Strengthened Decellularized Porcine Valves via Polyvinyl Alcohol as a Template Improving Processability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
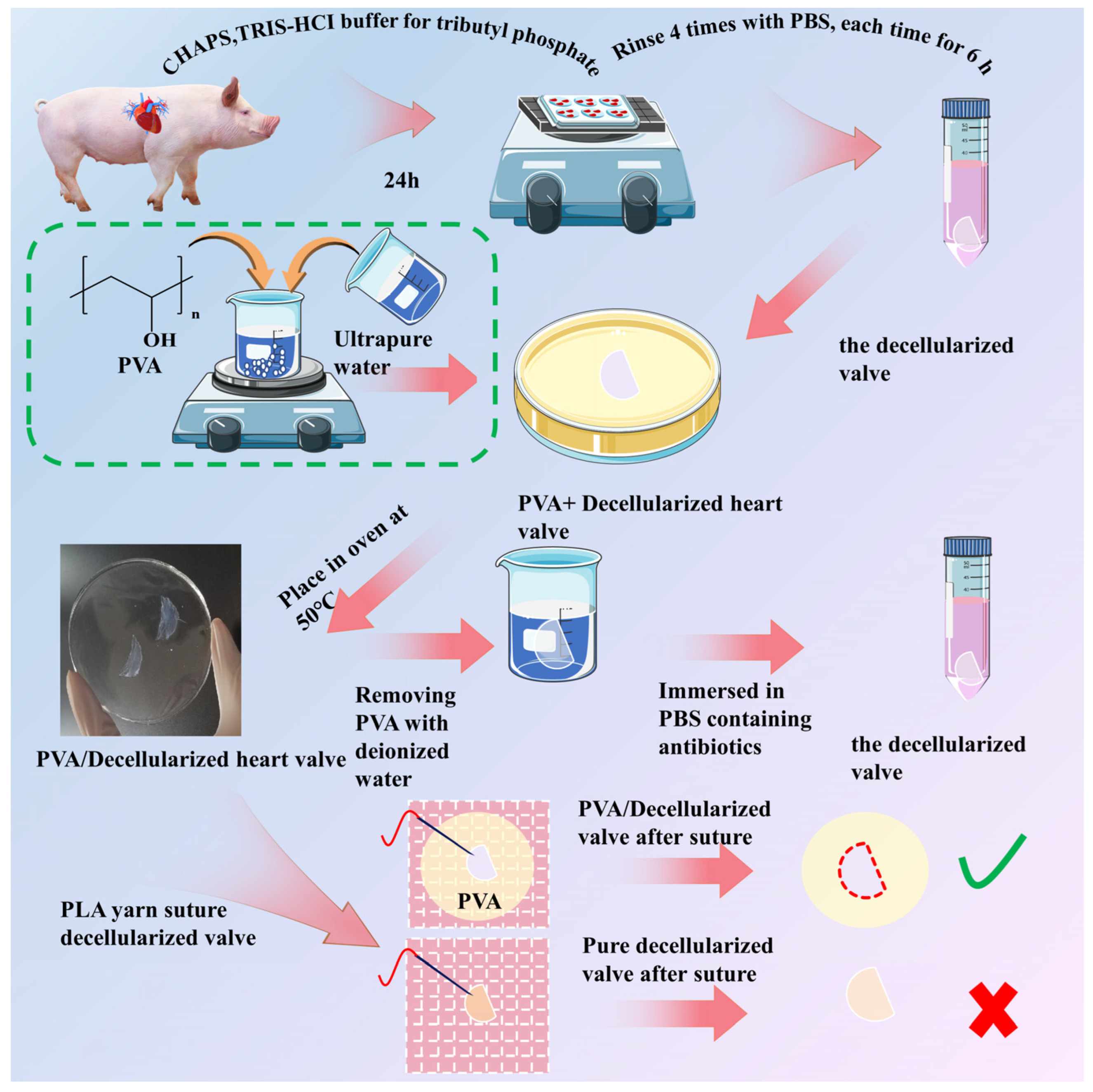
2.2. Preparation of Decellularized Valves
2.3. Preparation of Polyvinyl Alcohol (PVA) Solution [32]
2.4. Preparation of PVA/Decellularized Valves Composite Membranes
2.5. H&E Staining
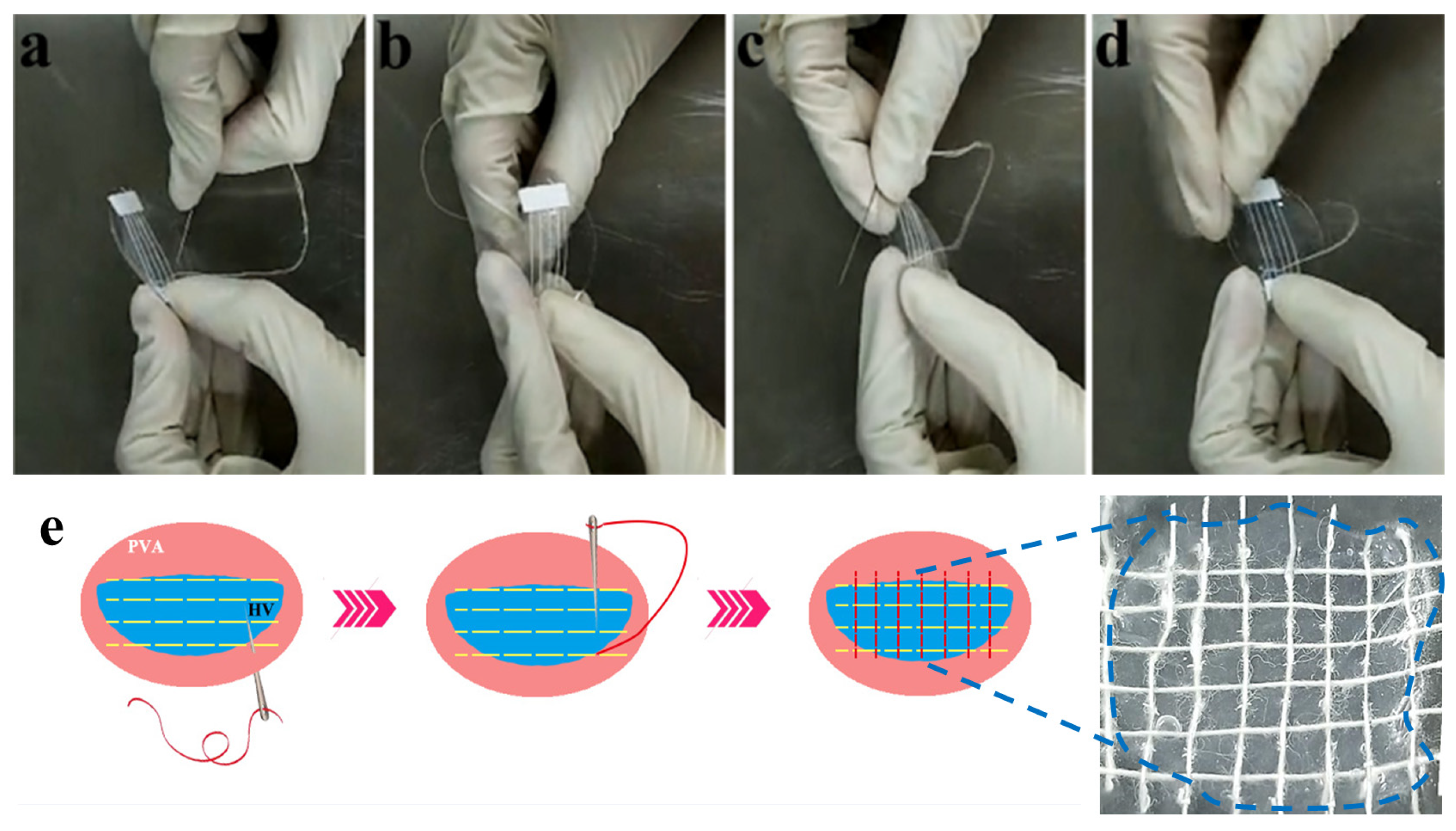
2.6. Yarn Sutured Decellularized Heart Valves
2.7. Surface Morphology Characterization
2.8. Flexibility Characterization
2.9. Mechanical Properties Characterization
2.10. Fourier Transform Infrared Spectrometer (FTIR) Analysis
2.11. Sterilization of Samples
2.12. Cell Culture
2.13. Cell Adhesion
2.14. Cell Proliferation
2.15. Cell Morphology
2.16. Statistical Analysis
3. Results
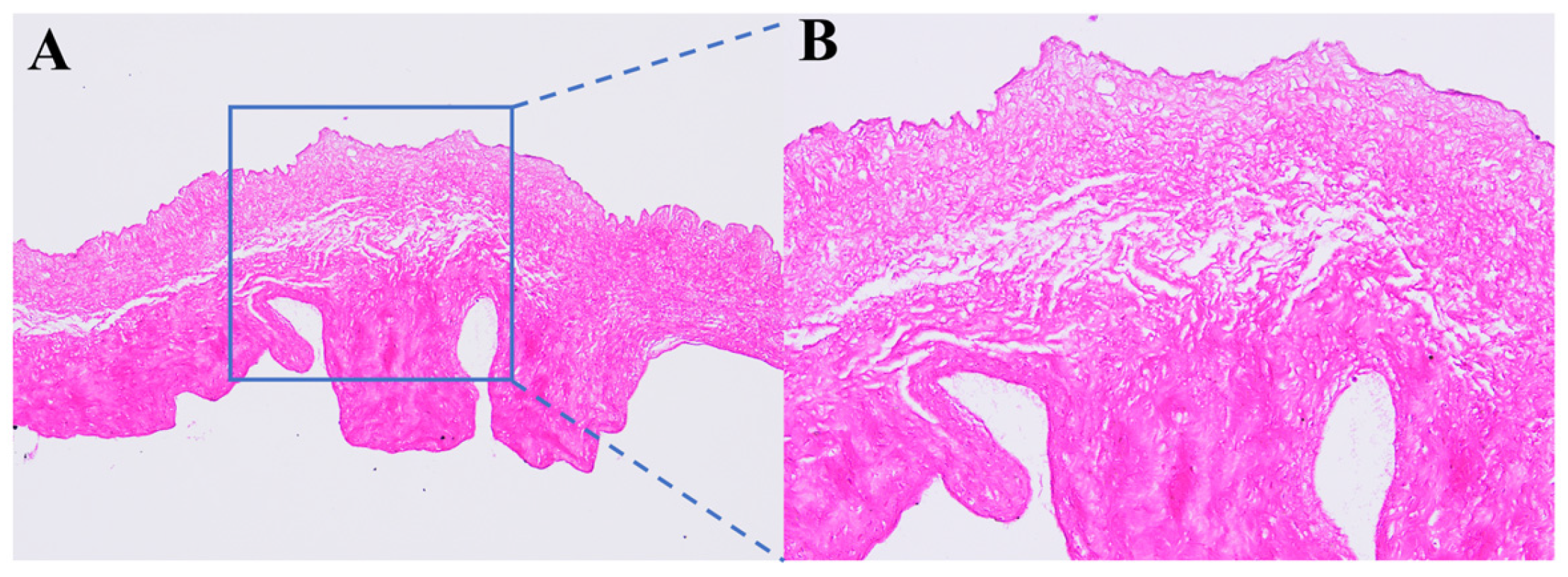
3.1. Histological Staining of Decellularized Valves Treated with PVA Solution
3.2. Microscopic Morphology of Decellularized Valves Treated with Different Concentrations of PVA
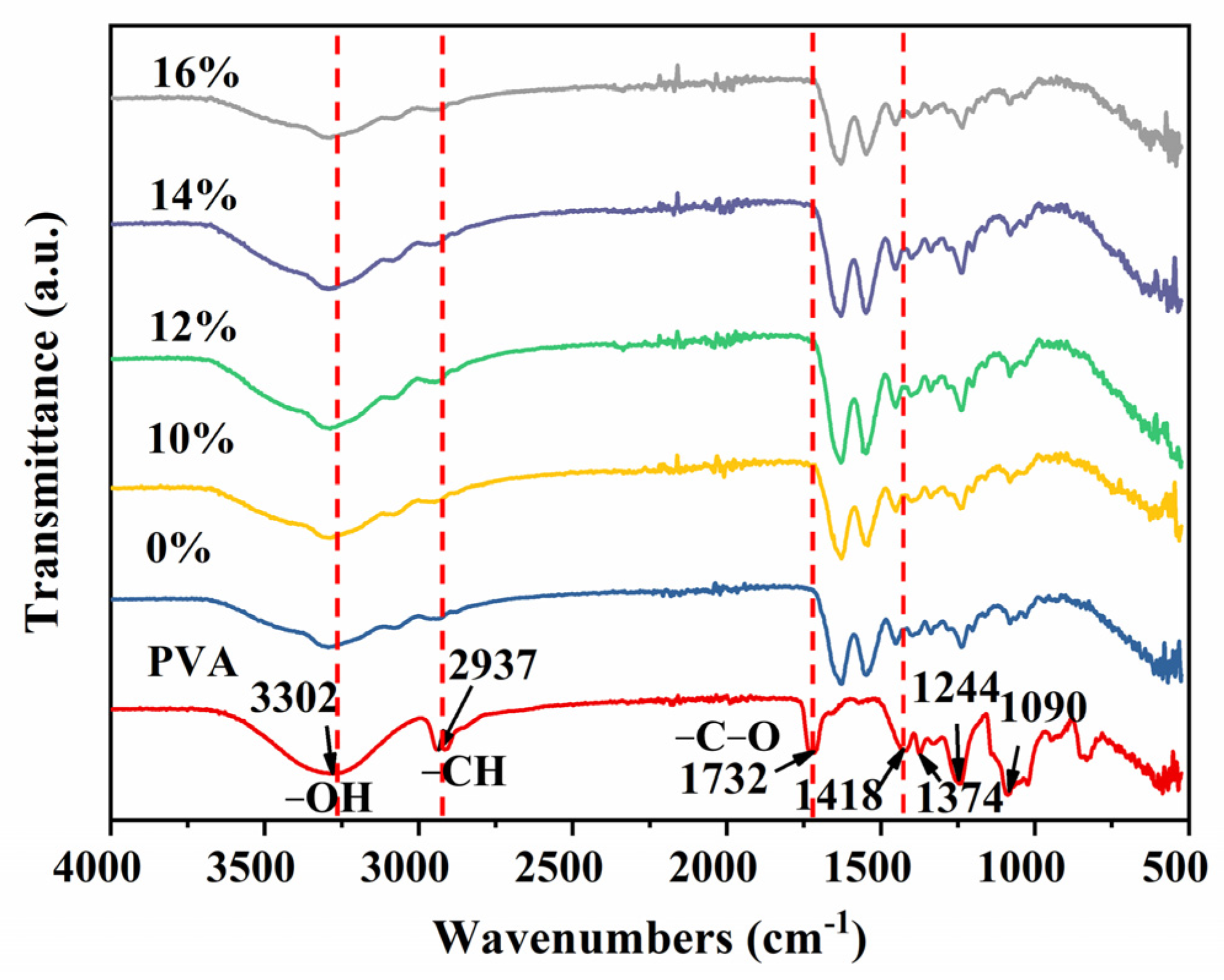
3.3. FTIR Analysis
3.4. Comparison of Flexibility of Decellularized Valves Treated with Different Concentrations of PVA
3.5. Comparison of Mechanical Properties of Decellularized Valves Treated with Different Concentrations of PVA
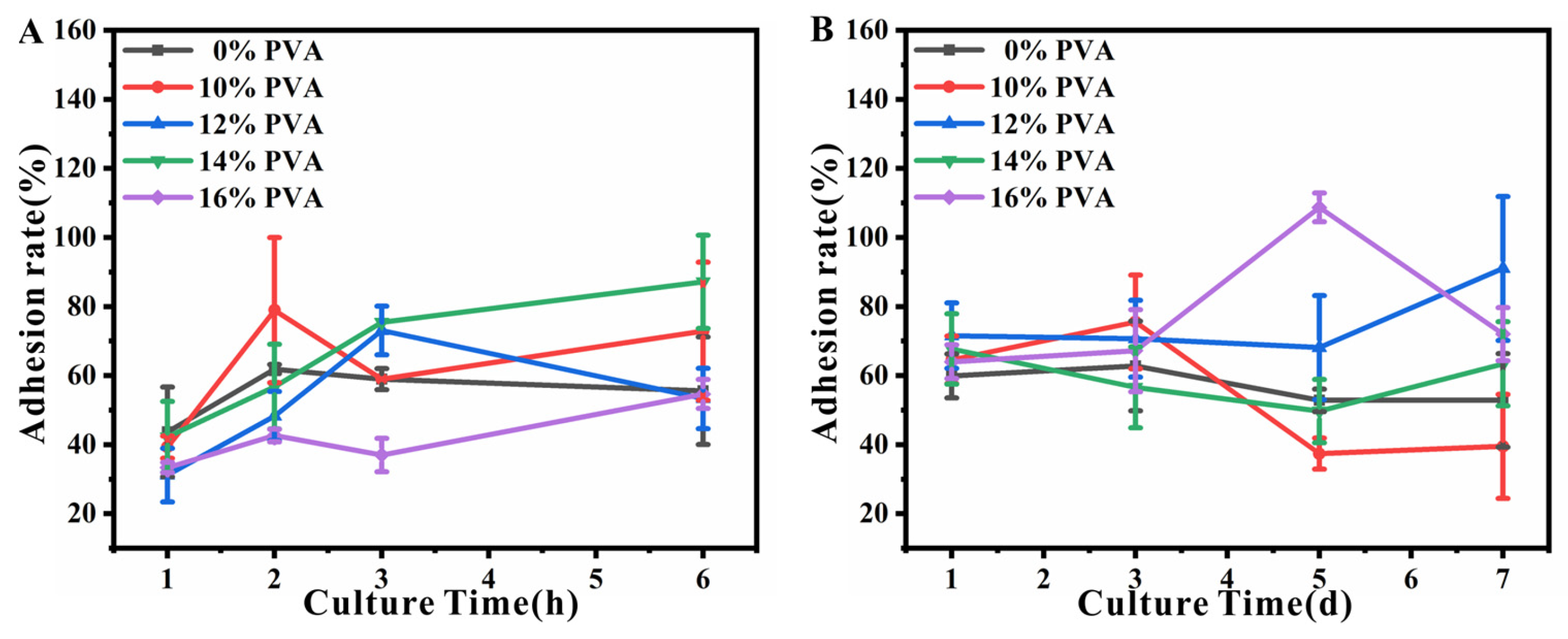
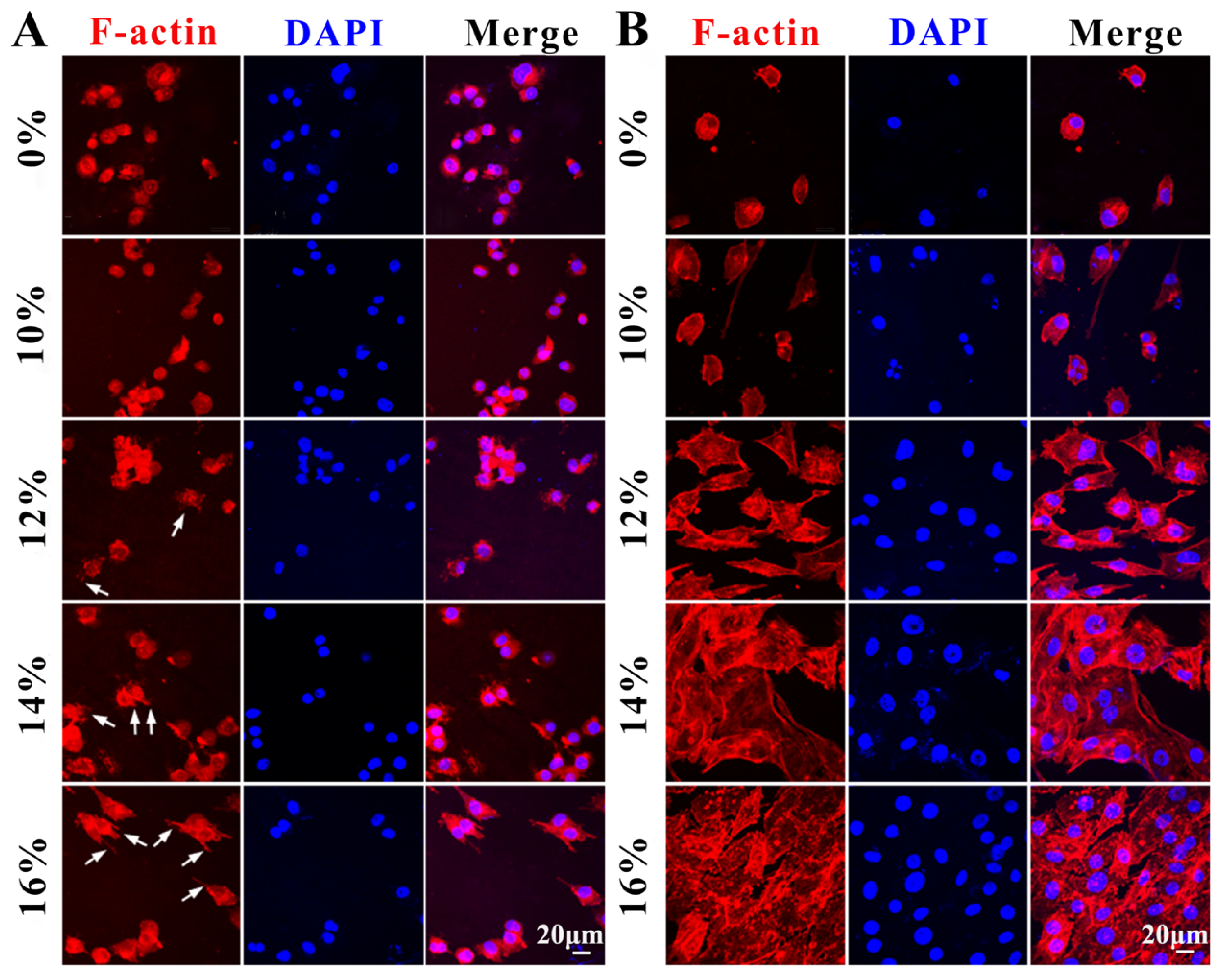
