Robust CA-GO-TiO2/PTFE Photocatalytic Membranes for the Degradation of the Azithromycin Formulation from Wastewaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Sample Preparation
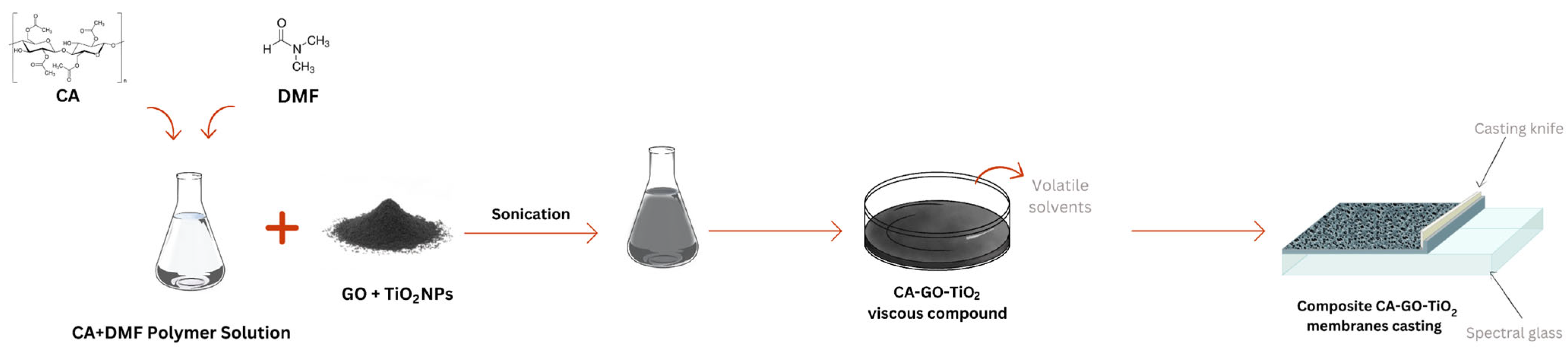
2.2.1. Sonochemical and Phase Inversion Methods for the Synthesis of TiO2-Decorated GO and Composite Membranes
2.2.2. Plasma Deposition of PTFE-like Films
2.3. Investigation of the Properties of the Thin-Film Composite Membranes
3. Results
3.1. The Morphological and Structural Properties of the CA-GO-TiO2 and CA-GO-TiO2/PTFE Membranes
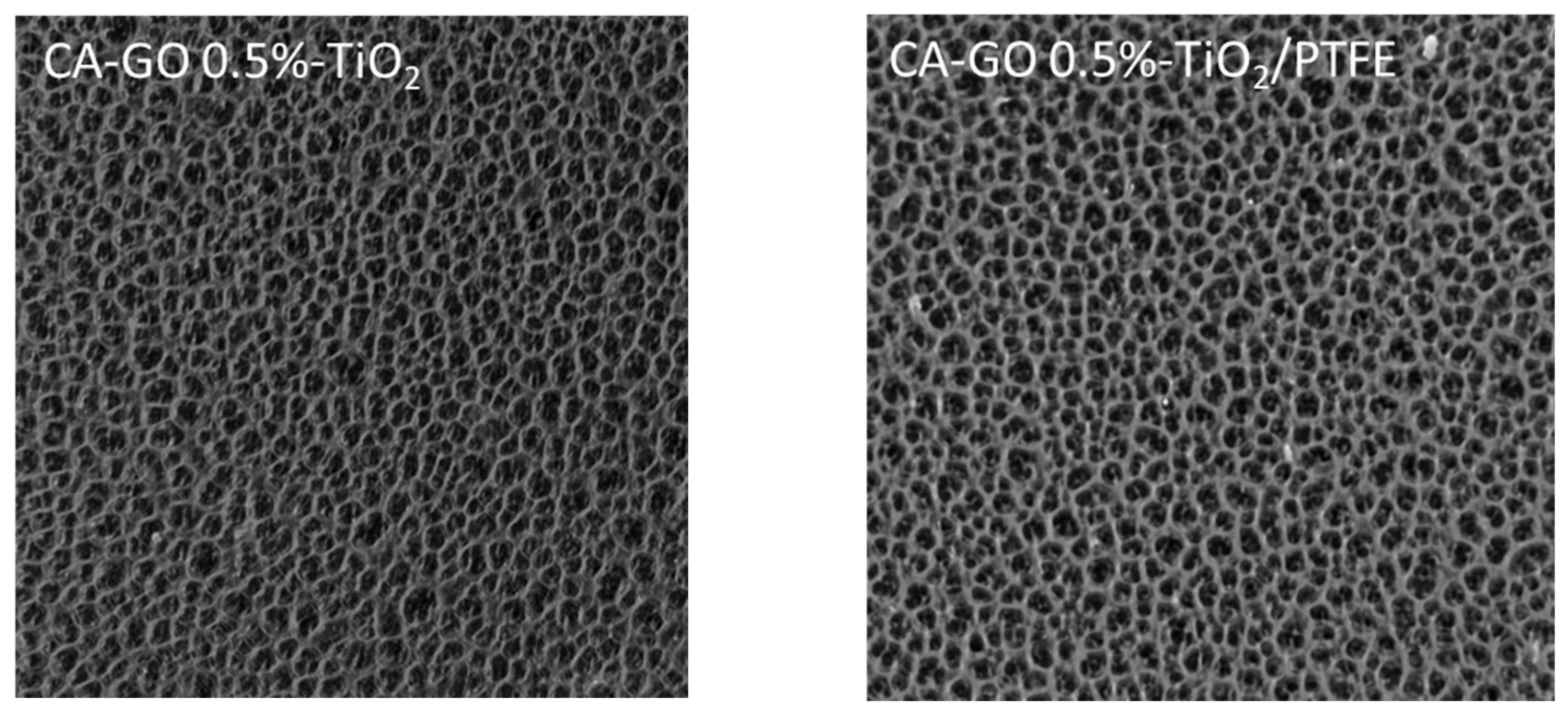
3.1.1. Surface Morphology
3.1.2. Structural Properties
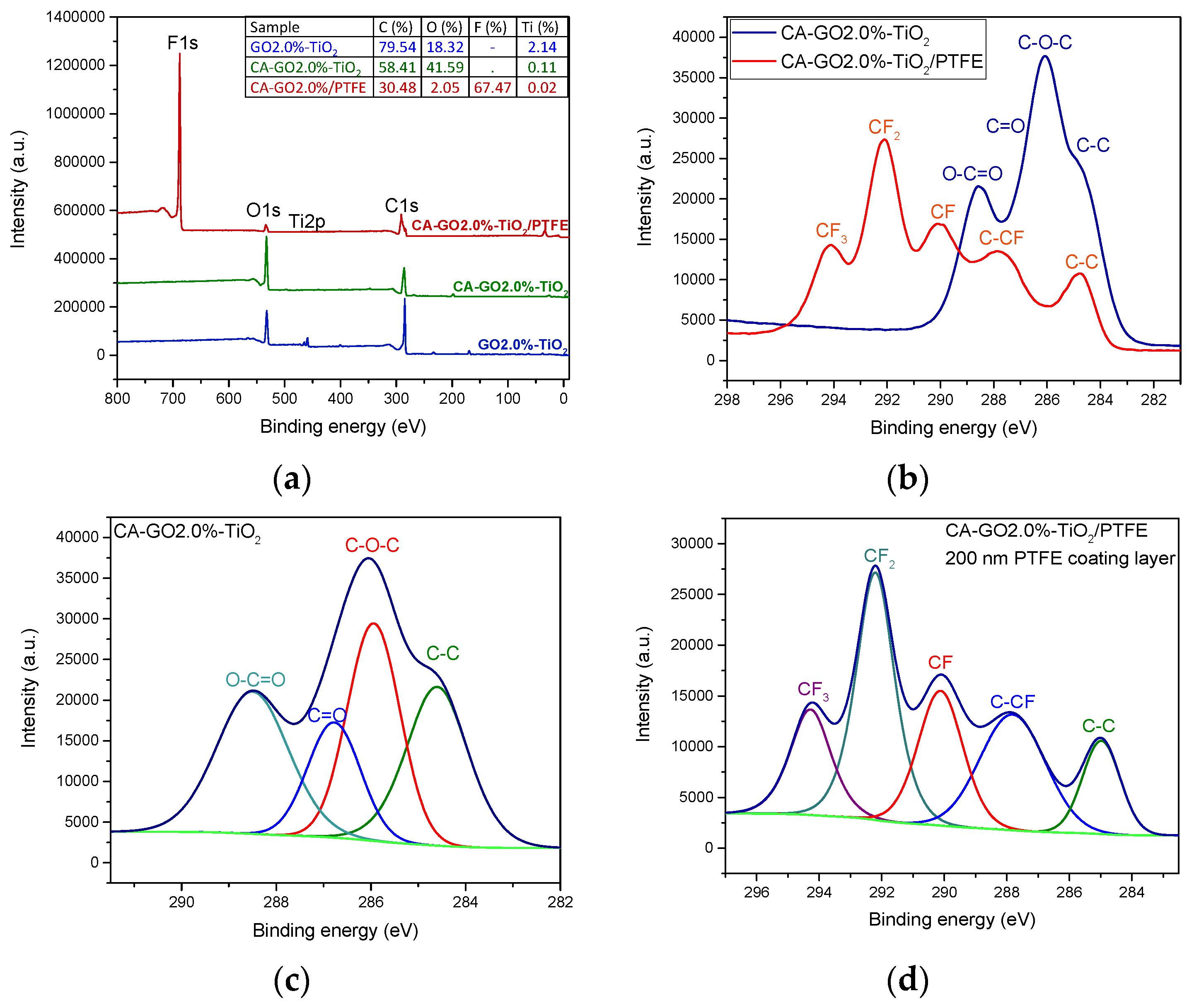
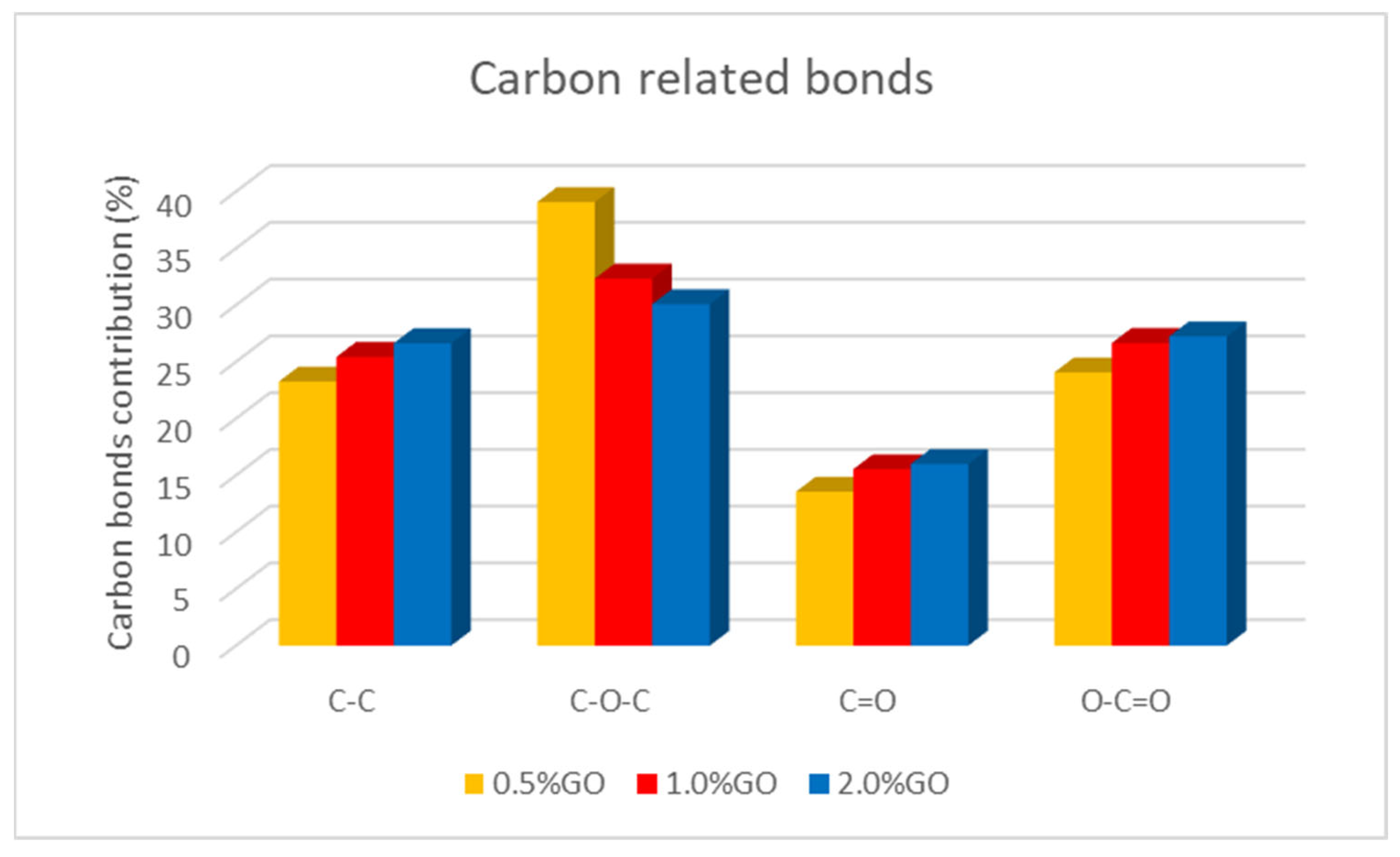
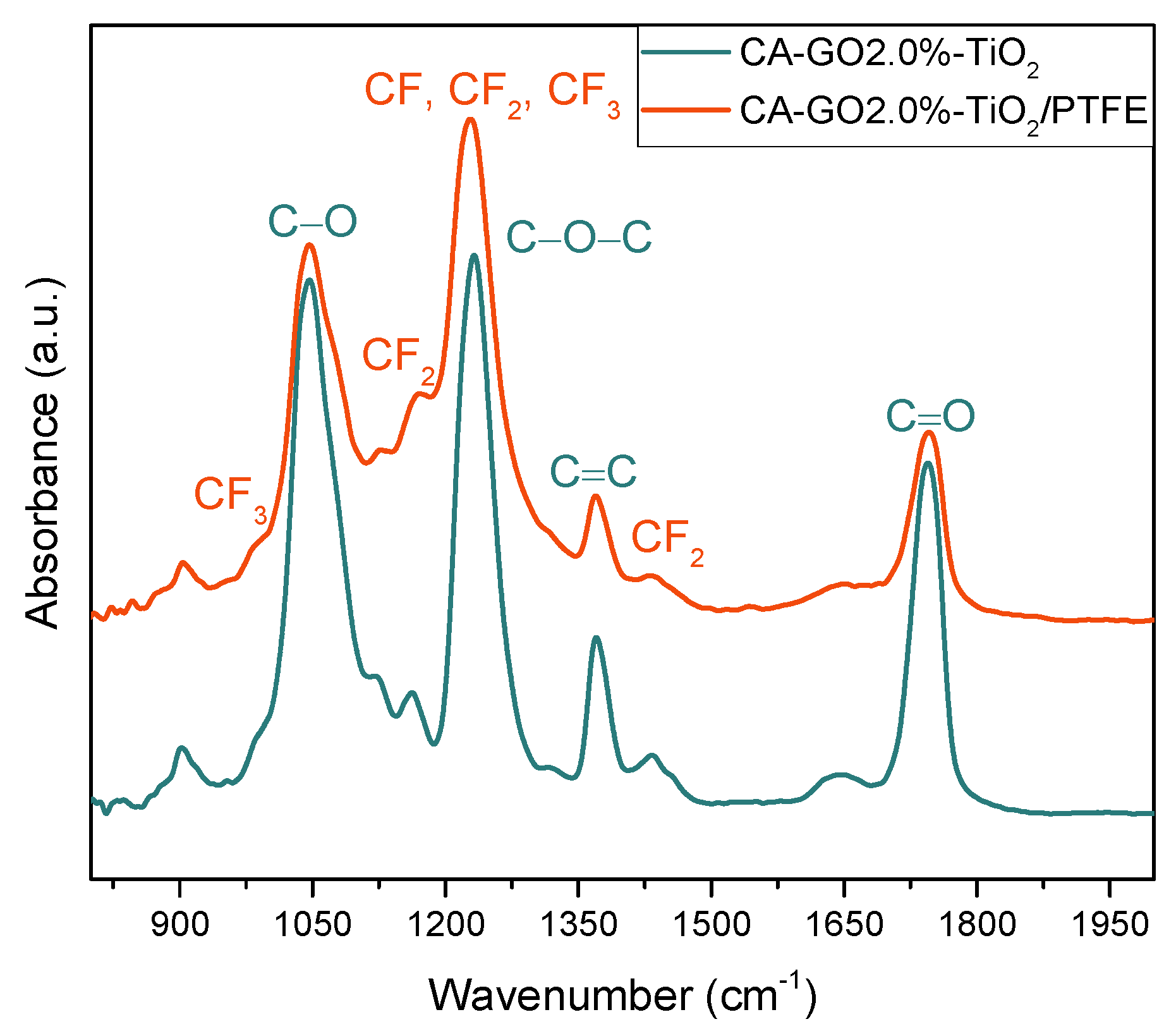
3.2. Chemical Characterization of the CA-GO-TiO2 and CA-GO-TiO2/PTFE Membranes
3.3. Stability Properties of the CA-GO-TiO2 and CA-GO-TiO2/PTFE Membranes
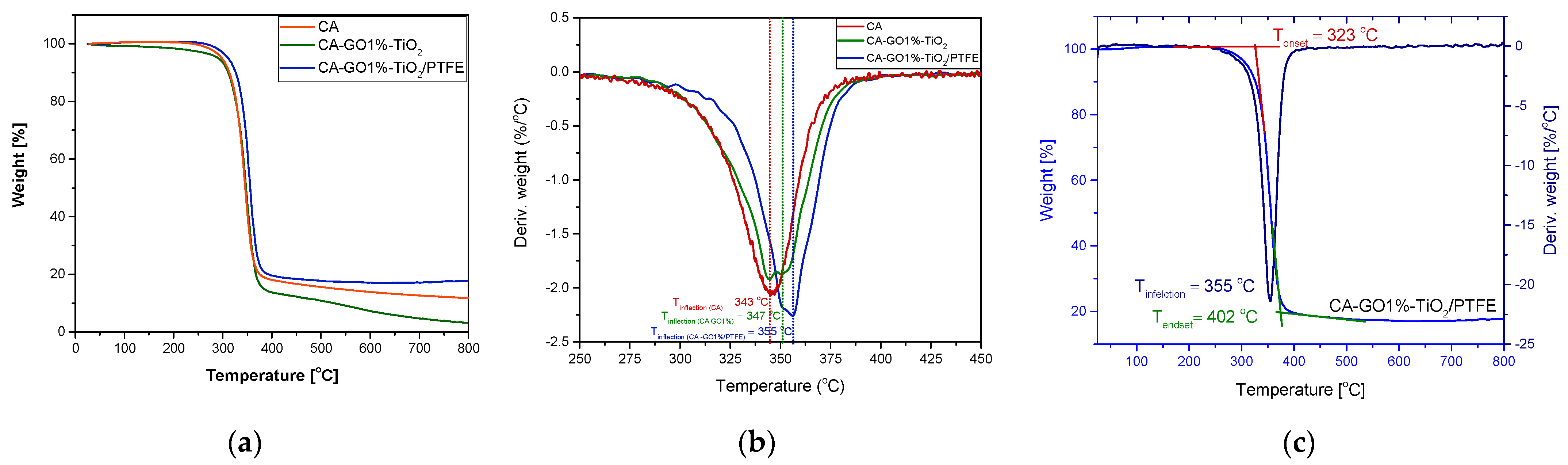
3.3.1. Thermal Properties
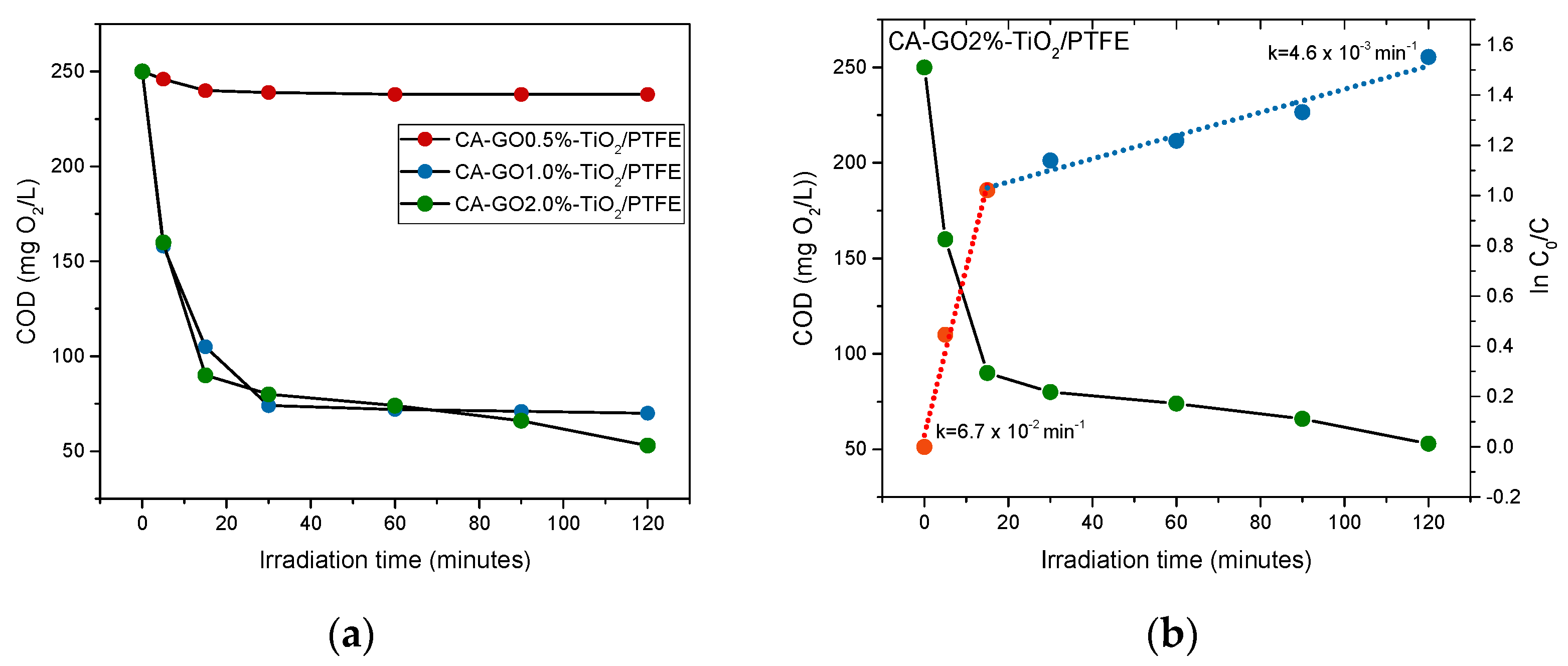
3.3.2. Stability of the Membranes with Respect to the Operation in Liquid Phase under UV Irradiation
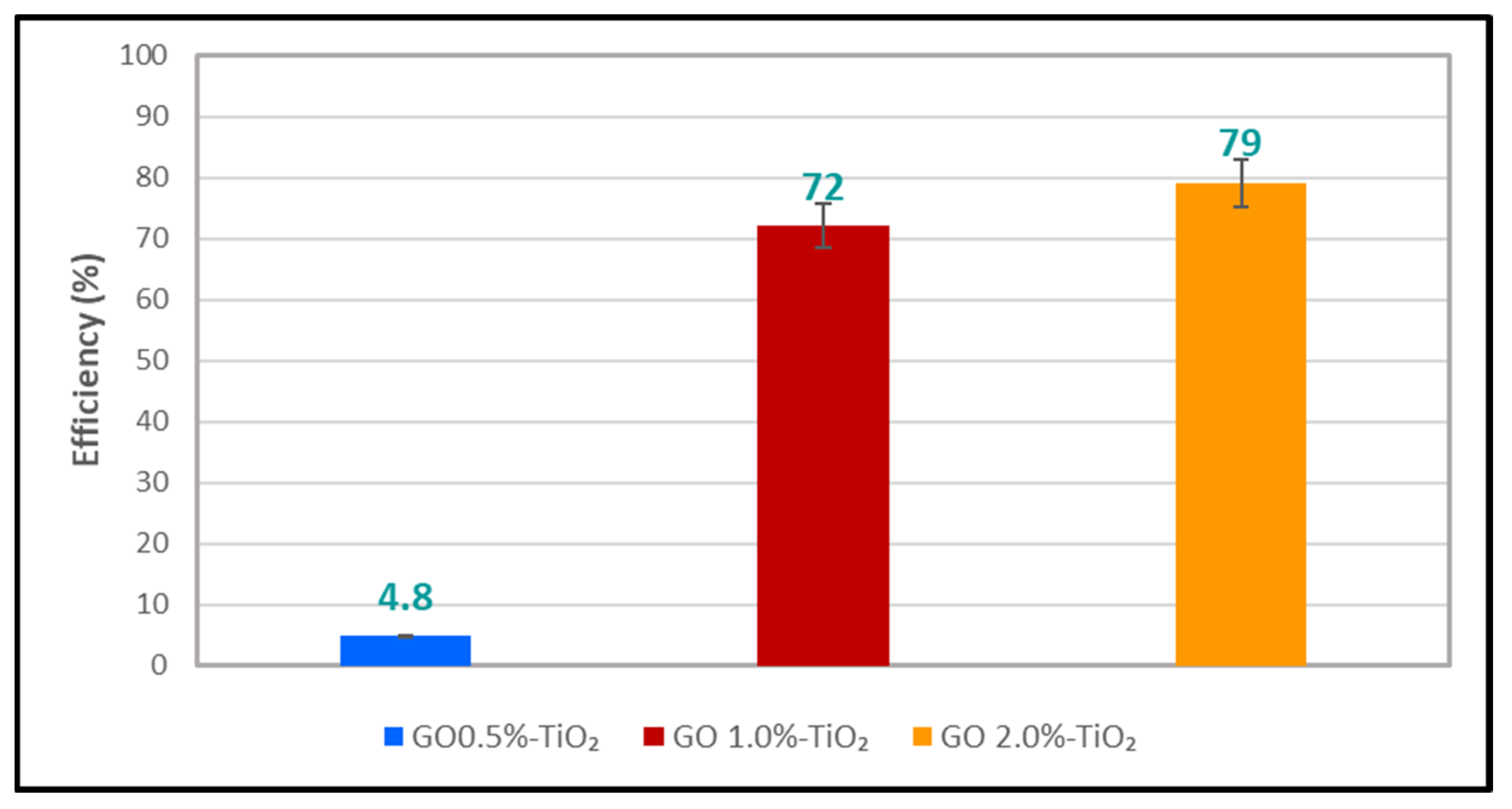
3.4. Assessment of Photocatalytic Performances of CA-GO-PTFE Thin-Film Composite Membranes in the Degradation of Azithromycin Formulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arun, J.; Nirmala, N.; Priyadharsini, P.; Dawn, S.; Santhosh, A.; Gopinath, K.; Govarthanan, M. A mini-review on bioderived carbon and its nanocomposites for removal of organic pollutants from wastewater. Mater. Lett. 2021, 310, 131476. [Google Scholar] [CrossRef]
- Paumo, H.K.; Dalhatou, S.; Katata-Seru, L.M.; Kamdem, B.P.; Tijani, J.O.; Vishwanathan, V.; Kane, A.; Bahadur, I. TiO2 assisted photocatalysts for degradation of emerging organic pollutants in water and wastewater. J. Mol. Liq. 2021, 331, 115458. [Google Scholar] [CrossRef]
- Singh, S.; Jayaram, R. Attainment of water and sanitation goals: A review and agenda for research. Sustain. Water Resour. Manag. 2022, 8, 146. [Google Scholar] [CrossRef]
- Malik, O.A.; Hsu, A.; Johnson, L.A.; de Sherbinin, A. A global indicator of wastewater treatment to inform the Sustainable Development Goals (SDGs). Environ. Sci. Policy 2015, 48, 172–185. [Google Scholar] [CrossRef]
