Protein-Aided Synthesis of Copper-Integrated Polyaniline Nanocomposite Encapsulated with Reduced Graphene Oxide for Highly Sensitive Electrochemical Detection of Dimetridazole in Real Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
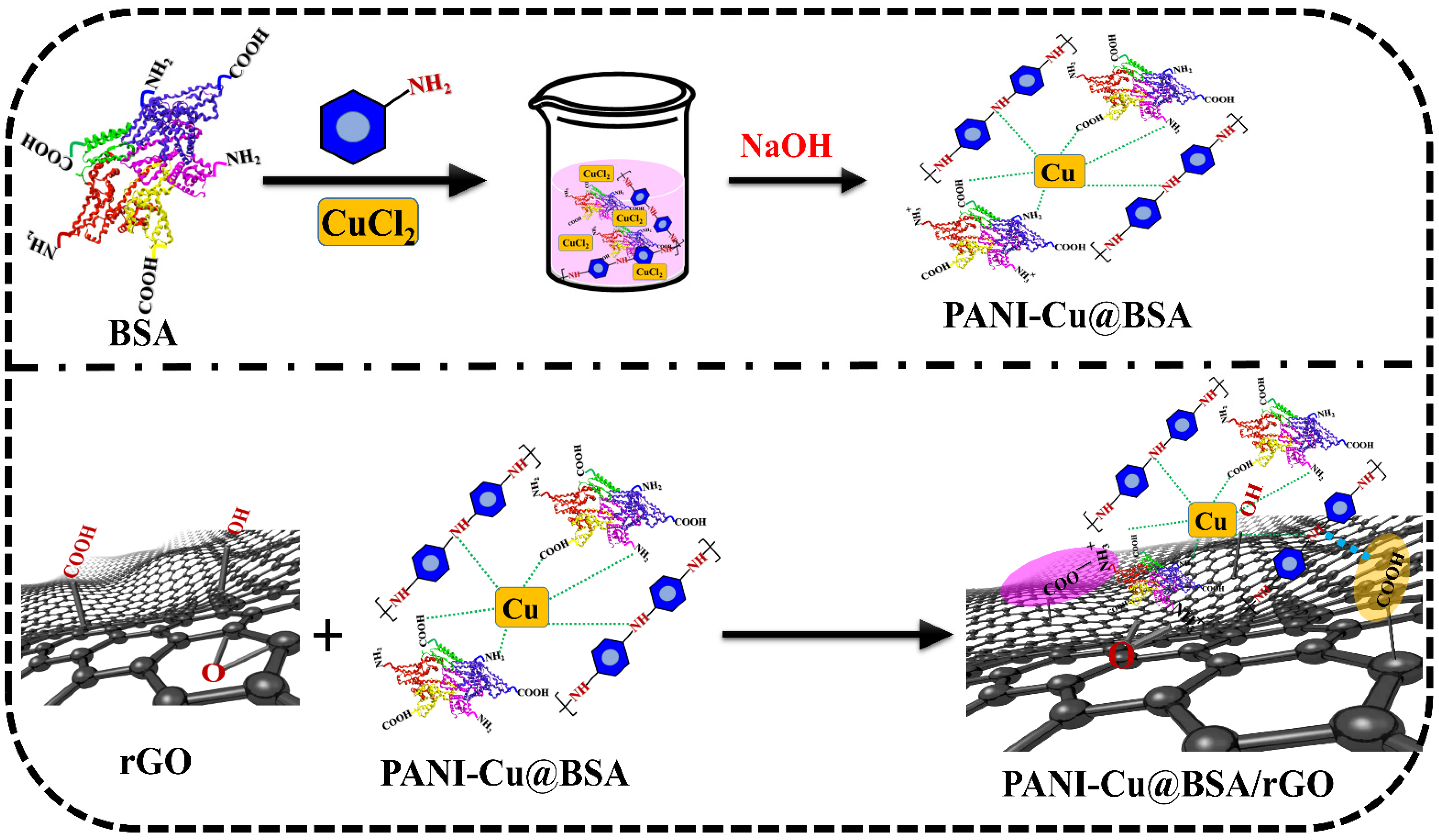
2.2. Preparation of PANI-Cu@BSA and PANI-Cu@BSA/rGO Nanocomposite
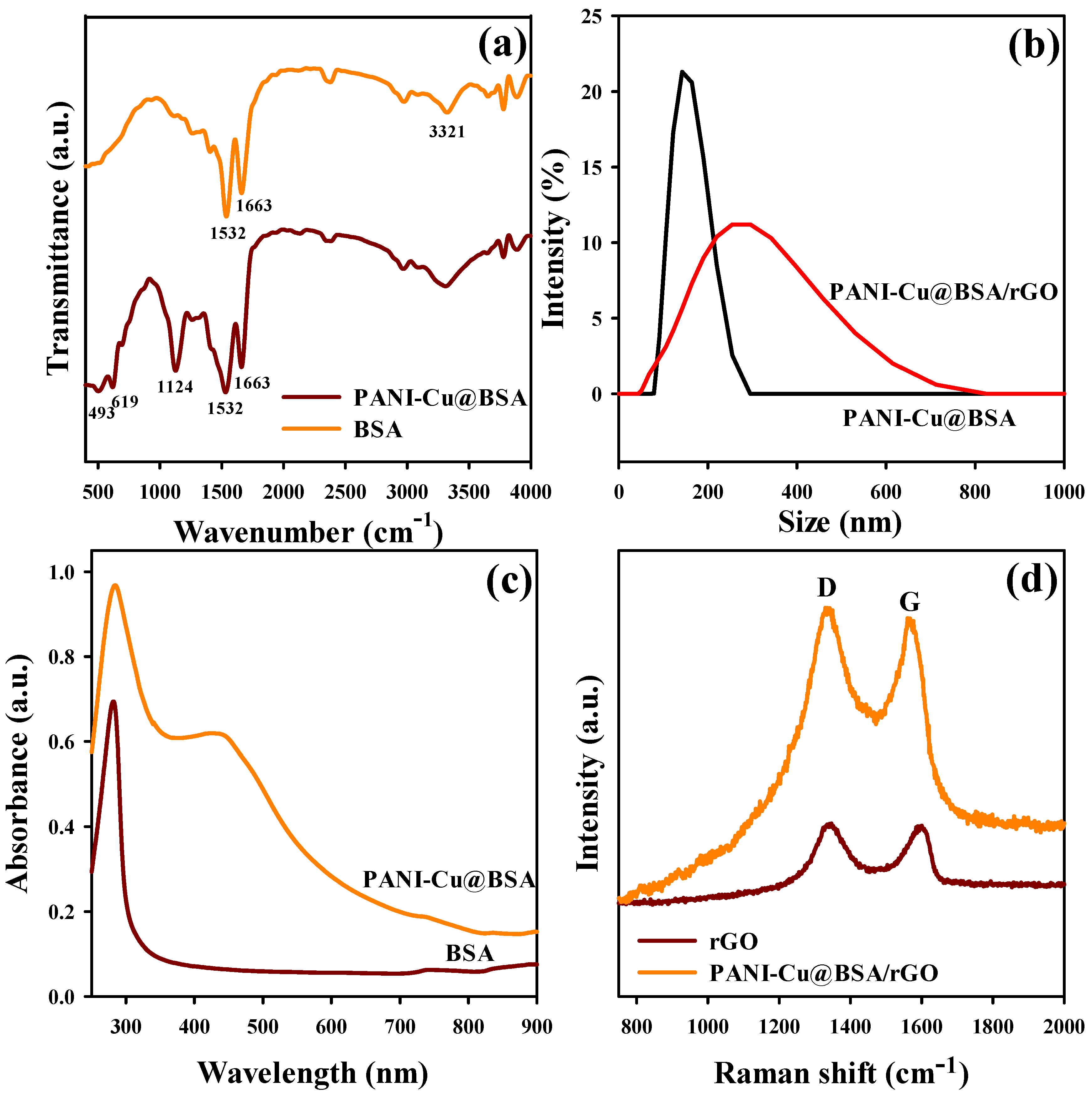
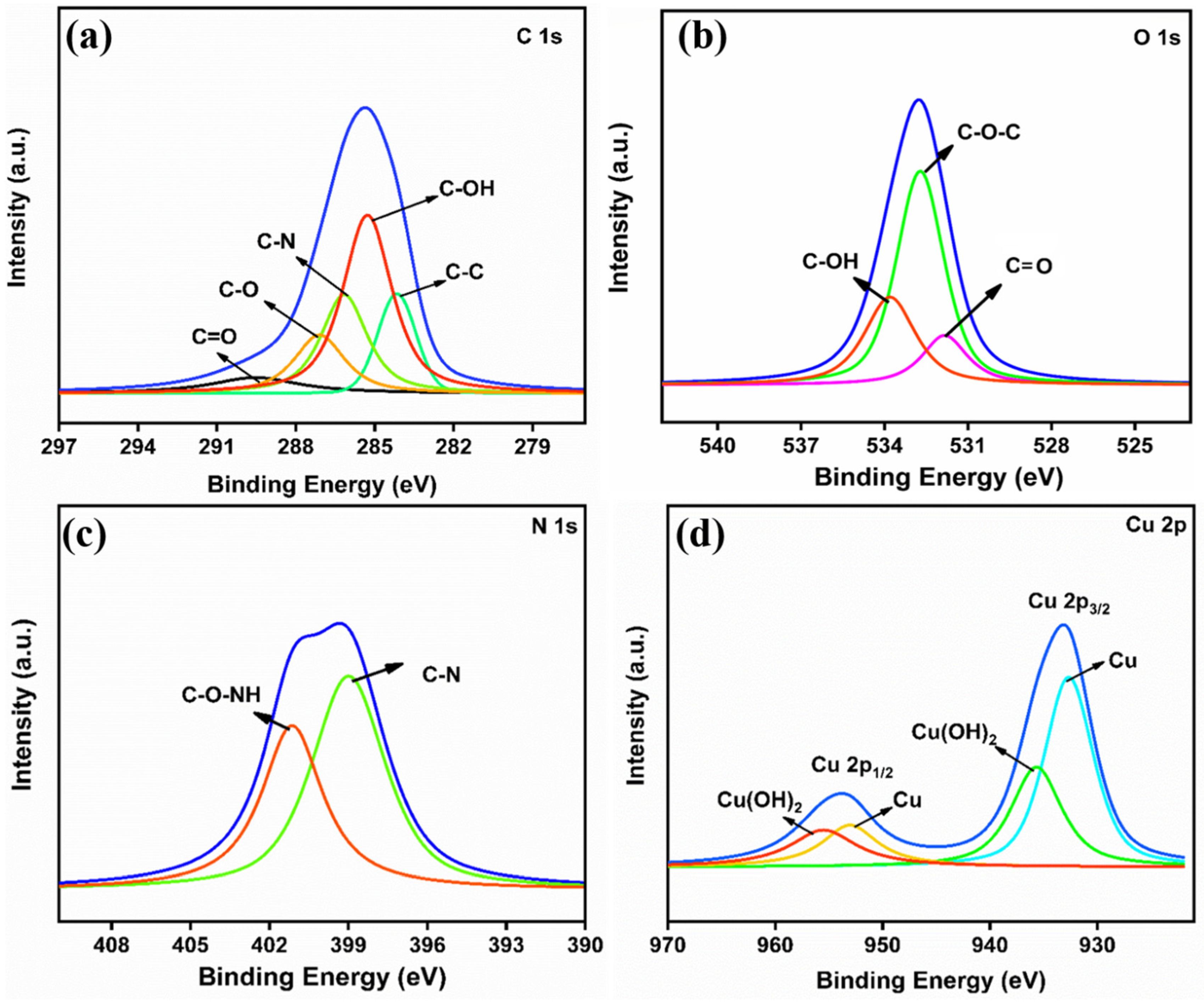
2.3. Characterization
2.4. Electrode Fabrication and Electrochemical Experiments
3. Results and Discussion
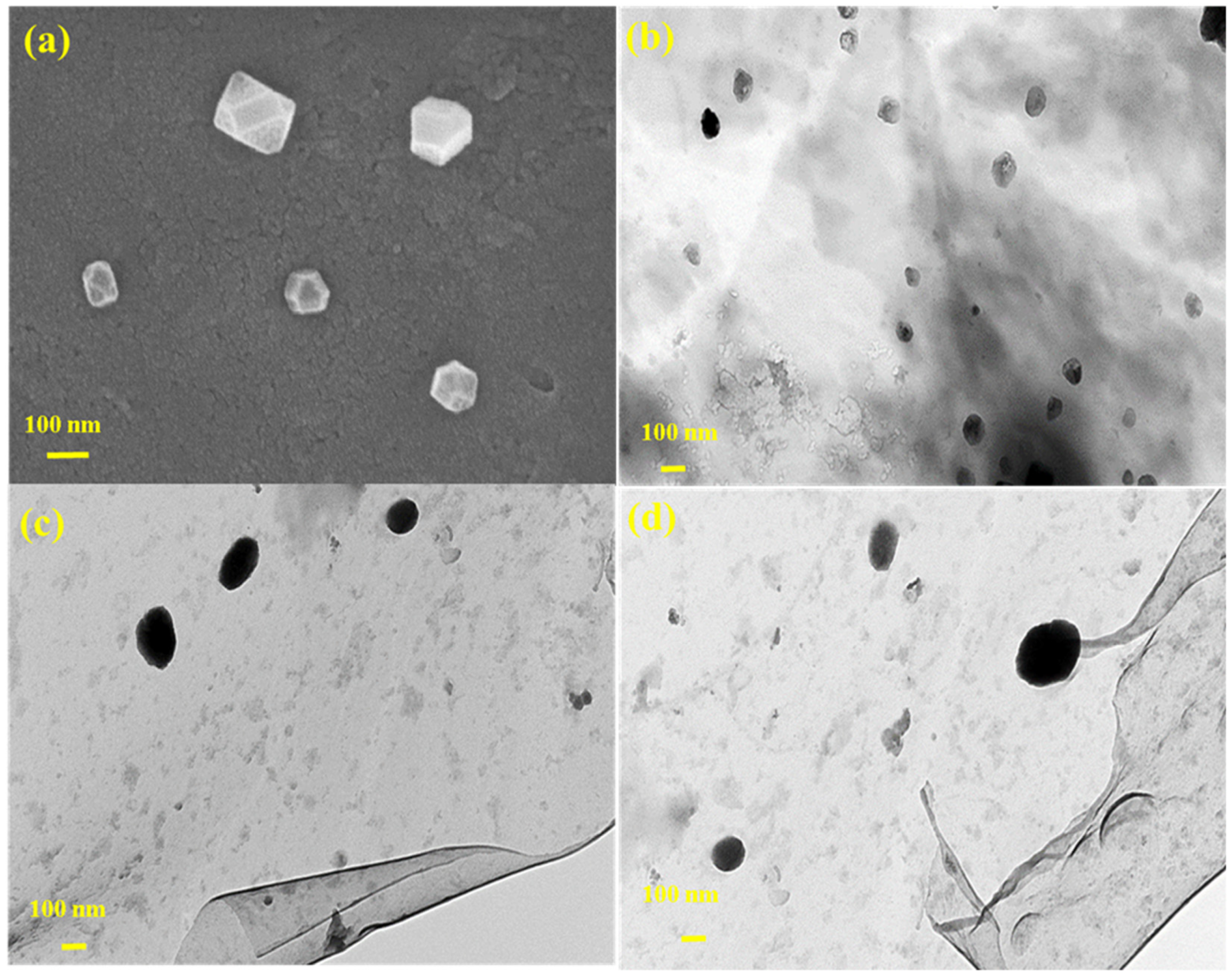
3.1. Morphological and Structural Characteristics of PANI-Cu@BSA/rGO Nanocomposite
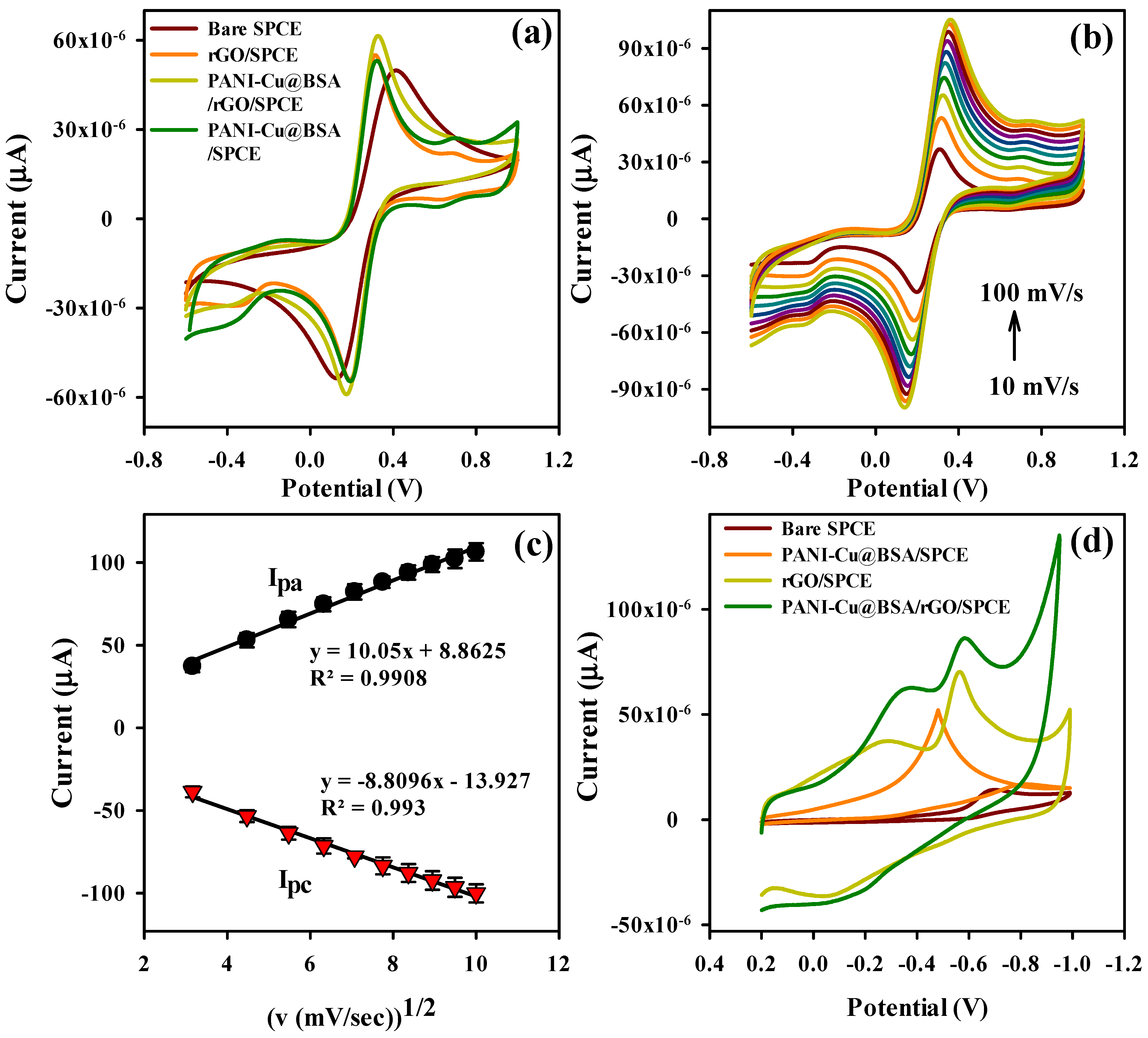
3.2. Electrochemical Characterizations of Modified SPCEs
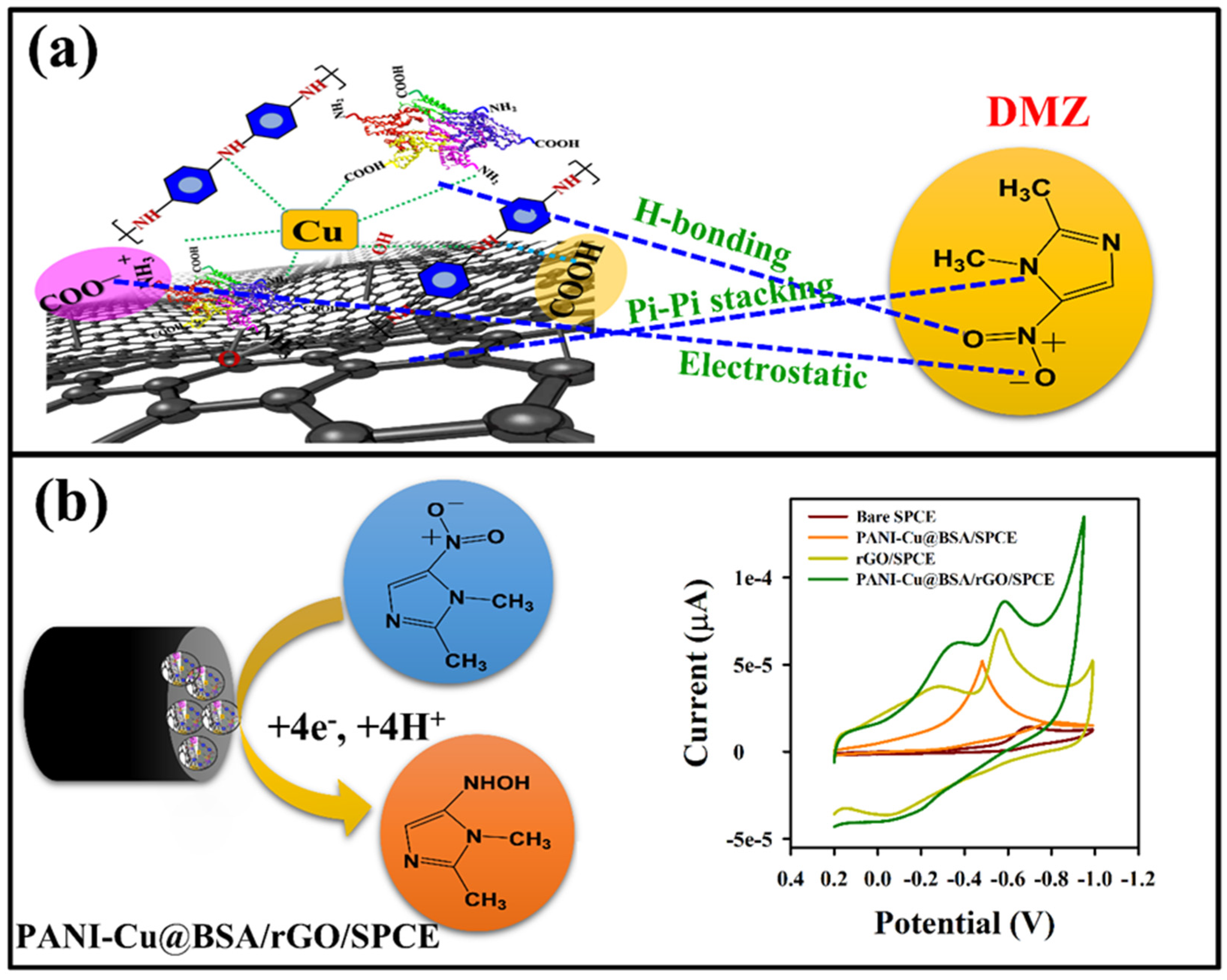
3.3. Electrocatalytic Reduction of DMZ on PANI-Cu@BSA/rGO/SPCE
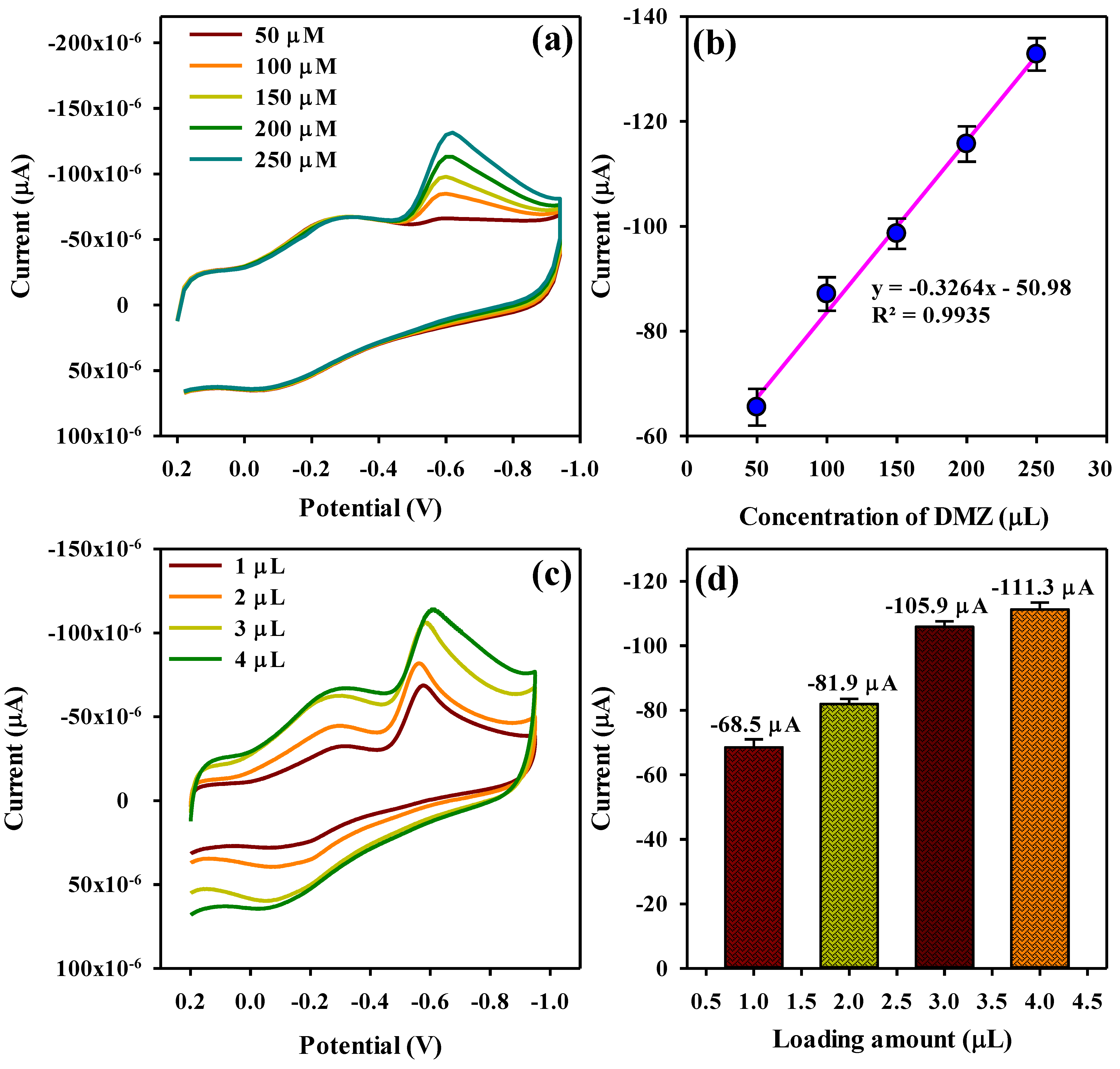
3.4. Quantitative Determination and Selectivity Studies of DMZ
3.5. Repeatability and Stability Studies
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Q.; Liu, J.; Zhu, W.; Dong, Z.; Liu, Z.; Cheng, L. Albumin-assisted synthesis of ultrasmall FeS2 nanodots for imaging-guided photothermal enhanced photodynamic therapy. ACS Appl. Mater. Interfaces 2018, 10, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Lamp, K.C.; Freeman, C.D.; Klutman, N.E.; Lacy, M.K. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin. Pharmacokinet. 1999, 36, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Re, J.L.; Meo, M.P.D.; Laget, M.; Guiraud, H.; Castegnaro, M.; Vanelle, P.; Dumenil, G. Evaluation of the genotoxic activity of metronidazole and dimetridazole in human lymphocytes by the comet assay. Mutat. Res. 1997, 375, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Behera, K.; Kumari, M.; Chang, Y.H.; Chiu, F.C. Chitosan/boron nitride nanobiocomposite films with improved properties for active food packaging applications. Int. J. Biol. Macromol. 