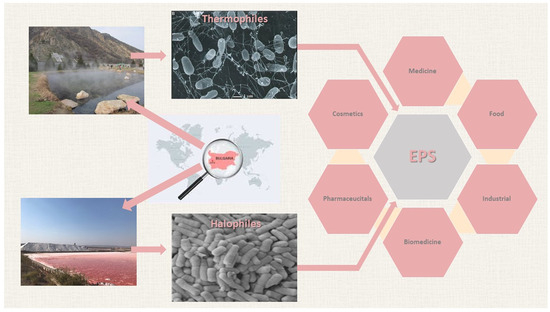
Exploring Extremophiles from Bulgaria: Biodiversity, Biopolymer Synthesis, Functional Properties, Applications
Abstract
:1. Introduction
2. Thermophiles
2.1. Diversity in Bulgarian Hot Springs
2.2. EPSs from Thermophilic Bacteria
2.3. Geobacillus tepidamans V264
2.4. Aeribacillus pallidus 418
2.5. Brevibacillus thermoruber 423
2.6. Brevibacillus thermoruber 438
| Thermophilic Bacilli (Specific Conditions) | Carbon Source | Monosaccharide Analysis | Properties and Activities | EPS Yield (g/L) | References |
|---|---|---|---|---|---|
| Bacillus sonoresis NTV10 (45 °C, pH 7.0) | Glycerol | Monosaccharide analysis: glucose/mannose/rhamnose in a relative ratio of 5.1:2.2:1 | Food processing, cosmetics, and pharmaceuticals | 1.597 | [65] |
| Bacillus haynesii CamB6 (55 °C, pH 5.8) | Glucose | Monosaccharide analysis: mannose/glucose/galactose (3.3/1.0/0.7 by relative ratio) | Antioxidant, flocculation capacities, additive for the food industry | 5.6 | [66] |
| Brevibacillus borstelensis (50 °C, pH 6.4) | Glucose | Monosaccharide analysis: glucose and galacturonic acid | Bioemulsifier, stabilizer, bisurfactant, viscosifier and binding agent | 1.88 ± 0.02 | [67,68] |
| Geobacillus sp. strain WSUCF1 (60 °C, pH 7.0) | Glucose | EPS 1, Monosaccharide analysis: α-(1,3)-D-mannose and α-(1,6)-D-glucose (1/0.21, by molar ratio) EPS-2, Monosaccharide analysis: α-(1,3)-D-mannose | Both showed antioxidant activities, non-cytotoxicity | 0.525 | [49] |
| Geobacillus thermodenitri-ficans ArzA-6 (65 °C, pH 7.0) | Fructose | Monosaccharide analysis: mannose/galactose/arabinose/fructose/glucose (1/0.13/0.1/0.06/0.05, by relative ratio) | Not tested | 0.27 | [69] |
| Geobacillus toebii ArzA-8 (65 °C, pH 7.0) | Fructose | Monosaccharide analysis: mannose/galactose/glucose/arabinose (1/0.5/0.2/0.05, by relative ratio) | Not tested | 0.22 | [69] |
| Geobacillus sp. TS3-9 (55 °C, pH 8.0) | Lactose | EPS 1, Monosaccharide analysis: mannose/trehalose/galactosamine/glucosamine/galactose/glucose/ribose (69.3/7.8/6.3/5.4/4.7/3.4/2.9, by molar ratio) EPS 2, Monosaccharide analysis: mannose/galactose/glucose/galactosamine/glucosamine/ribose/arabinose (33.9/17.9/15.5/11.7/8.1/5.3/4.9, by molar ratio) | Antioxidant activity, antitumor activity | 0.087 | [70] |
| Geobacillus tepidamans V264 (60 °C, pH 7.0) | Maltose | Monosaccharide analysis: glucose/galactose/fucose/fructose (1/0.07/0.04/0.02, by molar ratio) | Degradation temperature 280 °C, anti-cytotoxicity | 0.111 | [44] |
| Aeribacillus pallidus 418 (55 °C, pH 7.0) | Maltose | EPS 1, Monosaccharide analysis: mannose/trehalose/galactosamine/glucosamine/galactose/glucose/ribose (69.3/7.8/6.3/5.4/4.7/3.4/2.9, by molar ratio) EPS 2, Monosaccharide analysis: mannose/galactose/glucose/galactosamine/glucosamine/ribose/arabinose (33.9/17.9/15.5/11.7/8.1/5.3/4.9, by molar ratio) | Degradation temperature EPS1 176 °C, EPS2 226 °C, pseudoplastic rheological property, foaming ability, emulsifying activity | 0.17 | [33,34,35] |
| Brevibacillus thermoruber 423 (55 °C, pH 6.5) | Maltose | Monosaccharide analysis: glucose/galactose/mannose/galactosamine/mannosamine (57.7/16.3/9.2/14.2/2.4, by percentage of abundance) | Biocompatibility, non-cytotoxicity, stabilizing agents or thickeners | 0.897 | [56] |
| Brevibacillus thermoruber 438 (55 °C, pH 8.0) | Maltose | - | - | 0.078 | [64] |
3. Halophiles
3.1. Diversity in Bulgarian Salt Habitats
3.2. EPSs from Halophilic Bacteria
3.3. Chromohalobacter canadensis 28
| Halophiles (Specific Conditions) | Carbon Source | Monosaccharide Analysis | Properties and Activities | EPS Yield (g/L) | References |
|---|---|---|---|---|---|
| Chromohalobacter canadensis 28 (30 °C, pH 7.5, 150 g/L NaCl) | Lactose | Glucosamine/glucose/rhamnose/xylose/unknown sugar (36.7/32.3/25.4/1.7/3.9, by weight percentage) | Pseudoplastic rheological property; high swelling behavior; emulsifying and stabilizing activities; foaming ability | 2.4 | [95,99,100] |
