Superhydrophilic and Underwater Superoleophobic Copper Mesh Coated with Bamboo Cellulose Hydrogel for Efficient Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
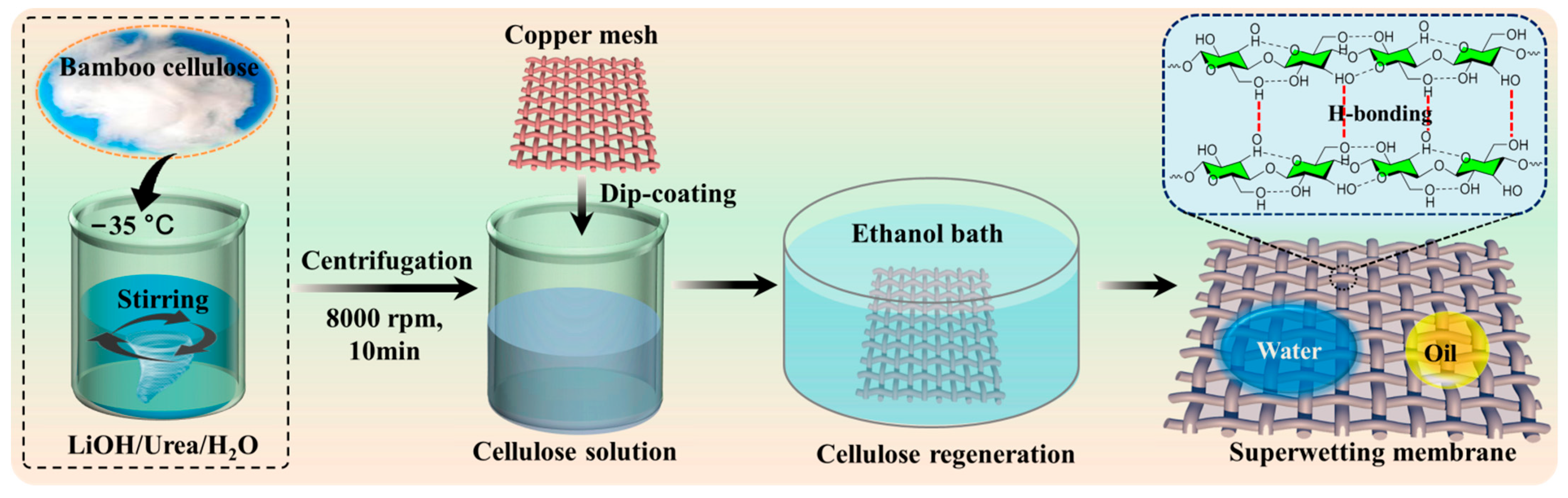
2.2. Fabrication of the Superhydrophilic/Superoleophobic Bamboo Cellulose Hydrogel-Coated Copper Mesh
2.3. Characterization
2.4. Oil/Water Separation Experiments of Super-Wetting Membrane
3. Results and Discussion
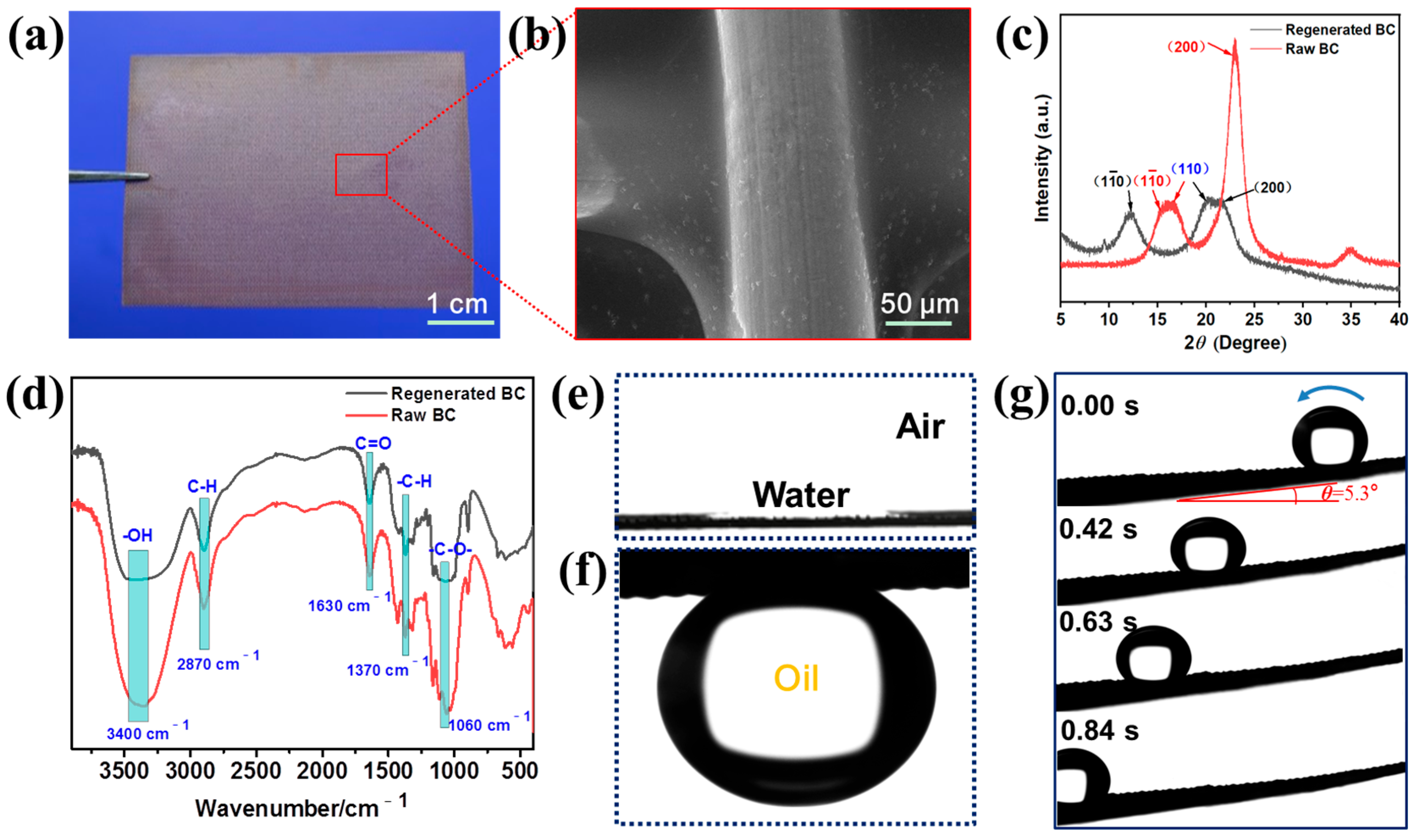
3.1. Micromorphologies, Chemical Compositions and Wettability of the Super-Wetting Membrane
3.2. Performances of Oil/Water Separation
3.3. Oil/Water Separation Mechanism of the Super-Wetting Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Peng, C.; Dai, M.; Lin, D.; Ali, I.; Alhewairini, S.S.; Zheng, X.; Chen, G.; Li, J.; Naz, I. Recent development of super-wettable materials and their applications in oil-water separation. J. Clean. Prod. 2020, 266, 121624. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Tummons, E.; Han, Q.; Tanudjaja, H.J.; Hejase, C.A.; Chew, J.W.; Tarabara, V.V. Membrane fouling by emulsified oil: A review. Sep. Purif. Technol. 2020, 248, 116919. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Electrospun nanofibrous membranes: An effective arsenal for the purification of emulsified oily wastewater. Adv. Funct. Mater. 2020, 30, 2002192. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, J.; Li, S.; Ge, M.; Teng, L.; Chen, Z.; Lai, Y. Advanced materials with special wettability toward intelligent oily wastewater remediation. ACS Appl. Mater. Interfaces 2021, 13, 67–87. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Brandão Costa Santos, N.; Marques Fagundes, F.; Leibsohn Martins, A.; Ribeiro Damasceno, J.J.; de Oliveira Arouca, F. Stability of oil well olefin drilling fluids: Solid–liquid sedimentation and rheological characterization. Part. Sci. Technol. 2020, 38, 203–209. [Google Scholar] [CrossRef]
- Yu, J.; Cao, C.; Pan, Y. Advances of adsorption and filtration techniques in separating highly viscous crude oil/water mixtures. Adv. Mater. Interfaces 2021, 8, 2100061. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, F.; Guo, Z. Superwetting surfaces for filtration separation of high-viscosity raw petroleum/water mixtures. J. Mater. Chem. A 2022, 10, 14273–14292. [Google Scholar] [CrossRef]
- Liu, C.; Peng, Y.; Huang, C.; Ning, Y.; Shang, J.; Li, Y. Bioinspired superhydrophobic/superhydrophilic janus copper foam for on-demand oil/water separation. ACS Appl. Mater. Interfaces 2022, 14, 11981–11988. [Google Scholar] [CrossRef]