3.6. Cytocompatibility of Decellularized Valves Treated with Different Concentrations of PVA
3.7. Processability of Decellularized Valves Treated with Different Concentrations of PVA—An In Vitro Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoen, F.J. Evolving Concepts of Cardiac Valve Dynamics. Circulation 2008, 118, 1864–1880. [Google Scholar] [CrossRef] [PubMed]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific Aortic Valve Disease: Not Simply a Degenerative Process. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.T.; Gilliland, T.; Maxfield, M.W.; Church, S.; Naito, Y.; Shinoka, T.; Breuer, C.K. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: From the bench to the clinic and back again. Regen. Med. 2012, 7, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, C. Heart valve disease in elderly. World J. Cardiol. 2019, 11, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Iung, B. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Schoen, F.J. Morphology, Clinicopathologic Correlations, and Mechanisms in Heart Valve Health and Disease. Cardiovasc. Eng. Technol. 2016, 9, 126–140. [Google Scholar] [CrossRef]
- van der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Lee, A.; Winlaw, D.S.; Dehghani, F. Materials and manufacturing perspectives in engineering heart valves: A review. Mater. Today Bio 2020, 5, 100038. [Google Scholar] [CrossRef]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2020, 18, 92–116. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.T.; Salacinski, H.J.; Hamilton, G.; Seifalian, A.M. The use of animal models in developing the discipline of cardiovascular tissue engineering: A review. Biomaterials 2004, 25, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Kheradvar, A.; Zareian, R.; Kawauchi, S.; Goodwin, R.L.; Rugonyi, S. Animal models for heart valve research and development. Drug Discov. Today Dis. Models 2017, 24, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Coyan, G.N.; da Mota Silveira-Filho, L.; Matsumura, Y.; Luketich, S.K.; Katz, W.; Badhwar, V.; Wagner, W.R.; D’Amore, A. Acute In Vivo Functional Assessment of a Biodegradable Stentless Elastomeric Tricuspid Valve. J. Cardiovasc. Transl. Res. 2020, 13, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Cesarovic, N.; Lipiski, M.; Falk, V.; Emmert, M.Y. Animals in cardiovascular research. Eur. Heart J. 2020, 41, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, S.R.; Mulderrig, S.; Della Barbera, M.; Vedovelli, L.; Bottigliengo, D.; Tessari, C.; Jockenhoevel, S.; Gregori, D.; Thiene, G.; Korossis, S.; et al. Bioengineered percutaneous heart valves for transcatheter aortic valve replacement: A comparative evaluation of decellularised bovine and porcine pericardia. Mater. Sci. Eng. C 2021, 123, 111936. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ding, J.; Zhu, Z.; Xu, J.; Yi, Y.; Li, Y.; Fan, H.; Bai, S.; Yang, J.; Tang, Y.; et al. Surface biofunctionalization of the decellularized porcine aortic valve with VEGF-loaded nanoparticles for accelerating endothelialization. Mater. Sci. Eng. C 2019, 97, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, C.; Li, M.; Ding, K.; Huang, X.; Liang, X.; Lei, Y.; Jiang, Q.; Wang, Y. Sodium lignosulfonate cross-linked bioprosthetic heart valve materials for enhanced cytocompatibility, improved hemocompatibility, and reduced calcification. Compos. Part B Eng. 2022, 234, 109669. [Google Scholar] [CrossRef]
- Jang, W.; Choi, S.; Kim, S.H.; Yoon, E.; Lim, H.-G.; Kim, Y.J. A Comparative Study on Mechanical and Biochemical Properties of Bovine Pericardium After Single or Double Crosslinking Treatment. Korean Circ. J. 2012, 42, 154–163. [Google Scholar] [CrossRef]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Duisit, J.; Amiel, H.; Wüthrich, T.; Taddeo, A.; Dedriche, A.; Destoop, V.; Pardoen, T.; Bouzin, C.; Joris, V.; Magee, D.; et al. Perfusion-decellularization of human ear grafts enables ECM-based scaffolds for auricular vascularized composite tissue engineering. Acta Biomater. 2018, 73, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, P.E.; Gaudiello, E.; Pippenger, B.; Jaquiery, C.; Klein, T.; Pigeot, S.; Todorov, A.; Feliciano, S.; Banfi, A.; Martin, I. Engineered Extracellular Matrices as Biomaterials of Tunable Composition and Function. Adv. Funct. Mater. 