Four-Component Statistical Copolymers by RAFT Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
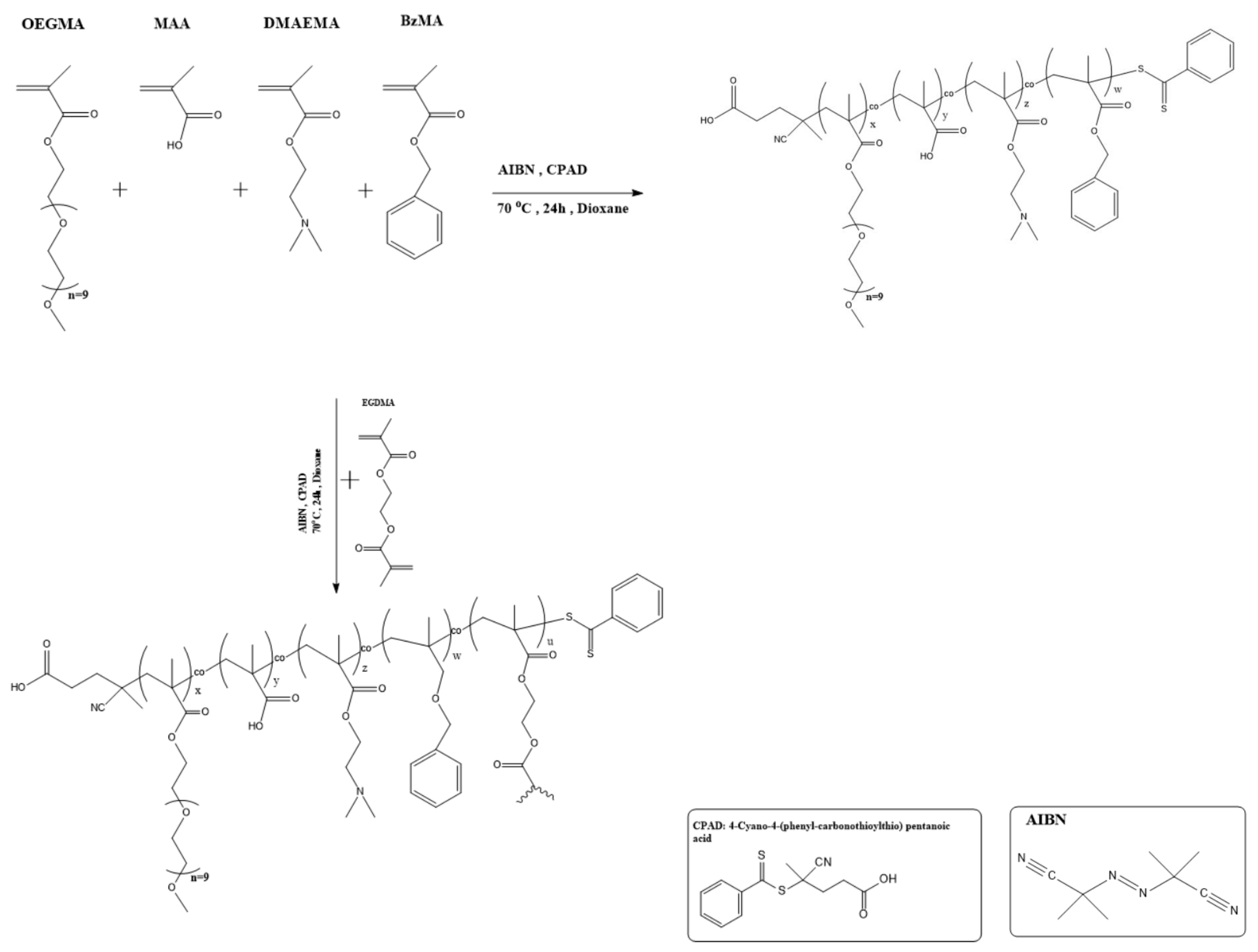
2.2. Synthesis of Linear and Hyperbranched Statistical Quaterpolymers
2.3. Self-Assembly of Quaterpolymers in Aqueous Media
2.4. FBS Interactions with Quaterpolymers
2.5. Characterization Methods
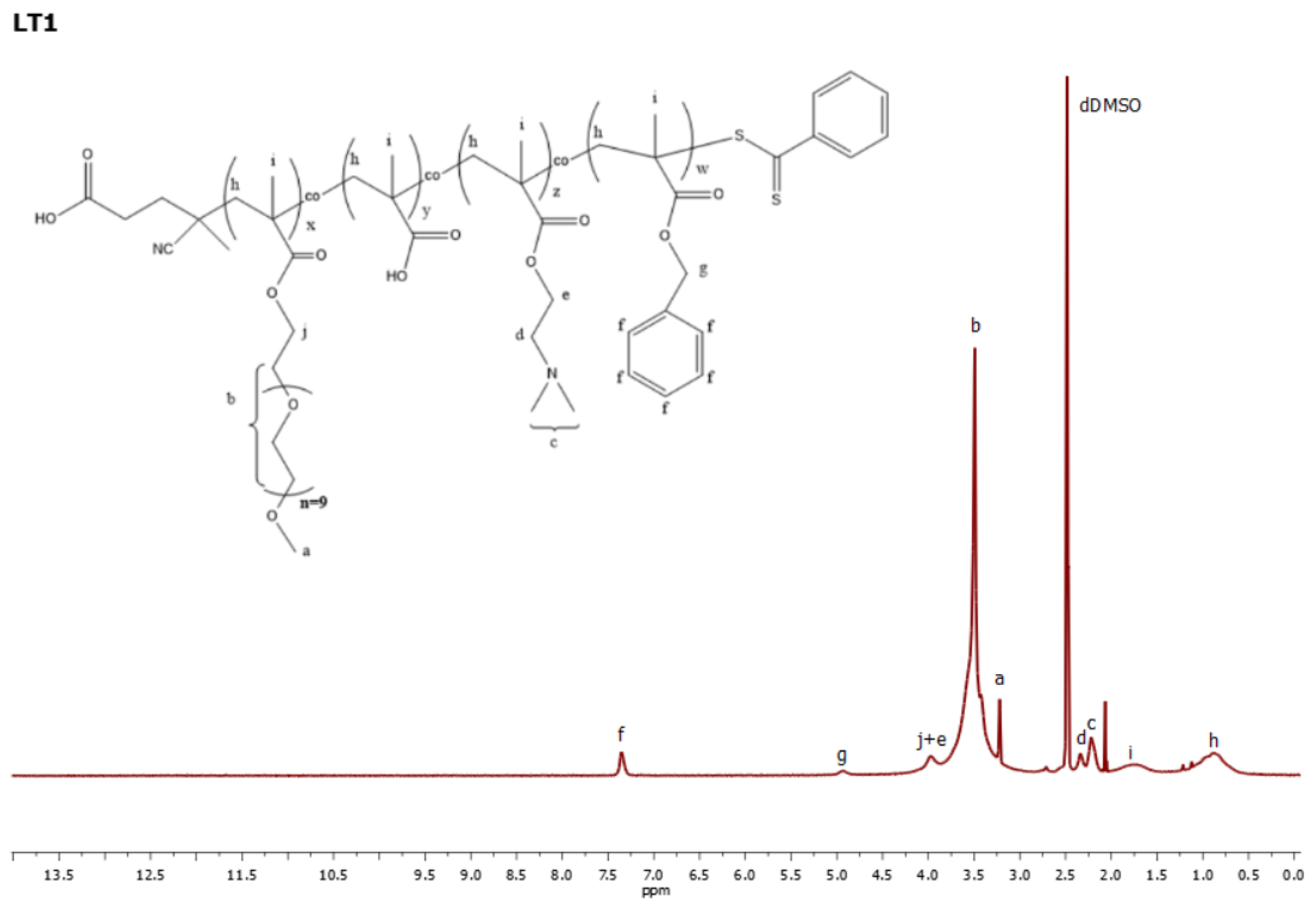
2.5.1. Proton Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
2.5.2. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.5.3. Dynamic Light Scattering (DLS)
2.5.4. Electrophoretic Light Scattering (ELS)
2.5.5. Fluorescence Spectroscopy (FS)
3. Results
3.1. Synthesis and Qualititative Characterization of Quaterpolymers
3.2. Physicochemical Characterization of the Quaterpolymer Assembly in Aqueous Media
3.2.1. Critical Aggregation Concentration (CAC) Determination
3.2.2. Structural Studies on Quaterpolymer Self-Assembly at Neutral pH
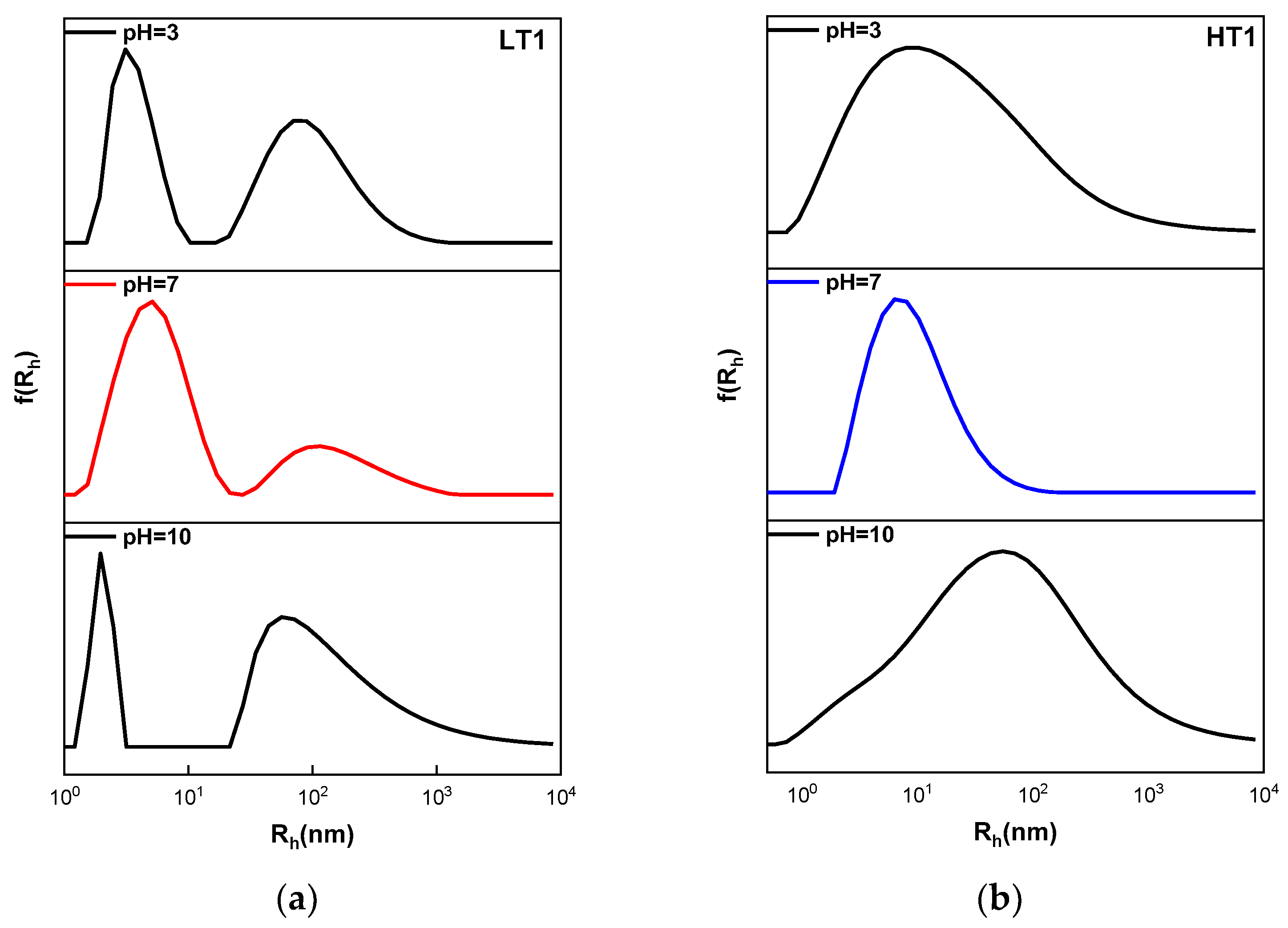
3.3. Influence of Solution pH on Quaterpolymer Assembly