2021, 186, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Umesh, N.M.; Jesila, J.A.; Wang, S.F.; Devi, K.S.S.; Govindasamy, M.; Alothman, A.A.; Alshgari, R.A. An enhanced electrochemical performance of in milk, pigeon meat and eggs samples using se nanorods capped with CO3O4 nanoflowers decorated on graphene oxide. Colloids Surf. B Biointerfaces 2021, 200, 111577–111587. [Google Scholar] [CrossRef] [PubMed]
- Stubbings, G.; Bigwood, T. The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC–MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) approach. Anal. Chim. Acta 2009, 637, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ming, T.; Lan, T.; Yu, M.; Wang, H.; Deng, J.; Kong, D.; Yang, S.; Shen, Z. Platinum black/gold nanoparticles/polyaniline modified electrochemical microneedle sensors for continuous in vivo monitoring of pH value. Polymers 2023, 15, 2796. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Bounegru, I. Chitosan-based electrochemical sensors for pharmaceuticals and clinical applications. Polymers 2023, 15, 3539. [Google Scholar] [CrossRef]
- Parshina, A.; Yelnikova, A.; Safronova, E.; Kolganova, T.; Bobreshova, O.; Yaroslavtsev, A. Potentiometric sensor arrays based on hybrid PFSA/CNTs membranes for the analysis of UV-degraded drugs. Polymers 2023, 15, 2682. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Bosman, A.W.; Brancart, J.; Vanderborght, B.; Clemens, F. Supramolecular self-healing sensor fiber composites for damage detection in piezoresistive electronic skin for soft robots. Polymers 2021, 13, 2983. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Balamurugan, K.; Chen, S.M.; Sukanya, R. Facile synthesis of neodymium stannate nanoparticles an effective electrocatalyst for the selective detection of dimetridazole in biological samples. Anal. Chim. Acta 2022, 1190, 339234–339247. [Google Scholar] [CrossRef] [PubMed]
