Enhancing Pectin Particles with Polymer Additives: Mitigating Rumen Degradation and Minimizing Yellowish Milk Color in Grazed Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis, Characterization, and In Vitro and In Vivo Analysis of PnP, PwP, and PwPL
2.2. Statistical Analysis
3. Results
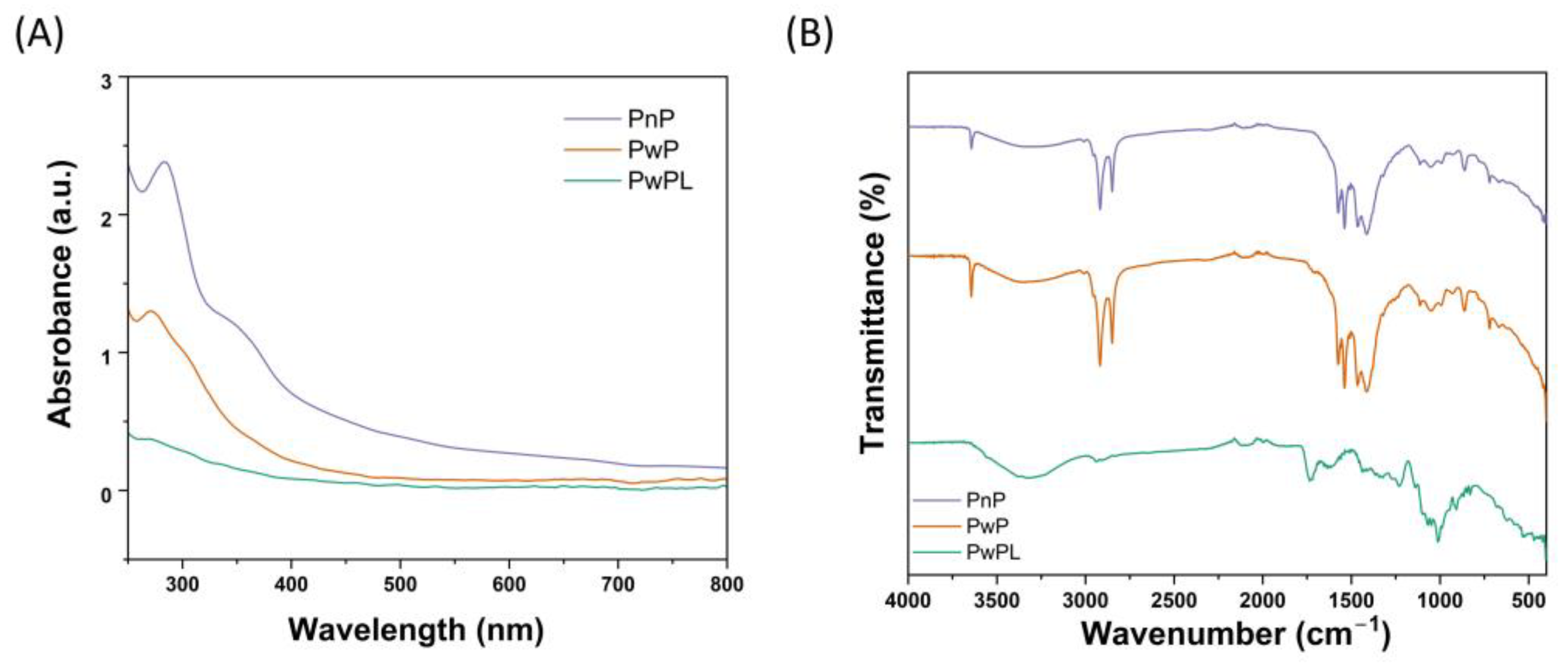
3.1. Characterization of PnP, PwP, and PwPL
3.2. In Vitro Measurement of the Bioaccessibility Rate between Pectin and Total Carotenes
3.3. Rate of Pectin Secretion Evaluated at Treatments and pH Differences
3.4. In Situ Degradability of Pectin with Two Protection Techniques
3.5. Effect of the Use of Protected Pectin on the Chemical Profile of Milk from Grazing Cows
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. Recent Progress in Preparation and Agricultural Application of Microcapsules. J. Biomed. Mater. Res. A 2019, 107, 2371–2385. [Google Scholar] [CrossRef]
- Naz, F.F.; Shah, K.U.; Niazi, Z.R.; Zaman, M.; Lim, V.; Alfatama, M. Polymeric Microparticles: Synthesis, Characterization and In Vitro Evaluation for Pulmonary Delivery of Rifampicin. Polymers 2022, 14, 2491. [Google Scholar] [CrossRef]
- Haider, M.; Elsayed, I.; Ahmed, I.S.; Fares, A.R. In Situ-Forming Microparticles for Controlled Release of Rivastigmine: In Vitro Optimization and In Vivo Evaluation. Pharmaceuticals 2021, 14, 66. [Google Scholar] [CrossRef]
- Fadia, P.; Tyagi, S.; Bhagat, S.; Nair, A.; Panchal, P.; Dave, H.; Dang, S.; Singh, S. Calcium Carbonate Nano- and Microparticles: Synthesis Methods and Biological Applications. 3 Biotech 2021, 11, 457. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. The Role of Microencapsulation in Food Application. Molecules 2022, 27, 1499. [Google Scholar] [CrossRef]
- de Carvalho Arjona, J.; das Graças Silva-Valenzuela, M.; Wang, S.-H.; Valenzuela-Diaz, F.R. Biodegradable Nanocomposite Microcapsules for Controlled Release of Urea. Polymers 2021, 13, 722. [Google Scholar] [CrossRef]
- Piñeiro-Vázquez, A.T.; Canul-Solís, J.R.; Alayón-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Solorio-Sánchez, F.J.; Ku-Vera, J.C. Potential of Condensed Tannins for the Reduction of Emissions of Enteric Methane and Their Effect on Ruminant Productivity. Arch. Med. Vet. 2015, 47, 263–272. [Google Scholar] [CrossRef]
- Khliji, S.; van de Ven, R.; Lamb, T.A.; Lanza, M.; Hopkins, D.L. Relationship between Consumer Ranking of Lamb Colour and Objective Measures of Colour. Meat Sci. 2010, 85, 224–229. [Google Scholar] [CrossRef]