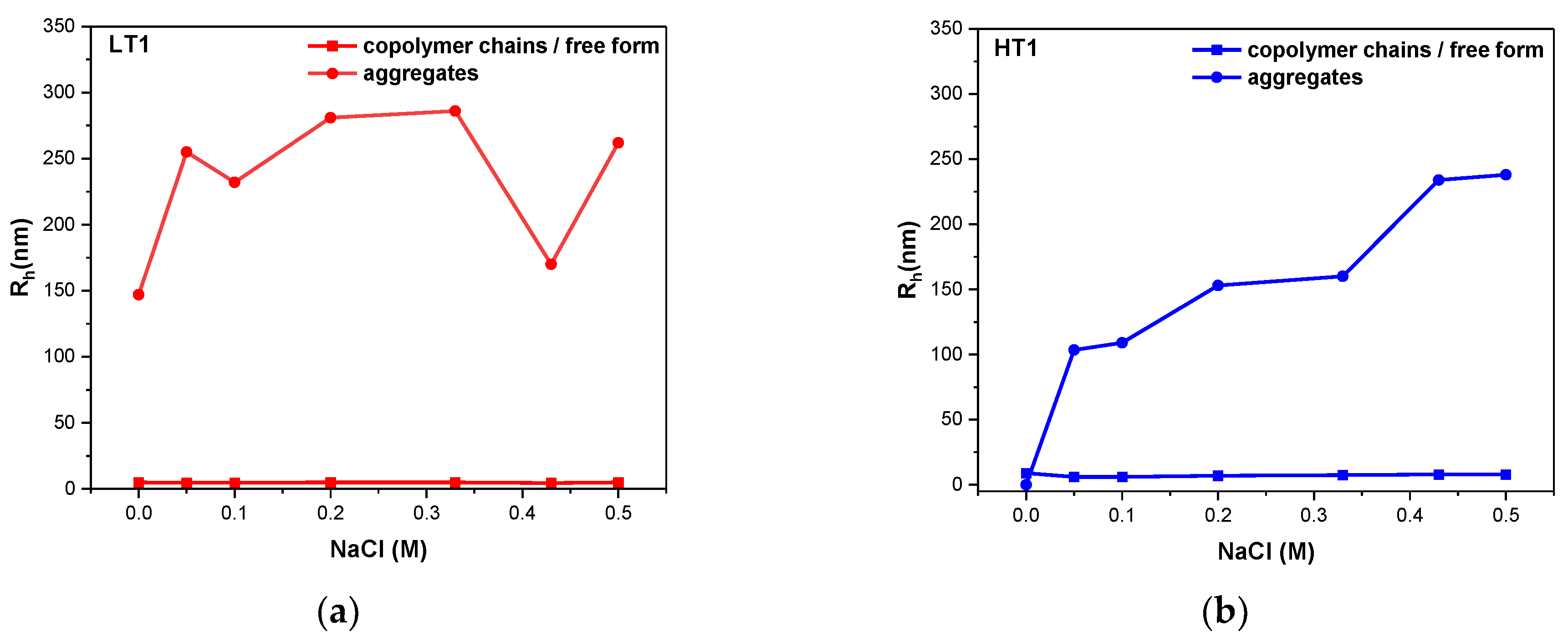
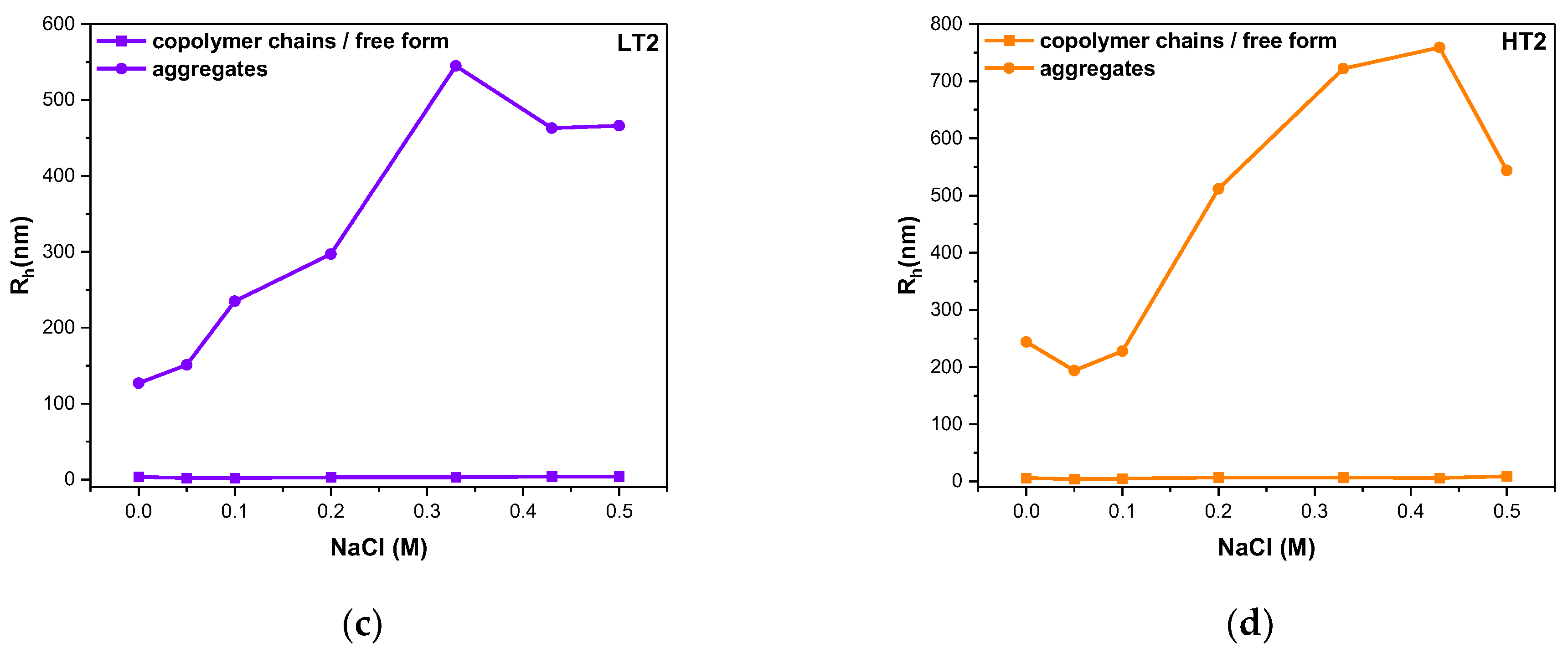
3.4. Ionic Strength Effects
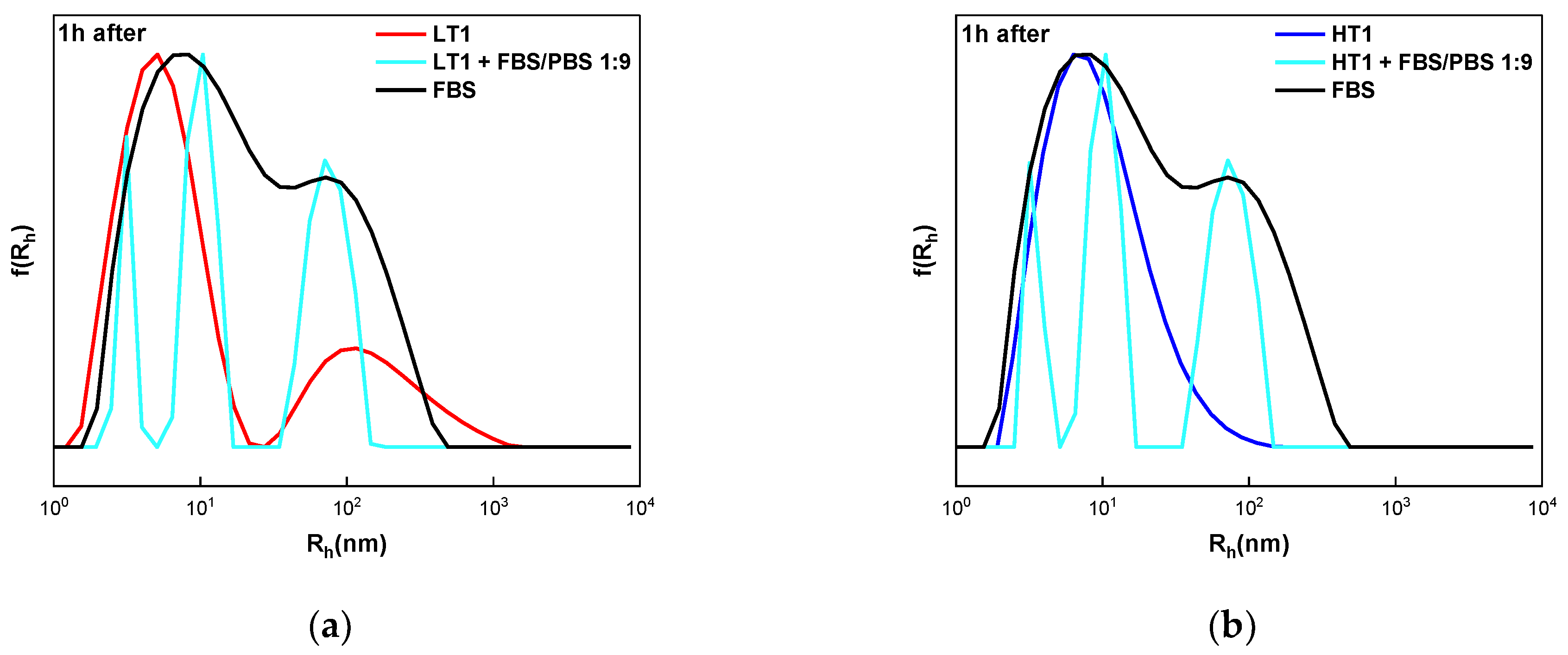
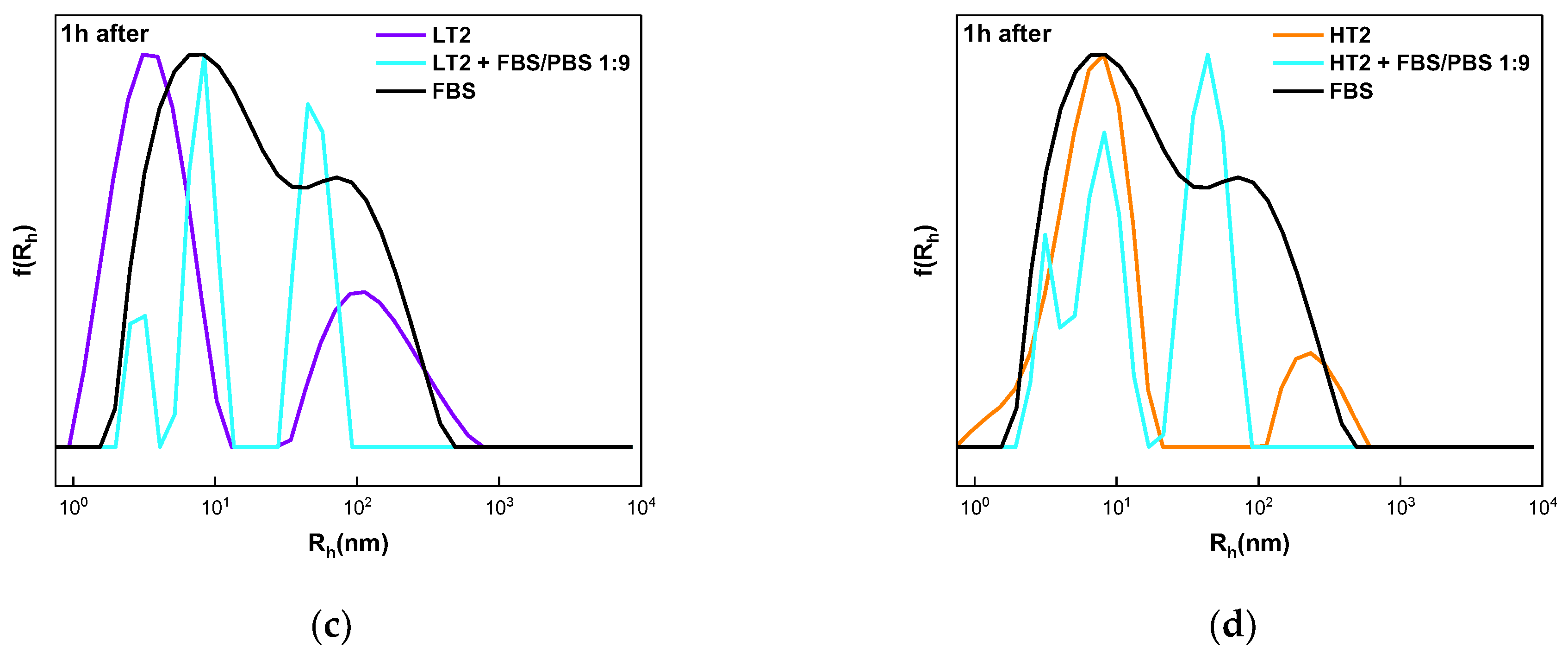
3.5. Interactions of Quaterpolymers’ FBS Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moad, G.; Rizzardo, E.; Thang, S. Radical addition-fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Qiao, B.; Olvera de la Cruz, M. Efficient encapsulation of proteins with random copolymers. Proc. Natl. Acad. Sci. USA 2018, 115, 6578–6583. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, Z.; Qi, X. Design and Application of Hybrid Polymer-Protein Systems in Cancer Therapy. Polymers 2023, 15, 2219. [Google Scholar] [CrossRef] [PubMed]
- Hilburg, S.L.; Ruan, Z.; Xu, T.; Alexander-Katz, A. Behavior of Protein-Inspired Synthetic Random Heteropolymers. Macromolecules 2020, 53, 9187–9199. [Google Scholar] [CrossRef]