| Halobacillus. sp. Strain EG1HP4QL (35 °C, pH 8.0) | Sucrose | Two polymers, a negatively charged and a neutral one (~3:1), in which mannose and glucose are the main neutral monosaccharide constituents | Emulsifying activity | 5.9 | [105] |
| Halomonas smyrnensis AAD6T (37 °C, pH 7.0, 137.2 g/L NaCl) | Sucrose | Fructose | Degradation temperature 253 °C; bioflocculating activity; anti-cytotoxicity; biocompatibility; antitumor activity after periodate oxidation | 1.073—flasks and 1.844—fermenter | [79,80,81,106,107,108] |
| Halomonas almeriensis M8T (32 °C, pH 7.0, 75 g/L) | Glucose | EPS 1, mannose/glucose/rhamnose (72/27.5/0.5, by weight percentage) EPS 2, mannose/glucose (70/30, by weight percentage) | Emulsifying activity; heavy metal binding capacity; pseudoplastic rheological property | 1.7 | [85] |
| Halomonas stenophila B100 (32 °C, pH 7.2, 75 g/L total salts) | Glucose | Glucose/galactose/mannose (44.5/40.5/15.0, by weight percentage) | Antitumor activity after oversulfation | 3.89 | [76,77] |
| Alteromonas hispanica F32T (32 °C, pH 7.2, 75 g/L total salts) | Glucose | Mannose/glucose/xylose/rhamnose (62.75/18.15/12.25/6.85, by molar percentage) | Emulsifying activity; heavy metal binding capacity; pseudoplastic rheological property | 1–1.5 | [89] |
| Halomonas eurihalina F2-7 (32 °C, pH 7.2, 75 g/L total salts) | Glucose | Glucose/mannose/rhamnose (2.9/1.5/1, by relative ratio) | Emulsifying activity; pseudoplastic rheological property | 1.6 | [78,109] |
| Halomonas ventosae A112T (32 °C, pH 7.2, 75 g/L total salts) | Glucose | Glucose/mannose/galactose (1.75/4/1, by molar ratio), and small quantities of xylose, arabinose, and galacturonic acid | Emulsifying activity; heavy metal binding capacity; biofilm formation capacity; pseudoplastic rheological property | 0.28 | [86] |
| Halomonas anticariensis FP35T (32 °C, pH 7.2, 75 g/L salts) | Glucose | Glucose/mannose/galacturonic acid (1/3/2.5, by molar ratio) | Emulsifying activity; heavy metal binding capacity; biofilm formation capacity; pseudoplastic rheological property | 0.29 | [86] |
4. Conclusions
Funding
Conflicts of Interest
References
- DasSarma, S.; DasSarma, P.; Laye, V.J.; Schwieterman, E.W. Extremophilic models for astrobiology: Haloarchaeal survival strategies and pigments for remote sensing. Extremophiles 2020, 24, 31–41. [Google Scholar] [CrossRef] [PubMed]
- MacElroy, R.D. Some comments on the evolution of extremophiles. Biosystems 1974, 6, 74–75. [Google Scholar] [CrossRef]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. “Bioplastics from microalgae”—Polyhydroxyalkanoate production by cyanobacteria. In Handbook of Microalgae-Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 597–645. [Google Scholar]
- Rampelotto, P.H. Extremophiles and extreme environments. Life 2013, 3, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Kambourova, M.; Radchenkova, N.; Nicolaus, B.; Tommonaro, G.; Poli, A.; Oner, E.T.; Yildiz, S.Y.; Arga, K.Y. Exopolysaccharides Synthesized by Thermophilic Microorganisms Isolated from Bulgarian Hot Springs. Acta Microbiol. Bulg. 2018, 34, 68–76. [Google Scholar]
- Kambourova, M. Thermostable enzymes and polysaccharides produced by thermophilic bacteria isolated from Bulgarian hot springs. Eng. Life Sci. 2018, 18, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Valli, V.; Molinaro, A.; Carteni, M.; De Rosa, M. Exopolysaccharides production in Lactobacillus bulgaricus and Lactobacillus casei exploiting microfiltration. J. Ind. Microbiol. Biotechnol. 2006, 33, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Bacterial extracellular polysaccharides. Can. J. Microbiol. 1988, 34, 415–420. [Google Scholar] [CrossRef]
- Ahmad, N.H.; Mustafa, S.; Che Man, Y.B. Microbial polysaccharides and their modification approaches: A review. Int. J. Food Prop. 2015, 18, 332–347. [Google Scholar] [CrossRef]
- Kambourova, M.; Oner, E.T.; Poli, A. Exopolysaccharides from prokaryotic microorganisms—Promising sources for white biotechnology processes. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 523–554. [Google Scholar]
- Sutherland, I.W. Microbial exopolysaccharides-structural subtleties and their consequences. Pure Appl. Chem. 1997, 69, 1911–1918. [Google Scholar] [CrossRef]