- Yan, X.; Xiao, X.; Au, C.; Mathur, S.; Huang, L.; Wang, Y.; Zhang, Z.; Zhu, Z.; Kipper, M.J.; Tang, J.; et al. Electrospinning nanofibers and nanomembranes for oil/water separation. J. Mater. Chem. A 2021, 9, 21659–21684. [Google Scholar] [CrossRef]
- Cheng, X.; Ye, Y.; Li, Z.; Chen, X.; Bai, Q.; Wang, K.; Zhang, Y.; Drioli, E.; Ma, J. Constructing environmental-friendly “oil-diode” Janus membrane for oil/water separation. ACS Nano 2022, 16, 4684–4692. [Google Scholar] [CrossRef]
- Dong, D.; Zhu, Y.; Fang, W.; Ji, M.; Wang, A.; Gao, S.; Lin, H.; Huang, R.; Jin, J. Double-defense design of super-anti-fouling membranes for oil/water emulsion separation. Adv. Funct. Mater. 2022, 32, 2113247. [Google Scholar] [CrossRef]
- Han, L.; Shen, L.; Lin, H.; Cheng, T.; Wen, J.; Zeng, Q.; Xu, Y.; Li, R.; Zhang, M.; Hong, H.; et al. Three dimension-printed membrane for ultrafast oil/water separation as driven by gravitation. Nano Energy 2023, 111, 108351. [Google Scholar] [CrossRef]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil–water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef]
- Tao, X.; Chen, X.; Cai, S.; Yan, F.; Li, S.; Jin, S.; Zhu, H. A multifunctional heterogeneous superwettable coating for water collection, oil/water separation and oil absorption. J. Hazard. Mater. 2023, 443, 130166. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Kongparakul, S.; Samart, C.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Fabrication and evaluation of nanocellulose sponge for oil/water separation. Carbohydr. Polym. 2018, 190, 184–189. [Google Scholar] [CrossRef]
- Ding, F.; Gao, M. Pore wettability for enhanced oil recovery, contaminant adsorption and oil/water separation: A review. Adv. Colloid Interface Sci. 2021, 289, 102377. [Google Scholar] [CrossRef]
- Fu, Y.; Chung, D.D.L. Coagulation of oil in water using sawdust, bentonite and calcium hydroxide to form floating sheets. Appl. Clay Sci. 2011, 53, 634–641. [Google Scholar] [CrossRef]
- Khor, C.M.; Wang, J.; Li, M.; Oettel, B.A.; Kaner, R.B.; Jassby, D.; Hoek, E.M.V. Performance, energy and cost of produced water treatment by chemical and electrochemical coagulation. Water 2020, 12, 3426. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, M.; Hong, S.K.; Park, C.; Cho, S.J.; Lim, G. Comprehensive electrokinetic-assisted separation of oil emulsion with ultrahigh flux. ACS Nano 2021, 15, 15815–15823. [Google Scholar] [CrossRef]
- Ghaffarian Khorram, A.; Fallah, N.; Nasernejad, B.; Afsham, N.; Esmaelzadeh, M.; Vatanpour, V. Electrochemical-based processes for produced water and oily wastewater treatment: A review. Chemosphere 2023, 338, 139565. [Google Scholar] [CrossRef]