- Bautkinováa, T.; Siftona, A.; Mawunya, K.E.; Dendisováb, M.; Kopeckýc, D.; Ulbrichd, P.; Mazúra, P.; Laachachie, A.; Hassouna, F. New approach for the development of reduced graphene oxide/polyaniline nanocomposites via sacrificial surfactant-stabilized reduced graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2020, 89, 124415–124428. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071–1800106. [Google Scholar] [CrossRef]
- Reddy, K.K.; Bandal, H.; Satyanarayana, M.; Goud, K.Y.; Gobi, K.V.; Jayaramudu, T.; Amalraj, J.; Kim, H. Recent trends in electrochemical sensors for vital biomedical markers using hybrid nanostructured materials. Adv. Sci. 2020, 7, 1902980–1903027. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ahmed, S.; Abdullah, A.Z.; Hakami, J.; Chaudhary, A.A.; Rudayni, H.A.; Khan, S.U.D.; Khan, A.; Basher, N.S. Nanostructured material-based optical and electrochemical detection of amoxicillin antibiotic. Luminescence 2023, 38, 1064–1086. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Asif, M.; Liu, Q.; Xiao, F.; Liu, H.; Xia, B.Y. Noble metal construction for electrochemical nonenzymatic glucose detection. Adv. Mater. Technol. 2023, 8, 220027–220041. [Google Scholar] [CrossRef]
- Sudha, V.; Murugadoss, G.; Thangamuthu, R. Structural and morphological tuning of Cu-based metal oxide nanoparticles by a facile chemical method and highly electrochemical sensing of sulphite. Sci. Rep. 2021, 11, 3413–3425. [Google Scholar] [CrossRef]
