Synthesis and Properties of Bio-Based Polycarbonates Containing Silicone Blocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
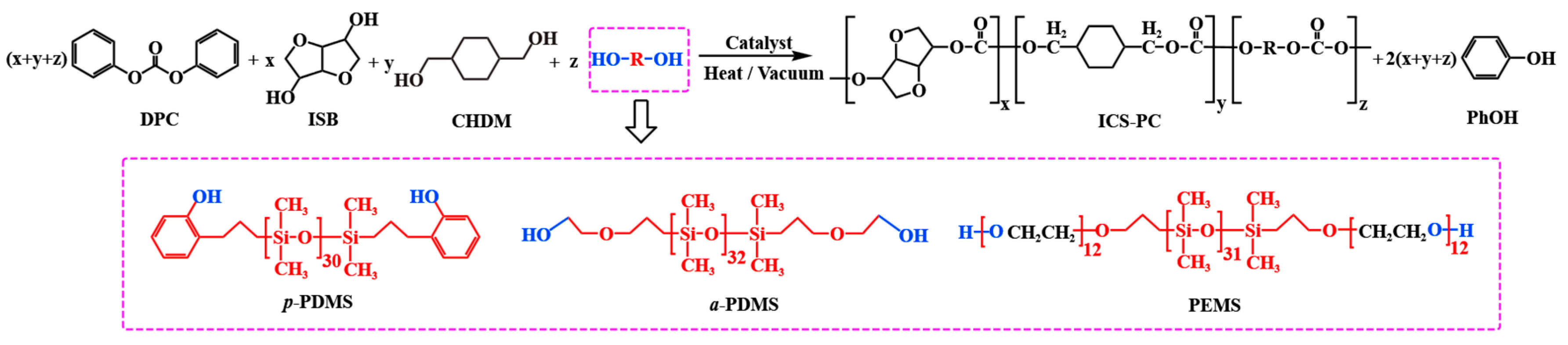
2.2. Synthesis of PEMS
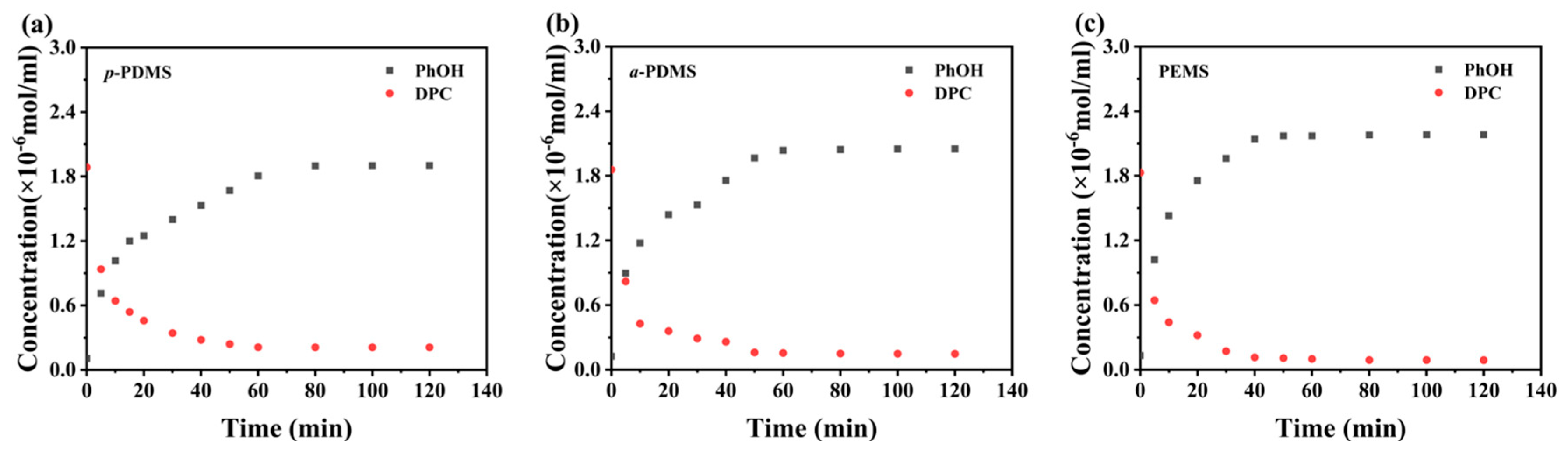
2.3. Experiments on the Kinetics of Transesterification Reactions
2.4. Synthesis of ICS-PC
2.5. Preparation of Copolymer Films
2.6. Characterizations
3. Results and Discussions
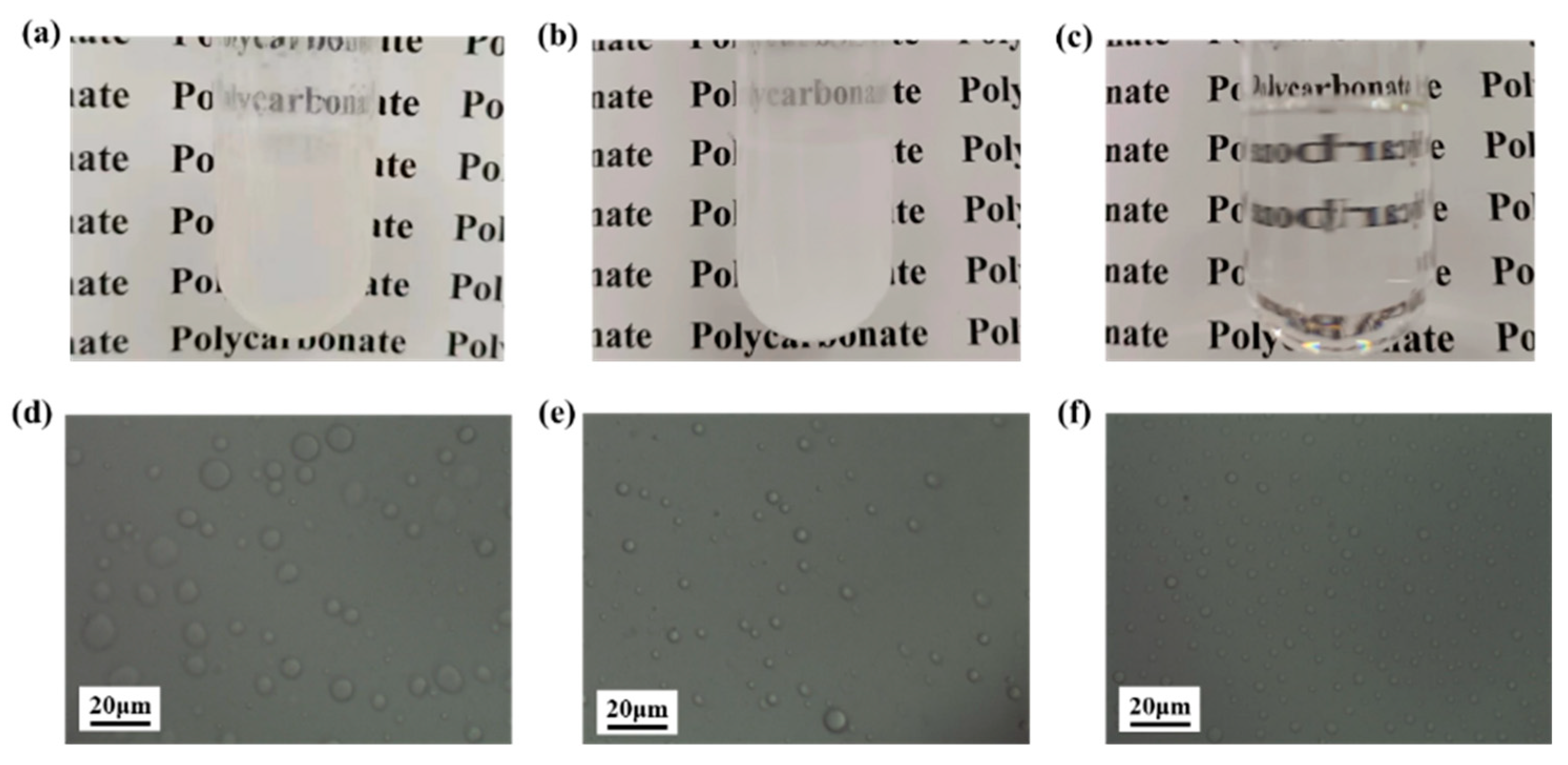
3.1. Compatibility Analysis of Silicone with Other Monomers
3.2. Effect of Silicones on Transesterification Reaction Rates
3.3. Analysis of Silicone Conversion
3.4. Physical Property Analysis of ICS-PC
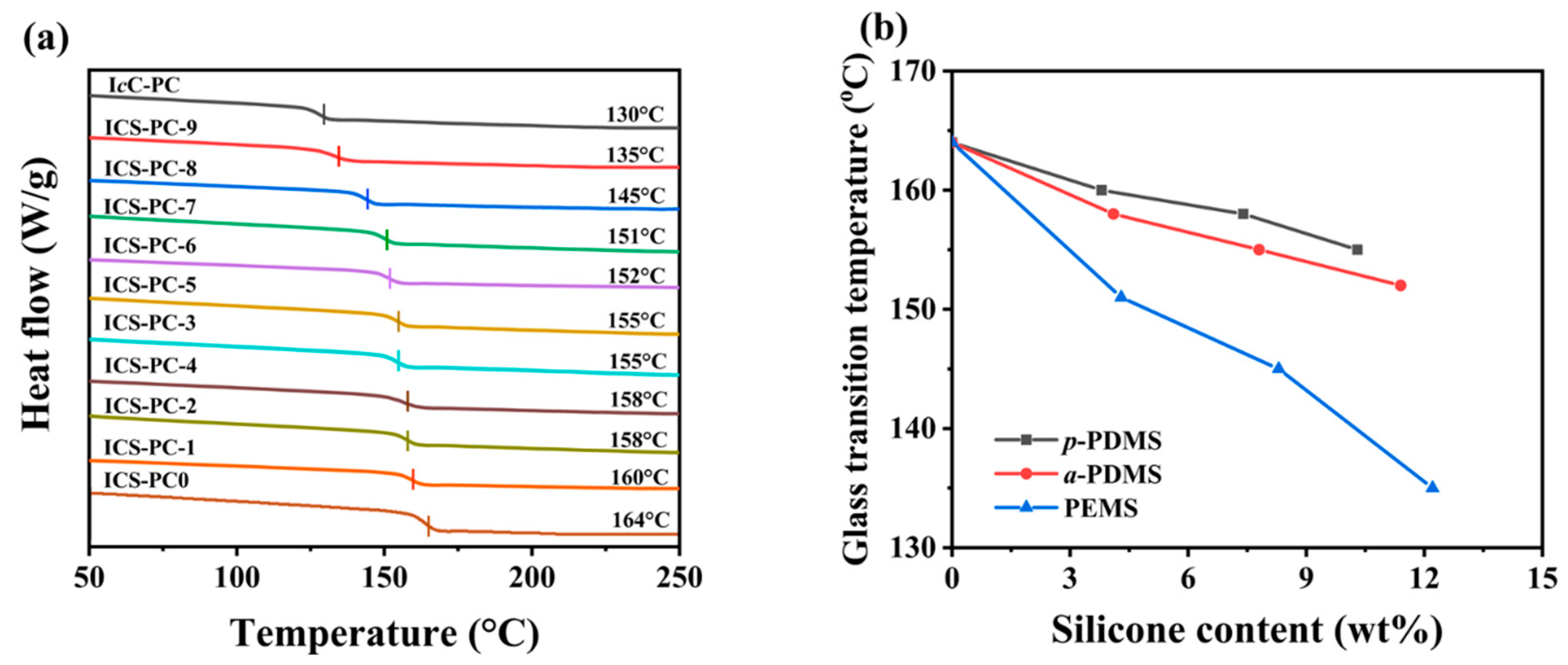
3.4.1. Glass Transition Temperature (Tg)
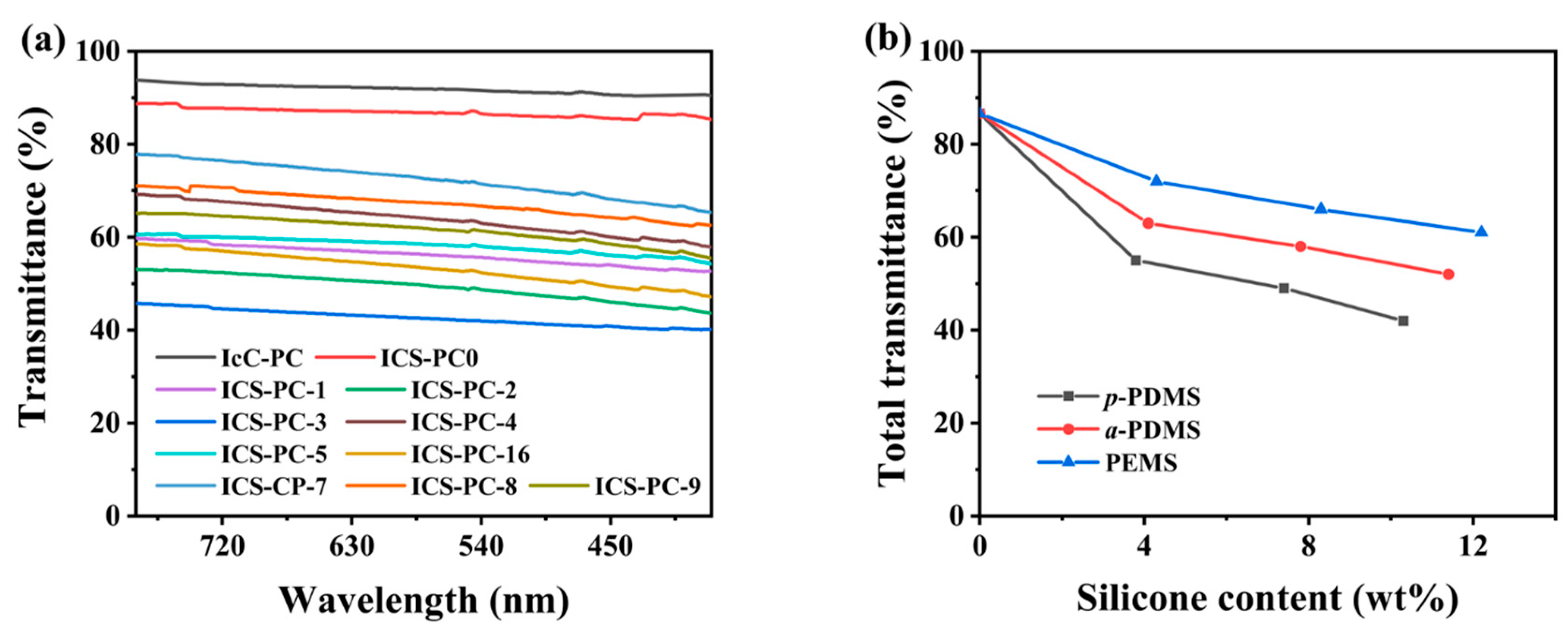
3.4.2. Optical Characterization
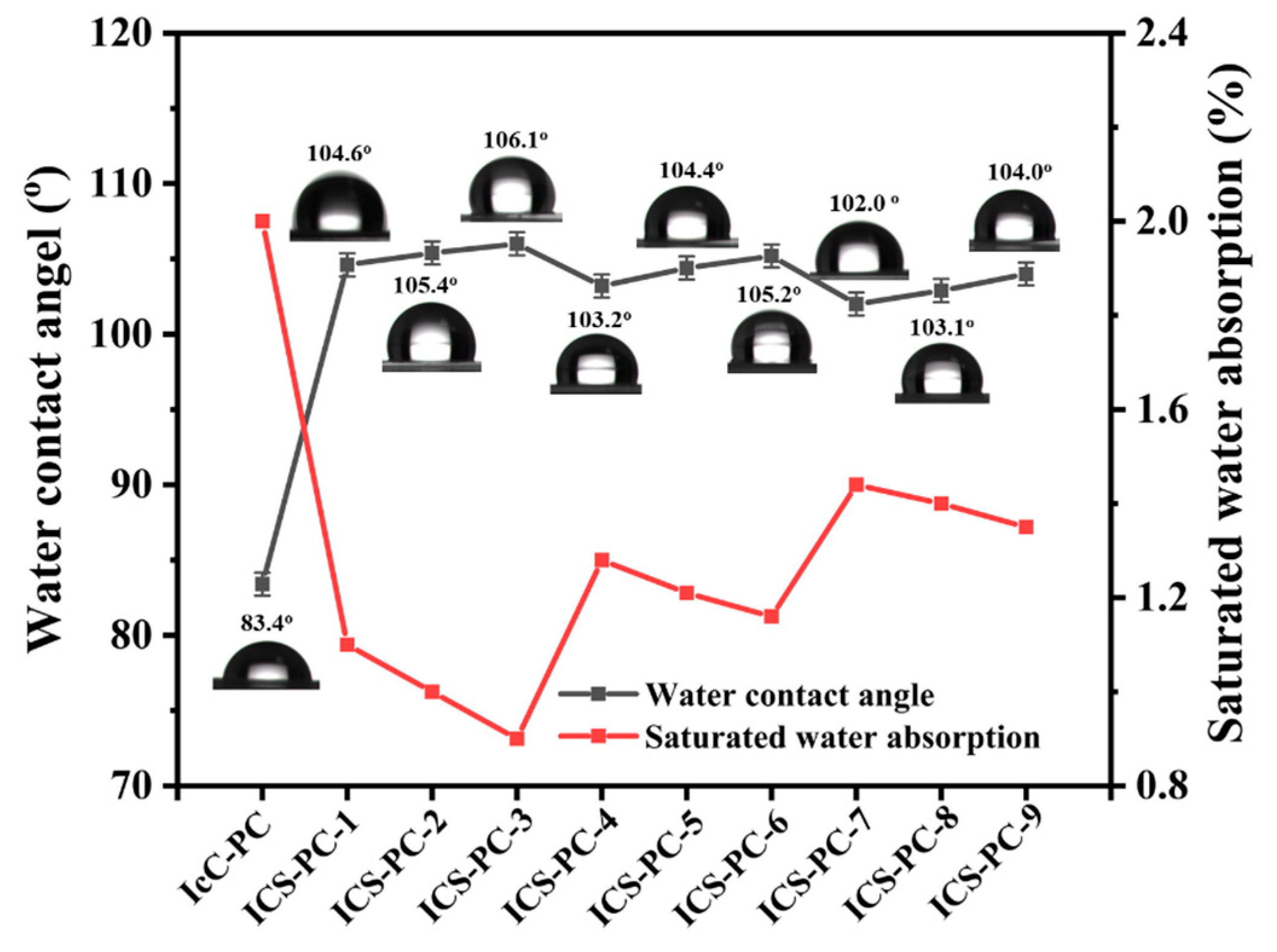
3.4.3. Hydrophobicity and Water Absorption
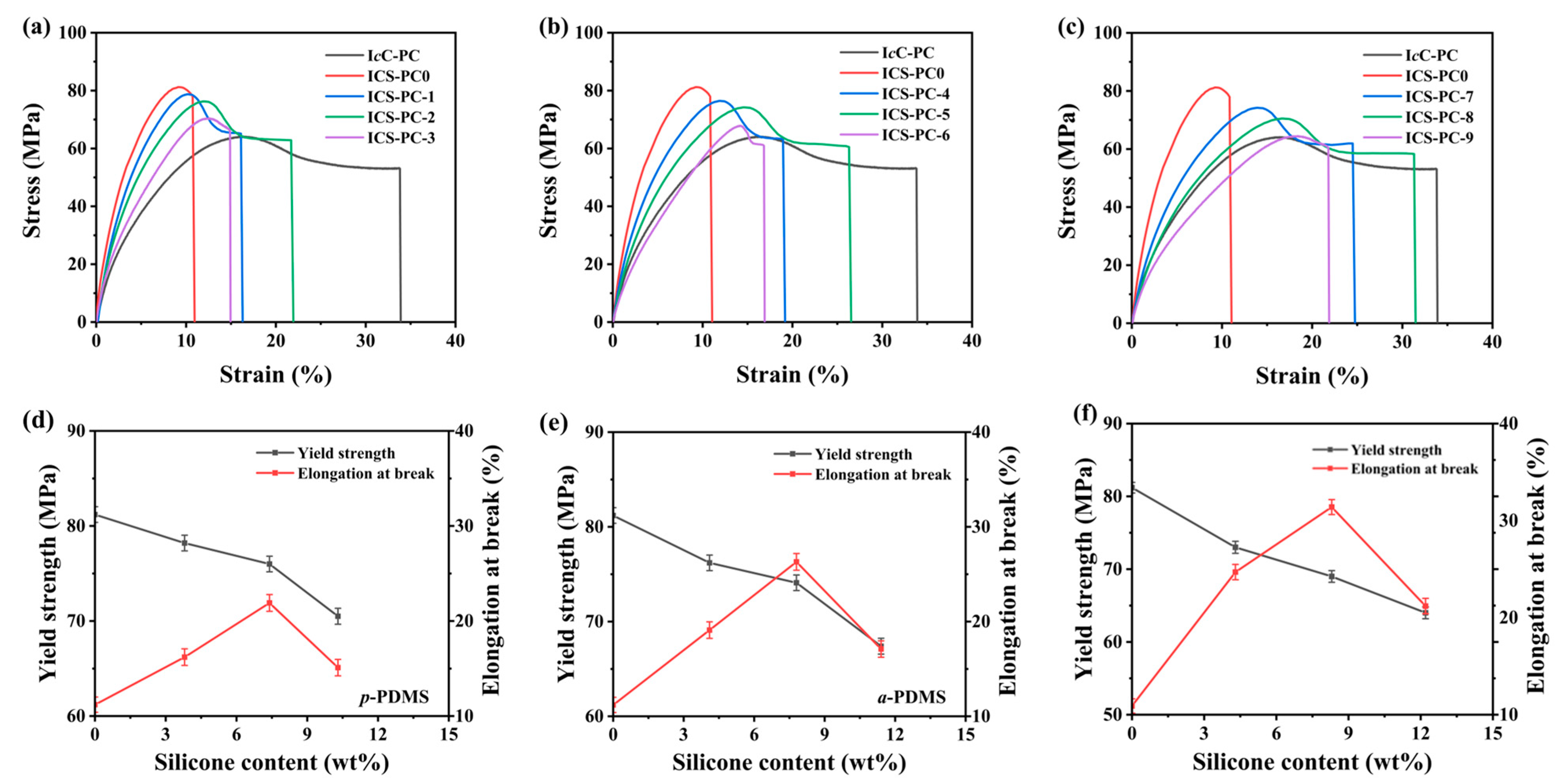
3.4.4. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Freitag, D.; Fengler, G.; Morbitzer, L. Routes to new aromatic polycarbonates with special material properties. Angew. Chem. Int. Ed. 1991, 30, 1598–1610. [Google Scholar] [CrossRef]
- Hotaka, T.; Kondo, F.; Niimi, R.; Togashi, F.; Morita, Y. Industrialization of automotive glazing by polycarbonate and hard-coating. Polym. J. 2019, 51, 1249–1263. [Google Scholar] [CrossRef]
