Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Technique
3. Results and Discussion
3.1. Curing Conditions
3.2. Dielectric Observation of the Examined Materials
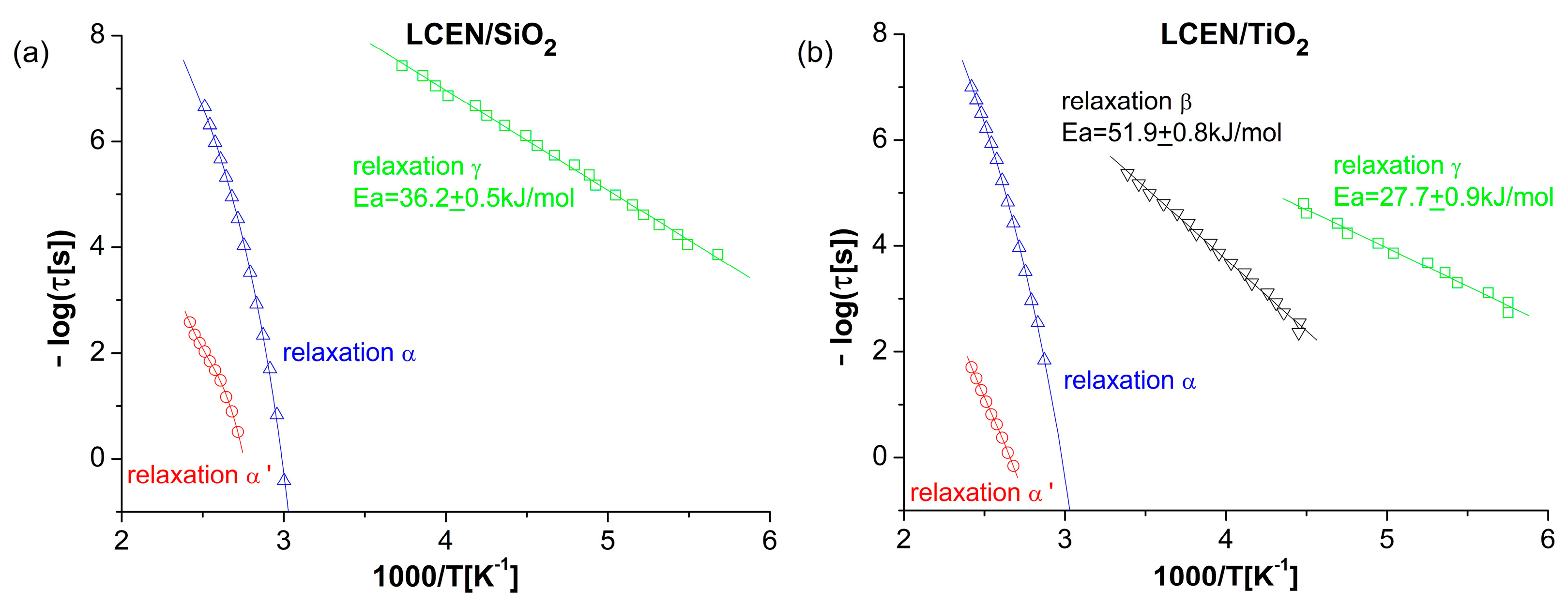
3.3. Properties of the Obtained Organic/Inorganic Matrices in Dielectric Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Wang, C.; Campbell, G.A.; Earls, J.D.; Priester, J.D. Effects of liquid crystalline structure formation on the curing kinetics of an epoxy resin. J. Polym. Sci. A Polym. Chem. 1997, 35, 1105–1124. [Google Scholar] [CrossRef]
- Castell, P.; Serra, A.; Galià, M.; Giamberini, M.; Carfagna, C. Anisotropic thermosets from liquid-crystalline azomethynic epoxy resins and primary aromatic diamines. J. Polym. Sci. A Polym. Chem. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, J. The effect of mesogenic length on the curing behavior and properties of liquid crystalline epoxy resins. Polymer 2006, 47, 3036–3042. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, J. Anisotropically ordered liquid crystalline epoxy network on carbon fiber surface. Polym. Bull. 2007, 59, 261–268. [Google Scholar] [CrossRef]