- Martinez-Martinez, L.; Timmerman, C.; Fleer, A.; Verhoef, J. Chemiluminescence of human polymorphonuclear leukocytes after stimulation with whole cells and cell-wall components of Staphylococcus epidermidis. J. Med. Microbiol. 1993, 39, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Moriello, V.S.; Esposito, E.; Lama, L.; Gambacorta, A.; Nicolaus, B. Exopolysaccharide production by a new Halomonas strain CRSS isolated from saline lake Cape Russell in Antarctica growing on complex and defined media. Biotechnol. Lett. 2004, 26, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.M.; Guezennec, J.; Bowman, J. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: A review. Mar. Biotechnol. 2005, 7, 253–271. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del Rocío López-Cuellar, M.; Rodríguez-Hernández, A.I. A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. From the adverse to diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Jindal, N.; Khattar, J.S. Microbial polysaccharides in food industry. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–123. [Google Scholar]
- Moscovici, M.; Balas, C. Bacterial polysaccharides versatile medical uses. In Polysaccharides of Microbial Origin: Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 859–891. [Google Scholar]
- Atanassova, M.; Derekova, A.; Mandeva, R.; Sjøholm, C.; Kambourova, M. Anoxybacillus bogrovensis sp. nov., a novel thermophilic bacterium isolated from a hot spring in Dolni Bogrov, Bulgaria. Int. J. Syst. Evol. Microbiol. 2008, 58, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, J.; Ljungdahl, L.G.; Demain, A.L. The importance of thermophilic bacteria in biotechnology. Crit. Rev. Biotechnol. 1985, 3, 39–108. [Google Scholar] [CrossRef]
- Stefanova, K.; Tomova, I.; Tomova, A.; Radchenkova, N.; Atanassov, I.; Kambourova, M. Archaeal and bacterial diversity in two hot springs from geothermal regions in Bulgaria as demostrated by 16S rRNA and GH-57 genes. Int. Microbiol. 2015, 18, 217–223. [Google Scholar]
- Derekova, A.; Mandeva, R.; Kambourova, M. Phylogenetic diversity of thermophilic carbohydrate degrading bacilli from Bulgarian hot springs. World J. Microbiol. Biotechnol. 2008, 24, 1697–1702. [Google Scholar] [CrossRef]
- Derekova, A.; Sjøholm, C.; Mandeva, R.; Kambourova, M. Anoxybacillus rupiensis sp. nov., a novel thermophilic bacterium isolated from Rupi basin (Bulgaria). Extremophiles 2007, 11, 577–583. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Khan, M.I.; Khattak, M.N.; Zhang, J.; Li, K.; Hassan, I.U. Diversity and distribution of thermophilic microorganisms and their applications in biotechnology. J. Basic Microbiol. 2022, 62, 95–108. [Google Scholar] [CrossRef]
- Mehta, D.; Satyanarayana, T. Diversity of Hot Environments and Thermophilic Microbes. In Thermophilic Microbes in Environmental and Industrial Biotechnology: Biotechnology of Thermophiles, 2nd ed.; Springer: Berlin, Germany, 2013; pp. 3–60. [Google Scholar]
- Nadaroglu, H.; Polat, M.S. Microbial extremozymes: Novel sources and industrial applications. In Microbial Extremozymes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 67–88. [Google Scholar]
- Nicolaus, B.; Manca, M.C.; Romano, I.; Lama, L. Production of an exopolysaccharide from two thermophilic archaea belonging to the genus Sulfolobus. FEMS Microbiol. Lett. 1993, 109, 203–206. [Google Scholar] [CrossRef]
- Rinker, K.D.; Kelly, R.M. Growth physiology of the hyperthermophilic archaeon Thermococcus litoralis: Development of a sulfur-free defined medium, characterization of an exopolysaccharide, and evidence of biofilm formation. Appl. Environ. Microbiol. 1996, 62, 4478–4485. [Google Scholar] [CrossRef]
- Rinker, K.D.; Kelly, R.M. Effect of carbon and nitrogen sources on growth dynamics and exopolysaccharide production for the hyperthermophilic archaeon Thermococcus litoralis and bacterium Thermotoga maritima. Biotechnol. Bioeng. 2000, 69, 537–547. [Google Scholar] [CrossRef]