- Ribaya-Mercado, J.D.; Lopez-Miranda, J.; Ordovas, J.M.; Blanco, M.C.; Fox, J.G.; Russell, R.M. Distribution of β-Carotene and Vitamin A in Lipoprotein Fractions of Ferret Serum. Ann. N. Y. Acad. Sci. 1993, 691, 232–237. [Google Scholar] [CrossRef]
- da Conceição dos Santos, R.; Alves, K.S.; Mezzomo, R.; Oliveira, L.R.S.; Cutrim, D.O.; Gomes, D.I.; Leite, G.P.; de Souza Araújo, M.Y. Performance of Feedlot Lambs Fed Palm Kernel Cake-Based Diets. Trop. Anim. Health Prod. 2016, 48, 367–372. [Google Scholar] [CrossRef]
- Prache, S. Developing a Diet Authentication System from the Composition of Milk and Meat in Sheep: A Review: PAPER PRESENTED AT THE 9TH ANNUAL LANGFORD FOOD INDUSTRY CONFERENCE, BRISTOL, 24–25 MAY 2006. J. Agric. Sci. 2007, 145, 435–444. [Google Scholar] [CrossRef]
- Erdman, J.W.; Fahey, G.C.; White, C.B. Effects of Purified Dietary Fiber Sources on Beta-Carotene Utilization by the Chick. J. Nutr. 1986, 116, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Sriwichai, W.; Berger, J.; Picq, C.; Avallone, S. Determining Factors of Lipophilic Micronutrient Bioaccessibility in Several Leafy Vegetables. J. Agric. Food Chem. 2016, 64, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In Vitro Bioaccessibility of Carotenoids, Flavonoids, and Vitamin C from Differently Processed Oranges and Orange Juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Gronowska-Senger, A.; Wolf, G. Effect of Dietary Protein on the Enzyme from Rat and Human Intestine Which Converts β-Carotene to Retinal12. J. Nutr. 1970, 100, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Monterrosa, R.G.; Ramirez-Bribiesca, J.E.; Guerrero-Legarreta, M.I.; Zinn, R.A. Influence of Pectin on Intestinal Digestion of Chromogens in Steers. Anim. Feed Sci. Technol. 2015, 207, 274–277. [Google Scholar] [CrossRef]
- Becker Peres, L.; Becker Peres, L.; de Araújo, P.H.H.; Sayer, C. Solid Lipid Nanoparticles for Encapsulation of Hydrophilic Drugs by an Organic Solvent Free Double Emulsion Technique. Colloids Surf. B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef]
- Hamman, J.H.; Enslin, G.M.; Kotzé, A.F. Oral Delivery of Peptide Drugs: Barriers and Developments. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2005, 19, 165–177. [Google Scholar] [CrossRef]
- Cerón, G.T.; Bribiesca, J.E.R.; Jimenez, L.M.; Monterrosa, R.G.C.; Mancera, H.A.Z.; Galván, M.M.C.; Ramos-Juárez, J.A.; Sánchez, D.H. Design of Pectin Microcapsules Protected with Shellac and Sodium Alginate with Sensibility of Degradation in Intestinal pH of Bovine. Rev. Chim. 2020, 70, 4233–4237. [Google Scholar]