- Hoekstra, E.J.; Catherine, S. Release of bisphenol A from polycarbonate—A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T. Human health risk on environmental exposure to Bisphenol-A: A review. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2006, 24, 225–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, X.B. Chemical recycling to monomers: Industrial bisphenol-A-polycarbonates to novel aliphatic polycarbonate materials. J. Polym. Sci. 2022, 60, 3256–3268. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, W.X.; Li, C.C.; Guan, G.H.; Zhang, D.; Xiao, Y.N.; Zheng, L.C. A high-molecular-weight and high-Tg poly(ester carbonate) partially based on isosorbide: Synthesis and structure-property relationships. Polym. Chem. 2015, 6, 633–642. [Google Scholar] [CrossRef]
- Wang, H.; Xu, F.; Zhang, Z.C.; Feng, M.; Jiang, M.; Zhang, S.J. Bio-based polycarbonates: Progress and prospects. RSC Sustain. 2023, 1, 2162–2179. [Google Scholar] [CrossRef]
- Zenner, M.D.; Xia, Y.; Chen, J.S.; Kessler, M.R. Polyurethanes from isosorbide-based diisocyanates. ChemSusChem 2013, 6, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Medimagh, R.; Smaidia, M.M.; Bennour, H.; Chatti, S. Synthesis and evaluation of the thermal properties of biosourced poly(ether)ureas and copoly(ether)ureas from 1,4:3,6-dianhydrohexitols. Polym. Int. 2015, 64, 513–520. [Google Scholar] [CrossRef]