- Jiang, T.; Hall, A.; Eres, M.; Hemmatian, Z.; Qiao, B.; Zhou, Y.; Ruan, Z.; Couse, A.D.; Heller, W.T.; Huang, H.; et al. Single-chain heteropolymers transport protons selectively and rapidly. Nature 2020, 577, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Muraoka, T.; Kinbara, K. Supramolecular Transmembrane Ion Channels Formed by Multiblock Amphiphiles. Acc. Chem. Res. 2021, 54, 3700–3709. [Google Scholar] [CrossRef] [PubMed]
- Jayapurna, I.; Ruan, Z.; Eres, M.; Jalagam, P.; Jenkins, S.; Xu, T. Sequence Design of Random Heteropolymers as Protein Mimics. Biomacromolecules 2023, 24, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, B.; Qiao, B.; Jiang, T.; DelRe, C.; Obadia, M.M.; Nguyen, T.D.; Smith, A.A.A.; Hall, A.; Sit, I.; Crosby, M.G.; et al. Random heteropolymers preserve protein function in foreign environments. Science 2018, 359, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Hilburg, S.L.; Alexander-Katz, A. Forced Unfolding of Protein-Inspired Single-Chain Random Heteropolymers. Macromolecules 2022, 55, 1295–1309. [Google Scholar] [CrossRef]