- Suzuki, N.; Kiba, S.; Yamauchi, Y. Fabrication of mesoporous silica KIT-6/polymer composite and its low thermal expansion property. Mater. Lett. 2011, 65, 544–547. [Google Scholar] [CrossRef]
- Paquet, C.; Kumacheva, E. Nanostructured polymers for photonics. Mater. Today 2008, 11, 48–56. [Google Scholar] [CrossRef]
- Agra-Kooijman, D.M.; Fisch, M.R.; Joshi, L.; Ren, W.; McMullan, P.J.; Griffin, A.C.; Kumar, S. Dual relaxation and structural changes under uniaxial strain in main-chain smectic-C liquid crystal elastomer. Phys. Chem. Chem. Phys. 2015, 17, 191–199. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, L.; Bae, J. Advances in 4D printing of liquid crystalline elastomers: Materials, techniques, and applications. Mater. Horiz. 2022, 9, 1825–1849. [Google Scholar] [CrossRef] [PubMed]
- Douglas, E.P. Structure-property relationships in liquid crystalline thermosets. J. Macromol. Sci. Polym. Rev. 2006, 46, 127–141. [Google Scholar] [CrossRef]
- Shiota, A.; Ober, C.K. Rigid rod and liquid crystalline thermosets. Prog. Polym. Sci. 1997, 22, 975–1000. [Google Scholar] [CrossRef]