- Santos, F.A.; Santos, J.E.; Theurer, C.B.; Huber, J.T. Effects of Rumen-Undegradable Protein on Dairy Cow Performance: A 12-Year Literature Review. J. Dairy Sci. 1998, 81, 3182–3213. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Oboh, I.O. PRESERVATIVES | Traditional Preservatives—Vegetable Oils. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 137–140. ISBN 978-0-12-384733-1. [Google Scholar]
- Sulaiman, N.S.; Sintang, M.D.; Mantihal, S.; Zaini, H.M.; Munsu, E.; Mamat, H.; Kanagaratnam, S.; Jahurul, M.H.A.; Pindi, W. Balancing Functional and Health Benefits of Food Products Formulated with Palm Oil as Oil Sources. Heliyon 2022, 8, e11041. [Google Scholar] [CrossRef]
- Saminathan, M.; Mohamed, W.N.W.; Noh, A.M.; Ibrahim, N.A.; Fuat, M.A.; Ramiah, S.K. Effects of Dietary Palm Oil on Broiler Chicken Productive Performance and Carcass Characteristics: A Comprehensive Review. Trop. Anim. Health Prod. 2022, 54, 64. [Google Scholar] [CrossRef]
- Mosley, S.A.; Mosley, E.E.; Hatch, B.; Szasz, J.I.; Corato, A.; Zacharias, N.; Howes, D.; McGuire, M.A. Effect of Varying Levels of Fatty Acids from Palm Oil on Feed Intake and Milk Production in Holstein Cows. J. Dairy Sci. 2007, 90, 987–993. [Google Scholar] [CrossRef]
- Wagh, A.S. Chapter 13 Calcium Phosphate Cements. In Chemically Bonded Phosphate Ceramics, 2nd ed.; Wagh, A.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 165–178. ISBN 978-0-08-100380-0. [Google Scholar]
- Mandal, B.K. Chapter 9—Scopes of Green Synthesized Metal and Metal Oxide Nanomaterials in Antimicrobial Therapy. In Nanobiomaterials in Antimicrobial Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Bucharest, Romania, 2016; pp. 313–341. ISBN 978-0-323-42864-4. [Google Scholar]
- Irimia-Vladu, M.; Głowacki, E.D.; Schwabegger, G.; Leonat, L.; Akpinar, H.Z.; Sitter, H.; Bauer, S.; Sariciftci, N.S. Natural Resin Shellac as a Substrate and a Dielectric Layer for Organic Field-Effect Transistors. Green Chem. 2013, 15, 1473–1476. [Google Scholar] [CrossRef]
- Thombare, N.; Kumar, S.; Kumari, U.; Sakare, P.; Yogi, R.K.; Prasad, N.; Sharma, K.K. Shellac as a Multifunctional Biopolymer: A Review on Properties, Applications and Future Potential. Int. J. Biol. Macromol. 2022, 215, 203–223. [Google Scholar] [CrossRef]
- Patil, D.; Patil, G.; Deshmukh, D.P.; Belgamwar, V.S.; Fursule, R.A. Chitosan Coated Mucoadhesive Multiparticulate Drug Delivery System for Gliclazide. Asian J. Pharm. Clin. Res. 2009, 2, 62–68. [Google Scholar]
- Agroalimentaria, S.N.D.S. Inocuidad y Calidad NOM 062 ZOO 1999. Available online: http://www.gob.mx/senasica/documentos/nom-062-zoo-1999 (accessed on 2 November 2023).