- Dorozhko, E.V.; Gashevskay, A.S.; Korotkova, E.I.; Barek, J.; Vyskocil, V.; Eremin, S.A.; Galunin, E.V.; Saqib, M. A copper nanoparticle-based electrochemical immunosensor for carbaryl detection. Talanta 2021, 228, 122174–122182. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent progress in hybrid conducting polymers and metal oxide nanocomposite for room-temperature gas sensor applications: A review. Sens. Actuators A Phys. 2023, 359, 114472–114502. [Google Scholar] [CrossRef]
- Khangarot, R.K.; Khandelwal, M.; Singh, R. Copper-Based Polymer Nanocomposites: Application as Sensors. In Metal Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 489–508. [Google Scholar]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-performance perovskite composite electrocatalysts enabled by controllable interface engineering. Small 2021, 17, 2101573–2101583. [Google Scholar] [CrossRef]
- Kushwaha, C.S.; Singh, P.; Shukla, S.K.; Chehimi, M.M. Advances in conducting polymer nanocomposite based chemical sensors: An overview. Mater. Sci. Eng. B 2022, 284, 115856–115890. [Google Scholar] [CrossRef]
- Kazemi, F.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. Biosensing applications of polyaniline (PANI)-based nanocomposites: A review. Polym. Rev. 2021, 61, 553–597. [Google Scholar] [CrossRef]
- Yang, D.; Wang, J.; Cao, Y.; Tong, X.; Hua, T.; Qin, R.; Shao, Y. Polyaniline-based biological and chemical sensors: Sensing mechanism, configuration design, and perspective. ACS Appl. Electron. Mater. 2023, 5, 593–611. [Google Scholar] [CrossRef]
- Firda, P.B.D.; Malik, Y.T.; Oh, J.K.; Wujcik, E.K.; Jeon, J.W. Enhanced chemical and electrochemical stability of polyaniline-based layer-by-layer films. Polymers 2021, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Goswami, B.; Mahanta, D. Fe3O4-polyaniline nanocomposite for non-enzymatic electrochemical detection of 2, 4-dichlorophenoxyacetic acid. ACS Omega 2021, 6, 17239–17246. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Sahoo, P.K.; Sharma, A.; Satpati, A.K. Interfacial polymerized RGO/MnFe2O4/polyaniline fibrous nanocomposite supported glassy carbon electrode for selective and ultrasensitive detection of nitrite. Sens. Actuators B Chem. 2020, 309, 127763–127775. [Google Scholar] [CrossRef]
