholar] [CrossRef] [PubMed]
- Bizeau, J.; Mertz, D. Design and applications of protein delivery systems in nanomedicine and tissue engineering. Adv. Colloid. Interface Sci. 2021, 287, 102334. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Kapoor, S.; Vali, S.; Senthil, V.; Nithya, D.; Venkataramanan, R.; Sharma, A.; Talwadkar, A.; Ray, A.; Bhatnagar, P.K.; et al. Dual epidermal growth factor receptor (EGFR)/insulin-like growth factor-1 receptor (IGF-1R) inhibitor: A novel approach for overcoming resistance in anticancer treatment. Eur. J. Pharmacol. 2011, 667, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, B.; Bizzoni, C.; Jansen, G.; Leamon, C.P.; Peters, G.J.; Low, P.S.; Matherly, L.H.; Figini, M. Folate receptors and transporters: Biological role and diagnostic/therapeutic targets in cancer and other diseases. J. Exp. Clin. Canc. Res. 2019, 38, 125. [Google Scholar] [CrossRef][Green Version]
- Jeon, Y.T.; Kim, Y.B.; Park, S.Y.; Kim, J.W.; Park, N.H.; Kang, S.B.; Song, Y.S. Gonadotropin-releasing hormone receptor expression in endometrial cancer. Int. J. Gynecol. Pathol. 2009, 28, 19–22. [Google Scholar] [CrossRef][Green Version]
- Shi, X.; Zhang, X.N.; Chen, J.; Cheng, Q.; Pei, H.; Louie, S.G.; Zhang, Y. A poly-ADP-ribose polymer-based antibody-drug conjugate. Chem. Sci. 2020, 11, 9303–9308. [Google Scholar] [CrossRef]
- Swaminathan, S.K.; Roger, E.; Toti, U.; Niu, L.; Ohlfest, J.R.; Panyam, J. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J. Control Release 2013, 171, 280–287. [Google Scholar] [CrossRef][Green Version]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery–New applications on the horizon. J. Control Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, W.P. In Situ Growth of Self-Assembled Protein-Polymer Nanovesicles for Enhanced Intracellular Protein Delivery. ACS Appl. Mater Inter. 2017, 9, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Du, Y.T.; Sun, Q.; Peng, Y.W.; Wang, R.D.; Zhou, Y.; Wang, Y.Q.; Zhang, C.L.; Qi, X.R. Albumin-Based Nanotheranostic Probe with Hypoxia Alleviating Potentiates Synchronous Multimodal Imaging and Phototherapy for Glioma. ACS Nano 2020, 14, 6191–6212. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, A.; Raffa, V.; Canfarotta, F.; Signore, G.; Piletsky, S.; MacDonald, M.P.; Cuschieri, A. In Vivo Recognition of Human Vascular Endothelial Growth Factor by Molecularly Imprinted Polymers. Nano Lett. 2017, 17, 2307–2312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Feng, J.; Xu, M.; Wang, J.; Zhou, S.; Liu, Y.; Liu, S.; Huang, Y.; Chen, Y.; Chen, L.; Song, Q.; et al. Sequential delivery of nanoformulated alpha-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials 2020, 241, 119907. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Kang, T.; Wang, J.; Yang, G.; Yang, Y.; Lin, X.; Wang, L.; Li, K.; Liu, J.; et al. NIR-II Absorbing Semiconducting Polymer-Triggered Gene-Directed Enzyme Prodrug Therapy for Cancer Treatment. Small 2021, 17, e2100501. [Google Scholar] [CrossRef]
- Price, R.; Poursaid, A.; Cappello, J.; Ghandehari, H. In vivo evaluation of matrix metalloproteinase responsive silk-elastinlike protein polymers for cancer gene therapy. J. Control Release 2015, 213, 96–102. [Google Scholar] [CrossRef][Green Version]