- Chatti, S.; Schwarz, G.; Kricheldorf, H.R. Cyclic and noncyclic polycarbonates of isosorbide (1,4:3,6-dianhydro-d-glucitol). Macromolecules 2006, 39, 9064–9070. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseaua, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhdrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Thomas, J.; Pati, R.S.; John, J.; Patil, M. A Comprehensive Outlook of Scope within Exterior Automotive Plastic Substrates and Its Coatings. Coatings 2023, 13, 1569. [Google Scholar] [CrossRef]
- Feng, X.H.; East, A.J.; Hammond, W.B.; Zhang, Y.; Jaffe, M. Overview of advances in sugar-based polymers. Polym. Adv. Technol. 2011, 22, 139–150. [Google Scholar] [CrossRef]
- Nelson, A.M.; Long, T.E. A perspective on emerging polymer technologies for bisphenol-A replacement. Polym. Int. 2012, 61, 1485–1491. [Google Scholar] [CrossRef]
- Rose, M.; Palkovits, R. Isosorbide as a renewable platform chemical for versatile applications-quo vadis? ChemSusChem 2012, 5, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Tundo, P.; Aricò, F.; Gauthier, G.; Rossi, L.; Rosamilia, A.E.; Bevinakatti, H.S.; Sievert, R.L.; Newman, C.P. Green synthesis of dimethyl isosorbide. ChemSusChem 2010, 3, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Chatti, S.; Kricheldorf, H.R.; Schwarz, G. Copolycarbonates of isosorbide and various Diols. J. Polym. Sci. Pol. Chem. 2006, 44, 3616–3628. [Google Scholar] [CrossRef]