- Mutharani, B.; Tsai, H.C.; Lai, J.Y.; Chen, S.M. Protein-assisted biomimetic synthesis of nanoscale gadolinium integrated polypyrrole for synergetic and ultrasensitive electrochemical assays of nicardipine in biological samples. Anal. Chim. Acta 2022, 1199, 339567–339580. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.B.; Gao, H.L.; Yao, H.B.; Liu, L.; Cölfen, H.; Liu, G.; Chen, S.M.; Li, S.K.; Yan, Y.X.; Liu, Y.Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and peptide-directed syntheses of inorganic materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef]
- Xionga, Y.; Sun, F.; Liu, P.; Yang, Z.; Cao, J.; Liu, H.; Liu, P.; Hu, J.; Xu, Z.; Yang, S. A biomimetic one-pot synthesis of versatile Bi2S3/FeS2 theranostic nanohybrids for tumor-targeted photothermal therapy guided by CT/MR dual-modal imaging. Chem. Eng. J. 2019, 378, 122172–122184. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Ke, H.; Wang, Q.; Lv, X.; Wu, H.; Tang, Y.; Yang, X.; Chen, C.; Zhao, Y.; et al. Protein-nanoreactor-assisted synthesis of semiconductor nanocrystals for efficient cancer theranostics. Adv. Mater. 2016, 28, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, J.; Xue, H.; Hu, Y.; Liu, Y.; Lin, G.; Liu, L.; Xu, R. Applications of human and bovine serum albumins in biomedical engineering: A review. Int. J. Biol. Macromol. 2023, 253, 126914–126933. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Askari, E.; Naghib, S.M.; Salehi, Z. Bovine serum albumin-functionalized graphene-decorated strontium as a potent complex nanoparticle for bone tissue engineering. Sci. Rep. 2022, 12, 12336–12349. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sheng, Q.; Zhang, S.; Zheng, X.; Zheng, J. One-pot synthesis of Fe3O4/polypyrrole/graphene oxide nanocomposites for electrochemical sensing of hydrazine. Microchim. Acta 2017, 184, 2219–2226. [Google Scholar] [CrossRef]