- Carfagna, C.; Amendola, C.E.; Giamberini, M. Liquid crystalline epoxy based thermosetting polymers. Prog. Polym. Sci. 1997, 22, 1607–1647. [Google Scholar] [CrossRef]
- Mohan, P. A critical review: The modification, properties, and applications of epoxy resins. Polym. Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Jeong, I.; Bin Kim, C.; Kang, D.G.; Jeong, K.U.; Jang, S.G.; You, N.H.; Ahn, S.; Lee, D.S.; Goh, M. Liquid crystalline epoxy resin with improved thermal conductivity by intermolecular dipole–dipole interactions. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 708–715. [Google Scholar] [CrossRef]
- Zhao, M.; Yuan, K.; Wang, Y.; Li, G.; Guo, J.; Gu, L.; Hu, W.; Zhao, H.; Tang, Z. Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nature 2016, 539, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Khan, S.; Chauhan, S.; Biradar, A.M. Metal oxide-nanoparticles and liquid crystal composites: A review of recent progress. J. Mol. Liq. 2020, 297, 112052. [Google Scholar] [CrossRef]
- Chou, T.-R.; Hsieh, J.; Chen, W.-T.; Chao, C.-Y. Influence of particle size on the ion effect of TiO2 nanoparticle doped nematic liquid crystal cell. Jpn. J. Appl. Phys. 2014, 53, 071701. [Google Scholar] [CrossRef]