- Li, L.; Raghupathi, K.; Song, C.; Prasad, P.; Thayumanavan, S. Self-assembly of random copolymers. Chem. Commun. 2014, 50, 13417–13432. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, Y.; Yan, D. Hyperbranched polymer vesicles: From self-assembly, characterization, mechanisms, and properties to applications. Chem. Soc. Rev. 2015, 44, 3874–3889. [Google Scholar] [CrossRef]
- Soultan, A.H.; Verheyen, T.; Smet, M.; De Borggraeve, W.M.; Patterson, J. Synthesis and peptide functionalization of hyperbranched poly(arylene oxindole) towards versatile biomaterials. Polym. Chem. 2018, 9, 2775–2784. [Google Scholar] [CrossRef]
- Chakraborty, A.K. Disordered heteropolymers: Models for biomimetic polymers and polymers with frustrating quenched disorder. Phys. Rep. 2001, 342, 1–61. [Google Scholar] [CrossRef]
- Pande, V.S.; Grosberg, A.Y.; Tanaka, T. Heteropolymer freezing and design: Towards physical models of protein folding. Rev. Mod. Phys. 2000, 72, 259–314. [Google Scholar] [CrossRef]
- Mann, S.K.; Dufour, A.; Glass, J.J.; De Rose, R.; Kent, S.J.; Such, G.K.; Johnston, A.P.R. Tuning the properties of pH responsive nanoparticles to control cellular interactions in vitro and ex vivo. Polym. Chem. 2016, 7, 6015–6024. [Google Scholar] [CrossRef]
- Lutz, J.-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Ozer, I.; Tomak, A.; Zareie, H.M.; Baran, Y.; Bulmus, V. Effect of Molecular Architecture on Cell Interactions and Stealth Properties of PEG. Biomacromolecules 2017, 18, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Bulmus, V.; Woodward, M.; Lin, L.; Murthy, N.; Stayton, P.; Hoffman, A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J. Control. Release 2003, 93, 105–120. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.E.H.; Hoffman, A.S.; Stayton, P.S. Rational design of composition and activity correlations for pH-sensitive and glutathione-reactive polymer therapeutics. J. Control. Release 2005, 101, 47–58. [Google Scholar] [CrossRef]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, S.H. RAFT Agent Design and Synthesis. Macromolecules 2012, 45, 5321–5342. [Google Scholar] [CrossRef]
