Vitrimer Nanocomposites for Highly Thermal Conducting Materials with Sustainability
Abstract
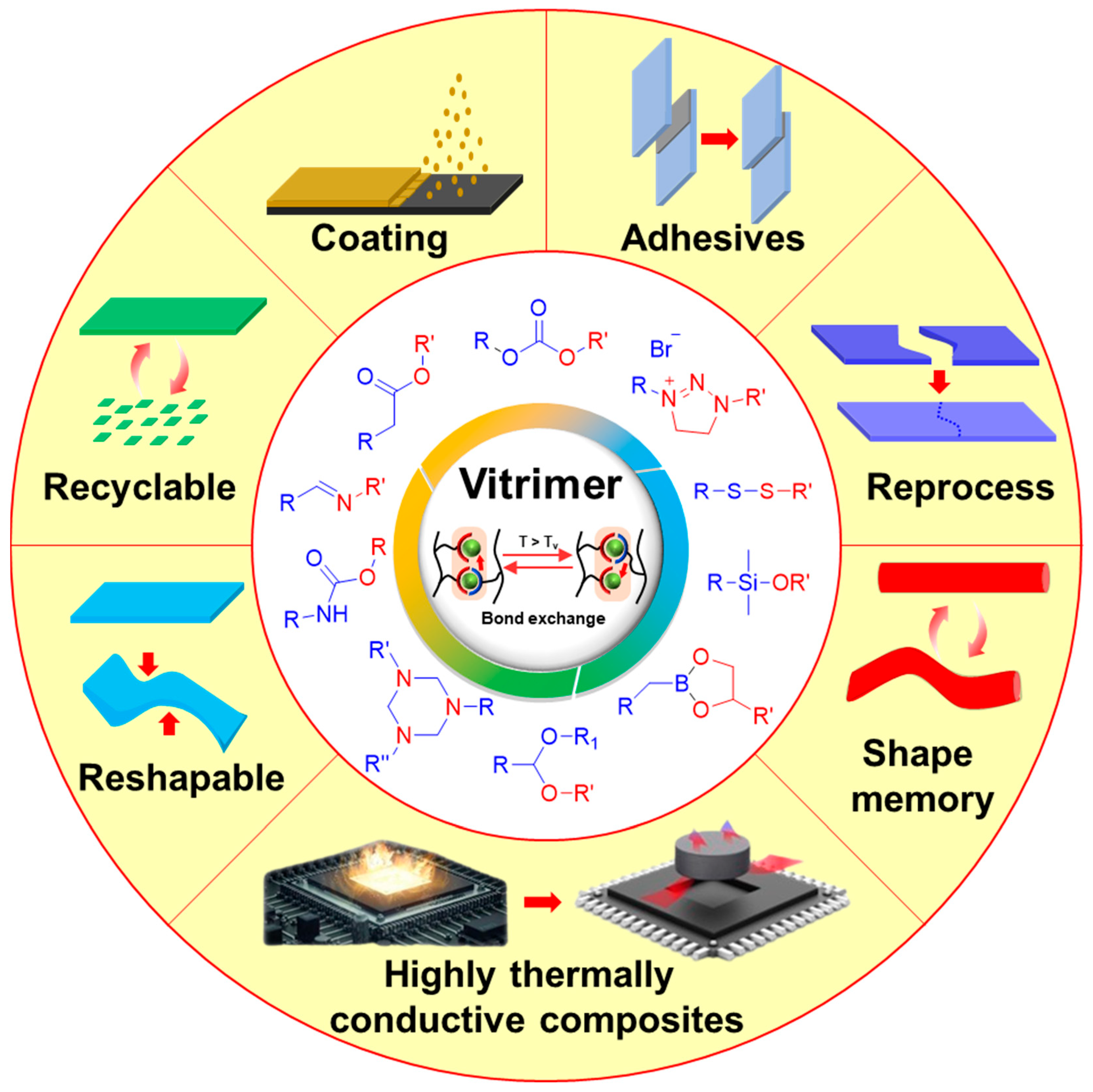
1. Introduction
2. Vitrimer-Assisted Filler Orientation for the Highly Thermal Conducting Pathway of Nanocomposites
3. Reprocessability and Recyclability of Vitrimer-Assisted Filler Nanocomposites
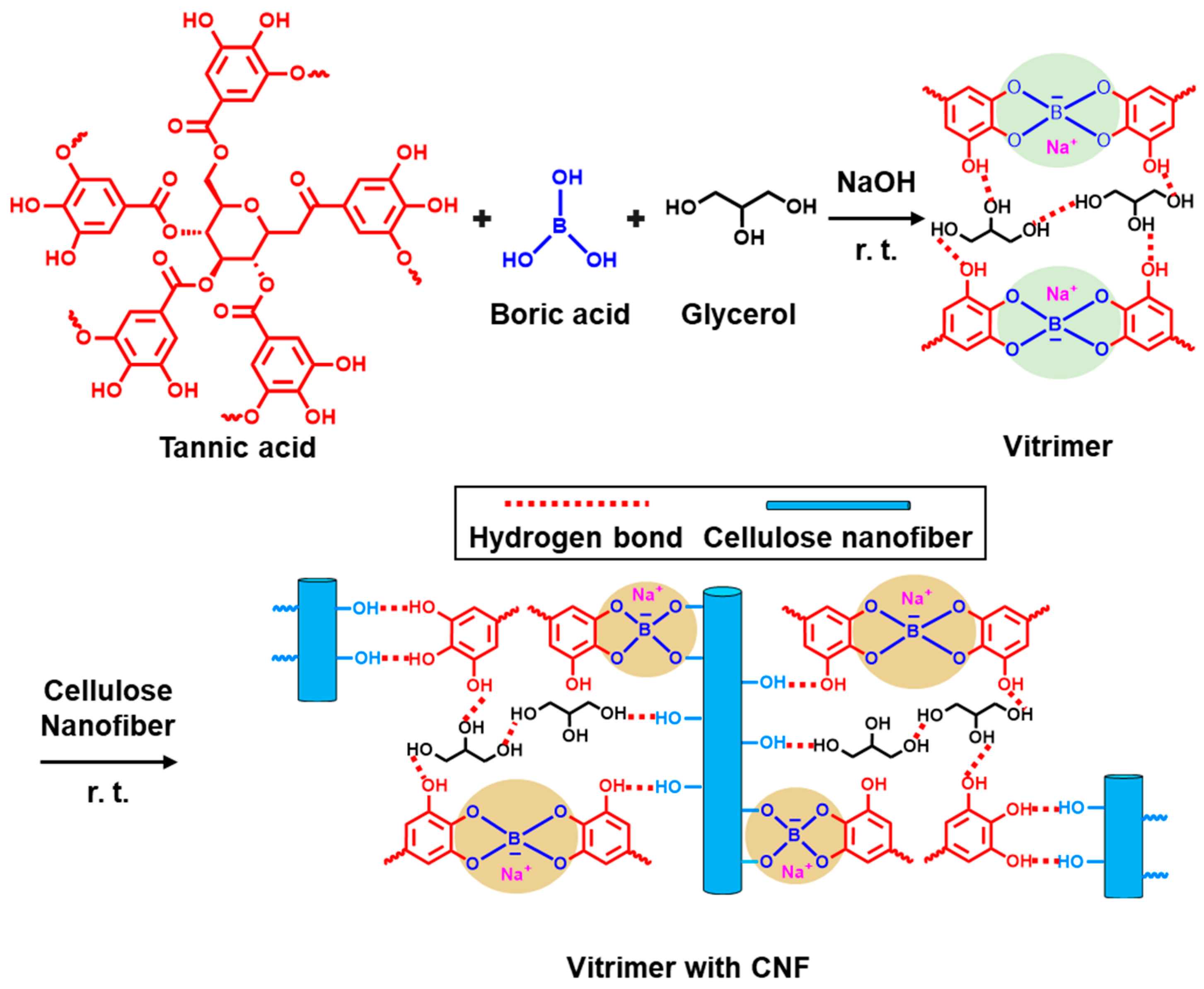
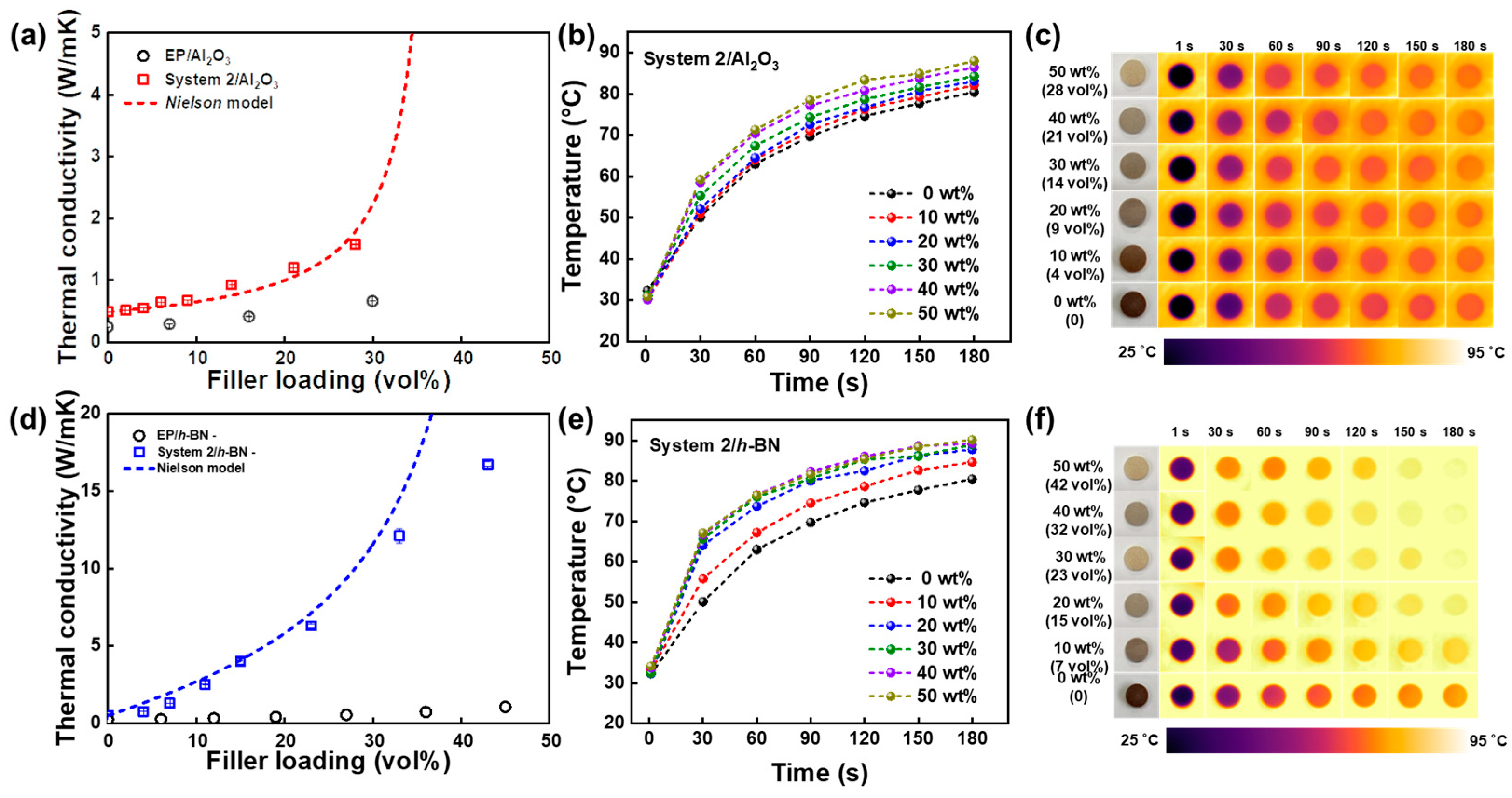
4. Natural Supramolecule-Based Vitrimer Nanocomposites Containing a Large Thermal Pathway
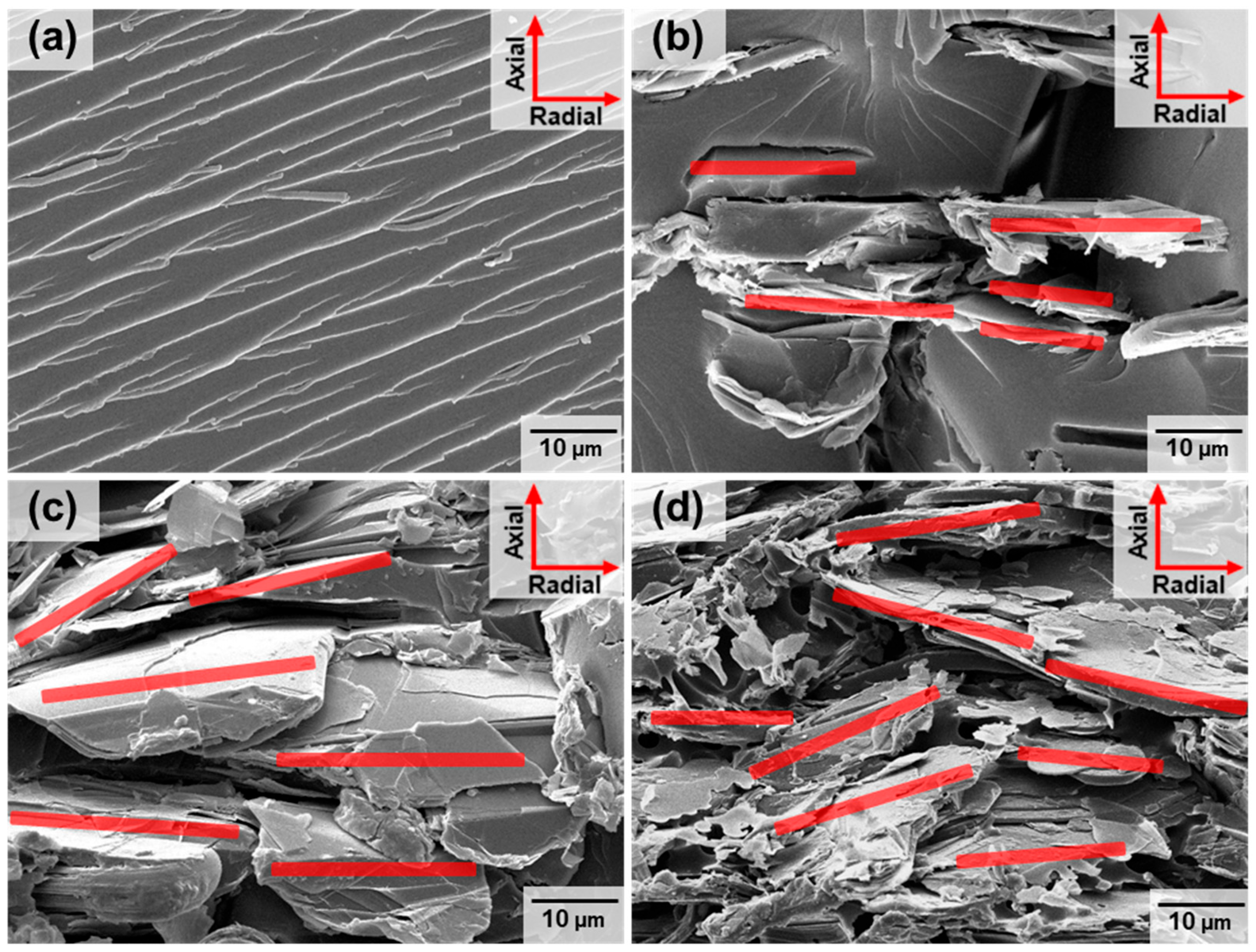
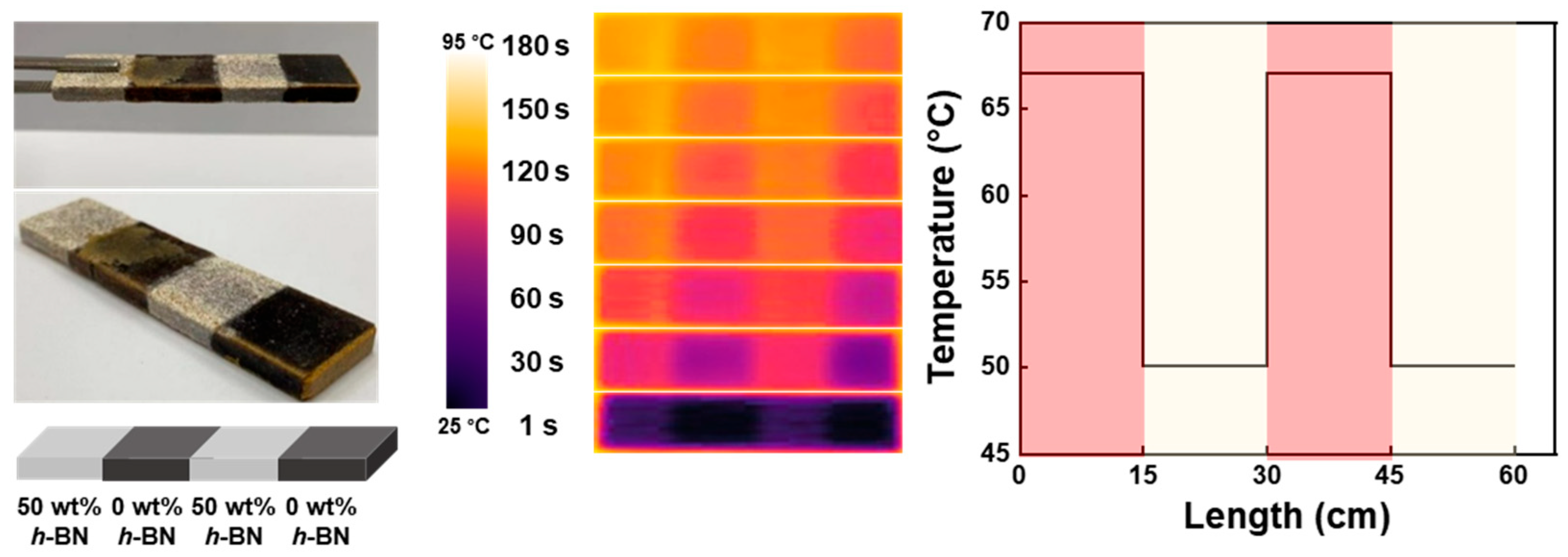
5. Thermal Grating Structure Using Reprocessability of Vitrimer
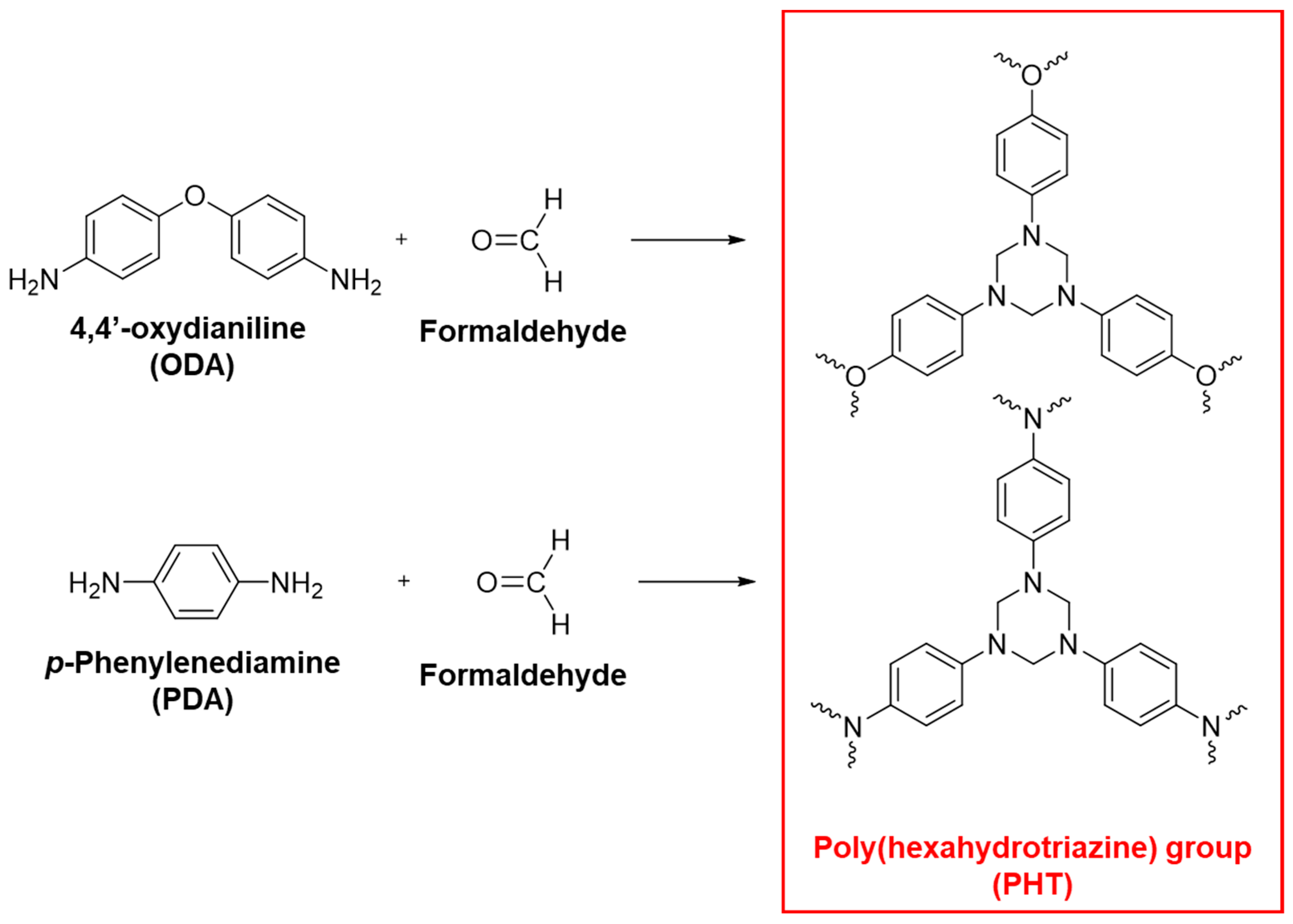
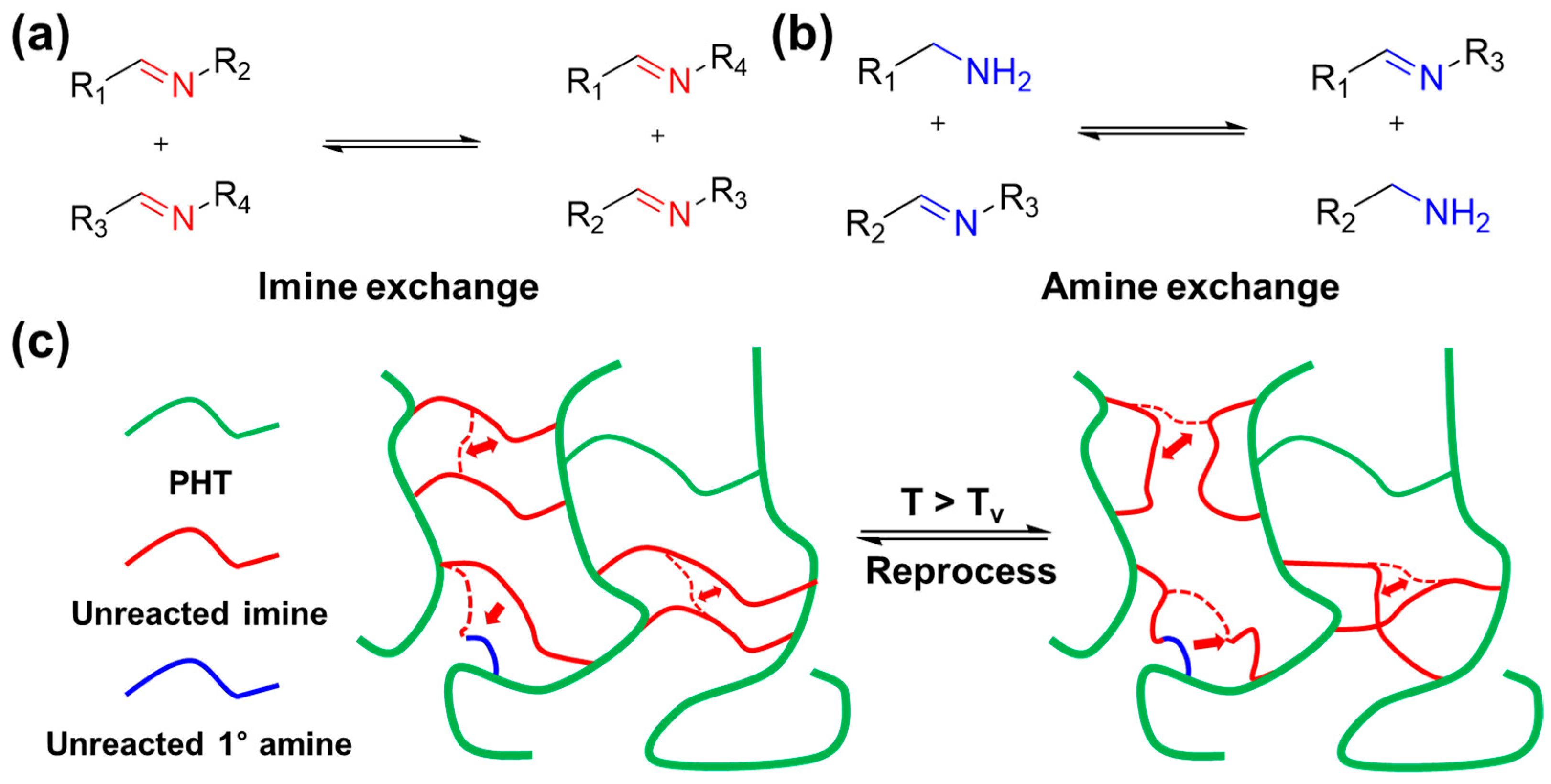
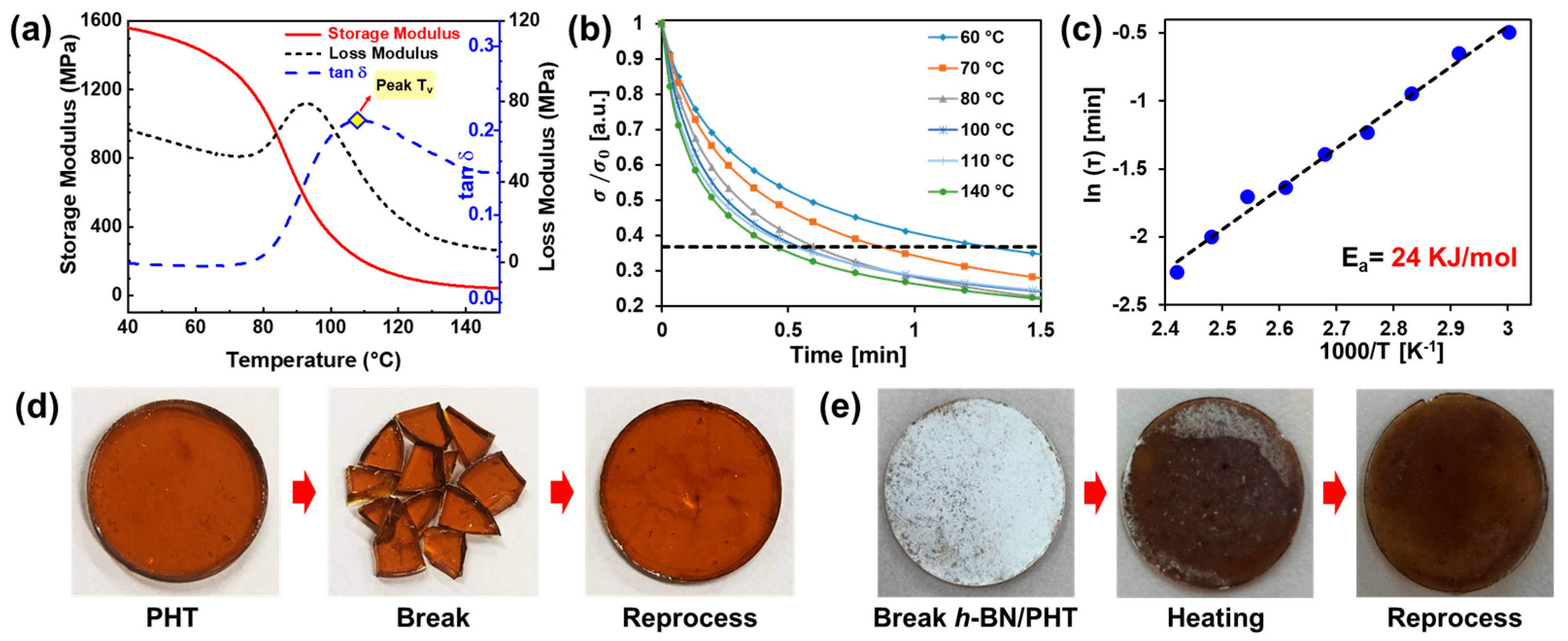
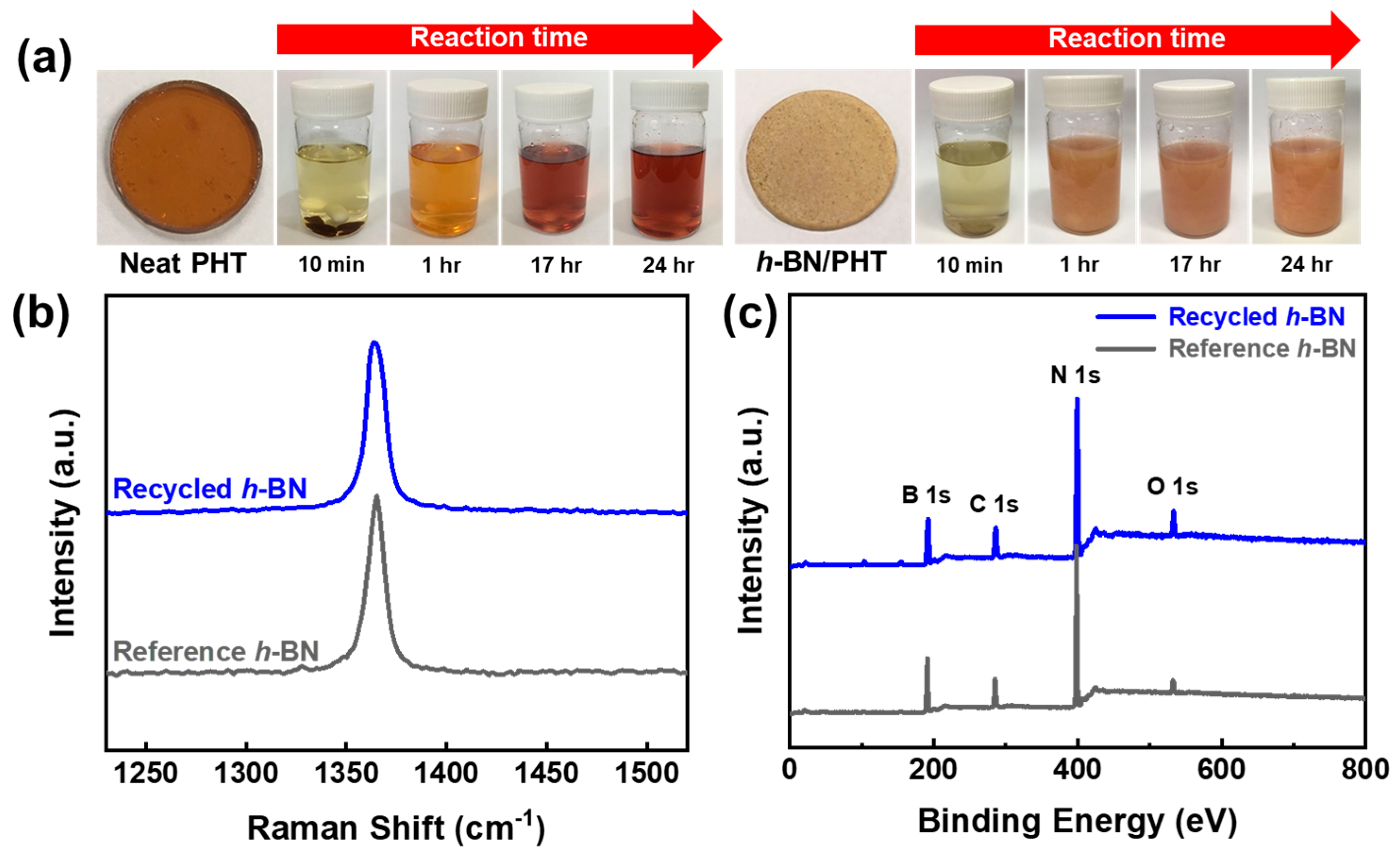
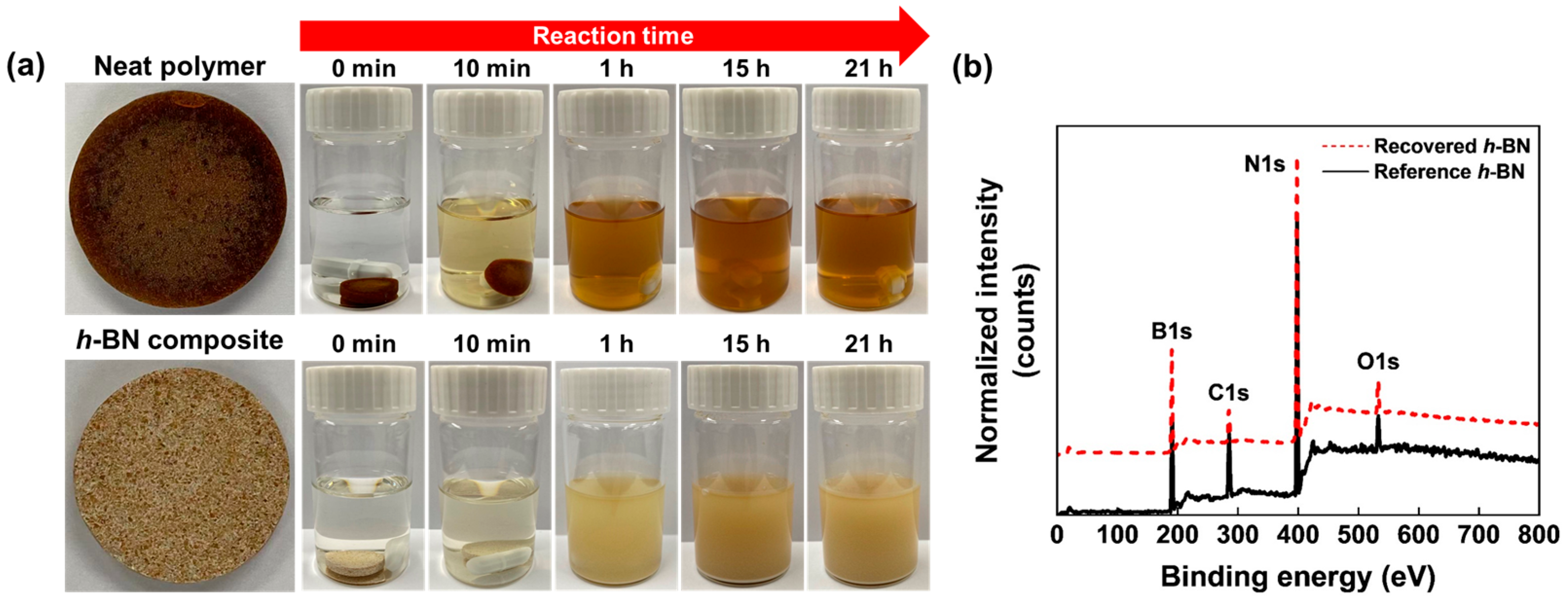
6. Recyclability of Vitrimer and Sustainability of Vitrimer Nanocomposites
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Sanka, R.V.S.P.; Binder, W.H.; Parthasarthy, V.; Rana, S.; Karak, N. Vitrimers: Associative Dynamic Covalent Adaptive Networks in Thermoset Polymers. Chem. Eng. J. 2020, 385, 123820. [Google Scholar] [CrossRef]
- Lucherelli, M.A.; Duval, A.; Avérous, L. Biobased Vitrimers: Towards Sustainable and Adaptable Performing Polymer Materials. Prog. Polym. Sci. 2022, 127, 101515. [Google Scholar] [CrossRef]
- Hammer, L.; Van Zee, N.J.; Nicolaÿ, R. Dually Crosslinked Polymer Networks Incorporating Dynamic Covalent Bonds. Polymers 2021, 13, 396. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, L.; Chen, Z.; Zhang, Y.; Xiao, P.; Yu, S.; Wu, Y.; Zhao, X. Multi-Functional Epoxy Vitrimers: Controllable Dynamic Properties, Multiple-Stimuli Response, Crack-Healing and Fracture-Welding. Compos. Sci. Technol. 2022, 221, 109364. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, P.; Lamm, M.E.; Sha, Y.; Kurnaz, L.B.; Ma, Y.; Zhou, Y. Plant Oil-Derived Vitrimers-Graphene Composites with Self-Healing Ability Triggered by Multiple Stimuli. Compos. B Eng. 2023, 259, 110704. [Google Scholar] [CrossRef]