- Vilhelmsson, O.; Sigurbjornsdottir, M.A.; Thorsteinsdottir, G.V.; Cascone, M.; Corso, D.; Tonietti, L.; Migliaccio, F.; Nappi, N.; Ricciardelli, A.; Selci, M. Diversity of Thermophilic Prokaryotes. In Thermophilic Anaerobes: Phylogeny, Physiology and Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 21–90. [Google Scholar]
- Nicolaus, B.; Lama, L.; Panico, A.; Moriello, V.S.; Romano, I.; Gambacorta, A. Production and characterization of exopolysaccharides excreted by thermophilic bacteria from shallow, marine hydrothermal vents of Flegrean Ares (Italy). Syst. Appl. Microbiol. 2002, 25, 319–325. [Google Scholar] [CrossRef]
- Draper, B.; Yee, W.L.; Pedrana, A.; Kyi, K.P.; Qureshi, H.; Htay, H.; Naing, W.; Thompson, A.J.; Hellard, M.; Howell, J. Reducing liver disease-related deaths in the Asia-Pacific: The important role of decentralised and non-specialist led hepatitis C treatment for cirrhotic patients. Lancet Reg. Health–West. Pac. 2022, 20, 100359. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Panchev, I.; Anzelmo, G.; Tomova, I.; Nicolaus, B.; Kuncheva, M.; Petrov, K.; Kambourova, M. Production and properties of two novel exopolysaccharides synthesized by a thermophilic bacterium Aeribacillus pallidus. Appl. Biochem. Biotechnol. 2013, 171, 31–43. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Martinov, M.; Kuncheva, M.; Panchev, I.; Vlaev, S.; Kambourova, M. Optimization of the aeration and agitation speed of Aeribacillus palidus 418 exopolysaccharide production and the emulsifying properties of the product. Process Biochem. 2014, 49, 576–582. [Google Scholar] [CrossRef]
- Radchenkova, N.; Panchev, I.; Vassilev, S.; Kuncheva, M.; Dobreva, S.; Kambourova, M. Continuous cultivation of a thermophilic bacterium Aeribacillus pallidus 418 for production of an exopolysaccharide applicable in cosmetic creams. J. Appl. Microbiol. 2015, 119, 1301–1309. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Zammuto, V.; Gugliandolo, C.; Madeleine-Perdrillat, C.; Spanò, A.; Magazù, S. Thermal restraint of a bacterial exopolysaccharide of shallow vent origin. Int. J. Biol. Macromol. 2018, 114, 649–655. [Google Scholar] [CrossRef]
- Corsaro, M.M.; Grant, D.W.; Marciano, E.C.; Parrilli, M. Structure determination of an exopolysaccharide from an alkaliphilic bacterium closely related to Bacillus spp. Eur. J. Biochem. 1999, 264, 610–618. [Google Scholar] [CrossRef]
- Nicolaus, B.; Moriello, V.; Maugeri, T.; Gugliandolo, C.; Gambacorta, A. Bacilli from shallow mediterraneae marine vents producers of exopolysaccharides. Recent Res. Devel. Microbiol. 2003, 7, 197–208. [Google Scholar]
- Arena, A.; Gugliandolo, C.; Stassi, G.; Pavone, B.; Iannello, D.; Bisignano, G.; Maugeri, T.L. An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: Antiviral activity on immunocompetent cells. Immunol. Lett. 2009, 123, 132–137. [Google Scholar] [CrossRef]
- Nicolaus, B.; Panico, A.; Manca, M.C.; Lama, L.; Gambacorta, A.; Maugeri, T.; Gugliandolo, C.; Caccamo, D. A thermophilic Bacillus isolated from an Eolian shallow hydrothermal vent able to produce exopolysaccharides. Syst. Appl. Microbiol. 2000, 23, 426–432. [Google Scholar] [CrossRef]
- Spanò, A.; Gugliandolo, C.; Lentini, V.; Maugeri, T.L.; Anzelmo, G.; Poli, A.; Nicolaus, B. A novel EPS-producing strain of Bacillus licheniformis isolated from a shallow vent off Panarea Island (Italy). Curr. Microbiol. 2013, 67, 21–29. [Google Scholar] [CrossRef]
- Lin, M.-H.; Yang, Y.-L.; Chen, Y.-P.; Hua, K.-F.; Lu, C.-P.; Sheu, F.; Lin, G.-H.; Tsay, S.-S.; Liang, S.-M.; Wu, S.-H. A novel exopolysaccharide from the biofilm of Thermus aquaticus YT-1 induces the immune response through Toll-like receptor. J. Biol. Chem. 2011, 286, 17736–17745. [Google Scholar] [CrossRef]
- Kambourova, M.; Mandeva, R.; Dimova, D.; Poli, A.; Nicolaus, B.; Tommonaro, G. Production and characterization of a microbial glucan, synthesized by Geobacillus tepidamans V264 isolated from Bulgarian hot spring. Carbohydr. Polym. 2009, 77, 338–343. [Google Scholar] [CrossRef]