- Li, W.; Zhu, M.; Ding, X.; Li, B.; Huang, W.; Cao, H.; Yang, Z.; Yang, H. Studies on electro-optical properties of polymer matrix/LC/SiO2 nanoparticles composites. J. Appl. Polym. Sci. 2009, 111, 1449–1453. [Google Scholar] [CrossRef]
- Rezaei Qazviniha, M.; Piri, F. Preparation, Identification, and Evaluation of the Thermal Properties of Novolac Resins Modified with TiO2, MgO, and V2O5 Oxides. Mech. Adv. Compos. Struct. 2024, 11, 1–9. [Google Scholar]
- Ota, S.; Harada, M. Filler surface adsorption of mesogenic epoxy for LC Epoxy/MgO composites with high thermal conductivity. Compos. Part C Open Access 2021, 4, 100087. [Google Scholar] [CrossRef]
- Uzay, Ç. Mechanical and thermal characterization of laminar carbon/epoxy composites modified with magnesium oxide microparticles. Polym. Compos. 2022, 43, 299–310. [Google Scholar] [CrossRef]
- Janus, M.; Kusiak-Nejman, E.; Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Zając, K.; Morawski, A.W. Bacterial inactivation on concrete plates loaded with modified TiO2 photocatalysts under visible light irradiation. Molecules 2019, 24, 3026. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.; Zatorska, J.; Czyżewski, A.; Bubacz, K.; Kusiak-Nejman, E.; Morawski, A.W. Self-cleaning properties of cement plates loaded with N, C-modified TiO2 photocatalysts. Appl. Surf. Sci. 2015, 330, 200–206. [Google Scholar] [CrossRef]
- Schwendler, L.A.; Lansarin, M.A. Polyhydroxybutyrate-co-hydroxyvalerate/titanium dioxide composite films: Green synthesis, characterization, and photocatalytic assessment. J. Polym. Res. 2023, 30, 169. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. (Eds.) Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Rault, J. Relaxation of glasses: The Kohlrausch exponent. J. Non-Cryst. Solids 2011, 357, 339–345. [Google Scholar] [CrossRef]
- Mohomed, K. A broad spectrum analysis of the dielectric properties of poly (2-hydroxyethyl methacrylate). Polymer 2005, 46, 3847–3855. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Dyre, J.C. The random free-energy barrier model for ac conduction in disordered solids. J. Appl. Phys. 1988, 64, 2456–2468. [Google Scholar] [CrossRef]
- Donth, E. The Glass Transition: Relaxation Dynamics in Liquids and Disordered Materials; Springer: New York, NY, USA, 2001. [Google Scholar]
- Kovacs, A.J. La contraction isotherme du volume des polymères amorphes. J. Polym. Sci. 1958, 30, 131–147. [Google Scholar] [CrossRef]
- Kranbuehl, D.; Delos, S.; Yi, E.; Mayer, J.; Jarvie, T.; Winfree, W.; Hou, T. Dynamic dielectric analysis: Nondestructive material evaluation and cure cycle monitoring. Polym. Eng. Sci. 1986, 26, 338–345. [Google Scholar] [CrossRef]
- Senturia, S.D.; Sheppard, N.F. Epoxy Resins and Composites IV. Adv. Polym. Sci. 1986, 80, 1–47. [Google Scholar]
- Fournier, J.; Williams, G.; Duch, C.; Aldridge, G.A. Changes in molecular dynamics during bulk polymerization of an epoxide− amine system as studied by dielectric relaxation spectroscopy. Macromolecules 1996, 29, 7097–7107. [Google Scholar] [CrossRef]
- Włodarska, M. Curing Reaction and Dielectric Properties of Rigid and Elastic Liquid Crystal Epoxy Networks Modified with Nanofillers. Int. J. Polym. Sci. 2018, 2018, 9578654. [Google Scholar] [CrossRef]
- Włodarska, M.; Mossety-Leszczak, B.; Kisiel, M.; Zając, W.; Okrasa, L. Changes in molecular relaxations and network properties of a triaromatic liquid crystal epoxy resin with nonterminal functional groups. J. Polym. Sci. 2023, 61, 3244–3255. [Google Scholar] [CrossRef]