- Gwenzi, W.; Simbanegavi, T.T.; Marumure, J.; Makuvara, Z. Chapter 13—Ecological Health Risks of Emerging Organic Con-taminants. In Emerging Contaminants in the Terrestrial-Aquatic-Atmosphere Continuum: Occurrence, Health Risks and Mitigation; Gwenzi, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–242. ISBN 9780323900515. [Google Scholar] [CrossRef]
- Peña, O.I.G.; Zavala, M.L.; Ruelas, H.C. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.; Mahapatra, S.; Ali, H. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Balu, S.; Chuaicham, C.; Balakumar, V.; Rajendran, S.; Sasaki, K.; Sekar, K.; Maruthapillai, A. Recent development on core-shell photo(electro)catalysts for elimination of organic compounds from pharmaceutical wastewater. Chemosphere 2022, 298, 134311. [Google Scholar] [CrossRef] [PubMed]
- Antonazzo, I.C.; Fornari, C.; Rozza, D.; Conti, S.; di Pasquale, R.; Cortesi, P.; Kaleci, S.; Ferrara, P.; Zucchi, A.; Maifredi, G.; et al. Azithromycin use and outcomes in patients with COVID-19: An observational real-world study. Int. J. Infect. Dis. 2022, 124, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Morales-Paredes, C.A.; Rodríguez-Díaz, J.M.; Boluda-Botella, N. Pharmaceutical compounds used in the COVID-19 pandemic: A review of their presence in water and treatment techniques for their elimination. Sci. Total Environ. 2021, 814, 152691. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Koyuncu, I.; Arikan, O.A.; Wiesner, M.R.; Rice, C. Removal of hormones and antibiotics by nanofiltration membranes. J. Membr. Sci. 2008, 309, 94–101. [Google Scholar] [CrossRef]
- Adams, C.; Wang, Y.; Loftin, K.; Meyer, M. Removal of Antibiotics from Surface and Distilled Water in Conventional Water Treatment Processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Xu, L.; Sun, Y.; Du, L.; Zhang, J. Removal of tetracycline hydrochloride from wastewater by nanofiltration enhanced by electro-catalytic oxidation. Desalination 2014, 352, 58–65. [Google Scholar] [CrossRef]
- Pojana, G.; Fantinati, A.; Marcomini, A. Occurrence of environmentally relevant pharmaceuticals in Italian drinking water treatment plants. Int. J. Environ. Anal. Chem. 2011, 91, 537–552. [Google Scholar] [CrossRef]