- VanFossen, A.L.; Lewis, D.L.; Nichols, J.D.; Kelly, R.M. Polysaccharide degradation and synthesis by extremely thermophilic anaerobes. Ann. N. Y. Acad. Sci. 2008, 1125, 322–337. [Google Scholar] [CrossRef]
- Duboc, P.; Mollet, B. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- Patel, A.; Prajapati, J. Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv. Dairy Res. 2013, 1, 1–7. [Google Scholar]
- Al Christopher, C.; da Silva, Í.G.; Pangilinan, K.D.; Chen, Q.; Caldona, E.B.; Advincula, R.C. High performance polymers for oil and gas applications. React. Funct. Polym. 2021, 162, 104878. [Google Scholar]
- Wang, J.; Salem, D.R.; Sani, R.K. Two new exopolysaccharides from a thermophilic bacterium Geobacillus sp. WSUCF1: Characterization and bioactivities. N. Biotechnol. 2021, 61, 29–39. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Wang, H. Eco Polymeric Materials and Natural Polymer. Polymers 2023, 15, 4021. [Google Scholar] [CrossRef]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- Zheng, C.; Li, Z.; Su, J.; Zhang, R.; Liu, C.; Zhao, M. Characterization and emulsifying property of a novel bioemulsifier by Aeribacillus pallidus YM-1. J. Appl. Microbiol. 2012, 113, 44–51. [Google Scholar] [CrossRef]
- Harirchi, S.; Etemadifar, Z.; Mahboubi, A.; Yazdian, F.; Taherzadeh, M.J. The effect of calcium/magnesium ratio on the biomass production of a novel thermoalkaliphilic Aeribacillus pallidus strain with highly heat-resistant spores. Curr. Microbiol. 2020, 77, 2565–2574. [Google Scholar] [CrossRef]
- Radchenkova, N. Production and Characterization of Exopolysaccharide(s) Synthesized from Thermophilic Strain Aeribacillus pallidus 418. Ph.D. Thesis, Bulgarian Academy of Science, Sofia, Bulgaria, 2014. [Google Scholar]
- Gongi, W.; Cordeiro, N.; Pinchetti, J.L.G.; Ben Ouada, H. Functional, rheological, and antioxidant properties of extracellular polymeric substances produced by a thermophilic cyanobacterium Leptolyngbya sp. J. Appl. Phycol. 2022, 34, 1423–1434. [Google Scholar] [CrossRef]
- Yasar Yildiz, S.; Anzelmo, G.; Ozer, T.; Radchenkova, N.; Genç, S.; Di Donato, P.; Nicolaus, B.; Toksoy Oner, E.; Kambourova, M. Brevibacillus themoruber: A promising microbial cell factory for exopolysaccharide production. J. Appl. Microbiol. 2014, 116, 314–324. [Google Scholar] [CrossRef]
- Yildiz, S.Y.; Radchenkova, N.; Arga, K.Y.; Kambourova, M.; Toksoy Oner, E. Genomic analysis of Brevibacillus thermoruber 423 reveals its biotechnological and industrial potential. Appl. Microbiol. Biotechnol. 2015, 99, 2277–2289. [Google Scholar] [CrossRef]
- Yaşar Yildiz, S.; Nikerel, E.; Toksoy Öner, E. Genome-scale metabolic model of a microbial cell factory (Brevibacillus thermoruber 423) with multi-industry potentials for exopolysaccharide production. OMICS 2019, 23, 237–246. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Banu, J.R.; Kumar, V.; Kumar, G.; Yang, Y.-H.; Bhatia, S.K. Microbial Exopolysaccharide Composites in Biomedicine and Healthcare: Trends and Advances. Polymers 2023, 15, 1801. [Google Scholar] [CrossRef]
- Synytsya, A.; Poučková, P.; Zadinova, M.; Troshchynska, Y.; Štětina, J.; Synytsya, A.; Saloň, I.; Kral, V. Hydrogels based on low-methoxyl amidated citrus pectin and flaxseed gum formulated with tripeptide glycyl-l-histidyl-l-lysine improve the healing of experimental cutting wounds in rats. Int. J. Biol. Macromol. 2020, 165, 3156–3168. [Google Scholar] [CrossRef]
- Pal, A.; Paknikar, K. Bioremediation of arsenic from contaminated water. In Microorganisms in Environmental Management: Microbes and Environment; Springer: Dordrecht, The Netherlands, 2012; pp. 477–523. [Google Scholar]
- Kumawat, T.K.; Kumawat, V.; Sharma, S.; Kandwani, N.; Biyani, M. Applications of EPS in environmental bioremediations. In Microbial Exopolysaccharides as Novel and Significant Biomaterials; Springer: Cham, Switzerland, 2021; pp. 285–302. [Google Scholar]
- Wang, Q.; Hu, Z.; Li, Z.; Liu, T.; Bian, G. Exploring the Application and Prospects of Synthetic Biology in Engineered Living Materials. Adv. Mater. 2023. [Google Scholar] [CrossRef]