- Reisinger, D.; Kaiser, S.; Rossegger, E.; Alabiso, W.; Rieger, B.; Schlögl, S. Introduction of Photolatent Bases for Locally Controlling Dynamic Exchange Reactions in Thermo-activated Vitrimers. Angew. Chem. Int. Ed. Engl. 2021, 60, 14302–14306. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently Crosslinked Polymers with Dynamic Network Topology. Prog. Polym. Sci. 2020, 104, 101233. [Google Scholar] [CrossRef]
- Perego, A.; Khabaz, F. Effect of Bond Exchange Rate on Dynamics and Mechanics of Vitrimers. J. Polym. Sci. 2021, 59, 2590–2602. [Google Scholar] [CrossRef]
- Krishnan, B.P.; Saalwaechter, K.; Adjedje, V.K.B.; Binder, W.H. Design, Synthesis and Characterization of Vitrimers with Low Topology Freezing Transition Temperature. Polymers 2022, 14, 2456. [Google Scholar] [CrossRef]
- Hubbard, A.M.; Ren, Y.; Konkolewicz, D.; Sarvestani, A.; Picu, C.R.; Kedziora, G.S.; Roy, A.; Varshney, V.; Nepal, D. Vitrimer Transition Temperature Identification: Coupling Various Thermomechanical Methodologies. ACS Appl. Polym. Mater. 2021, 3, 1756–1766. [Google Scholar] [CrossRef]
- Xia, J.; Kalow, J.A.; Olvera de la Cruz, M. Structure, Dynamics, and Rheology of Vitrimers. Macromolecules 2023, 56, 8080–8093. [Google Scholar] [CrossRef]
- Chen, M.; Si, H.; Zhang, H.; Zhou, L.; Wu, Y.; Song, L.; Kang, M.; Zhao, X.-L. The Crucial Role in Controlling the Dynamic Properties of Polyester-Based Epoxy Vitrimers: The Density of Exchangeable Ester Bonds (υ). Macromolecules 2021, 54, 10110–10117. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Wang, Z.; Mao, Y.; Jin, L.; Zhang, K.; Xia, Y.; Gao, H. Amine-Cured Glycidyl Esters as Dual Dynamic Epoxy Vitrimers. Macromolecules 2022, 55, 523–534. [Google Scholar] [CrossRef]
- Reddy, K.S.K.; Chen, Y.-C.; Jeng, R.-J.; Abu-Omar, M.M.; Lin, C.-H. Upcycling Waste Polycarbonate to Poly(Carbonate Imine) Vitrimers with High Thermal Properties and Unprecedented Hydrolytic Stability. ACS Sustain. Chem. Eng. 2023, 11, 8580–8591. [Google Scholar] [CrossRef]
- Zhou, W.; Chang, Y.-C.; Liu, T.; Fei, M.-E.; Hao, C.; Zhao, B.; Zhang, M.; Zhang, J. Biobased Aliphatic Polyurethane Vitrimer with Superior Mechanical Performance and Fluorescence-Based Defect Diagnostic Function. ACS Appl. Polym. Mater. 2023, 5, 3129–3137. [Google Scholar] [CrossRef]