- Hashemi, P.; Bagheri, H.; Afkhami, A.; Ardakani, Y.H.; Madrakian, T. Fabrication of a novel aptasensor based on three-dimensional reduced graphene oxide/polyaniline/gold nanoparticle composite as a novel platform for high sensitive and specific cocaine detection. Anal. Chim. Acta 2017, 996, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Bai, W.; Zhai, J.; Zhou, S.; Cui, C.; Wang, W.; Cheng, C.; Ren, E.; Xiao, H.; Zhou, M.; Zhang, J.; et al. Graphene oxide nanosheets and Ni nanoparticles coated on glass fabrics modified with bovine serum albumin for electromagnetic shielding. ACS Appl. Nano Mater. 2022, 5, 8491–8501. [Google Scholar] [CrossRef]
- Manna, R.; Srivastava, S.K. Reduced graphene oxide/Fe3O4/polyaniline ternary composites as a superior microwave absorber in the shielding of electromagnetic pollution. ACS Omega 2021, 6, 9164–9175. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Zeng, J.; Cheng, Z.; Liu, Z. Albumin-NIR dye self-assembled nanoparticles for photoacoustic pH imaging and pH-responsive photothermal therapy effective for large tumors. Biomaterials 2016, 98, 23–30. [Google Scholar] [CrossRef]
- Strozyk, M.S.; Chanana, M.; Santos, I.P.; Juste, J.P.; Marz, L.M.L. Protein/polymer-based dual-responsive gold nanoparticles with pH-dependent thermal sensitivity. Adv. Funct. Mater. 2012, 22, 1436–1444. [Google Scholar] [CrossRef]
- Bhatt, M.; Maity, D.; Hingu, V.; Suresh, E.; Ganguly, B.; Paul, P. Functionalized calix[4]arene as colorimetric dual sensor for Cu(II) and cysteine in aqueous media: Experimental and computational study. New J. Chem. 2017, 41, 12541–12553. [Google Scholar] [CrossRef]