- Weyermann, J.; Lochmann, D.; Georgens, C.; Zimmer, A. Albumin-protamine-oligonucleotide-nanoparticles as a new antisense delivery system. Part 2: Cellular uptake and effect. Eur. J. Pharm. Biopharm. 2005, 59, 431–438. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control Release 2020, 325, 52–71. [Google Scholar] [CrossRef]
- Gobo, N.R.S.; Brocksom, T.J.; Zukerman-Schpector, J.; de Oliveira, K.T. Synthesis of an Octa-tert-butylphthalocyanine: A Low-Aggregating and Photochemically Stable Photosensitizer. Eur. J. Org. Chem. 2013, 2013, 5028–5031. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Liu, Z.Y.; Wang, S.; Zheng, B.; Guo, W.S.; Yang, W.T.; Gong, X.Q.; Wu, X.L.; Wang, H.J.; Chang, J. A Protein-Polymer Bioconjugate-Coated Upconversion Nanosystem for Simultaneous Tumor Cell Imaging, Photodynamic Therapy, and Chemotherapy. ACS Appl. Mater. Inter. 2016, 8, 32688–32698. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Hu, D.H.; Zheng, M.B.; Zhao, P.F.; Liu, H.L.; Gao, D.Y.; Gong, P.; Gao, G.H.; Zhang, P.F.; Ma, Y.F.; et al. Smart Human Serum Albumin-Indocyanine Green Nanoparticles Generated by Programmed Assembly for Dual-Modal Imaging-Guided Cancer Synergistic Phototherapy. ACS Nano 2014, 8, 12310–12322. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, L.J.; Du, Z.; Ma, R.; Yan, T.; Alimu, G.; Zhang, X.L.; Alifu, N.; Ma, C.L. Polymer encapsulated clinical ICG nanoparticles for enhanced photothermal therapy and NIR fluorescence imaging in cervical cancer. RSC Adv. 2021, 11, 20850–20858. [Google Scholar] [CrossRef]