- Miyashita, M.; Yamaguchi, M. Effect of water absorption on the structure and properties of isosorbide-based polycarbonate. Polymer 2020, 202, 122713. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Dan, Y.; Jiang, L.; Li, G.X. The effect of crystallization on hydrolytic stability of polycarbonate. Polym. Degrad. Stab. 2013, 98, 1465–1472. [Google Scholar] [CrossRef]
- Hein, C.; Patil, R.; Zhou, B.; Lin, G.Y.; Schmidt, C.; Zoller, D.; Ma, S.; Hassman, C. Polycarbonate Copolymers, Articles Formed Therefrom, and Methods of Manufacture. W.O. Patent 2018164706A1, 13 September 2018. [Google Scholar]
- Chun, B.; Bahn, H.; Hwang, Y.; Park, J.; Hong, M.; Lee, K.; Ko, U.; Son, Y. Copolycarbonate and Composition Containing Same. W.O. Patent 2016089118A2, 9 June 2016. [Google Scholar]
- Kimura, T.; Tando, K.; Oda, K. Polycarbonate-Polydiorganosiloxane Copolymer, Resin Composition of Polycarbonate- Polydiorganosiloxane Copolymer, and Production Method for Resin Composition of Polycarbonate-Polydiorganosiloxane Copolymer. W.O. Patent 2019124556A1, 27 June 2019. [Google Scholar]
- Yilgor, I.; Yilgor, E. Thermal stabilities of end groups in hydroxyalkyl terminated polydimethylsiloxane oligomers. Polym. Bull. 1998, 40, 525–532. [Google Scholar] [CrossRef]