- Lu, H.; Pan, Z.; Wang, H.; Liu, Y.; Dai, P.; Yang, Q. Fiber coalescence treatment of oily wastewater: A new theory and application. J. Hazard. Mater. 2021, 412, 125188. [Google Scholar] [CrossRef]
- Yao, X.; Hou, X.; Qi, G.; Zhang, R. Preparation of superhydrophobic polyimide fibrous membranes with controllable surface structure for high efficient oil-water emulsion and heavy oil separation. J. Environ. Chem. Eng. 2022, 10, 107470. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation mechanism and construction of surfaces with special wettability for oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Zhong, Y.; Ma, X.; Lv, W.; Zhao, H.; Zhu, J.; Yan, N. Multifunctional superhydrophilic/underwater superoleophobic lignin-based polyurethane foam for highly efficient oil-water separation and water purification. Sep. Purif. Technol. 2023, 311, 123284. [Google Scholar] [CrossRef]
- Hailan, S.M.; Ponnamma, D.; Krupa, I. The separation of oil/water mixtures by modified melamine and polyurethane foams: A review. Polymers 2021, 13, 4142. [Google Scholar] [CrossRef]
- Wang, J.M.; Wang, L.D.; Feng, L. One-step fabrication of fluoropolymer transparent films with superhydrophobicity by dry method. J. Appl. Polym. Sci. 2011, 120, 524–529. [Google Scholar] [CrossRef]
- Chen, C.; Du, C.; Weng, D.; Mahmood, A.; Feng, D.; Wang, J. Robust superhydrophobic polytetrafluoroethylene nanofibrous coating fabricated by self-assembly and its application for oil/water separation. ACS Appl. Nano Mater. 2018, 1, 2632–2639. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, W.; Zheng, H.; Zhao, C.; Liu, D. Laser-engineered superhydrophobic polydimethylsiloxane for highly efficient water manipulation. ACS Appl. Mater. Interfaces 2021, 13, 48163–48170. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, X.; Chao, Y.; Chen, D.; Liao, Y. Super-hydrophobic magnetic fly ash coated polydimethylsiloxane (MFA@PDMS) sponge as an absorbent for rapid and efficient oil/water separation. Polymers 2022, 14, 3726. [Google Scholar] [CrossRef]
- Wang, C.J.; Kuan, W.-F.; Lin, H.P.; Shchipunov, Y.A.; Chen, L.-J. Facile hydrophilic modification of polydimethylsiloxane-based sponges for efficient oil–water separation. J. Ind. Eng. Chem. 2021, 96, 144–155. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Wahid, F.; Cui, J.X.; Wang, Y.Y.; Zhong, C. Cellulose-based special wetting materials for oil/water separation: A review. Int. J. Biol. Macromol. 2021, 185, 890–906. [Google Scholar] [CrossRef]