- Rong, P.F.; Huang, P.; Liu, Z.G.; Lin, J.; Jin, A.; Ma, Y.; Niu, G.; Yu, L.; Zeng, W.B.; Wang, W.; et al. Protein-based photothermal theranostics for imaging-guided cancer therapy. Nanoscale 2015, 7, 16330–16336. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Yang, J.; Luo, L.H.; Jiang, M.S.; Qin, B.; Yin, H.; Zhu, C.Q.; Yuan, X.L.; Zhang, J.L.; Luo, Z.Y.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef][Green Version]
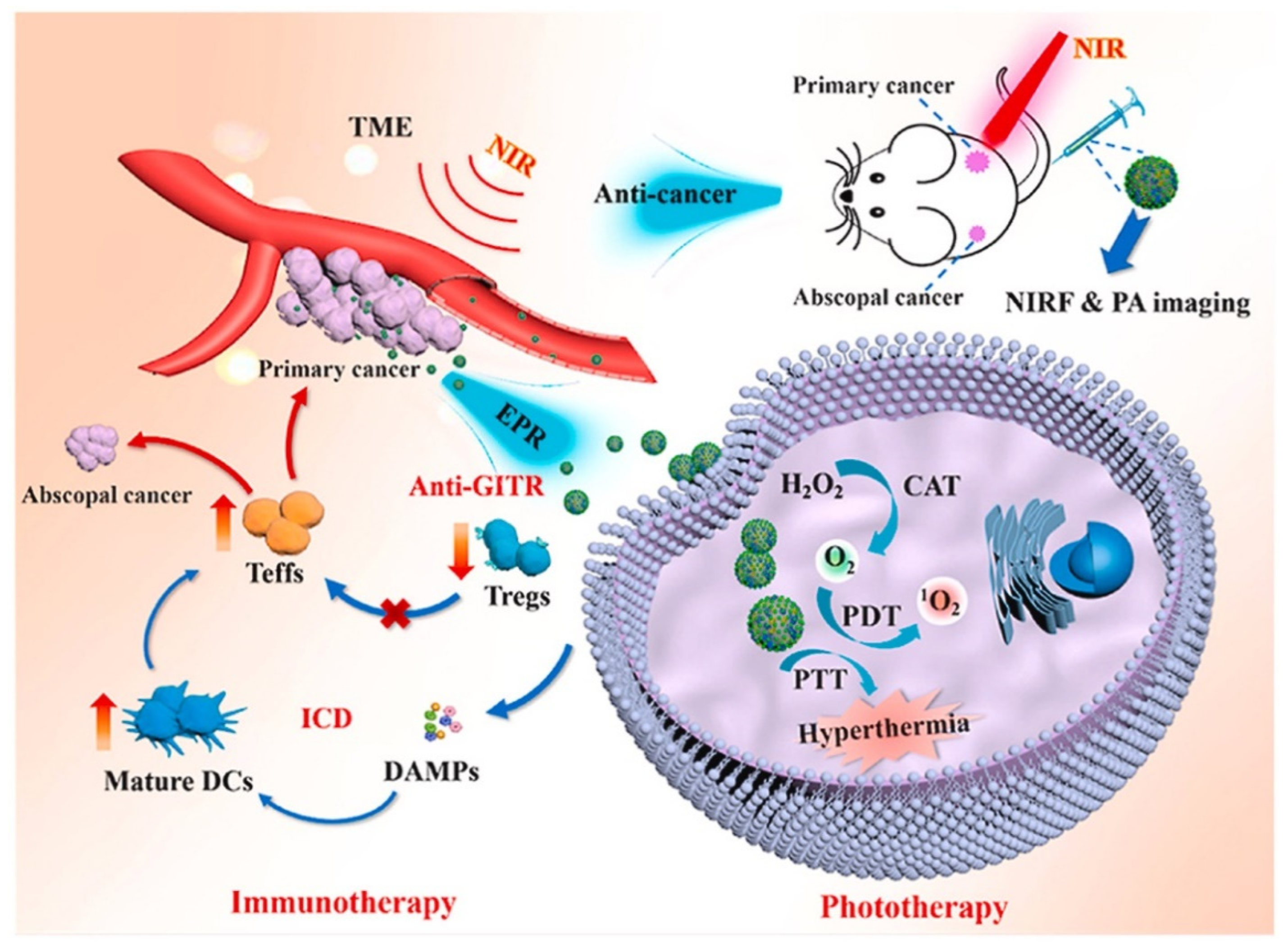
- Sun, Q.; Yang, Z.Z.; Lin, M.; Peng, Y.W.; Wang, R.D.; Du, Y.T.; Zhou, Y.; Li, J.J.; Qi, X.R. Phototherapy and anti-GITR antibody-based therapy synergistically reinvigorate immunogenic cell death and reject established cancers. Biomaterials 2021, 269, 120648. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.W.; Yang, Z.J.; Xu, J.; Xu, L.G.; Liang, C.; Han, X.; Liu, Z. Nanoparticle-Enhanced Radiotherapy to Trigger Robust Cancer Immunotherapy. Adv. Mater. 2019, 31, e1802228. [Google Scholar] [CrossRef]
- Zhang, R.; Song, X.J.; Liang, C.; Yi, X.; Song, G.S.; Chao, Y.; Yang, Y.; Yang, K.; Feng, L.Z.; Liu, Z. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials 2017, 138, 13–21. [Google Scholar] [CrossRef]
- Chen, L.; Chen, F.K.; Niu, H.T.; Li, J.D.; Pu, Y.Z.; Yang, C.H.; Wang, Y.; Huang, R.; Li, K.; Lei, Y.J.; et al. Chimeric Antigen Receptor (CAR)-T Cell Immunotherapy Against Thoracic Malignancies: Challenges and Opportunities. Front. Immunol. 2022, 13, 871661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.; Chan, S.L.; Choo, S.P.; Kudo, M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020, 72, 307–319. [Google Scholar] [CrossRef][Green Version]