- Niu, W.; Cao, X.; Wang, Y.; Yao, B.; Zhao, Y.; Cheng, J.; Wu, S.; Zhang, S.; He, X. Photonic Vitrimer Elastomer with Self-healing, High Toughness, Mechanochromism, and Excellent Durability Based on Dynamic Covalent Bond. Adv. Funct. Mater. 2021, 31, 2009017. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, X.; Shi, Z.; Berrocal, J.A.; Weder, C. Closed-Loop Recycling of Vinylogous Urethane Vitrimers. Angew. Chem. Int. Ed. Engl. 2023, 135, e202306188. [Google Scholar] [CrossRef]
- Lu, N.; Li, Q.; Ma, S.; Wang, B.; Xu, X.; Wang, S.; Ye, J.; Qiu, J.; Zhu, J. Scalable and Facile Synthesis of Acetal Covalent Adaptable Networks with Readily Adjustable Properties. Eur. Polym. J. 2021, 147, 110291. [Google Scholar] [CrossRef]
- Boucher, D.; Madsen, J.; Yu, L.; Huang, Q.; Caussé, N.; Pébère, N.; Ladmiral, V.; Negrell, C. Polystyrene Hybrid-Vitrimer Based on the Hemiacetal Ester Exchange Reaction. Macromolecules 2021, 54, 6772–6779. [Google Scholar] [CrossRef]
- Luo, C.; Wang, W.; Yang, W.; Liu, X.; Lin, J.; Zhang, L.; He, S. High-Strength and Multi-Recyclable Epoxy Vitrimer Containing Dual-Dynamic Covalent Bonds Based on the Disulfide and Imine Bond Metathesis. ACS Sustain. Chem. Eng. 2023, 11, 14591–14600. [Google Scholar] [CrossRef]
- Liu, J.; Bernaerts, K.V. Preparation of Lignin-Based Imine Vitrimers and Their Potential Application as Repairable, Self-Cleaning, Removable and Degradable Coatings. J. Mater. Chem. A Mater. Energy Sustain. 2024. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.; Li, T.; Wang, G.; Jia, Z.; Dong, X.; Xu, Y.; Ma, Q.; Zhang, D.; Ding, H.; et al. Mechanically Robust, Recyclable, and Self-healing Polyimine Networks. Adv. Sci. 2023, 10, 2300958. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Jiang, Z.; Li, Y.; Jing, X. Boronic Ester Based Vitrimers with Enhanced Stability via Internal Boron–Nitrogen Coordination. J. Am. Chem. Soc. 2020, 142, 21852–21860. [Google Scholar] [CrossRef]
- Sangaletti, D.; Ceseracciu, L.; Marini, L.; Athanassiou, A.; Zych, A. Biobased Boronic Ester Vitrimer Resin from Epoxidized Linseed Oil for Recyclable Carbon Fiber Composites. Resour. Conserv. Recycl. 2023, 198, 107205. [Google Scholar] [CrossRef]
- Gosecki, M.; Gosecka, M. Boronic Acid Esters and Anhydrates as Dynamic Cross-Links in Vitrimers. Polymers 2022, 14, 842. [Google Scholar] [CrossRef]