2017, 27, 1605486. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Findeisen, K.; Morticelli, L.; Goecke, T.; Kolbeck, L.; Ramm, R.; Höffler, H.K.; Brandes, G.; Korossis, S.; Haverich, A.; Hilfiker, A. Toward acellular xenogeneic heart valve prostheses: Histological and biomechanical characterization of decellularized and enzymatically deglycosylated porcine pulmonary heart valve matrices. Xenotransplantation 2020, 27, e12617. [Google Scholar] [CrossRef]
- Callington, A.; Long, Q.; Mohite, P.; Simon, A.; Mittal, T.K. Computational fluid dynamic study of hemodynamic effects on aortic root blood flow of systematically varied left ventricular assist device graft anastomosis design. J. Thorac. Cardiovasc. Surg. 2015, 150, 696–704. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Conconi, M.T.; Borgio, L.; Di Liddo, R.; Sartore, L.; Dalzoppo, D.; AmistÀ, P.; Lora, S.; Parnigotto, P.P.; Grandi, C. Evaluation of vascular grafts based on polyvinyl alcohol cryogels. Mol. Med. Rep. 2014, 10, 1329–1334. [Google Scholar] [CrossRef]
- Dahl, S.L.M.; Koh, J.; Prabhakar, V.; Niklason, L.E. Decellularized Native and Engineered Arterial Scaffolds for Transplantation. Cell Transplant. 2003, 12, 659–666. [Google Scholar] [CrossRef]