- Pérez, E.P. Procedimiento de Fabricación de Jabones Cálcicos Para Alimentación Animal 2007. Patents 2007. Available online: http://www.google.com/patents/WO2007077266A1?cl=es (accessed on 26 May 2022).
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Changes in Vitamin C, Phenolic, and Carotenoid Profiles Throughout in Vitro Gastrointestinal Digestion of a Blended Fruit Juice. J. Agric. Food Chem. 2013, 61, 1859–1867. [Google Scholar] [CrossRef]
- Vera-Vázquez, F.J.; López-Garrido, S.; Guerrero-Legarreta, I.; Mota-Rojas, D.; Bautista-Martínez, Y.; Cruz-Monterrosa, R.G. Degradabilidad de carotenoides totales en alfalfa (Medicago sativa L.) en cultivo in vitro con líquido ruminal. Ecosistemas Recur. Agropecu. 2021, 8, 8. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The Estimation of Protein Degradability in the Rumen from Incubation Measurements Weighted According to Rate of Passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Salih, A.M.; Ahmad, M.B.; Ibrahim, N.A.; Dahlan, K.Z.H.M.; Tajau, R.; Mahmood, M.H.; Yunus, W.M.Z.W. Synthesis of Radiation Curable Palm Oil–Based Epoxy Acrylate: NMR and FTIR Spectroscopic Investigations. Molecules 2015, 20, 14191–14211. [Google Scholar] [CrossRef]
- Demir, D.; Ceylan, S.; Göktürk, D.; Bölgen, N. Extraction of Pectin from Albedo of Lemon Peels for Preparation of Tissue Engineering Scaffolds. Polym. Bull. 2021, 78, 2211–2226. [Google Scholar] [CrossRef]
- Yan, G.; Cao, Z.; Devine, D.; Penning, M.; Gately, N.M. Physical Properties of Shellac Material Used for Hot Melt Extrusion with Potential Application in the Pharmaceutical Industry. Polymers 2021, 13, 3723. [Google Scholar] [CrossRef]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical Characterization of Nanoparticle Size and Surface Modification Using Particle Scattering Diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An Overview on Concepts, Methods, Properties and Applications in Foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Del Piano, M.; Carmagnola, S.; Ballarè, M.; Sartori, M.; Orsello, M.; Balzarini, M.; Pagliarulo, M.; Tari, R.; Anderloni, A.; Strozzi, G.P.; et al. Is Microencapsulation the Future of Probiotic Preparations? The Increased Efficacy of Gastro-Protected Probiotics. Gut Microbes 2011, 2, 120–123. [Google Scholar] [CrossRef]
- López-Fernández, S.G.; Fernández Michel, S.G.; Armenta Corral, R.I.; García-Galaz, A.; Moreno-Rivas, S.C.; Vázquez-Moreno, L.; Ramos-Clamont Montfort, G. Caracterización del co-encapsulamiento de Lactobacillus plantarum y ácidos grasos omega-3 en una matriz de alginato-pectina. Biotecnia 2019, 21, 38–46. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Yan, X. Effects of Adding Methods of Fluorane Microcapsules and Shellac Resin Microcapsules on the Preparation and Properties of Bifunctional Waterborne Coatings for Basswood. Polymers 2022, 14, 3919. [Google Scholar] [CrossRef]