- Li, J.; Aung, H.H.; Du, B. Curing regime-modulating insulation performance of anhydride-cured epoxy resin: A review. Molecules 2023, 28, 547. [Google Scholar] [CrossRef] [PubMed]

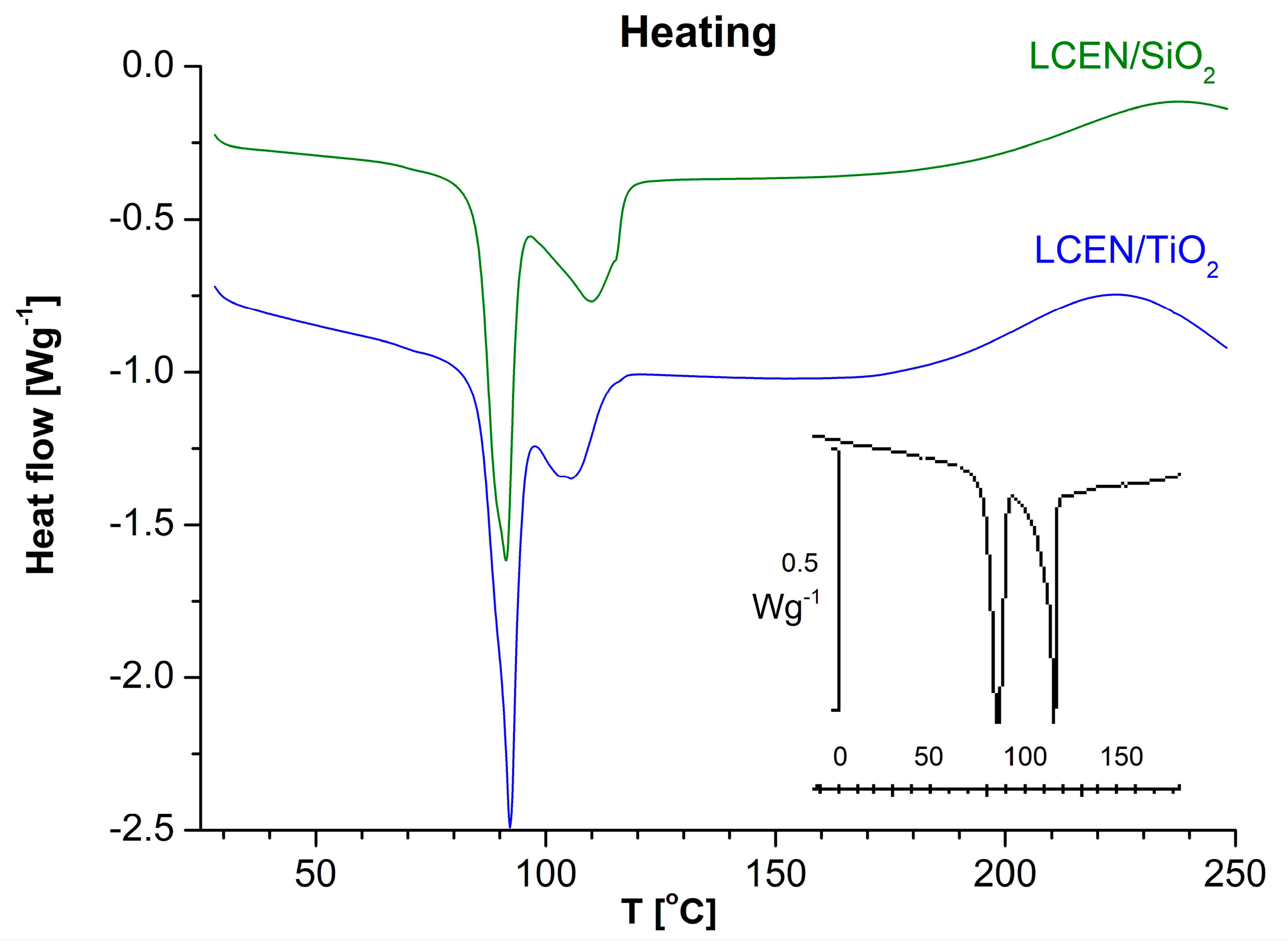
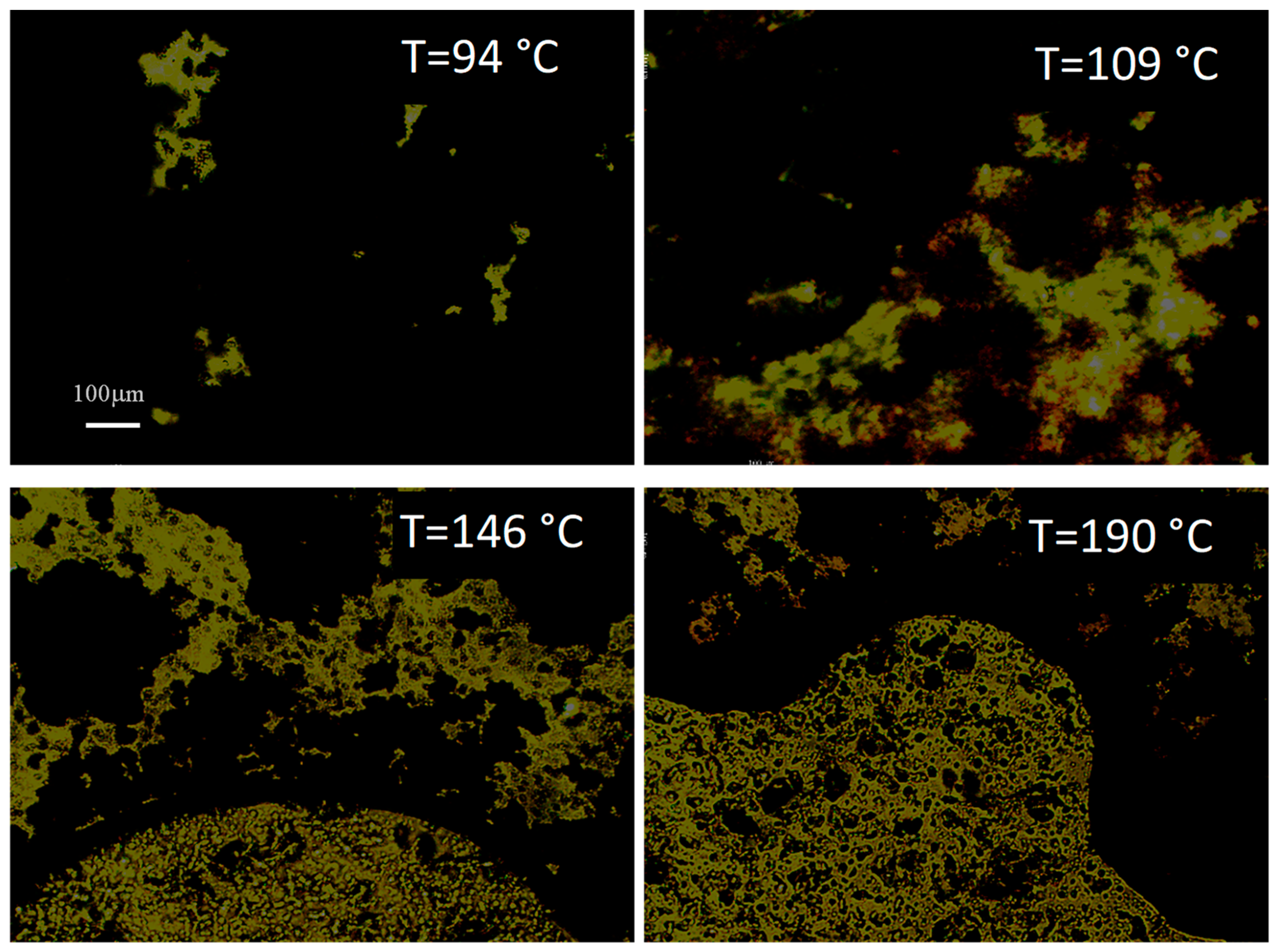
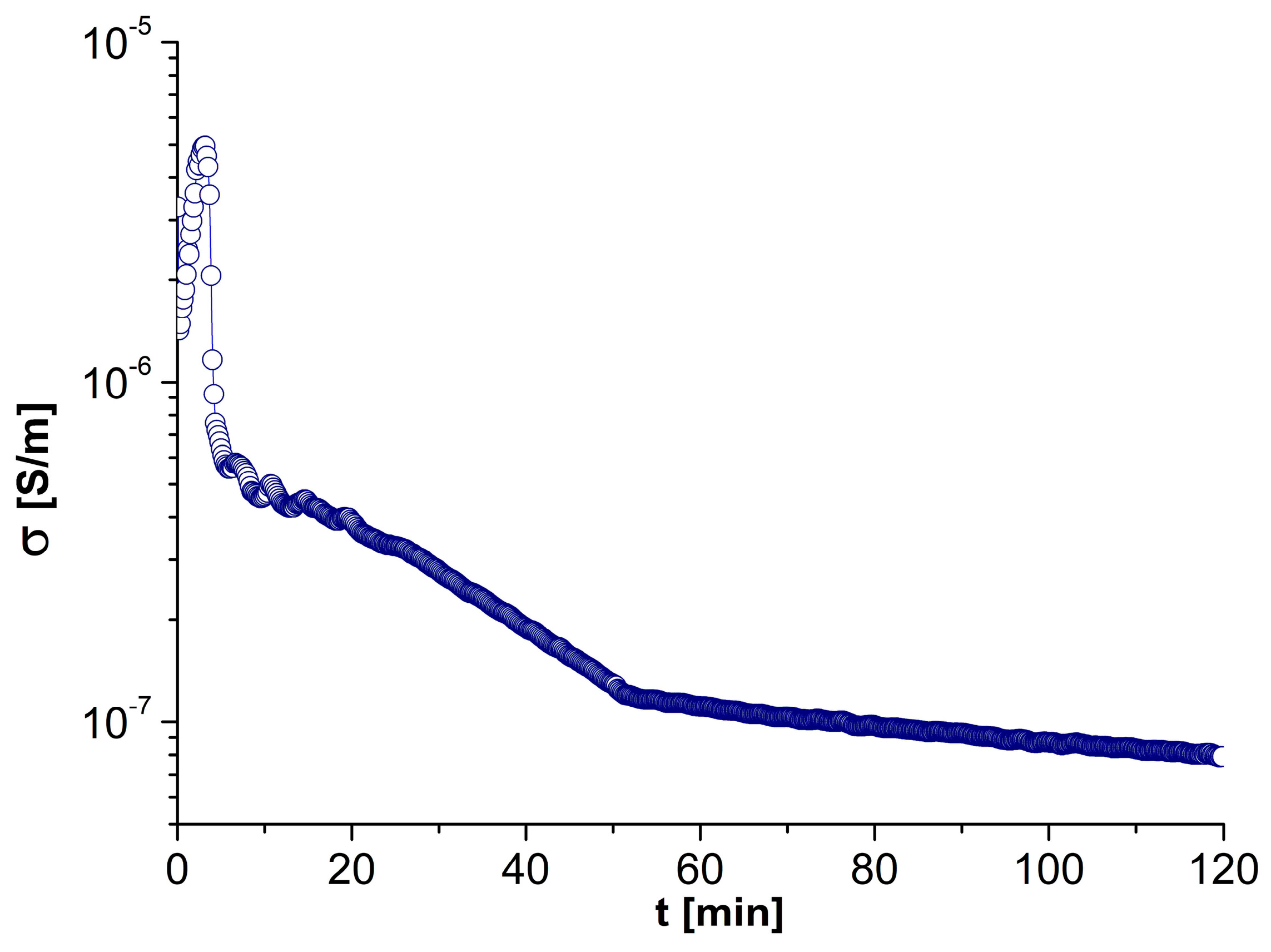

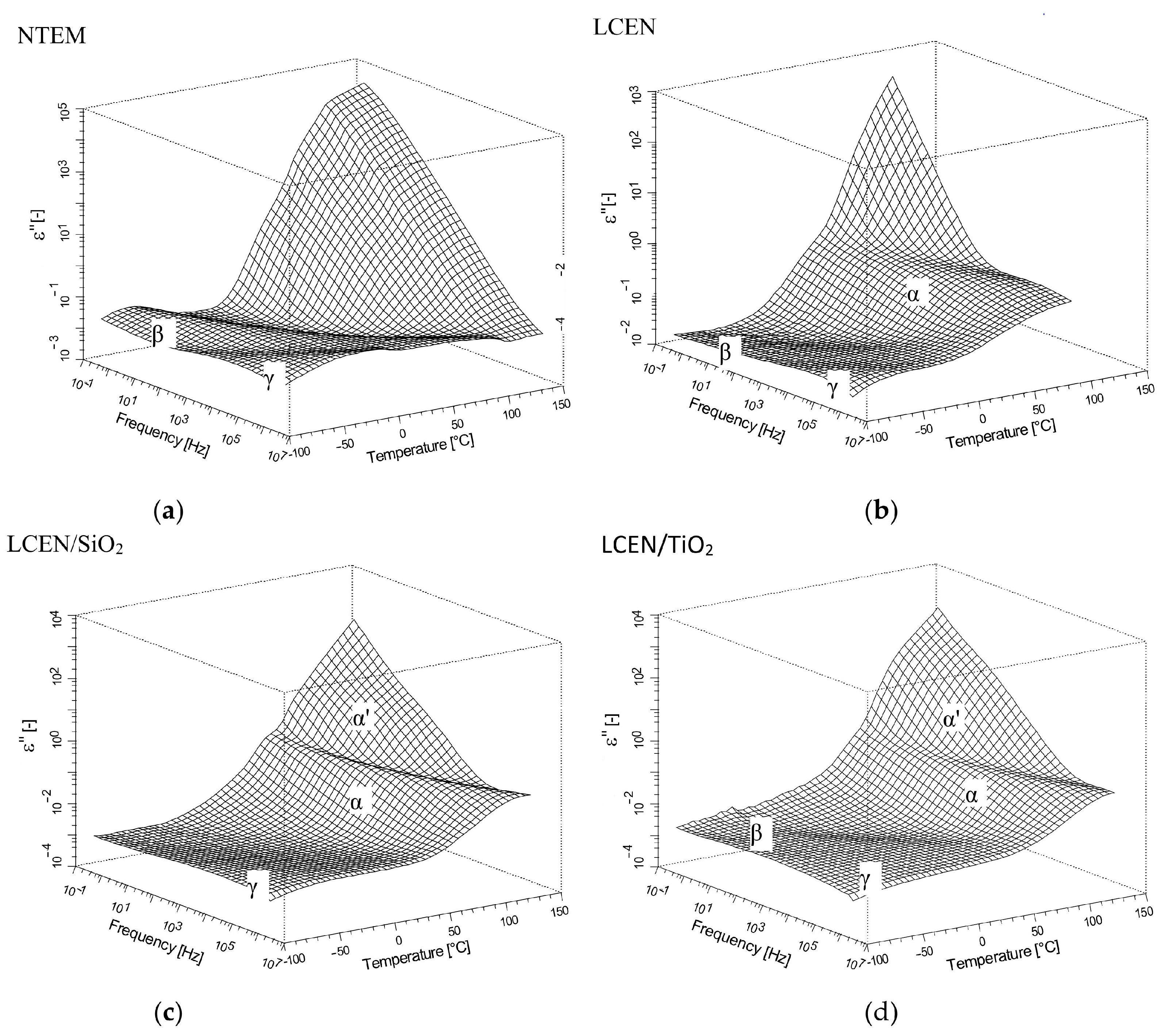
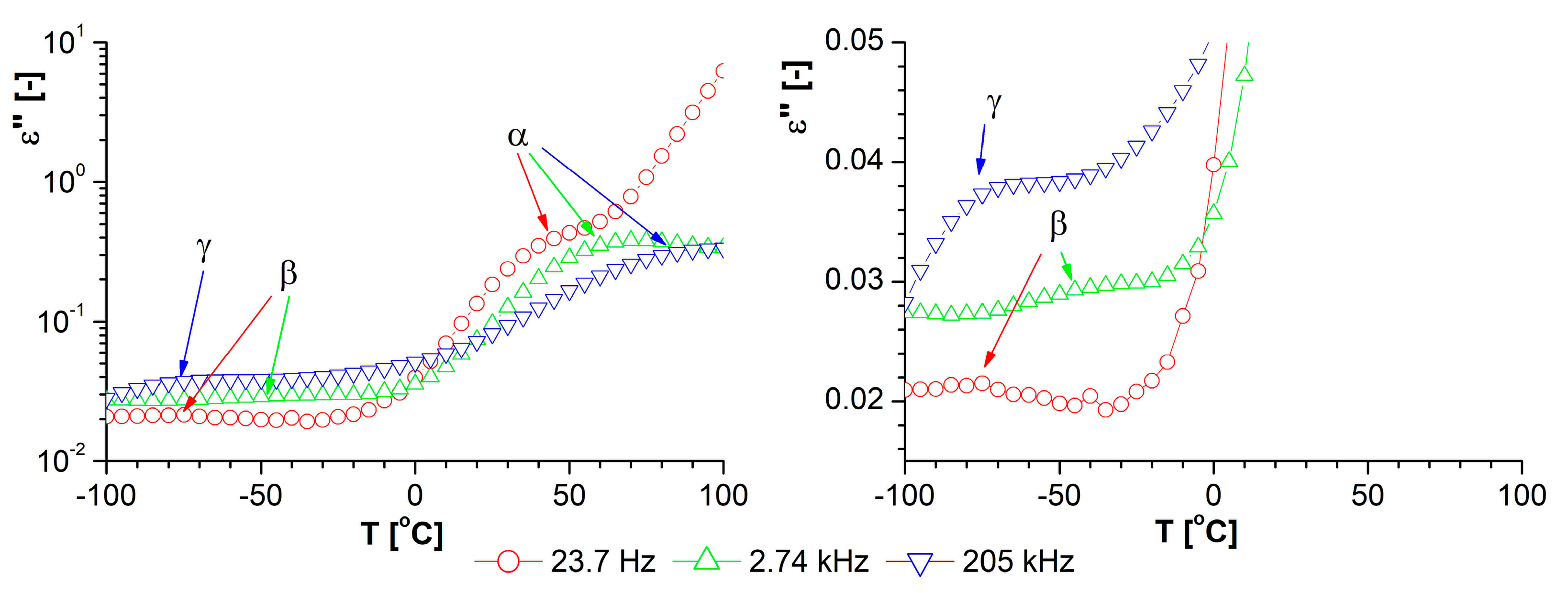
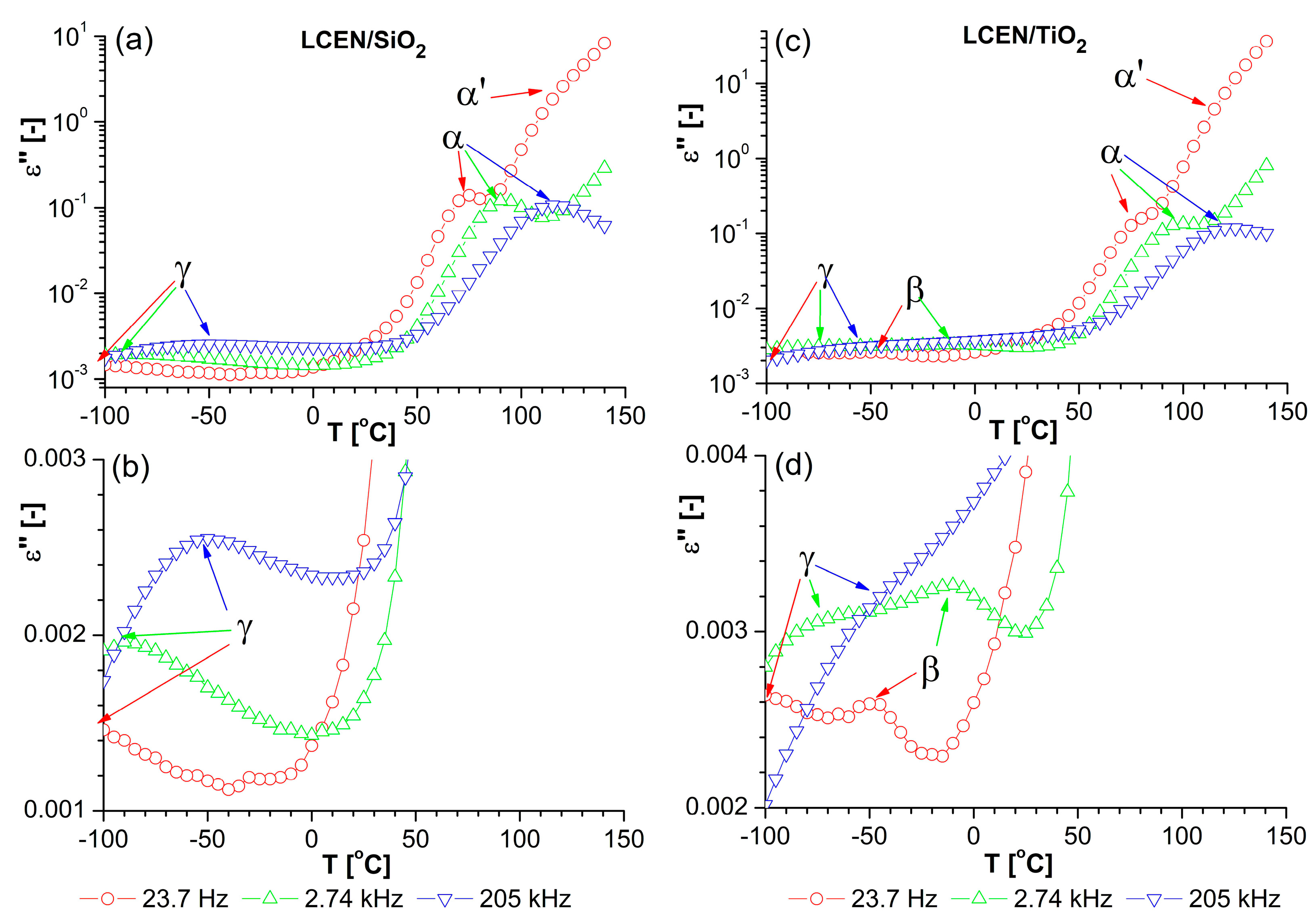
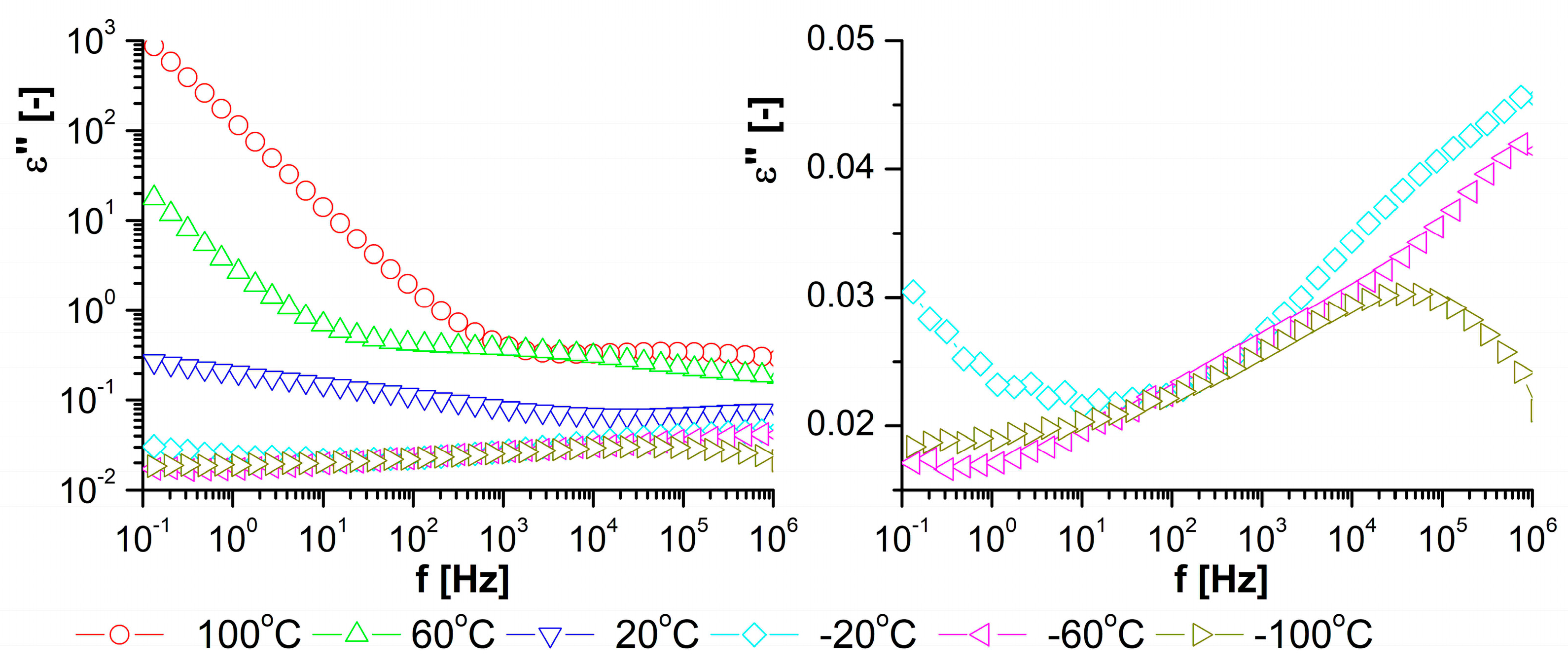
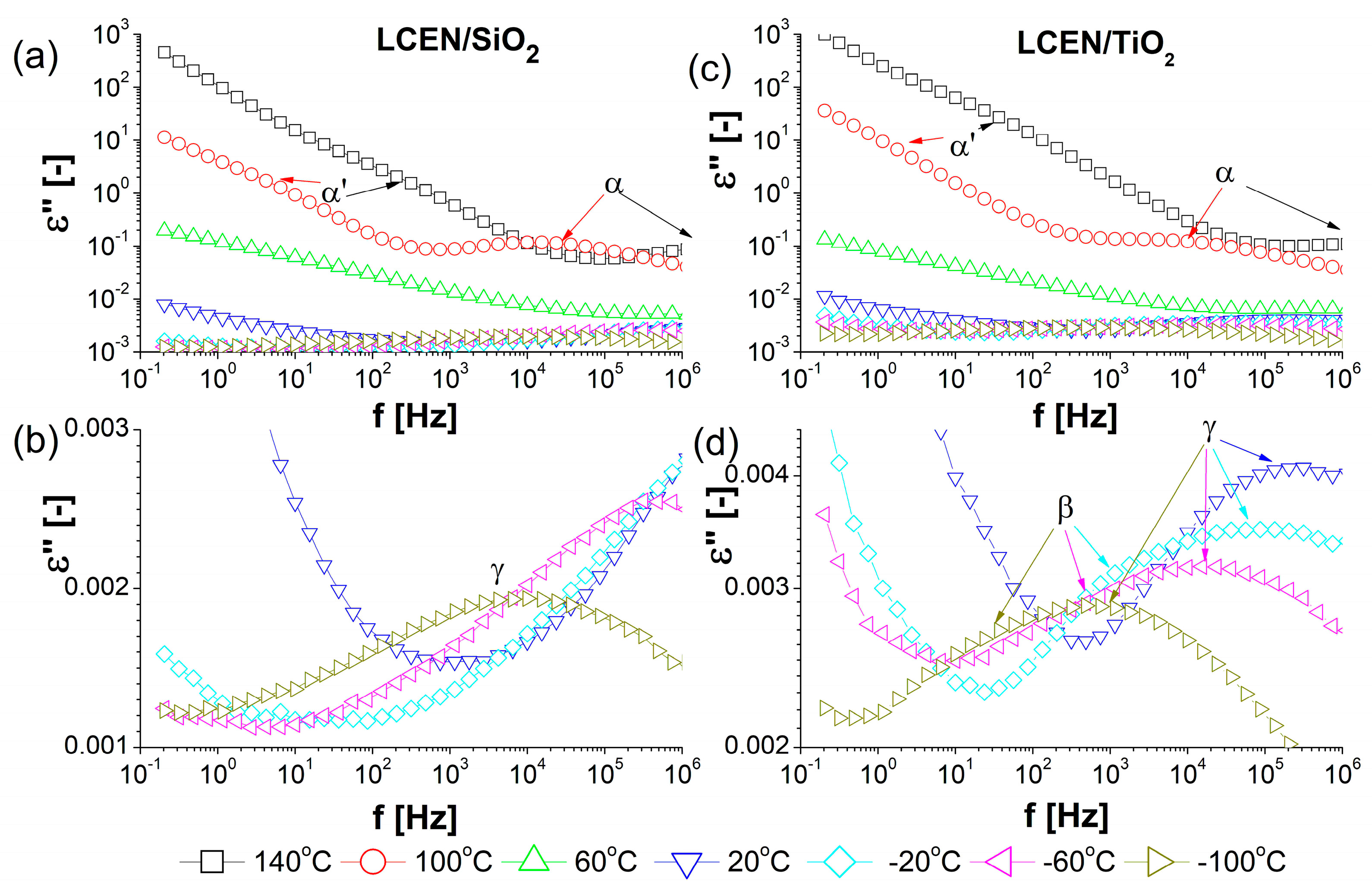
| Phase Transition | Temperature [°C] | Stage |
|---|---|---|
| Solid (S) to Nematic (N) Nematic (N) to Isotropic liquid (I) | 117 142 | heating |
| Isotropic liquid (I) to Nematic (N) Nematic (N) to Solid (S) | 140 113 | cooling |
| Mixture | Acronym | Percentage of Oxides in the Mixture |
|---|---|---|
| NTEM/DDM | LCEN | 0 |
| NTEM/DDM + SiO2 | LCEN/SiO2 | 8.6% |
| NTEM/DDM + TiO2 | LCEN/TiO2 | 9.7% |
| Sample Acronym | Curing Temperature [°C] | Curing Time [h] |
|---|---|---|
| LCEN | 185 | 3 |
| LCEN/SiO2 | 195 | 3 |
| LCEN/TiO2 | 190 | 2 |