- Formon, G.J.M.; Storch, S.; Delplanque, A.Y.-G.; Bresson, B.; Van Zee, N.J.; Nicolaÿ, R. Overcoming the Tradeoff between Processability and Mechanical Performance of Elastomeric Vitrimers. Adv. Funct. Mater. 2023, 33, 2306065. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zhang, S.; Zhu, W.; Li, S.; Zhang, Y.; Hu, Y.; Zhou, G. Facile Approach for the Preparation of Robust and Thermally Stable Silyl Ether Cross-Linked Poly(Ethylene-Vinyl Acetate) Vitrimers. ACS Appl. Polym. Mater. 2023, 5, 8379–8386. [Google Scholar] [CrossRef]
- Zhao, B.; Hang, G.; Li, L.; Zheng, S. Nanocomposites of Polyethylene with Polyhedral Oligomeric Silsesquioxane: From Thermoplastics to Vitrimers through Silyl Ether Metathesis. Mater. Today Chem. 2022, 24, 100759. [Google Scholar] [CrossRef]
- Roig, A.; Agizza, M.; Serra, À.; De la Flor, S. Disulfide Vitrimeric Materials Based on Cystamine and Diepoxy Eugenol as Bio-Based Monomers. Eur. Polym. J. 2023, 194, 112185. [Google Scholar] [CrossRef]
- Si, H.; Zhou, L.; Wu, Y.; Song, L.; Kang, M.; Zhao, X.; Chen, M. Rapidly Reprocessable, Degradable Epoxy Vitrimer and Recyclable Carbon Fiber Reinforced Thermoset Composites Relied on High Contents of Exchangeable Aromatic Disulfide Crosslinks. Compos. B Eng. 2020, 199, 108278. [Google Scholar] [CrossRef]
- Jourdain, A.; Asbai, R.; Anaya, O.; Chehimi, M.M.; Drockenmuller, E.; Montarnal, D. Rheological Properties of Covalent Adaptable Networks with 1,2,3-Triazolium Cross-Links: The Missing Link between Vitrimers and Dissociative Networks. Macromolecules 2020, 53, 1884–1900. [Google Scholar] [CrossRef]
- Debsharma, T.; Amfilochiou, V.; Wróblewska, A.A.; De Baere, I.; Van Paepegem, W.; Du Prez, F.E. Fast Dynamic Siloxane Exchange Mechanism for Reshapable Vitrimer Composites. J. Am. Chem. Soc. 2022, 144, 12280–12289. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Liu, X.; Zhang, Q.; Feng, P.; Chi, Y.; Jian, X.; Song, Y.; Xu, J. Dual Rapid Self-Healing and Easily Recyclable Carbon Fiber Reinforced Vitrimer Composites. Chem. Eng. J. 2024, 480, 148147. [Google Scholar] [CrossRef]
- Cvek, M.; Sevcik, J.; Vilcakova, J.; Athanassiou, A.; Zych, A. Self-Healing Recyclable Bio-Based Magnetic Composites with Boronic Ester Vitrimer Matrix. Appl. Mater. Today 2023, 35, 101997. [Google Scholar] [CrossRef]