- Liu, M.K.; Liu, Y.Y.; Bao, D.D.; Zhu, G.; Yang, G.H.; Geng, J.F.; Li, H.T. Effective Removal of Tetracycline Antibiotics from Water using Hybrid Carbon Membranes. Sci. Rep. 2017, 7, srep43717. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H.; Giorno, L.; Drioli, E. An Introduction to Membrane Science and Technology; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Huang, H.-H.; Joshi, R.K.; De Silva, K.K.H.; Badam, R.; Yoshimura, M. Fabrication of reduced graphene oxide membranes for water desalination. J. Membr. Sci. 2018, 572, 12–19. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yuan, J.; Xiang, W.; Xin, X.; Liu, H.; Luo, H.; Li, L.; Chen, M.; Zhong, D.; et al. Photocatalytic optical fibers for degradation of organic pollutants in wastewater: A review. Environ. Chem. Lett. 2020, 19, 1335–1346. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Toxicity changes of wastewater during various advanced oxidation processes treatment: An overview. J. Clean. Prod. 2021, 315, 128202. [Google Scholar] [CrossRef]
- Pandele, A.M.; Iovu, H.; Orbeci, C.; Tuncel, C.; Miculescu, F.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Surface modified cellulose acetate membranes for the reactive retention of tetracycline. Sep. Purif. Technol. 2020, 249, 117145. [Google Scholar] [CrossRef]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Parmar, R. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An Overview of the Applications of Graphene-Based Materials in Supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Hunge, Y.; Yadav, A.; Dhodamani, A.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason. Sonochem. 2019, 61, 104849. [Google Scholar] [CrossRef] [PubMed]
- Voicu, S.I.; Thakur, V.K. Graphene-based composite membranes for nanofiltration: Performances and future perspectives. Emergent Mater. 2021, 5, 1429–1441. [Google Scholar] [CrossRef]
- Nandi, D.; Mohan, V.B.; Bhowmick, A.K.; Bhattacharyya, D. Metal/metal oxide decorated graphene synthesis and application as supercapacitor: A review. J. Mater. Sci. 2020, 55, 6375–6400. [Google Scholar] [CrossRef]