- Lipe, D.N.; Shaffer, S. CAR-T and checkpoint inhibitors: Toxicities and antidotes in the emergency department. Clin. Toxicol. 2021, 59, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.G.; Zhao, Q. Nanomedicine-Combined Immunotherapy for Cancer. Curr. Med. Chem. 2020, 27, 5716–5729. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Ribas, A.; Hu-Lieskovan, S. Combining targeted therapy with immunotherapy. Can 1 + 1 equal more than 2? Semin. Immunol. 2016, 28, 73–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Charych, D.H.; Hoch, U.; Langowski, J.L.; Lee, S.R.; Addepalli, M.K.; Kirk, P.B.; Sheng, D.W.; Liu, X.F.; Sims, P.W.; VanderVeen, L.A.; et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin. Cancer Res. 2016, 22, 680–690. [Google Scholar] [CrossRef][Green Version]
- Javia, A.; Vanza, J.; Bardoliwala, D.; Ghosh, S.; Misra, L.A.; Patel, M.; Thakkar, H. Polymer-drug conjugates: Design principles, emerging synthetic strategies and clinical overview. Int. J. Pharm. 2022, 623, 121863. [Google Scholar] [CrossRef]
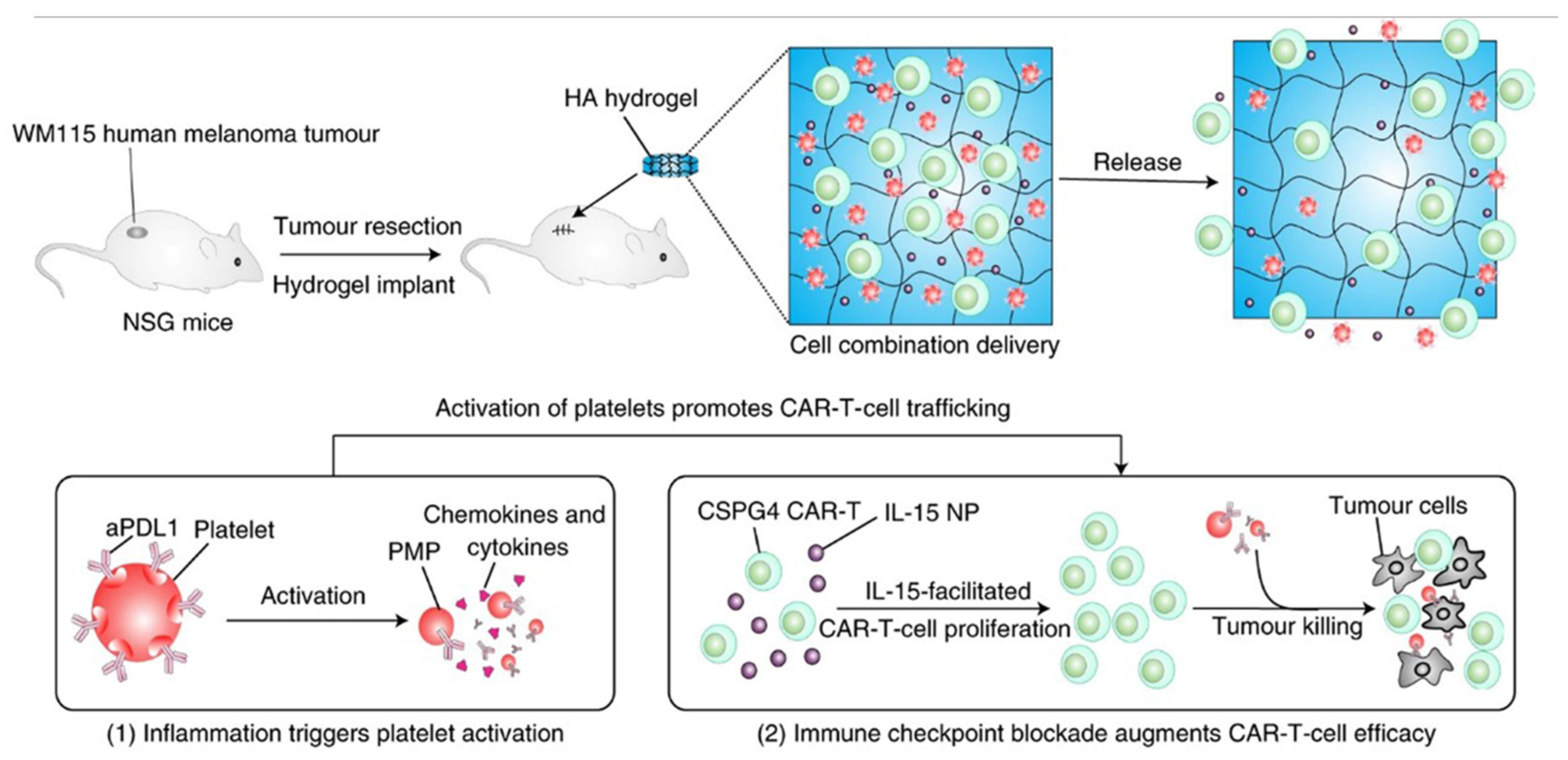
- Hu, Q.Y.; Li, H.J.; Archibong, E.; Chen, Q.; Ruan, H.T.; Ahn, S.; Dukhovlinova, E.; Kang, Y.; Wen, D.; Dotti, G.; et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat. Biomed. Eng. 2021, 5, 1038–1047. [Google Scholar] [CrossRef]
- Fang, T.; Li, R.; Li, Z.Y.; Cho, J.; Guzman, J.S.; Kamm, R.D.; Ploegh, H.L. Remodeling of the Tumor Microenvironment by a Chemokine/Anti-PD-L1 Nanobody Fusion Protein. Mol. Pharmaceut. 2019, 16, 2838–2844. [Google Scholar] [CrossRef]
- Neek, M.; Kim, T.I.; Wang, S.W. Protein-based nanoparticles in cancer vaccine development. Nanomed.-Nanotechnol. 2019, 15, 164–174. [Google Scholar] [CrossRef]