- Tanpichai, S.; Boonmahitthisud, A.; Soykeabkaew, N.; Ongthip, L. Review of the recent developments in all-cellulose nanocomposites: Properties and applications. Carbohydr. Polym. 2022, 286, 119192. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent advances in cellulose-based hydrogels for tissue engineering applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Liu, W.; Liu, K.; Du, H.; Zheng, T.; Zhang, N.; Xu, T.; Pang, B.; Zhang, X.; Si, C.; Zhang, K. Cellulose nanopaper: Fabrication, functionalization, and applications. Nanomicro Lett. 2022, 14, 104. [Google Scholar] [CrossRef]
- Ning, D.; Lu, Z.; Tian, C.; Yan, N.; Xie, F.; Li, N.; Hua, L. Superwettable cellulose acetate-based nanofiber membrane with spider-web structure for highly efficient oily water purification. Int. J. Biol. Macromol. 2023, 253, 126865. [Google Scholar] [CrossRef]
- Firmanda, A.; Fahma, F.; Syamsu, K.; Suprihatin, S.; Purnawati, R.; Mahardika, M.; Suryanegara, L.; Saito, Y.; Wood, K.; Sinaga, R. Cellulose and its composite for sustainable oils/water (O/W) separation: From cellulose sponge to 3D printed nanocellulose. J. Environ. Chem. Eng. 2023, 11, 110359. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Cai, M.; Xu, Q.; Zhang, J.; Cao, X.; Zhang, J.; Chen, Y. Advanced superhydrophobic and multifunctional nanocellulose aerogels for oil/water separation: A review. Carbohydr. Polym. 2023, 300, 120242. [Google Scholar] [CrossRef]
- Liu, C.-H.; Shang, J.-P.; Su, X.; Zhao, S.; Peng, Y.; Li, Y.-B. Fabrication of superhydrophobic/superoleophilic bamboo cellulose foam for oil/water separation. Polymers 2022, 14, 5162. [Google Scholar] [CrossRef]
- Fan, B.; Wu, L.; Ming, A.; Liu, Y.; Yu, Y.; Cui, L.; Zhou, M.; Wang, Q.; Wang, P. Highly compressible and hydrophobic nanofibrillated cellulose aerogels for cyclic oil/water separation. Int. J. Biol. Macromol. 2023, 242, 125066. [Google Scholar] [CrossRef]
- Ling, H.; Wang, L.; Lin, Q.; Huang, Q.; Zhang, X.; Ren, J.; Li, N.; Zhou, C.; Lin, Z.; Zhou, J.; et al. Antimicrobial cellulose paper tuned with chitosan fibers for high-flux oil/water separation. Carbohydr. Polym. 2023, 312, 120794. [Google Scholar] [CrossRef]
- Dai, Q.; Li, D.; Sun, Y.; Wang, H.; Lu, Y.; Yang, D. Low temperature-resistant superhydrophobic and elastic cellulose aerogels derived from seaweed solid waste as efficient oil traps for oil/water separation. Chemosphere 2023, 336, 139179. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Tao, F.; An, Y.; Zhong, Y.; Liu, Z.; Hu, Z.; Zhang, X.; Wang, X. An overview of biomass-based oil/water separation materials. Sep. Purif. Technol. 2023, 316, 123767. [Google Scholar] [CrossRef]