- Paek, S.-M.; Yoo, E.; Honma, I. Enhanced Cyclic Performance and Lithium Storage Capacity of SnO2/Graphene Nanoporous Electrodes with Three-Dimensionally Delaminated Flexible Structure. Nano Lett. 2008, 9, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, X.; Liu, J.; Geng, D.; Zhang, Y.; Banis, M.N.; Li, Y.; Yang, J.; Li, R.; Sun, X.; et al. Tin Oxide with Controlled Morphology and Crystallinity by Atomic Layer Deposition onto Graphene Nanosheets for Enhanced Lithium Storage. Adv. Funct. Mater. 2012, 22, 1647–1654. [Google Scholar] [CrossRef]
- Wang, D.; Choi, D.; Li, J.; Yang, Z.; Nie, Z.; Kou, R.; Hu, D.; Wang, C.; Saraf, L.V.; Zhang, J.; et al. Self-Assembled TiO2–Graphene Hybrid Nanostructures for Enhanced Li-Ion Insertion. ACS Nano 2009, 3, 907–914. [Google Scholar] [CrossRef]
- Zhu, J.; Sharma, Y.K.; Zeng, Z.; Zhang, X.; Srinivasan, M.; Mhaisalkar, S.; Zhang, H.; Hng, H.H.; Yan, Q. Cobalt Oxide Nanowall Arrays on Reduced Graphene Oxide Sheets with Controlled Phase, Grain Size, and Porosity for Li-Ion Battery Electrodes. J. Phys. Chem. C 2011, 115, 8400–8406. [Google Scholar] [CrossRef]
- Madhu, R.; Dinesh, B.; Chen, S.-M.; Saraswathi, R.; Mani, V. An electrochemical synthesis strategy for composite based ZnO microspheres–Au nanoparticles on reduced graphene oxide for the sensitive detection of hydrazine in water samples. RSC Adv. 2015, 5, 54379–54386. [Google Scholar] [CrossRef]
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.M.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla-Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone–TiO2 decorated MWCNT composite membranes by sonochemical method. Appl. Phys. A 2020, 126, 233. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose Acetate-Based Materials for Water Treatment in the Context of Circular Economy. Water 2023, 15, 1860. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.-L.; Zhao, J.-Q.; Tang, W.; Pu, C.-S. Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Ameduri, B.M. Fluoropolymers: The Right Material for the Right Applications. Chem. A Eur. J. 2018, 24, 18830–18841. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, W. Evaporation of polytetrafluoroethylene by electron bombardment of the bulk material. Thin Solid Films 1974, 24, 101–111. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Optimizing polyamide thin film composite membrane covalently bonded with modified mesoporous silica nanoparticles. J. Membr. Sci. 2013, 428, 341–348. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef]