- Zhang, S.M.; Zhang, H.X.; Zhang, W.Y.; Wu, Z.Q.; Chen, F.; Fu, Q. Toughening of polycarbonate through reactive melt blending: Effect of hydroxyl content and viscosity of hydroxyl-terminated polydimethysiloxane. Chin. J. Polym. Sci. 2014, 32, 823–833. [Google Scholar] [CrossRef]
- Zhou, W.J.; Osby, J. Siloxane modification of polycarbonate for superior flow and impact toughness. Polymer 2010, 51, 1990–1999. [Google Scholar] [CrossRef]
- Vaughn, H.A. Organopolysiloxane-Polycarbonate Block Copolymers. U.S. Patent 3189662A, 15 June 1965. [Google Scholar]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly (dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Malmgren-Hansen, B.; Olesen, S.; Pommer, K.; Funch, L.W.; Pedersen, E. Survey of Chemical Substances in Consumer Products Survey No. 32—2003 Emission and Evalution of Chemical Substances from Selected Electrical and Electronic Products; The Danish Environmental Protection Agency: Copenhagen, DA, USA, 2003. [Google Scholar]
- Woodburm, K.; Drottar, K.; Domoradzki, J.; Durham, J.; Mcnett, D.; Jezowski, R. Determination of the dietary biomagnification of octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane with the rainbow trout (Oncorhynchus mykiss). Chemosphere 2013, 93, 779–788. [Google Scholar] [CrossRef]
- Annett, K.; Wolfgang, E.; Walter, K. Preparation of Polysiloxane/Polycarbonate Block Cocondensation Product. J.P. Patent 10251408A, 22 September 1998. [Google Scholar]
