Polydimethylsiloxane Surface Modification of Microfluidic Devices for Blood Plasma Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. PDMS Samples Preparation
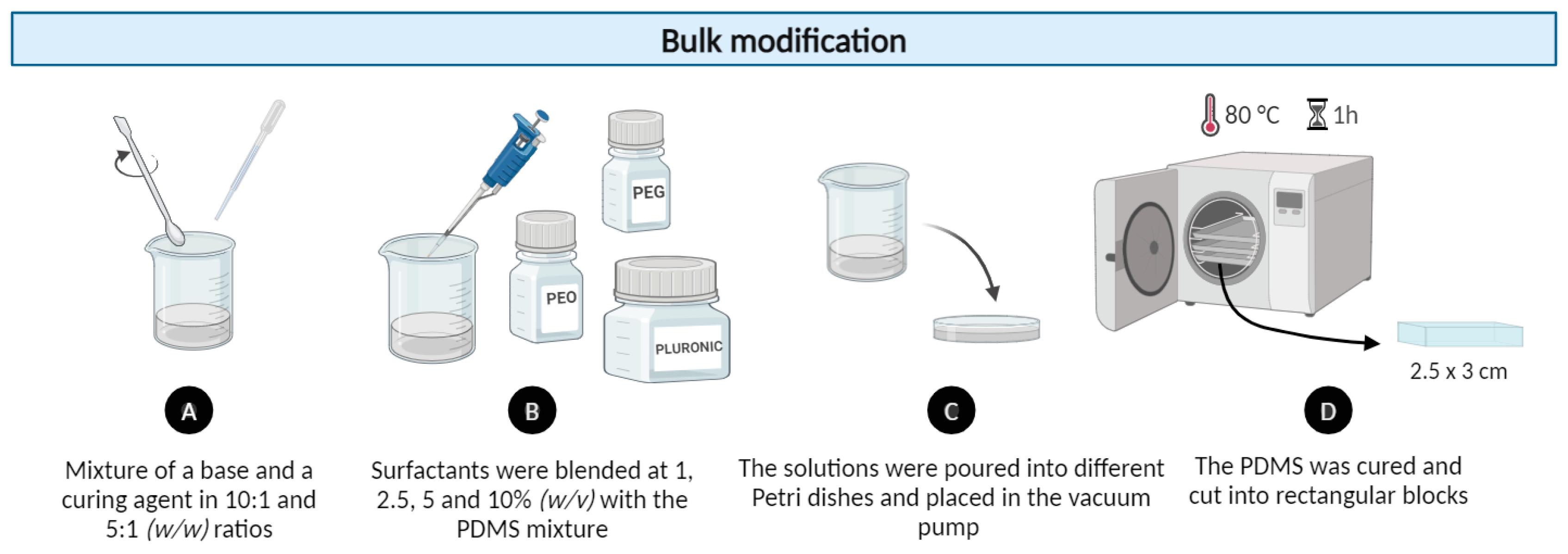
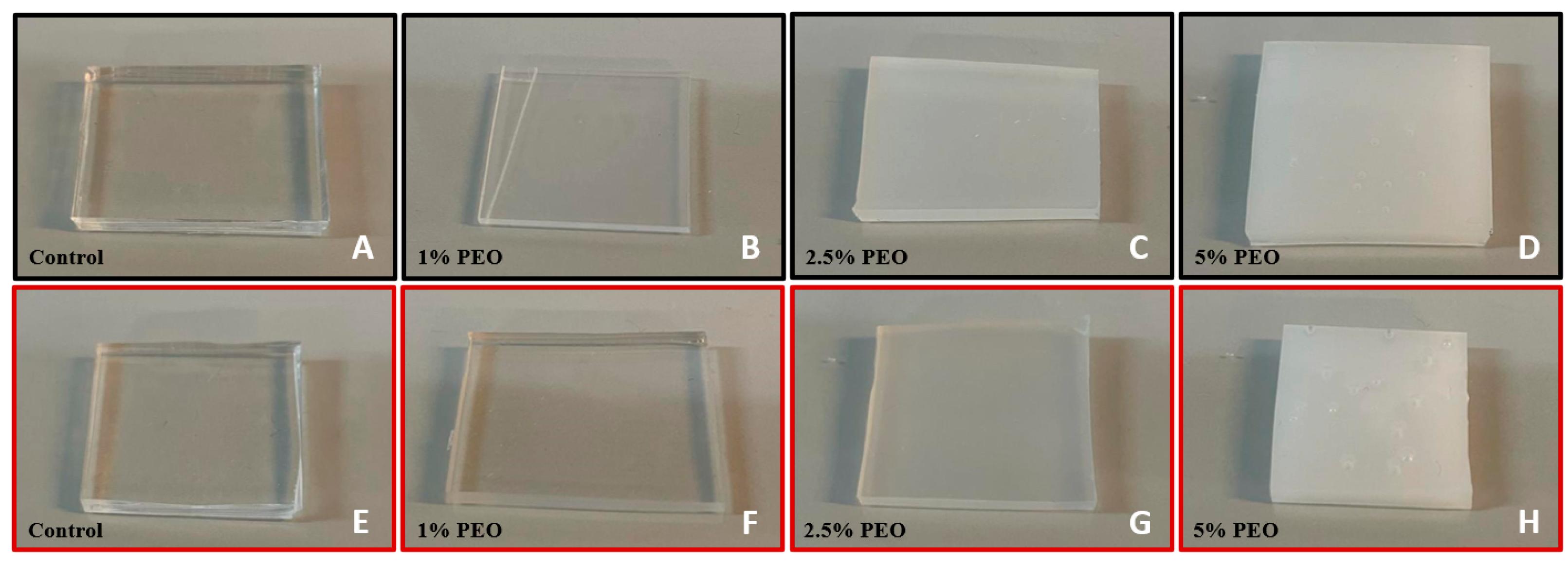
2.1.1. Bulk PDMS Modification Method
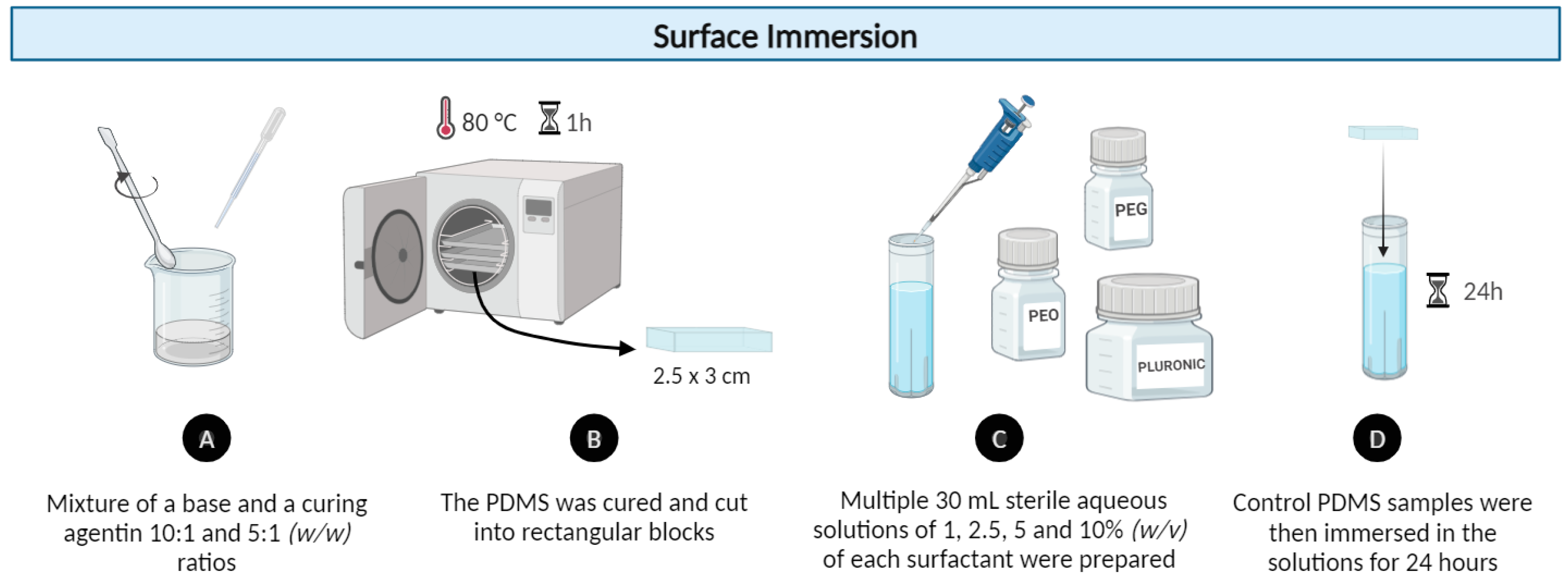