- Leong, J.-Y.; Lam, W.-H.; Ho, K.-W.; Voo, W.-P.; Lee, M.F.-X.; Lim, H.-P.; Lim, S.-L.; Tey, B.-T.; Poncelet, D.; Chan, E.-S. Advances in Fabricating Spherical Alginate Hydrogels with Controlled Particle Designs by Ionotropic Gelation as Encapsulation Systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Huang, X.; Gänzle, M.; Zhang, H.; Zhao, M.; Fang, Y.; Nishinari, K. Microencapsulation of Probiotic Lactobacilli with Shellac as Moisture Barrier and to Allow Controlled Release. J. Sci. Food Agric. 2021, 101, 726–734. [Google Scholar] [CrossRef]
- Farag, Y.; Leopold, C.S. Development of Shellac-Coated Sustained Release Pellet Formulations. Eur. J. Pharm. Sci. 2011, 42, 400–405. [Google Scholar] [CrossRef]
- Yoshimaru, T.; Takahashi, H.; Matsumoto, K. Microencapsulation of L-Lysine for Improving the Balance of Amino Acids in Ruminants. J. Fac. Agric. Kyushu Univ. 2000, 44, 359–365. [Google Scholar] [CrossRef]
- Schell, D.; Beermann, C. Fluidized Bed Microencapsulation of Lactobacillus Reuteri with Sweet Whey and Shellac for Improved Acid Resistance and In-Vitro Gastro-Intestinal Survival. Food Res. Int. 2014, 62, 308–314. [Google Scholar] [CrossRef]
- Jacob, R.; Bruno, F. Review on Shell Materials Used in the Encapsulation of Phase Change Materials for High Temperature Thermal Energy Storage. Renew. Sustain. Energy Rev. 2015, 48, 79–87. [Google Scholar] [CrossRef]
- Paharia, A.; Yadav, A.K.; Rai, G.; Jain, S.K.; Pancholi, S.S.; Agrawal, G.P. Eudragit-Coated Pectin Microspheres of 5-Fluorouracil for Colon Targeting. AAPS PharmSciTech 2007, 8, 12. [Google Scholar] [CrossRef]
- Ben Messaoud, G.; Sánchez-González, L.; Probst, L.; Jeandel, C.; Arab-Tehrany, E.; Desobry, S. Physico-Chemical Properties of Alginate/Shellac Aqueous-Core Capsules: Influence of Membrane Architecture on Riboflavin Release. Carbohydr. Polym. 2016, 144, 428–437. [Google Scholar] [CrossRef]
- Soyeurt, H.; Dehareng, F.; Gengler, N.; McParland, S.; Wall, E.; Berry, D.P.; Coffey, M.; Dardenne, P. Mid-Infrared Prediction of Bovine Milk Fatty Acids across Multiple Breeds, Production Systems, and Countries. J. Dairy Sci. 2011, 94, 1657–1667. [Google Scholar] [CrossRef]
- Calvopiña, A.I.; León, V. Estudio de La Suplementación de Tres Niveles de Grasa Sobrepasante en la Alimentación de Vacas Lactantes Holstein Friesian. Rumipamba 2007, 21, 1–12. [Google Scholar]
- Palmquist, D.L.; Jenkins, T.C. Fat in Lactation Rations1, 2: Review. J. Dairy Sci. 1980, 63, 1–14. [Google Scholar] [CrossRef]
- Fahey, J.; Mee, J.F.; Murphy, J.J.; O’Callaghan, D. Effects of Calcium Salts of Fatty Acids and Calcium Salt of Methionine Hydroxy Analogue on Plasma Prostaglandin F2α Metabolite and Milk Fatty Acid Profiles in Late Lactation Holstein–Friesian Cows. Theriogenology 2002, 58, 1471–1482. [Google Scholar] [CrossRef]
- Rodriguez, C.E.; Gómez, D.F. Efecto de la suplementación con diferentes dosis de grasa protegida sobre parámetros productivos y composicionales de la leche bovina. Zootec. Trop. 2013, 31, 299–310. [Google Scholar]