- Jiyunia, J.; Makurosuk, P.; Deibisu, G. Method of Preparing Block Copolysiloxane Carbonate. J.P. Patent 08311206A, 26 November 1996. [Google Scholar]
- Koenig, A.; Ebert, W.; Koehler, W. Process for the Preparation of Polysiloxane-Polycarbonate Block Cocondensates. D.E. Patent 19710081A1, 17 September 1998. [Google Scholar]
- Zhou, Z.B.; Wu, G.Z. Preparation of Bisphenol-A and Polydimethylsiloxane (PDMS) Block Copolycarbonates by Melt Polycondensation: Effects of PDMS Chain Length on Conversion and Miscibility. Polymers 2021, 13, 2660. [Google Scholar] [CrossRef]
- He, M.J.; Chen, W.X.; Dong, X.X. Polymer Physics, 3rd ed.; Fundan University Press: Shanghai, China, 2007. [Google Scholar]
- Shinobu, Y.; Takafumi, A.; Masami, S. Polycarbonate/Polyorganosiloxane Copolymer and Resin Composition Including Said Copolymer. W.O. Patent 2021112259A1, 10 June 2021. [Google Scholar]
- Yilgor, I.; Riffle, J.S.; McGrath, J.E. Reactive Difunctional Siloxane Oligomers; American Chemical Society Press: Washington, DC, USA, 1985. [Google Scholar]
- Venderbosch, R.W.; Goedmakers, J.; Hurst, J.D. Translucent Thermoplastic Composition, Method for Making the Composition and Articles Molded Therefrom. U.S. Patent 2005187372A1, 25 August 2005. [Google Scholar]