| Sample | Tg [K] from DSC | Tg [K] from BDS | Tv [K] | D | m | Ea′ (Tg) [kJ mol−1] |
|---|---|---|---|---|---|---|
| LCEN | 283 [35] | 289 ± 10 | 182 ± 9 | 23.9 ± 4.5 | 47.7 | 264 |
| LCEN/TiO2 | 322.7 | 323 ± 8 | 239 ± 7 | 14.6 ± 1.9 | 69.4 | 429 |
| LCEN/SiO2 | 319.1 | 326 ± 5 | 271 ± 4 | 7.1 ± 0.8 | 90.1 | 562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrasa, L.; Włodarska, M.; Kisiel, M.; Mossety-Leszczak, B. Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide. Polymers 2024, 16, 1320. https://doi.org/10.3390/polym16101320
Okrasa L, Włodarska M, Kisiel M, Mossety-Leszczak B. Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide. Polymers. 2024; 16(10):1320. https://doi.org/10.3390/polym16101320
Chicago/Turabian StyleOkrasa, Lidia, Magdalena Włodarska, Maciej Kisiel, and Beata Mossety-Leszczak. 2024. "Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide" Polymers 16, no. 10: 1320. https://doi.org/10.3390/polym16101320
APA StyleOkrasa, L., Włodarska, M., Kisiel, M., & Mossety-Leszczak, B. (2024). Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide. Polymers, 16(10), 1320. https://doi.org/10.3390/polym16101320