- Luo, J.; Zhao, X.; Ju, H.; Chen, X.; Zhao, S.; Demchuk, Z.; Li, B.; Bocharova, V.; Carrillo, J.-M.Y.; Keum, J.K.; et al. Highly Recyclable and Tough Elastic Vitrimers from a Defined Polydimethylsiloxane Network. Angew. Chem. Int. Ed. Engl. 2023, 62, e202310989. [Google Scholar] [CrossRef]
- Tratnik, N.; Tanguy, N.R.; Yan, N. Recyclable, Self-Strengthening Starch-Based Epoxy Vitrimer Facilitated by Exchangeable Disulfide Bonds. Chem. Eng. J. 2023, 451, 138610. [Google Scholar] [CrossRef]
- Ma, J.; Porath, L.E.; Haque, M.F.; Sett, S.; Rabbi, K.F.; Nam, S.; Miljkovic, N.; Evans, C.M. Ultra-Thin Self-Healing Vitrimer Coatings for Durable Hydrophobicity. Nat. Commun. 2021, 12, 5210. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Zhang, H.; Bi, L.; Jiang, J.; Chen, Y. Reprocessable, Self-Adhesive, and Recyclable Carbon Fiber-Reinforced Composites Using a Catalyst-Free Self-Healing Bio-Based Vitrimer Matrix. ACS Sustain. Chem. Eng. 2021, 9, 16281–16290. [Google Scholar] [CrossRef]
- Liu, J.; Pich, A.; Bernaerts, K.V. Preparation of Lignin-Based Vinylogous Urethane Vitrimer Materials and Their Potential Use as on-Demand Removable Adhesives. Green Chem. 2024. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, Z.; Luo, S.; Jin, Y.; Qiu, L.; Zhang, W. Malleable and Recyclable Conductive MWCNT-Vitrimer Composite for Flexible Electronics. ACS Appl. Nano Mater. 2020, 3, 4845–4850. [Google Scholar] [CrossRef]
- Ye, C.; Li, W.; Voet, V.S.D.; Folkersma, R.; Pei, Y.; Loos, K. Flexible Vitrimers for Self-healable Triboelectric Nanogenerators. Adv. Mater. Technol. 2023, 8, 202201670. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Wang, R.; Yang, X.; He, X.; Ye, H.; Cheng, J.; Yuan, C.; Zhang, Y.-F.; Ge, Q. Solvent-free Upcycling Vitrimers through Digital Light Processing-based 3D Printing and Bond Exchange Reaction. Adv. Funct. Mater. 2022, 32, 202111030. [Google Scholar] [CrossRef]
- Joe, J.; Shin, J.; Choi, Y.-S.; Hwang, J.H.; Kim, S.H.; Han, J.; Park, B.; Lee, W.; Park, S.; Kim, Y.S.; et al. A 4D Printable Shape Memory Vitrimer with Repairability and Recyclability through Network Architecture Tailoring from Commercial Poly(ε-caprolactone). Adv. Sci. 2021, 8, 202103682. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.; Konuray, O.; Benet, G.; Fernández-Francos, X.; Morancho, J.M.; Ramis, X. Optimization and Testing of Hybrid 3D Printing Vitrimer Resins. Polymers 2022, 14, 5102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Png, Z.M.; Ng, S.H.; Tham, G.X.; Ye, E.; Goh, S.S.; Loh, X.J.; Li, Z. Vitrimers: Current Research Trends and Their Emerging Applications. Mater. Today 2021, 51, 586–625. [Google Scholar] [CrossRef]