- Wilhelm, M.; Zhao, C.L.; Wang, Y.; Xu, R.; Winnik, M.A.; Mura, J.L.; Riess, G.; Croucher, M.D. Poly(styrene-ethylene oxide) block copolymer micelle formation in water: A fluorescence probe study. Macromolecules 1991, 24, 1033–1040. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, C.; Zhu, J.; Pochan, D.J.; Jia, X. Hybrid, elastomeric hydrogels crosslinked by multifunctional block copolymer micelles. Soft Matter 2010, 6, 5293–5297. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Eisenberg, A. Polymer Vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Das, T.; Ghorai, U.K.; Bandyopadhyay, A. Copolymers from methyl methacrylate and butyl acrylate with hyperbranched architecture. J. Appl. Polym. Sci. 2017, 134, 45356. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. Multi-responsive poly(oligo(ethylene glycol)methyl methacrylate)-co-poly(2-(diisopropylamino)ethyl methacrylate) hyperbranched copolymers via reversible addition fragmentation chain transfer polymerization. Polym. Chem. 2021, 12, 6582–6593. [Google Scholar] [CrossRef]
- Luo, S.; Han, M.; Cao, Y.; Ling, C.; Zhang, Y. Temperature- and pH-responsive unimolecular micelles with a hydrophobic hyperbranched core. Colloid Polym. Sci. 2011, 289, 1243–1251. [Google Scholar] [CrossRef]
- Balafouti, A.; Pispas, S. Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate H-P(MAA-co-LMA): Synthetic Aspects and Interactions with Biorelevant Compounds. Pharmaceutics 2023, 15, 1198. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Jiang, G.; Wang, R.; Hu, R.; Xi, X.; Zhou, Y.; Wang, S.; Wang, T. Synthesis of biomimetic hyperbranched zwitterionic polymers as targeting drug delivery carriers. J. Appl. Polym. Sci. 2013, 128, 3289–3294. [Google Scholar] [CrossRef]







| Quaterpolymer | pH | I90° (kHz) | PDI | Rh (nm) | ζ-Potential (mV) |
|---|---|---|---|---|---|
| LT1 | 3 | 28.5 | 0.5 | 4 (44%)/95 (56%) | +16.2 |
| 7 | 29 | 0.5 | 5 (75%)/147 (25%) | −11 | |
| 10 | 30 | 0.59 | 2 (21%)/135 (68%) | −51.5 | |
| HT1 | 3 | 40 | 0.55 | 18 | +36.5 |
| 7 | 50 | 0.43 | 9 | −5 | |