- Selvi, S.V.; Rajaji, U.; Chen, S.M.; Jebaranjitham, N. Floret-like manganese doped tin oxide anchored reduced graphene oxide for electrochemical detection of dimetridazole in milk and egg samples. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127733–127746. [Google Scholar] [CrossRef]
- Mutharani, B.; Behera, K.; Chang, Y.H.; Chiu, F.C. Devising a universal tailored monomer molecular strategy for SiOx/carbon hollow spheres as a synergistic electrocatalyst in azathioprine sensing. Mater. Today Chem. 2022, 26, 101058–101071. [Google Scholar] [CrossRef]
- Ma, X.; Li, J.; Luo, J.; Liu, C.; Li, S. Electrochemical sensor for the determination of dimetridazole using a 3D Cu2O/ErGO-modified electrode. Anal. Methods 2018, 10, 3380–3385. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Mokhtari, F.; Shadjou, N.; Eftekhari, A.; Mokhtarzadeh, A.; Gharamaleki, V.J.; Mahboob, S. Poly arginine-graphene quantum dots as a biocompatible and non-toxic nanocomposite: Layer-by-layer electrochemical preparation, characterization and non-invasive malondialdehyde sensory application in exhaled breath condensate. Mater. Sci. Eng. C 2017, 75, 247–258. [Google Scholar] [CrossRef]


| Modified Electrode Material | Method | Linear Range (μM) | LOD | Ref. |
|---|---|---|---|---|
| Se-Co3O4@GO-NC/GCE | DPV | 0.02–83.72 | 3.4 nM | [5] |
| DM/Nd2Sn2O7/GCE | DPV | 0.01-1453 | 6.0 nM | [11] |
| Mn-SnO@rGO/GCE | DPV | 0.009–1291 | 2.0 nM | [43] |
| Cu2O/ErGO/GCE | DPV | 0.03–0.15 | 3.6 nM | [45] |
| PARG-GQDs/GCE | DPV | 0.03–0.15 | 3.6 nM | [46] |
| PANI-Cu@BSA/rGO/SPCE | LSV | 0.79–2057 | 1.78 nM | This work |
| Samples | Loaded (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Human blood serum | 0.01 | 0.009 | 90.0 | 2.16 |
| 0.1 | 0.095 | 95.0 | 2.38 | |
| 1 | 0.97 | 97.0 | 1.32 | |
| 5 | 4.6 | 92.0 | 2.12 | |