- Schenk, V.; Labastie, K.; Destarac, M.; Olivier, P.; Guerre, M. Vitrimer Composites: Current Status and Future Challenges. Mater. Adv. 2022, 3, 8012–8029. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Ji, Y.; Wei, Y. Functional Epoxy Vitrimers and Composites. Prog. Mater. Sci. 2021, 120, 100710. [Google Scholar] [CrossRef]
- Biswal, A.K.; Nandi, A.; Wang, H.; Vashisth, A. Ultrasonic Welding of Fiber Reinforced Vitrimer Composites. Compos. Sci. Technol. 2023, 242, 110202. [Google Scholar] [CrossRef]
- Fei, M.; Chang, Y.-C.; Hao, C.; Shao, L.; Liu, W.; Zhao, B.; Zhang, J. Highly Engineerable Schiff Base Polymer Matrix with Facile Fiber Composite Manufacturability and Hydrothermal Recyclability. Compos. B Eng. 2023, 248, 110366. [Google Scholar] [CrossRef]
- Cui, Y.; Qin, Z.; Wu, H.; Li, M.; Hu, Y. Flexible Thermal Interface Based on Self-Assembled Boron Arsenide for High-Performance Thermal Management. Nat. Commun. 2021, 12, 1284. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal Conductivity of Polymer-Based Composites: Fundamentals and Applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, K.; Feng, Y.; Liang, L.; Chi, M.; Zhang, Z.; Chen, X. Recent Advances in Thermal-Conductive Insulating Polymer Composites with Various Fillers. Compos. Part A Appl. Sci. Manuf. 2024, 178, 107998. [Google Scholar] [CrossRef]
- Li, R.; Yang, X.; Li, J.; Shen, Y.; Zhang, L.; Lu, R.; Wang, C.; Zheng, X.; Chen, H.; Zhang, T. Review on Polymer Composites with High Thermal Conductivity and Low Dielectric Properties for Electronic Packaging. Mater. Today Phys. 2022, 22, 100594. [Google Scholar] [CrossRef]
- Yang, J.; Shen, X.; Yang, W.; Kim, J.-K. Templating Strategies for 3D-Structured Thermally Conductive Composites: Recent Advances and Thermal Energy Applications. Prog. Mater. Sci. 2023, 133, 101054. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors Affecting Thermal Conductivities of the Polymers and Polymer Composites: A Review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- García, J.M.; Jones, G.O.; Virwani, K.; McCloskey, B.D.; Boday, D.J.; ter Huurne, G.M.; Horn, H.W.; Coady, D.J.; Bintaleb, A.M.; Alabdulrahman, A.M.S.; et al. Recyclable, Strong Thermosets and Organogels via Paraformaldehyde Condensation with Diamines. Science 2014, 344, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, F.; Wan, J.; Yang, L.; Huang, Y.; Hu, Z. Recyclable Tough Thermosets with an Imide-Hexahydrotriazine Structure. Polym. Chem. 2022, 13, 3063–3075. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, S.; Lee, S.-K.; Ahn, S.; Jang, S.G.; You, N.-H.; Kim, C.B.; Goh, M. Optimizing Filler Network Formation in Poly(Hexahydrotriazine) for Realizing High Thermal Conductivity and Low Oxygen Permeation. Polymer 2019, 179, 121639. [Google Scholar] [CrossRef]
- Feng, M.; Pan, Y.; Zhang, M.; Gao, Q.; Liu, C.; Shen, C.; Liu, X. Largely Improved Thermal Conductivity of HDPE Composites by Building a 3D Hybrid Fillers Network. Compos. Sci. Technol. 2021, 206, 108666. [Google Scholar] [CrossRef]
- Shin, H.; Ahn, S.; Kim, D.; Lim, J.K.; Kim, C.B.; Goh, M. Recyclable Thermoplastic Hexagonal Boron Nitride Composites with High Thermal Conductivity. Compos. B Eng. 2019, 163, 723–729. [Google Scholar] [CrossRef]
- Tanimoto, M.; Yamagata, T.; Miyata, K.; Ando, S. Anisotropic Thermal Diffusivity of Hexagonal Boron Nitride-Filled Polyimide Films: Effects of Filler Particle Size, Aggregation, Orientation, and Polymer Chain Rigidity. ACS Appl. Mater. Interfaces 2013, 5, 4374–4382. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.; Shin, H.; Kim, C.B. Manipulating Bond Exchange Rates in Vitrimer-hexagonal Boron Nitride Nanohybrids via Heat Capacity Enhancement. J. Appl. Polym. Sci. 2021, 138, 50079. [Google Scholar] [CrossRef]
- Duan, P.; Zhao, H.; Chen, Q.; Liu, M.; Wu, X.; Ganesan, V.; Wei, Y.; Liu, J. Insights into Uniaxial Tension and Relaxation of Nanorod-Filled Polymer Vitrimer Nanocomposites: A Molecular Dynamics Simulation. Macromolecules 2023, 56, 4468–4481. [Google Scholar] [CrossRef]