- Radchenkova, N.; Tomova, A.; Kambourova, M. Biosynthesis of an exopolysaccharide produced by Brevibacillus thermoruber. Biotechnol. Biotechnol. Equip. 2011, 25, 77–79. [Google Scholar] [CrossRef]
- Vikromvarasiri, N.; Nakasaki, K. Exopolysaccharide production from glycerol by Bacillus sonorensis NTV10 under thermophilic condition. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Banerjee, A.; Breig, S.; Gómez, A.; Sánchez-Arévalo, I.; Faune, P.; Sarkar, S.; Bandopadhyay, R.; Vuree, S.; Cornejo, J.; Tapia, J.; et al. Optimization and Characterization of a Novel Exopolysaccharide from Bacillus haynesii CamB6 for Food Applications. Biomolecules 2022, 12, 834. [Google Scholar] [CrossRef]
- Dhagat, S.; Jujjavarapu, S. Simulated annealing and artificial neural network as optimization tools to enhance yields of bioemulsifier and exopolysaccharides by thermophilic Brevibacillus borstelensis. J. Envrion. Chem. Eng. 2021, 9, 105499. [Google Scholar] [CrossRef]
- Srivastava, N.; Kumari, S.; Kurmi, S.; Pinnaka, A.K.; Choudhury, A.R. Isolation, purification, and characterization of a novel exopolysaccharide isolated from marine bacteria Brevibacillus borstelensis M42. Arch Microbiol. 2022, 204, 399. [Google Scholar] [CrossRef]
- Panosyan, H.; Di Donato, P.; Poli, A.; Nicolaus, B. Production and characterization of exopolysaccharides by Geobacillus thermodenitrificans ArzA-6 and Geobacillus toebii Arz A-8 strains isolated from an Armenian geothermal spring. Extremophiles 2018, 22, 725–737. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Yang, L.; Liang, X.; Zhang, F.; Linhardt, J. Structural characterization and bioactivity of exopolysaccharide synthesized by Geobacillus sp. TS3-9 isolated from radioactive radon hot spring. Adv. Biotechnol. Microbiol. 2017, 4, 1–8. [Google Scholar]
- Kambourova, M.; Tomova, I.; Boyadzhieva, I.; Radchenkova, N.; Vasileva-Tonkova, E. Phylogenetic analysis of the bacterial community in a crystallizer pond, Pomorie salterns, Bulgaria. Biotechnol. Biotechnol. Equip. 2017, 31, 325–332. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Oren, A. Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front. Microbiol. 2013, 4, 315. [Google Scholar] [CrossRef]
- Li, P.; Liu, Z.; Xu, R. Chemical characterisation of the released polysaccharide from the cyanobacterium Aphanothece halophytica GR02. J. Appl. Phycol. 2001, 13, 71–77. [Google Scholar] [CrossRef]
- Arias, S.; Del Moral, A.; Ferrer, M.R.; Tallon, R.; Quesada, E.; Bejar, V. Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 2003, 7, 319–326. [Google Scholar] [CrossRef]
- Amjres, H.; Bejar, V.; Quesada, E.; Abrini, J.; Llamas, I. Halomonas rifensis sp. nov., an exopolysaccharide-producing, halophilic bacterium isolated from a solar saltern. Int. J. Syst. Evol. Microbiol. 2011, 61, 2600–2605. [Google Scholar] [CrossRef]
- Amjres, H.; Béjar, V.; Quesada, E.; Carranza, D.; Abrini, J.; Sinquin, C.; Ratiskol, J.; Colliec-Jouault, S.; Llamas, I. Characterization of haloglycan, an exopolysaccharide produced by Halomonas stenophila HK30. Int. J. Biol. Macromol. 2015, 72, 117–124. [Google Scholar] [CrossRef]
- Béjar, V.; Llamas, I.; Calvo, C.; Quesada, E. Characterization of exopolysaccharides produced by 19 halophilic strains of the species Halomonas eurihalina. J. Biotechnol. 1998, 61, 135–141. [Google Scholar] [CrossRef]
- Finore, I.; Di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation technologies for the optimization of marine microbial exopolysaccharide production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef]
- Küçükaşik, F.; Kazak, H.; Güney, D.; Finore, I.; Poli, A.; Yenigün, O.; Nicolaus, B.; Öner, E.T. Molasses as fermentation substrate for levan production by Halomonas sp. Appl. Microbiol. Biotechnol. 2011, 89, 1729–1740. [Google Scholar] [CrossRef]
- Poli, A.; Nicolaus, B.; Denizci, A.A.; Yavuzturk, B.; Kazan, D. Halomonas smyrnensis sp. nov., a moderately halophilic, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbiol. 2013, 63, 10–18. [Google Scholar] [CrossRef]