| 10 | 10.0 | 100.0 | 1.58 | |
| 15 | 14.5 | 96.6 | 3.21 | |
| Rat blood serum | 0.01 | 0.0101 | 101.0 | 1.91 |
| 0.1 | 0.099 | 99.0 | 1.81 | |
| 1 | 1.01 | 101.0 | 2.16 | |
| 5 | 5.0 | 100.0 | 1.51 | |
| 10 | 9.8 | 98.0 | 2.98 | |
| 15 | 15.1 | 100.6 | 3.12 | |
| Egg | 0.01 | 0.0099 | 99.0 | 2.08 |
| 0.1 | 0.098 | 98.5 | 1.52 | |
| 1 | 0.97 | 97.0 | 2.48 | |
| 5 | 4.9 | 98.0 | 1.85 | |
| 10 | 10 | 100 | 3.48 | |
| 15 | 14.95 | 99.6 | 2.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behera, K.; Mutharani, B.; Chang, Y.-H.; Kumari, M.; Chiu, F.-C. Protein-Aided Synthesis of Copper-Integrated Polyaniline Nanocomposite Encapsulated with Reduced Graphene Oxide for Highly Sensitive Electrochemical Detection of Dimetridazole in Real Samples. Polymers 2024, 16, 162. https://doi.org/10.3390/polym16010162
Behera K, Mutharani B, Chang Y-H, Kumari M, Chiu F-C. Protein-Aided Synthesis of Copper-Integrated Polyaniline Nanocomposite Encapsulated with Reduced Graphene Oxide for Highly Sensitive Electrochemical Detection of Dimetridazole in Real Samples. Polymers. 2024; 16(1):162. https://doi.org/10.3390/polym16010162
Chicago/Turabian StyleBehera, Kartik, Bhuvanenthiran Mutharani, Yen-Hsiang Chang, Monika Kumari, and Fang-Chyou Chiu. 2024. "Protein-Aided Synthesis of Copper-Integrated Polyaniline Nanocomposite Encapsulated with Reduced Graphene Oxide for Highly Sensitive Electrochemical Detection of Dimetridazole in Real Samples" Polymers 16, no. 1: 162. https://doi.org/10.3390/polym16010162
APA StyleBehera, K., Mutharani, B., Chang, Y.-H., Kumari, M., & Chiu, F.-C. (2024). Protein-Aided Synthesis of Copper-Integrated Polyaniline Nanocomposite Encapsulated with Reduced Graphene Oxide for Highly Sensitive Electrochemical Detection of Dimetridazole in Real Samples. Polymers, 16(1), 162. https://doi.org/10.3390/polym16010162