- Yeh, Y.-J.; Chu, J.P.; You, J.-D.; Chang, T.-H.; Liou, J.R.; Chiang, W.-H.; Yiu, P.; Hsueh, C.-H.; Shen, Y.-L.; Tung, K.-L. Tunable nanostructured stainless-steel coating for high-selective and high-permeable separation membranes for oil/water emulsions. NPJ Clean Water 2023, 6, 17. [Google Scholar] [CrossRef]
- Hu, X.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Ramakrishna, S.; Wang, X.; Yao, J. Preparation of high elastic bacterial cellulose aerogel through thermochemical vapor deposition catalyzed by solid acid for oil-water separation. Carbohydr. Polym. 2023, 305, 120538. [Google Scholar] [CrossRef]
- Lu, J.; Bai, T.; Wang, D.; Yu, H.; Wang, Q.; Niu, Z.; Hu, Y.; Liu, X.; Han, G.; Cheng, W. Electrospun polyacrylonitrile membrane in situ modified with cellulose nanocrystal anchoring TiO2 for oily wastewater recovery. Adv. Fiber Mater. 2023, 5, 2055–2068. [Google Scholar] [CrossRef]
- Ding, Z.; Tian, Z.; Ji, X.; Dai, H.; Si, C. Bio-inspired catalytic one-step prepared r-siloxane cellulose composite membranes with highly efficient oil separation. Adv. Compos. Hybrid Mater. 2022, 5, 2138–2153. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Chen, J.-T.; Hao, B.; Wang, R.; Ma, P.-C. Preparation of cellulose-coated cotton fabric and its application for the separation of emulsified oil in water. Carbohydr. Polym. 2020, 240, 116318. [Google Scholar] [CrossRef]
- Qiao, A.; Huang, R.; Penkova, A.; Qi, W.; He, Z.; Su, R. Superhydrophobic, elastic and anisotropic cellulose nanofiber aerogels for highly effective oil/water separation. Sep. Purif. Technol. 2022, 295, 121266. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Li, J.; Xie, F.; Yang, H.; Wang, C.; Fahlman, B.D.; Li, W. Natural microfibrils/regenerated cellulose-based carbon aerogel for highly efficient oil/water separation. J. Hazard. Mater. 2023, 454, 131397. [Google Scholar] [CrossRef]
- Qiao, A.; Huang, R.; Wu, J.; Qi, W.; Su, R. Anisotropic cellulose nanocrystalline sponge sheets with ultrahigh water fluxes and oil/water selectivity. Carbohydr. Polym. 2023, 312, 120807. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, Y.; Yu, W. Effects of extraction methods on morphology, structure and properties of bamboo cellulose. Ind. Crops Prod. 2021, 169, 113640. [Google Scholar] [CrossRef]