- Lybaert, L.; Vanparijs, N.; Fierens, K.; Schuijs, M.; Nuhn, L.; Lambrecht, B.N.; De Geest, B.G. A Generic Polymer-Protein Ligation Strategy for Vaccine Delivery. Biomacromolecules 2016, 17, 874–881. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pan, C.; Wu, J.; Qing, S.; Zhang, X.; Zhang, L.L.; Yue, H.; Zeng, M.; Wang, B.; Yuan, Z.; Qiu, Y.F.; et al. Biosynthesis of Self-Assembled Proteinaceous Nanoparticles for Vaccination. Adv. Mater. 2020, 32, e2002940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Fei, Z.; Dai, H.; Fan, Q.; Yang, Q.; Chen, Y.; Wang, B.; Wang, C. 3D Printing Scaffold Vaccine for Antitumor Immunity. Adv. Mater. 2021, 33, e2106768. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Hu, W.W.; Yan, Y.N.; Zhang, Z.C.; Chen, Y.X.; Yao, X.F.; Teng, L.; Wang, X.Y.; Chai, D.F.; Zheng, J.N.; et al. Using PAMPs and DAMPs as adjuvants in cancer vaccines. Hum. Vaccines Immunother. 2021, 17, 5546–5557. [Google Scholar] [CrossRef]
- Huang, L.X.; Rong, Y.; Tang, X.; Yi, K.Z.; Qi, P.; Hou, J.X.; Liu, W.H.; He, Y.; Gao, X.; Yuan, C.H.; et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Zhu, S.; Gu, Z.J.; Chen, C.Y.; Zhao, Y.L. Toxicity of manufactured nanomaterials. Particuology 2022, 69, 31–48. [Google Scholar] [CrossRef]




| Category | Example | References |
|---|---|---|
| Covalent conjugation | Amide bond | [80,81] |
| Thiourea bond | [84] | |
| Thioether bond | [89] | |
| Disulfide bond | [91] | |
| Non-covalent conjugation | Electrostatic adsorption | [108,109] |
| Hydrogen bond | [111] | |
| Hydrophobic interaction | [112] | |