- Dijkman, P.E.; Driessen-Mol, A.; Frese, L.; Hoerstrup, S.P.; Baaijens, F.P.T. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 2012, 33, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, C.V.L.; de Barros, H.E.A.; Freitas, H.R.; Bufalo, T.C.E.; Dias, E.S.; de Oliveira, J.E.; Marconcini, J.M. PVA/zein nanofibers obtained by solution blow spinning. J. Mater. Sci. 2023, 58, 13518–13529. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; El Damrawi, G.; Al-Shahawy, A. Some studies on calcium phosphate embedded in polyvinyl alcohol matrix before and after γ-irradiation. Phys. B Condens. Matter 2010, 405, 808–816. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Kalambettu, A.; Damodaran, A.; Dharmalingam, S.; Vallam, M.T. Evaluation of Biodegradation of Pineapple Leaf Fiber Reinforced PVA Composites. J. Nat. Fibers 2014, 12, 39–51. [Google Scholar] [CrossRef]
- Akodad, M.; Sellers, S.; Landes, U.; Meier, D.; Tang, G.H.L.; Gada, H.; Rogers, T.; Caskey, M.; Rutkin, B.; Puri, R.; et al. Balloon-Expandable Valve for Treatment of Evolut Valve Failure. JACC Cardiovasc. Interv. 2022, 15, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qiao, W.; Wang, C.; Wang, H.; Zhou, Y.; Gu, S.; Xu, W.; Zhuang, Y.; Shi, J.; Yang, H. Effect of poly (lactic acid) porous membrane prepared via phase inversion induced by water droplets on 3T3 cell behavior. Int. J. Biol. Macromol. 2021, 183, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Schakenraad, J.; Busscher, H.J. Cell—Polymer interactions: The influence of protein adsorption. Colloids Surf. 1989, 42, 331–343. [Google Scholar] [CrossRef]