- Poli, A.; Esposito, E.; Orlando, P.; Lama, L.; Giordano, A.; de Appolonia, F.; Nicolaus, B.; Gambacorta, A. Halomonas alkaliantarctica sp. nov., isolated from saline lake Cape Russell in Antarctica, an alkalophilic moderately halophilic, exopolysaccharide-producing bacterium. Syst. Appl. Microbiol. 2007, 30, 31–38. [Google Scholar] [CrossRef]
- Chikkanna, A.; Ghosh, D.; Kishore, A. Expression and characterization of a potential exopolysaccharide from a newly isolated halophilic thermotolerant bacteria Halomonas nitroreducens strain WB. PeerJ 2018, 6, e4684. [Google Scholar] [CrossRef]
- Gonzalez-Domenech, C.M.; Martínez-Checa, F.; Quesada, E.; Bejar, V. Halomonas cerina sp. nov., a moderately halophilic, denitrifying, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbiol. 2008, 58, 803–809. [Google Scholar] [CrossRef]
- Llamas, I.; Amjres, H.; Mata, J.A.; Quesada, E.; Béjar, V. The potential biotechnological applications of the exopolysaccharide produced by the halophilic bacterium Halomonas almeriensis. Molecules 2012, 17, 7103–7120. [Google Scholar] [CrossRef]
- Mata, J.A.; Béjar, V.; Llamas, I.; Arias, S.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E. Exopolysaccharides produced by the recently described halophilic bacteria Halomonas ventosae and Halomonas anticariensis. Res. Microbiol. 2006, 157, 827–835. [Google Scholar] [CrossRef]
- Romano, I.; Lama, L.; Nicolaus, B.; Poli, A.; Gambacorta, A.; Giordano, A. Halomonas alkaliphila sp. nov., a novel halotolerant alkaliphilic bacterium isolated from a salt pool in Campania (Italy). J. Gen. Appl. Microbiol. 2006, 52, 339–348. [Google Scholar] [CrossRef]
- Romano, I.; Lama, L.; Nicolaus, B.; Poli, A.; Gambacorta, A.; Giordano, A. Oceanobacillus oncorhynchi subsp. incaldanensis subsp. nov., an alkalitolerant halophile isolated from an algal mat collected from a sulfurous spring in Campania (Italy), and emended description of Oceanobacillus oncorhynchi. Int. J. Syst. Evol. Microbiol. 2006, 56, 805–810. [Google Scholar] [CrossRef]
- Mata, J.; Béjar, V.; Bressollier, P.; Tallon, R.; Urdaci, M.; Quesada, E.; Llamas, I. Characterization of exopolysaccharides produced by three moderately halophilic bacteria belonging to the family Alteromonadaceae. J. Appl. Microbiol. 2008, 105, 521–528. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Panico, A.; Lama, L.; Gambacorta, A.; Nicolaus, B. A halophilic thermotolerant Bacillus isolated from a marine hot spring able to produce a new exopolysaccharide. Biotechnol. Lett. 2002, 24, 515–519. [Google Scholar] [CrossRef]
- Casillo, A.; Parrilli, E.; Sannino, F.; Mitchell, D.E.; Gibson, M.I.; Marino, G.; Lanzetta, R.; Parrilli, M.; Cosconati, S.; Novellino, E. Structure-activity relationship of the exopolysaccharide from a psychrophilic bacterium: A strategy for cryoprotection. Carbohydr. Polym. 2017, 156, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shi, X.; Gao, X.; Liang, J.; Zhang, X.-H. Salipiger nanhaiensis sp. nov., a bacterium isolated from deep sea water. Int. J. Syst. Evol. Microbiol. 2015, 65, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Llamas, I.; Mata, J.A.; Tallon, R.; Bressollier, P.; Urdaci, M.C.; Quesada, E.; Béjar, V. Characterization of the exopolysaccharide produced by Salipiger mucosus A3T, a halophilic species belonging to the Alphaproteobacteria, isolated on the Spanish mediterranean seaboard. Mar. Drugs 2010, 8, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Silvi, S.; Barghini, P.; Aquilanti, A.; Juarez-Jimenez, B.; Fenice, M. Physiologic and metabolic characterization of a new marine isolate (BM39) of Pantoea sp. producing high levels of exopolysaccharide. Microb. Cell Factories 2013, 12, 1–12. [Google Scholar] [CrossRef]
- Radchenkova, N.; Boyadzhieva, I.; Atanasova, N.; Poli, A.; Finore, I.; Di Donato, P.; Nicolaus, B.; Panchev, I.; Kuncheva, M.; Kambourova, M. Extracellular polymer substance synthesized by a halophilic bacterium Chromohalobacter canadensis 28. Appl. Microbiol. Biotechnol. 2018, 102, 4937–4949. [Google Scholar] [CrossRef]