- Chen, X.; Lee, H.F.; Gardella, J. Effects of structure and annealing on the surface composition of multiblock copolymers of bisphenol A polycarbonate and poly(dimethylsiloxane). Macromolecules 1993, 26, 4601–4605. [Google Scholar] [CrossRef]
- Song, Z.Q.; Xu, F.; Wang, H.; Zhang, Z.C.; Feng, M.; Zhang, Y.W.; Yang, Z.; Xie, J.X.; Su, D.; Li, T. Design and synthesis of isosorbide-based copolycarbonates with high transparency and low hygroscopicity for optical applications. J. Appl. Polym. 2023, 140, e54009. [Google Scholar] [CrossRef]
- Pang, X.Y.; Ge, X.; Ji, J.Y.; Liang, W.J.; Liu, R.L.; Chen, X.J.; Yin, G.Q.; Ge, J.F. Improving oxygen permeability and thermostability of polycarbonate via copolymerization modification with bio-phenol polysiloxane. Polymers 2019, 11, 1302. [Google Scholar] [CrossRef]
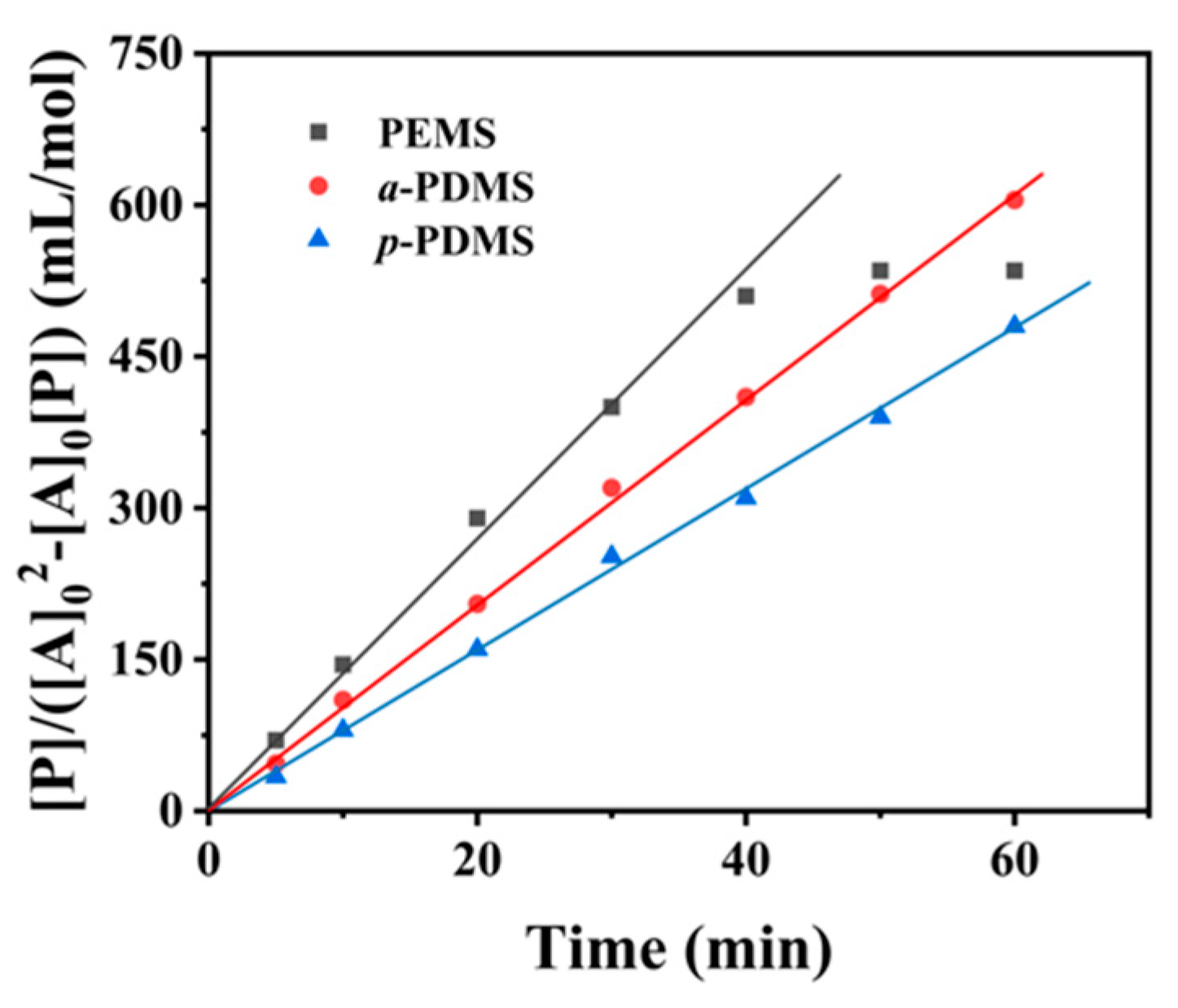
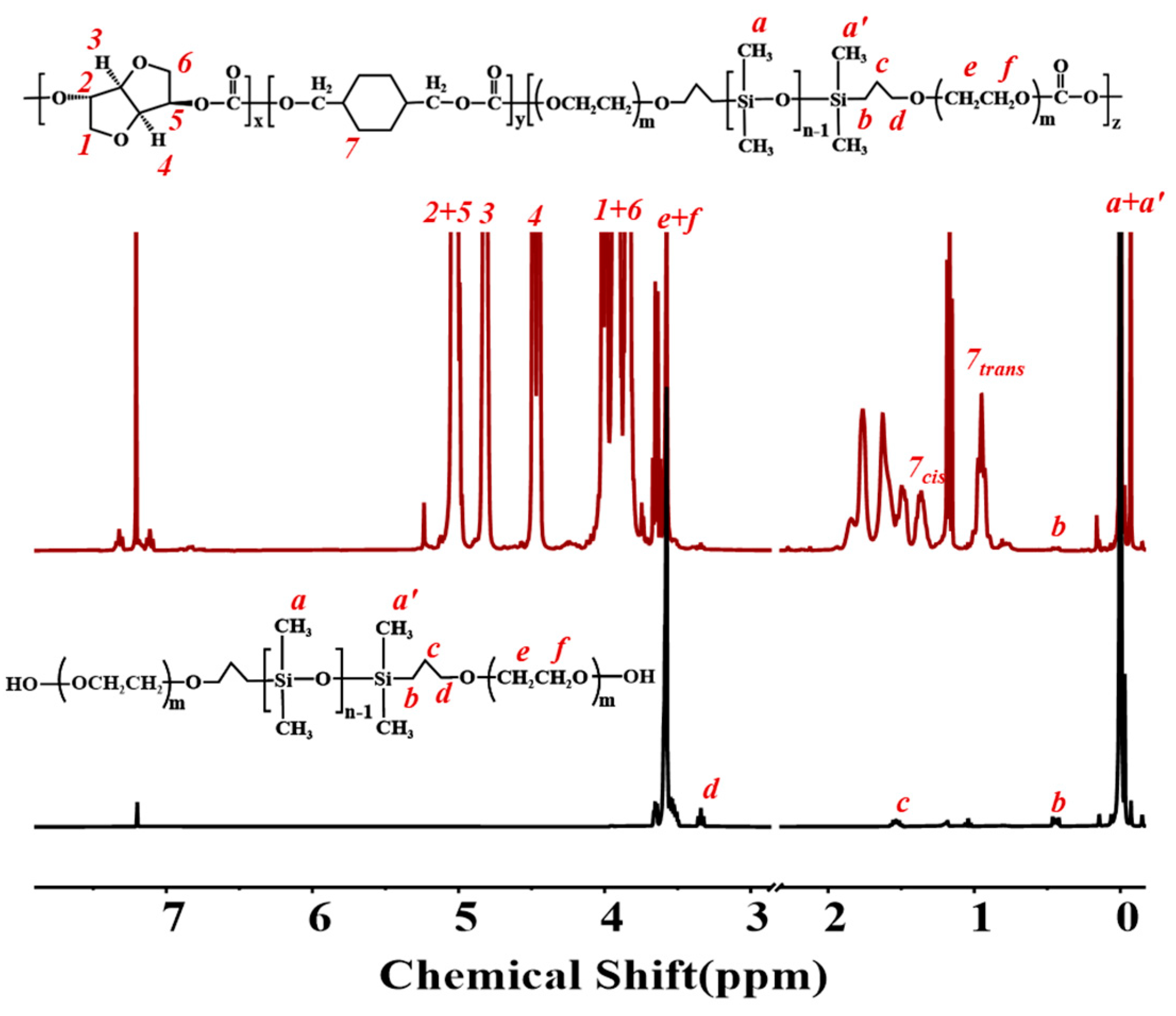


| Silicone | [PhOH] 1 (×10−6 mol/mL) | [DPC] 1 (×10−6 mol/mL) | k′ |
|---|---|---|---|
| PEMS | 2.18 | 0.08 | 13.33 |
| a-PDMS | 2.05 | 0.15 | 10.24 |
| p-PDMS | 1.90 | 0.21 | 8.00 |
| Sample 1 | ISB/CHDM | Silicone | Feed Ratio 2 (wt%) | Mη (kg/mol) | Tg (°C) | ΔC (%) | W3 (wt%) | Conversion 3 (%) |
|---|---|---|---|---|---|---|---|---|
| IcC-PC | 70/30 | / | 0 | 35.1 | 130 | 0.10 | / | / |
| ICS-PC-0 | 90/10 | / | 0 | 32.8 | 164 | 0.11 | / | / |
| ICS-PC-1 | 90/10 | p-PDMS | 5 | 31.2 | 160 | 0.26 | 3.8 | 76.9 |