- Jhang, S.-R.; Lin, H.-Y.; Liao, Y.-S.; Chou, J.-P.; Wu, J.M. Local dipole enhancement of space-charge piezophototronic catalysts of core-shell polytetrafluoroethylene@TiO2 nanospheres. Nano Energy 2022, 102, 107619. [Google Scholar] [CrossRef]
- Hubert, J.; Mertens, J.; Dufour, T.; Vandencasteele, N.; Reniers, F.; Viville, P.; Lazzaroni, R.; Raes, M.; Terryn, H. Synthesis and texturization processes of (super)-hydrophobic fluorinated surfaces by atmospheric plasma. J. Mater. Res. 2015, 30, 3177–3191. [Google Scholar] [CrossRef]
- Kravets, L.I.; Yarmolenko, M.A.; Rogachev, A.V.; Gainutdinov, R.V.; Altynov, V.A.; Lizunov, N.E. Formation of Hydrophobic and Superhydrophobic Coatings on Track-Etched Membrane Surfaces to Create Composite Membranes for Water Desalination. Colloid J. 2022, 84, 427–444. [Google Scholar] [CrossRef]
- Limb, S.J.; Labelle, C.B.; Gleason, K.K.; Edell, D.J.; Gleason, E.F. Growth of fluorocarbon polymer thin films with high CF2 fractions and low dangling bond concentrations by thermal chemical vapor deposition. Appl. Phys. Lett. 1996, 68, 2810–2812. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Kita, R.; Tsuchiya, K.; Iwamori, S. Optical characteristics of poly(tetrafluoroethylene) thin film prepared by a vacuum evaporation. Jpn. J. Appl. Phys. 2016, 55, 02BB04. [Google Scholar] [CrossRef]
- Biederman, H. Organic films prepared by polymer sputtering. J. Vac. Sci. Technol. A 2000, 18, 1642–1648. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Pandele, A.; Voicu, S.; Kravets, L.; Dinescu, G. Composite polyethylene terephthalate track membranes with thin teflon-like layers: Preparation and surface properties. Appl. Surf. Sci. 2019, 476, 452–459. [Google Scholar] [CrossRef]
- Drábik, M.; Polonskyi, O.; Kylián, O.; Čechvala, J.; Artemenko, A.; Gordeev, I.; Choukourov, A.; Slavínská, D.; Matolínová, I.; Biederman, H. Super-Hydrophobic Coatings Prepared by RF Magnetron Sputtering of PTFE. Plasma Process. Polym. 2010, 7, 544–551. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Altynov, V.; Lizunov, N.; Kravets, L.; Dinescu, G. Synthesis and characterization of porous composite membranes with hydrophilic/hydrophobic sides. Thin Solid Films 2017, 630, 92–99. [Google Scholar] [CrossRef]