- Schroeder, G.F.; Gagliostro, G.A.; Bargo, F.; Delahoy, J.E.; Muller, L.D. Effects of Fat Supplementation on Milk Production and Composition by Dairy Cows on Pasture: A Review. Livest. Prod. Sci. 2004, 86, 1–18. [Google Scholar] [CrossRef]
- Zalzano, E.; Rojas-Bourrillon, A.; Murillo-Barrantes, J. Efecto de La Suplementación de β-Caroteno en Vacas Lecheras Sobre Concentraciones en Sangre y Calostro, Reproducción y Salud de la Ubre. Nutr. Anim. Trop. 2022, 16, 53–81. [Google Scholar] [CrossRef]
- Park, Y.W. Goat Milk Chemistry and Nutrition. In Handbook of Milk of Non-Bovine Mammals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 42–83. ISBN 978-1-119-11031-6. [Google Scholar]
- von Lintig, J.; Vogt, K. Filling the Gap in Vitamin A Research: Molecular identification of an enzyme cleaving β-carotene to retinal*. J. Biol. Chem. 2000, 275, 11915–11920. [Google Scholar] [CrossRef]
- Leveille, G.A.; Sauberlich, H.E. Mechanism of the Cholesterol-Depressing Effect of Pectin in the Cholesterol-Fed Rat. J. Nutr. 1966, 88, 209–214. [Google Scholar] [CrossRef]
- Orellano, M.S.; Longo, G.S.; Porporatto, C.; Correa, N.M.; Falcone, R.D. Role of Micellar Interface in the Synthesis of Chitosan Nanoparticles Formulated by Reverse Micellar Method. Colloids Surf. Physicochem. Eng. Asp. 2020, 599, 124876. [Google Scholar] [CrossRef]
- Pramanik, D.; Ganguly, M. Formulation and evaluation of a pectin based controlled drug delivery system containing metronidazole. RJLBPCS 2017, 3, 16–25. [Google Scholar]
- Escobar, C. Si La Leche Es Blanca, ¿Por Qué El Queso Es Amarillo? Rev. Química 2021, 35, 26–29. [Google Scholar]
- Saikia, C.; Gogoi, P. Chitosan: A Promising Biopolymer in Drug Delivery Applications. J. Mol. Genet. Med. 2015, s4, 899–910. [Google Scholar] [CrossRef]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-Lowering Properties of Different Pectin Types in Mildly Hyper-Cholesterolemic Men and Women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Hicks, K.B. Pectin in Controlled Drug Delivery—A Review. Cellulose 2007, 14, 15–24. [Google Scholar] [CrossRef]
- Wathoni, N.; Shan, C.Y.; Yi Shan, W.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and Antioxidant Activity of Pectin from Indonesian Mangosteen (Garcinia mangostana L.) Rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef]
- Evans, C.E.L. Dietary Fibre and Cardiovascular Health: A Review of Current Evidence and Policy. Proc. Nutr. Soc. 2020, 79, 61–67. [Google Scholar] [CrossRef]
- Zhou, M.; Bi, J.; Chen, J.; Wang, R.; Richel, A. Impact of Pectin Characteristics on Lipid Digestion under Simulated Gastrointestinal Conditions: Comparison of Water-Soluble Pectins Extracted from Different Sources. Food Hydrocoll. 2021, 112, 106350. [Google Scholar] [CrossRef]


| pH | PnP | PwP | PwPL | SE |
|---|---|---|---|---|
| 2 | 99.685 a,x | 18.961 c,xy | 21.979 b,xy | 1.013 |