- Da Costa Correia, V.; Ardanuy, M.; Claramunt, J.; Savastano, H. Assessment of chemical and mechanical behavior of bamboo pulp and nanofibrillated cellulose exposed to alkaline environments. Cellulose 2019, 26, 9269–9285. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, Q.; Wang, X.; Yu, Y.; Yu, W.; Huang, Y. Bamboo cellulose fibers prepared by different drying methods: Structure-property relationships. Carbohydr. Polym. 2022, 296, 119926. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Li, J.; Xu, J.; Zeng, J.; Li, M.; Li, X.; Li, Y. Efficient preparation of high-purity cellulose from moso bamboo by p-toluenesulfonic acid pretreatment. Int. J. Biol. Macromol. 2023, 245, 125395. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, Y.; Xie, Y.; Wang, H.; Wang, T.; Tang, W.; Jin, Z.; Ling, Z.; Yong, Q. Allomorphic regulation of bamboo cellulose by mild alkaline peroxide for holocellulose nanofibrils production. Int. J. Biol. Macromol. 2022, 223, 49–56. [Google Scholar] [CrossRef]
- Liu, C.; Liu, M.; Liu, W.; Li, Z.; Xu, F. Interlaminar fracture property of moso bamboo strips influenced by fiber distributions in bamboo internode and node. Compos. Struct. 2022, 294, 115777. [Google Scholar] [CrossRef]
- Shang, J.-P.; Liang, P.; Peng, Y.; Xu, D.-F.; Li, Y.-B. One-Step treatment for upgrading bleached bamboo pulp to dissolving pulp high solvency in green alkali/urea aqueous solution. Polymers 2023, 15, 1475. [Google Scholar] [CrossRef]
- Youngblood, J.P.; McCarthy, T.J. Ultrahydrophobic polymer surfaces prepared by simultaneous ablation of polypropylene and sputtering of poly(tetrafluoroethylene) using radio frequency plasma. Macromolecules 1999, 32, 6800–6806. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Zhao, S.; Huang, C.; Deng, F.; Liu, J.; Liu, C.; Li, Y. Superhydrophilic and Underwater Superoleophobic Copper Mesh Coated with Bamboo Cellulose Hydrogel for Efficient Oil/Water Separation. Polymers 2024, 16, 14. https://doi.org/10.3390/polym16010014
Peng Y, Zhao S, Huang C, Deng F, Liu J, Liu C, Li Y. Superhydrophilic and Underwater Superoleophobic Copper Mesh Coated with Bamboo Cellulose Hydrogel for Efficient Oil/Water Separation. Polymers. 2024; 16(1):14. https://doi.org/10.3390/polym16010014
Chicago/Turabian StylePeng, Yun, Shuang Zhao, Chuanlin Huang, Feifei Deng, Jie Liu, Chunhua Liu, and Yibao Li. 2024. "Superhydrophilic and Underwater Superoleophobic Copper Mesh Coated with Bamboo Cellulose Hydrogel for Efficient Oil/Water Separation" Polymers 16, no. 1: 14. https://doi.org/10.3390/polym16010014
APA StylePeng, Y., Zhao, S., Huang, C., Deng, F., Liu, J., Liu, C., & Li, Y. (2024). Superhydrophilic and Underwater Superoleophobic Copper Mesh Coated with Bamboo Cellulose Hydrogel for Efficient Oil/Water Separation. Polymers, 16(1), 14. https://doi.org/10.3390/polym16010014