- Feng, X.; Fan, J.; Li, A.; Li, G. Biobased Tannic Acid Cross-Linked Epoxy Thermosets with Hierarchical Molecular Structure and Tunable Properties: Damping, Shape Memory, and Recyclability. ACS Sustain. Chem. Eng. 2020, 8, 874–883. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Y.; Han, J.; Zhen, Y.; Hu, C.; Liu, D. Physicochemical Dual Cross-Linking Conductive Polymeric Networks Combining High Strength and High Toughness Enable Stable Operation of Silicon Microparticle Anodes. Adv. Mater. 2023, 35, 2301320. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic Acid: A Crosslinker Leading to Versatile Functional Polymeric Networks: A Review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hong, Y.; Hong, J.; Yu, A.; Lee, M.W.; Lee, J.; Goh, M. Bio-Based Boronic Ester Vitrimer for Realizing Sustainable and Highly Thermally Conducting Nanocomposites. Compos. B Eng. 2022, 244, 110181. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, M.; Coasne, B.; Keten, S.; Derome, D.; Carmeliet, J. Hygromechanics of Softwood Cellulosic Nanocomposite with Intermolecular Interactions at Fiber-Matrix Interface Investigated with Molecular Dynamics. Compos. B Eng. 2022, 228, 109449. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Y.; Niu, Z.; Wu, R.; Fan, W.; Dai, Q.; He, J.; Bai, C. Boronic Ester Bonds Crosslinked Vitrimer Elastomers with Mechanical Robustness, Shape Memory, Self-Healing and Recyclability Properties. Compos. Sci. Technol. 2022, 228, 109621. [Google Scholar] [CrossRef]



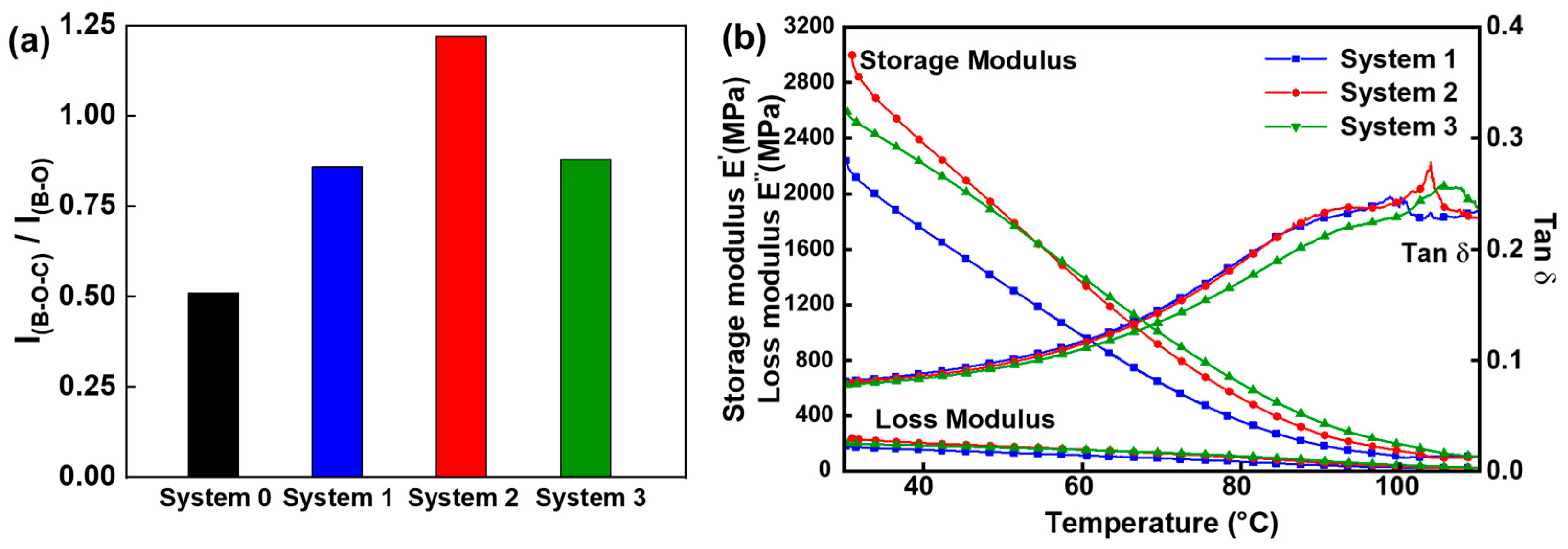


| System 0 | System 1 | System 2 | System 3 | |
|---|---|---|---|---|
| Tannic acid (g) | 3 | 3 | 3 | 3 |
| Boric acid (g) | 0.6 | 0.6 | 0.6 | 0.6 |
| Glycerol (mL) | 0.1 | 0.1 | 0.1 | 0.1 |
| NaOH (mL) | − | 3.5 | 3.5 | 3.5 |
| Cellulose nano-fiber | − | − | 0.6 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Goh, M. Vitrimer Nanocomposites for Highly Thermal Conducting Materials with Sustainability. Polymers 2024, 16, 365. https://doi.org/10.3390/polym16030365
Hong Y, Goh M. Vitrimer Nanocomposites for Highly Thermal Conducting Materials with Sustainability. Polymers. 2024; 16(3):365. https://doi.org/10.3390/polym16030365
Chicago/Turabian StyleHong, Younggi, and Munju Goh. 2024. "Vitrimer Nanocomposites for Highly Thermal Conducting Materials with Sustainability" Polymers 16, no. 3: 365. https://doi.org/10.3390/polym16030365
APA StyleHong, Y., & Goh, M. (2024). Vitrimer Nanocomposites for Highly Thermal Conducting Materials with Sustainability. Polymers, 16(3), 365. https://doi.org/10.3390/polym16030365