| 10 | 55 | 0.5 | 51 | −55 | |
| LT2 | 3 | 23.5 | 0.52 | 3 (44%)/133 (56%) | +30 |
| 7 | 25 | 0.52 | 4 (68%)/127 (32%) | −6 | |
| 10 | 34 | 0.56 | 3 (20%)/99 (76%) | −48 | |
| HT2 | 3 | 38 | 0.54 | 6 (68%)/95 (32%) | +40 |
| 7 | 44 | 0.46 | 6 (85%)/244 (15%) | −7 | |
| 10 | 38.5 | 0.52 | 19 | −51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagenas, D.; Pispas, S. Four-Component Statistical Copolymers by RAFT Polymerization. Polymers 2024, 16, 1321. https://doi.org/10.3390/polym16101321
Vagenas D, Pispas S. Four-Component Statistical Copolymers by RAFT Polymerization. Polymers. 2024; 16(10):1321. https://doi.org/10.3390/polym16101321
Chicago/Turabian StyleVagenas, Dimitrios, and Stergios Pispas. 2024. "Four-Component Statistical Copolymers by RAFT Polymerization" Polymers 16, no. 10: 1321. https://doi.org/10.3390/polym16101321
APA StyleVagenas, D., & Pispas, S. (2024). Four-Component Statistical Copolymers by RAFT Polymerization. Polymers, 16(10), 1321. https://doi.org/10.3390/polym16101321