- Senna, M.; Šepelák, V.; Shi, J.; Bauer, B.; Feldhoff, A.; Laporte, V.; Becker, K.-D. Introduction of oxygen vacancies and fluorine into TiO2 nanoparticles by co-milling with PTFE. J. Solid State Chem. 2012, 187, 51–57. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Y.; Li, H.; Cao, G.; Ouyang, F.; Zhu, R. Photocatalytic Oxidation of Toluene on Fluorine Doped TiO2/SiO2 Catalyst Under Simulant Sunlight in a Flat Reactor. Catalysts 2018, 8, 596. [Google Scholar] [CrossRef]
- Fessi, N.; Nsib, M.F.; Cardenas, L.; Guillard, C.; Dappozze, F.; Houas, A.; Parrino, F.; Palmisano, L.; Ledoux, G.; Amans, D.; et al. Surface and Electronic Features of Fluorinated TiO2 and Their Influence on the Photocatalytic Degradation of 1-Methylnaphthalene. J. Phys. Chem. C 2020, 124, 11456–11468. [Google Scholar] [CrossRef]
- Raicopol, M.D.; Andronescu, C.; Voicu, S.I.; Vasile, E.; Pandele, A.M. Cellulose acetate/layered double hydroxide adsorptive membranes for efficient removal of pharmaceutical environmental contaminants. Carbohydr. Polym. 2019, 214, 204–212. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Ion, V.; Marascu, V.; Matei, E.; Stancu, C.; Dinescu, G. Combining Fluorinated Polymers with Ag Nanoparticles as a Route to Enhance Optical Properties of Composite Materials. Polymers 2020, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Serbanescu, O.S.; Pandele, A.M.; Miculescu, F.; Voicu, S.I. Synthesis and Characterization of Cellulose Acetate Membranes with Self-Indicating Properties by Changing the Membrane Surface Color for Separation of Gd(III). Coatings 2020, 10, 468. [Google Scholar] [CrossRef]
- Greenberg, A.E.; Clesceri, L.S.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1992. [Google Scholar]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New J. Chem. 2016, 40, 2547–2553. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Aldeligan, S.H.; Alsubaie, F.S. Synthesis and Characterization of Cellulose Triacetate Obtained from Date Palm (Phoenix dactylifera L.) Trunk Mesh-Derived Cellulose. Molecules 2022, 27, 1434. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Ahmed, D.F.; Isawi, H.; Badway, N.A.; Elbayaa, A.; Shawky, H. Graphene oxide incorporated cellulose triacetate/cellulose acetate nanocomposite membranes for forward osmosis desalination. Arab. J. Chem. 2021, 14, 102995. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2010, 19, 152–165. [Google Scholar] [CrossRef]
- Bhalekar, M.R.; Harinarayana, D.; Madgulkar, A.R.; Pandya, S.J.; Jain, D.K. Improvement of Photostability in Formulation: A Review. Asian J. Chem. 2008, 20, 5095–5108. [Google Scholar]
- Lupu, G.-I.; Orbeci, C.; Bobirică, C.; Bobirică, L.; Lazăr, E.S.; Pandele-Cusu, J.; Verziu, M.N.; Pîrvu, C.; Irodia, R.-G. Photocatalytic degradation of azithromycin formulation in aqueous solution by doped titanium dioxide/fiberglass-rubberized silicone photocatalytic membrane. Sustain. Environ. Res. 2023, 33, 36. [Google Scholar] [CrossRef]
- Sharma, M.; Rajput, D.; Kumar, V.; Jatain, I.; Aminabhavi, T.M.; Mohanakrishna, G.; Kumar, R.; Dubey, K.K. Photocatalytic degradation of four emerging antibiotic contaminants and toxicity assessment in wastewater: A comprehensive study. Environ. Res. 2023, 231, 116132. [Google Scholar] [CrossRef]
- Nippes, R.P.; Macruz, P.D.; da Silva, G.N.; Scaliante, M.H.N.O. A critical review on environmental presence of pharmaceutical drugs tested for the covid-19 treatment. Process Saf. Environ. Prot. 2021, 152, 568–582. [Google Scholar] [CrossRef] [PubMed]



| Parameter, Unit | Value |
|---|---|
| Reactor volume, L | 1.5 |
| Recirculation flow rate, L/min | 2.0 |
| Volume of AZT working solution, L | 2.0 |
| UV lamp | High-pressure mercury lamp; power 120 W |
| pH of AZT working solution | 3 |
| H2O2/AZT molar ratio | 1 |
| Concentration of GO-TiO2 in the Sample | Surface Porosity (%) | |
|---|---|---|
| CA-GO-TiO2 Samples | CA-GO-TiO2/PTFE Samples | |
| 0.5% | 25.05 | 15.23 |
| 1.0% | 24.14 | 13.24 |
| 2.0% | 20.86 | 12.52 |
| Sample | Structure Thickness (μm) | Closed Porosity (%) | Specific Surface Area (1/μm) | Total Porosity * (%) |
|---|---|---|---|---|
| CA-GO0.5%-TiO2 | 10.90 | 0.10 | 0.31 | 96 |
| CA-GO1.0%-TiO2 | 11.50 | 0.25 | 0.28 | 89 |
| CA-GO2.0%-TiO2 | 12.70 | 0.24 | 0.27 | 90 |
| Averaged values CA-GO-TiO2 | 11.7 | 0.19 | 0.28 | 91.6 |