2.1.2. Modification by Surface Immersion Method
2.2. Contact Angle (CA) Measurements
2.3. Microchannel Fabrication for Capillary Studies
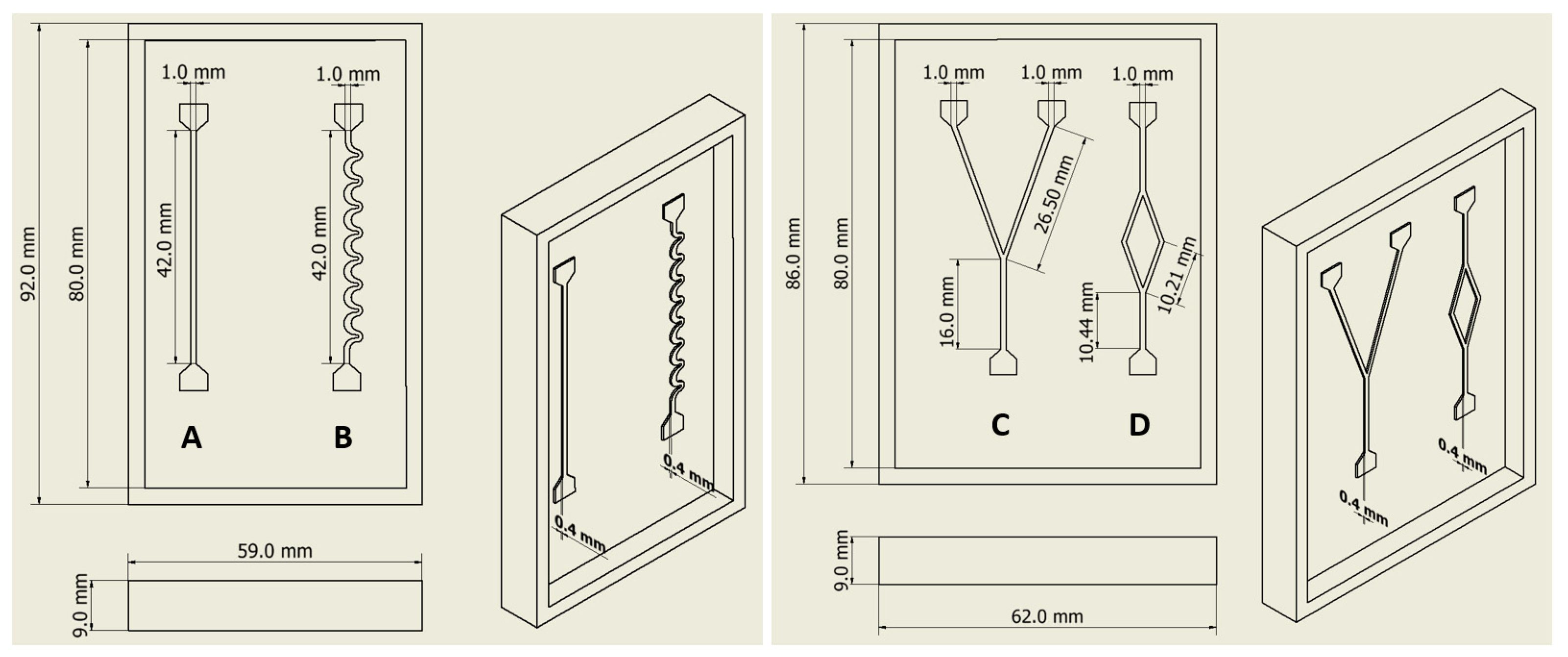