| ICS-PC-2 | 10 | 30.4 | 158 | 0.30 | 7.4 | 74.0 | ||
| ICS-PC-3 | 15 | 30.0 | 155 | 0.35 | 10.3 | 68.4 | ||
| ICS-PC-4 | 90/10 | a-PDMS | 5 | 31.4 | 158 | 0.24 | 4.1 | 81.0 |
| ICS-PC-5 | 10 | 31.3 | 155 | 0.27 | 7.8 | 78.0 | ||
| ICS-PC-6 | 15 | 30.1 | 152 | 0.32 | 11.4 | 76.2 | ||
| ICS-PC-7 | 90/10 | PEMS | 5 | 31.2 | 151 | 0.19 | 4.3 | 86.4 |
| ICS-PC-8 | 10 | 30.1 | 145 | 0.22 | 8.3 | 83.0 | ||
| ICS-PC-9 | 15 | 29.5 | 135 | 0.25 | 12.2 | 81.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, H.; Fang, W.; Lu, T.; Wang, J.; Wu, G. Synthesis and Properties of Bio-Based Polycarbonates Containing Silicone Blocks. Polymers 2024, 16, 1318. https://doi.org/10.3390/polym16101318
Liu M, Wang H, Fang W, Lu T, Wang J, Wu G. Synthesis and Properties of Bio-Based Polycarbonates Containing Silicone Blocks. Polymers. 2024; 16(10):1318. https://doi.org/10.3390/polym16101318
Chicago/Turabian StyleLiu, Mengjuan, Hui Wang, Wei Fang, Tao Lu, Jinsen Wang, and Guozhang Wu. 2024. "Synthesis and Properties of Bio-Based Polycarbonates Containing Silicone Blocks" Polymers 16, no. 10: 1318. https://doi.org/10.3390/polym16101318
APA StyleLiu, M., Wang, H., Fang, W., Lu, T., Wang, J., & Wu, G. (2024). Synthesis and Properties of Bio-Based Polycarbonates Containing Silicone Blocks. Polymers, 16(10), 1318. https://doi.org/10.3390/polym16101318