| CA-GO0.5%-TiO2/PTFE | 17.20 | 0.14 | 0.19 | 88 |
| CA-GO1.0%-TiO2/PTFE | 11.30 | 0.29 | 0.31 | 84 |
| CA-GO2.0%-TiO2/PTFE | 10.90 | 0.39 | 0.30 | 91 |
| Averaged values CA-GO-TiO2/PTFE | 13.13 | 0.27 | 0.26 | 87.6 |
| Sample/ Significant T (°C) | CA | CA-GO1%-TiO2 | CA-GO1%-TiO2/PTFE |
|---|---|---|---|
| Tonset | 308 | 312 | 323 |
| Tinflection | 343 | 347 | 355 |
| Tendset | 365 | 367 | 402 |
| Pseudo-First-Order Rate Constants (k, min−1) | |||
|---|---|---|---|
| Sample Codes | Degradation Stage | ||
| 1st Stage | 2nd Stage | Residual Sum of Squares for the 2nd Stage (r2) | |
| CA-GO0.5%-TiO2/PTFE | 2.7 × 10−3 | 7.0 × 10−5 | 1.7 ×10−5 |
| CA-GO1%-TiO2/PTFE | 3.8 × 10−2 | 6.0 × 10−4 | 6.3 ×10−2 |
| CA-GO2%-TiO2/PTFE | 6.7 × 10−2 | 4.6 × 10−3 | 5.4 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satulu, V.; Pandele, A.M.; Ionica, G.-I.; Bobirică, L.; Bonciu, A.F.; Scarlatescu, A.; Bobirică, C.; Orbeci, C.; Voicu, S.I.; Mitu, B.; et al. Robust CA-GO-TiO2/PTFE Photocatalytic Membranes for the Degradation of the Azithromycin Formulation from Wastewaters. Polymers 2024, 16, 1368. https://doi.org/10.3390/polym16101368
Satulu V, Pandele AM, Ionica G-I, Bobirică L, Bonciu AF, Scarlatescu A, Bobirică C, Orbeci C, Voicu SI, Mitu B, et al. Robust CA-GO-TiO2/PTFE Photocatalytic Membranes for the Degradation of the Azithromycin Formulation from Wastewaters. Polymers. 2024; 16(10):1368. https://doi.org/10.3390/polym16101368
Chicago/Turabian StyleSatulu, Veronica, Andreea Madalina Pandele, Giovanina-Iuliana Ionica, Liliana Bobirică, Anca Florina Bonciu, Alexandra Scarlatescu, Constantin Bobirică, Cristina Orbeci, Stefan Ioan Voicu, Bogdana Mitu, and et al. 2024. "Robust CA-GO-TiO2/PTFE Photocatalytic Membranes for the Degradation of the Azithromycin Formulation from Wastewaters" Polymers 16, no. 10: 1368. https://doi.org/10.3390/polym16101368
APA StyleSatulu, V., Pandele, A. M., Ionica, G.-I., Bobirică, L., Bonciu, A. F., Scarlatescu, A., Bobirică, C., Orbeci, C., Voicu, S. I., Mitu, B., & Dinescu, G. (2024). Robust CA-GO-TiO2/PTFE Photocatalytic Membranes for the Degradation of the Azithromycin Formulation from Wastewaters. Polymers, 16(10), 1368. https://doi.org/10.3390/polym16101368