- Ota, T.; Taketani, S.; Iwai, S.; Miyagawa, S.; Furuta, M.; Hara, M.; Uchimura, E.; Okita, Y.; Sawa, Y. Novel Method of Decellularization of Porcine Valves Using Polyethylene Glycol and Gamma Irradiation. Ann. Thorac. Surg. 2007, 83, 1501–1507. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Wang, Z.; Li, Q.; Tian, N. Simultaneous Enhancement of Strength and Toughness of PLA Induced by Miscibility Variation with PVA. Polymers 2018, 10, 1178. [Google Scholar] [CrossRef]
- Cai, P.; Lu, S.; Yu, J.; Xiao, L.; Wang, J.; Liang, H.; Huang, L.; Han, G.; Bian, M.; Zhang, S.; et al. Injectable nanofiber-reinforced bone cement with controlled biodegradability for minimally-invasive bone regeneration. Bioact. Mater. 2023, 21, 267–283. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; Batista, N.A.; Bavaresco, V.P.; Baranauskas, V.; Ceragioli, H.J.; Peterlevitz, A.C.; Mariolani, J.R.L.; Santana, M.H.A.; Belangero, W.D. In vivo evaluation of hydrogels of polyvinyl alcohol with and without carbon nanoparticles for osteochondral repair. Carbon 2012, 50, 2091–2099. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; Batista, N.A.; Bavaresco, V.P.; Baranauskas, V.; Ceragioli, H.J.; Peterlevitz, A.C.; Santos, A.R.; Belangero, W.D. Polyvinyl alcohol associated with carbon nanotube scaffolds for osteogenic differentiation of rat bone mesenchymal stem cells. Carbon 2012, 50, 450–459. [Google Scholar] [CrossRef]