| Host–guest interaction | [113] |
| Category | Polymer | Protein | Reference |
|---|---|---|---|
| Targeted therapy | poly-ADP-ribose polymer | Anti-HER2 antibody | [118] |
| PLGA | anti-CD133 antibody | [119] | |
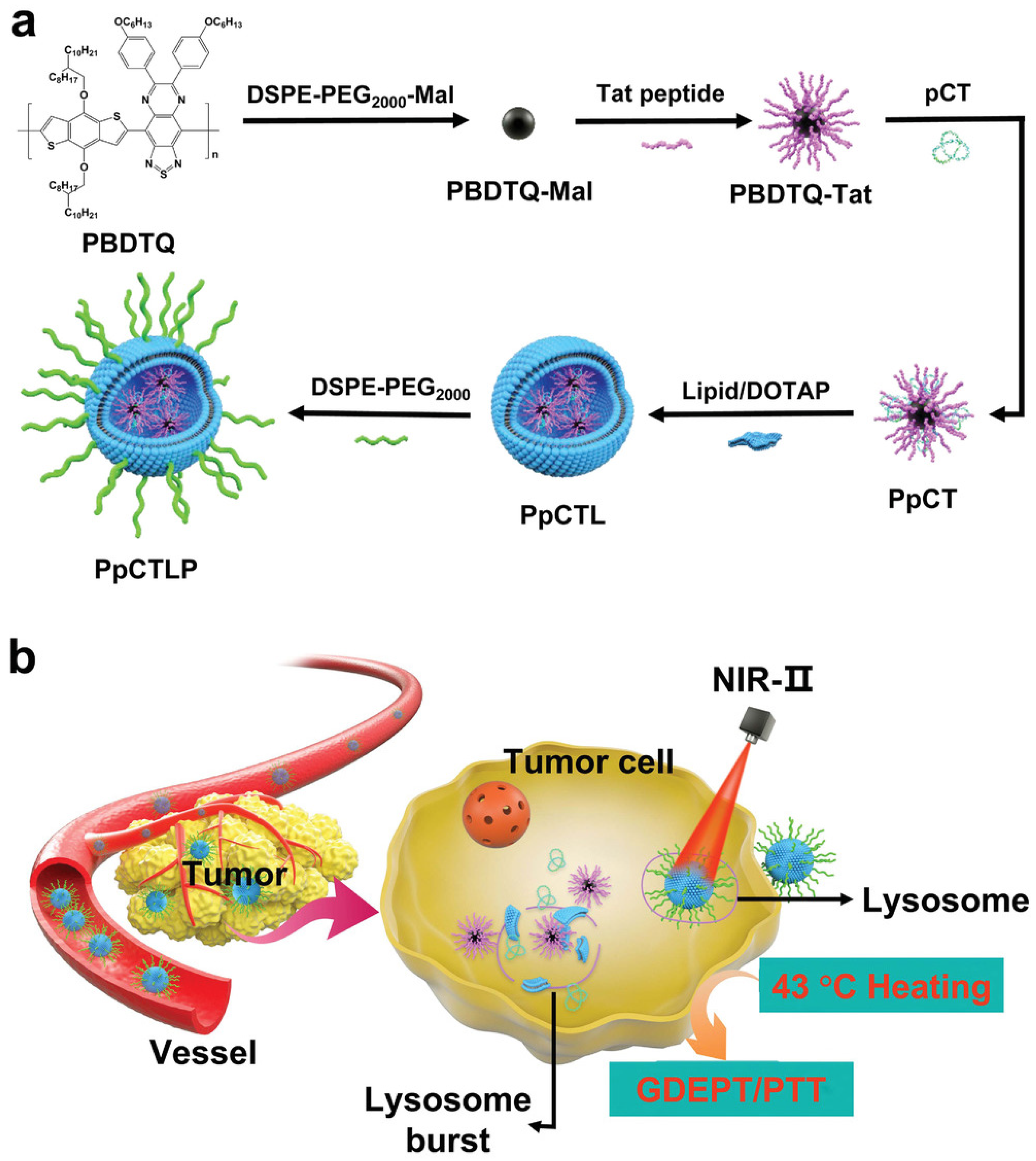
| Gene therapy | PBDTQ nanoparticles | Tat peptide, CD, TK | [127] |
| Silk-elastinlike protein polymers | MMP | [128] | |
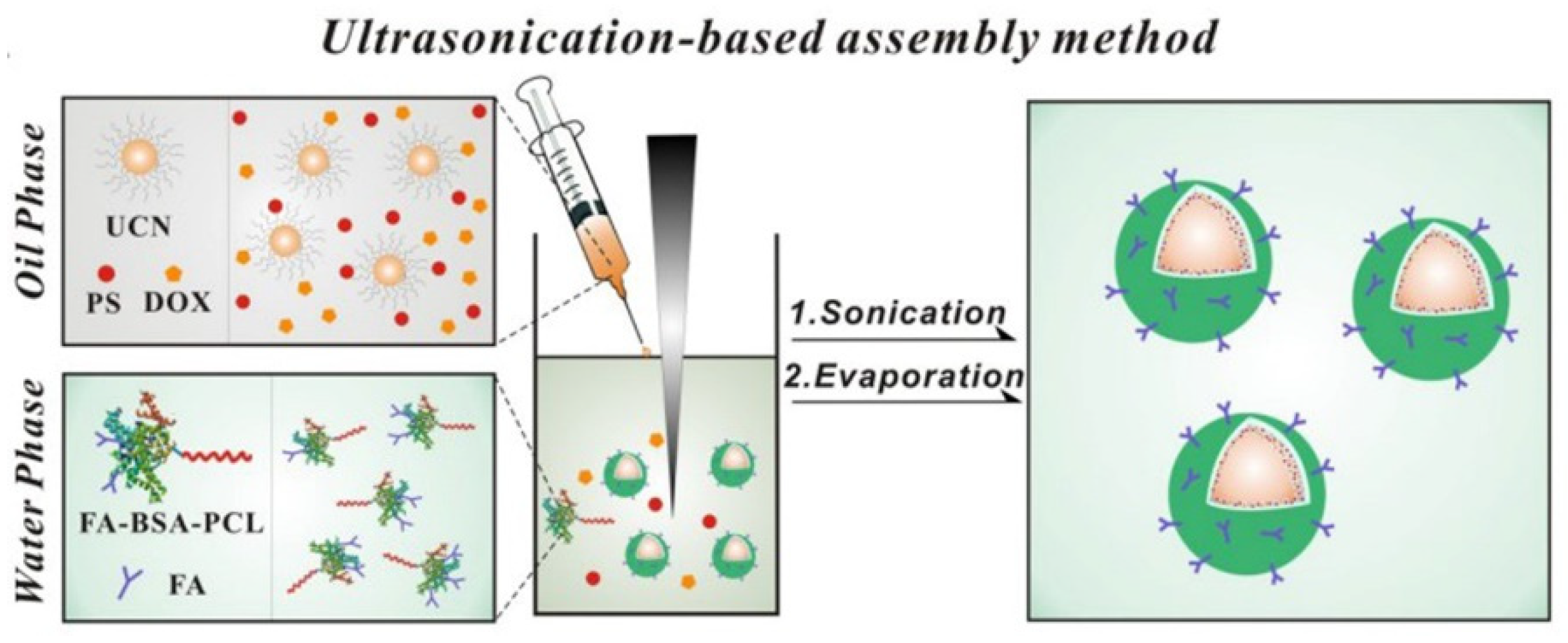
| Phototherapy | Upconversion nanoparticles | BSA | [135] |
| Gold nanospheres | Hb, pardaxin peptides | [139] | |
| PDA nanoparticles | CAT and DTA-1 | [140] | |
| Immunotherapy | PEG | IL-2 | [148] |
| Hyaluronic acid hydrogel | CSPG4, IL-15, aPDL1 | [150] | |
| Vaccines | Polymeric conjugates based on HPMA | OVA | [153] |
| 3D printed scaffolds | Antigen | [155] | |
| DC derived exosomes | Human neutrophil elastase, TLR3 agonist | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Yang, Z.; Qi, X. Design and Application of Hybrid Polymer-Protein Systems in Cancer Therapy. Polymers 2023, 15, 2219. https://doi.org/10.3390/polym15092219
Sun Q, Yang Z, Qi X. Design and Application of Hybrid Polymer-Protein Systems in Cancer Therapy. Polymers. 2023; 15(9):2219. https://doi.org/10.3390/polym15092219
Chicago/Turabian StyleSun, Qi, Zhenzhen Yang, and Xianrong Qi. 2023. "Design and Application of Hybrid Polymer-Protein Systems in Cancer Therapy" Polymers 15, no. 9: 2219. https://doi.org/10.3390/polym15092219
APA StyleSun, Q., Yang, Z., & Qi, X. (2023). Design and Application of Hybrid Polymer-Protein Systems in Cancer Therapy. Polymers, 15(9), 2219. https://doi.org/10.3390/polym15092219