- Singh, S.; Sran, K.S.; Pinnaka, A.K.; Choudhury, A.R. Purification, characterization and functional properties of exopolysaccharide from a novel halophilic Natronotalea sambharensis sp. nov. Int. J. Biol. Macromol. 2019, 136, 547–558. [Google Scholar] [CrossRef]
- Gan, L.; Li, X.; Zhang, H.; Zhang, R.; Wang, H.; Xu, Z.; Peng, B.; Tian, Y. Preparation, characterization and functional properties of a novel exopolysaccharide produced by the halophilic strain Halomonas saliphila LCB169T. Int. J. Biol. Macromol. 2020, 156, 372–380. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Molecular characterization and biocompatibility of Exopolysaccharide produced by moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast. Polymers 2022, 14, 3986. [Google Scholar] [CrossRef]
- Radchenkova, N.; Hasköylü, M.E.; Vassilev, S.; Yıldız, S.Y.; Boyadzhieva, I.; Oner, E.T.; Kambourova, M. Improved exopolymer production by Chromohalobacter canadensis cultures for its potential cosmeceutical applications. Microorganisms 2020, 8, 1935. [Google Scholar] [CrossRef]
- Radchenkova, N.; Boyadzhieva, I.; Hasköylü, M.E.; Atanasova, N.; Yıldız, S.Y.; Kuncheva, M.J.; Panchev, I.; Kisov, H.; Vassilev, S.; Oner, E.T. High bioreactor production and emulsifying activity of an unusual exopolymer by Chromohalobacter canadensis. Eng. Life Sci. 2020, 20, 357–367. [Google Scholar]
- Trabelsi, I.; Ktari, N.; Triki, M.; Bkhairia, I.; Slima, S.B.; Aydi, S.S.; Aydi, S.; Abdeslam, A.; Salah, R.B. Physicochemical, techno-functional, and antioxidant properties of a novel bacterial exopolysaccharide in cooked beef sausage. Int. J. Biol. Macromol. 2018, 111, 11–18. [Google Scholar] [CrossRef]
- Hinchliffe, J.D.; Parassini Madappura, A.; Syed Mohamed, S.M.D.; Roy, I. Biomedical applications of bacteria-derived polymers. Polymers 2021, 13, 1081. [Google Scholar] [CrossRef]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Larsson, M.; Huang, W.-T.; Chiou, S.-H.; Nicholls, S.J.; Chao, J.-I.; Liu, D.-M. The use of polymer-based nanoparticles and nanostructured materials in treatment and diagnosis of cardiovascular diseases: Recent advances and emerging designs. Prog. Polym. Sci. 2016, 57, 153–178. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Konnova, S.; Sigida, E.N.; Lyubun, E.V.; Muratova, A.; Fedonenko, Y.P.; Elbanna, K. Bioremediation potential of a halophilic Halobacillus sp. strain, EG1HP4QL: Exopolysaccharide production, crude oil degradation, and heavy metal tolerance. Extremophiles 2020, 24, 157–166. [Google Scholar] [CrossRef]
- Poli, A.; Kazak, H.; Gürleyendağ, B.; Tommonaro, G.; Pieretti, G.; Öner, T.; Nicolaus, B. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohyd. Polym. 2009, 78, 651–657. [Google Scholar] [CrossRef]
- Sarilmiser, K.; Oner, T. Investigation of anti-cancer activity of linear and aldehyde-activated levan from Halomonas smyrnensis AAD6T. Biochem. Eng. J. 2014, 92, 28–34. [Google Scholar] [CrossRef]
- Sam, S.; Kucukasik, F.; Yenigun, O.; Nicolaus, B.; Oner, E.T.; Yukselen, M.A. Flocculating performances of exopolysaccharides produced by a halophilic bacterial strain cultivated on agro-industrial waste. Bioresour. Technol. 2011, 102, 1788–1794. [Google Scholar] [CrossRef]
- Martínez-Checa, F.; Toledo, F.L.; El Mabrouki, K.; Quesada, E.; Calvo, C. Characteristics of bioemulsifier V2-7 synthesized in culture media added of hydrocarbons: Chemical composition, emulsifying activity and rheological properties. Bioresour. Technol. 2007, 98, 3130–3135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaşar Yıldız, S.; Radchenkova, N. Exploring Extremophiles from Bulgaria: Biodiversity, Biopolymer Synthesis, Functional Properties, Applications. Polymers 2024, 16, 69. https://doi.org/10.3390/polym16010069
Yaşar Yıldız S, Radchenkova N. Exploring Extremophiles from Bulgaria: Biodiversity, Biopolymer Synthesis, Functional Properties, Applications. Polymers. 2024; 16(1):69. https://doi.org/10.3390/polym16010069
Chicago/Turabian StyleYaşar Yıldız, Songül, and Nadja Radchenkova. 2024. "Exploring Extremophiles from Bulgaria: Biodiversity, Biopolymer Synthesis, Functional Properties, Applications" Polymers 16, no. 1: 69. https://doi.org/10.3390/polym16010069