- Batista, N.A.; Rodrigues, A.A.; Bavaresco, V.P.; Mariolani, J.R.L.; Belangero, W.D. Polyvinyl Alcohol Hydrogel Irradiated and Acetalized for Osteochondral Defect Repair: Mechanical, Chemical, and Histological Evaluation after Implantation in Rat Knees. Int. J. Biomater. 2012, 2012, 582685. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wang, C.; Wang, H.; Xiao, J.; Zhou, Y.; Gu, S.; Xu, W.; Yang, H. Strengthened Decellularized Porcine Valves via Polyvinyl Alcohol as a Template Improving Processability. Polymers 2024, 16, 16. https://doi.org/10.3390/polym16010016
Chen Q, Wang C, Wang H, Xiao J, Zhou Y, Gu S, Xu W, Yang H. Strengthened Decellularized Porcine Valves via Polyvinyl Alcohol as a Template Improving Processability. Polymers. 2024; 16(1):16. https://doi.org/10.3390/polym16010016
Chicago/Turabian StyleChen, Qingqing, Chaorong Wang, Han Wang, Jinfeng Xiao, Yingshan Zhou, Shaojin Gu, Weilin Xu, and Hongjun Yang. 2024. "Strengthened Decellularized Porcine Valves via Polyvinyl Alcohol as a Template Improving Processability" Polymers 16, no. 1: 16. https://doi.org/10.3390/polym16010016
APA StyleChen, Q., Wang, C., Wang, H., Xiao, J., Zhou, Y., Gu, S., Xu, W., & Yang, H. (2024). Strengthened Decellularized Porcine Valves via Polyvinyl Alcohol as a Template Improving Processability. Polymers, 16(1), 16. https://doi.org/10.3390/polym16010016