| 3 | 99.535 a,x | 23.380 c,x | 27.581 b,x | 1.287 |
| 5 | 97.337 a,x | 14.451 c,y | 18.408 b,y | 1.567 |
| 7 | 92.816 a,y | 8.163 c,z | 13.311 b,z | 1.614 |
| SE | 0.99 | 1.131 | 1.121 |
| Treatment | 24 h | 48 h |
|---|---|---|
| PnP | 99.42 a | 99.23 a |
| PwP | 27.59 b | 29.23 b |
| PwPL | 16.75 b | 18.60 b |
| SE | 6.24 | 6.08 |
| p value | 0.0001 | 0.0001 |
| Treatments | Fat | Total Solids | Density | Protein | Lactose | Degrees Brix | pH |
|---|---|---|---|---|---|---|---|
| NP | 3.295 b | 6.957 b | 28.150 | 3.120 | 4.417 | 9.095 | 7.200 |
| PwPL | 4.588 a | 8.200 a | 31.002 | 3.210 | 4.860 | 8.647 | 7.297 |
| SE | 0.379 | 0.409 | 1.098 | 0.104 | 0.166 | 0.295 | 0.034 |
| p value | 0.001 | 0.0033 | 0.0516 | 0.391 | 0.094 | 0.1337 | 0.064 |
| Treatments | L | A | B | C |
|---|---|---|---|---|
| NP | 80.5169 | −5.5418 a | 7.5685 a | 81.0884 |
| PwPL | 80.1193 | −2.9015 b | 2.8063 b | 80.2273 |
| SE | 0.9972 | 0.1986 | 0.3275 | 0.9995 |
| p value | 0.6918 | 0.0001 | 0.0001 | 0.3916 |
| Treatments | Glucose | Cholesterol | Lactate | Triglycerides |
|---|---|---|---|---|
| NP | 56.80 a | 167.10 a | 3.13 a | 106.50 |
| PwPL | 42.60 b | 151.10 b | 1.46 b | 103.90 |
| SE | 4.68 | 1.06 | 0.24 | 1.57 |
| p value | 0.0071 | 0.0001 | 0.0001 | 0.1162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Vázquez, F.; Ramírez-Bribiesca, J.E.; Cruz-Monterrosa, R.G.; Crosby-Galvan, M.M.; Barcena-Gama, J.R.; Ramírez, D.T.; Mejía-Méndez, J.L.; Vallejo-Hernández, L.H.; López-Mena, E.R. Enhancing Pectin Particles with Polymer Additives: Mitigating Rumen Degradation and Minimizing Yellowish Milk Color in Grazed Cows. Polymers 2024, 16, 106. https://doi.org/10.3390/polym16010106
Vera-Vázquez F, Ramírez-Bribiesca JE, Cruz-Monterrosa RG, Crosby-Galvan MM, Barcena-Gama JR, Ramírez DT, Mejía-Méndez JL, Vallejo-Hernández LH, López-Mena ER. Enhancing Pectin Particles with Polymer Additives: Mitigating Rumen Degradation and Minimizing Yellowish Milk Color in Grazed Cows. Polymers. 2024; 16(1):106. https://doi.org/10.3390/polym16010106
Chicago/Turabian StyleVera-Vázquez, Francisco, Jacinto Efrén Ramírez-Bribiesca, Rosy G. Cruz-Monterrosa, María M. Crosby-Galvan, José Ricardo Barcena-Gama, Diana Tamara Ramírez, Jorge L. Mejía-Méndez, Laura H. Vallejo-Hernández, and Edgar R. López-Mena. 2024. "Enhancing Pectin Particles with Polymer Additives: Mitigating Rumen Degradation and Minimizing Yellowish Milk Color in Grazed Cows" Polymers 16, no. 1: 106. https://doi.org/10.3390/polym16010106
APA StyleVera-Vázquez, F., Ramírez-Bribiesca, J. E., Cruz-Monterrosa, R. G., Crosby-Galvan, M. M., Barcena-Gama, J. R., Ramírez, D. T., Mejía-Méndez, J. L., Vallejo-Hernández, L. H., & López-Mena, E. R. (2024). Enhancing Pectin Particles with Polymer Additives: Mitigating Rumen Degradation and Minimizing Yellowish Milk Color in Grazed Cows. Polymers, 16(1), 106. https://doi.org/10.3390/polym16010106