2.3.1. Microchannel Design and 3D Printing of the Molds
2.3.2. PDMS Replica Molding and Double Casting Procedure
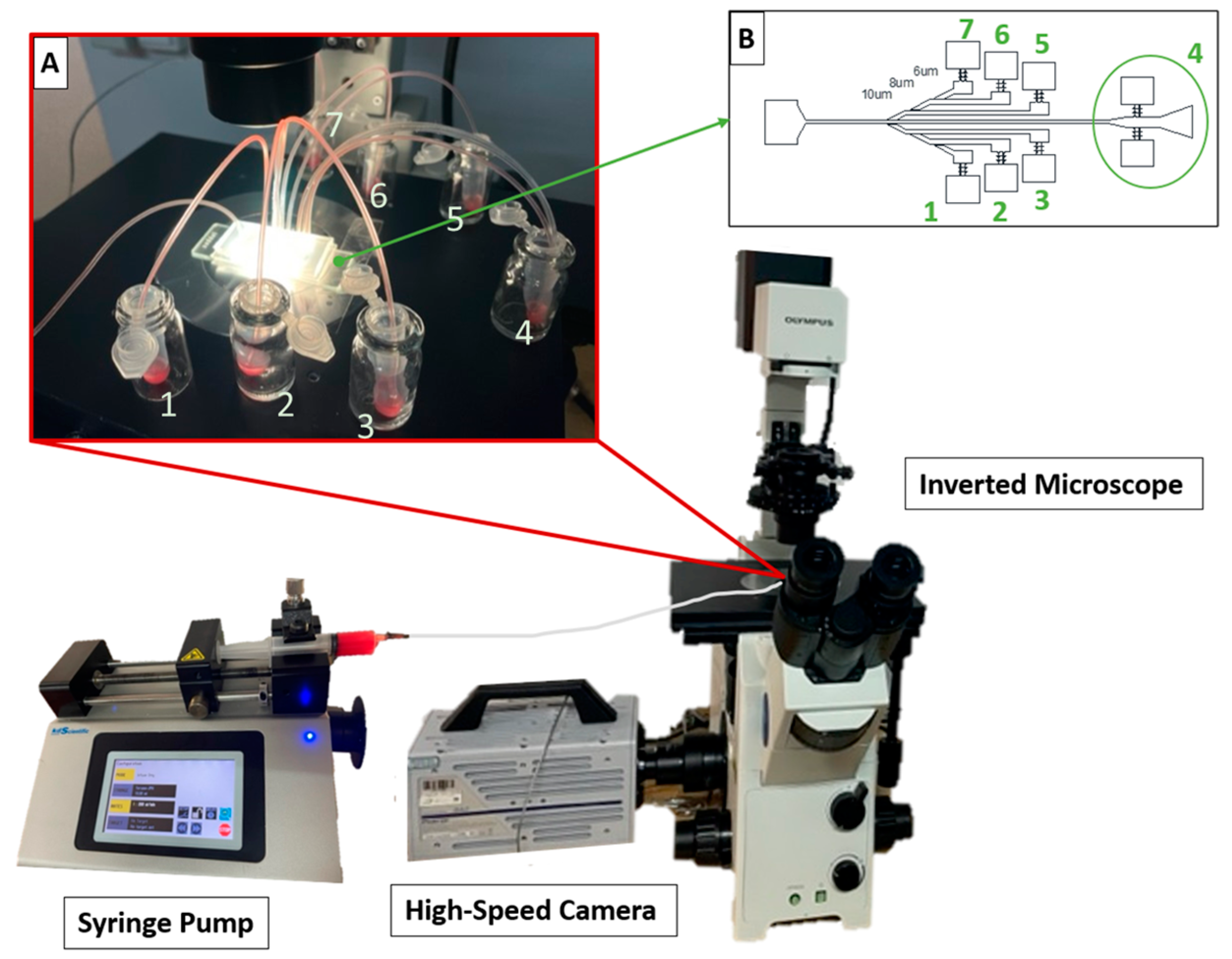
2.4. Blood Flow Experiments
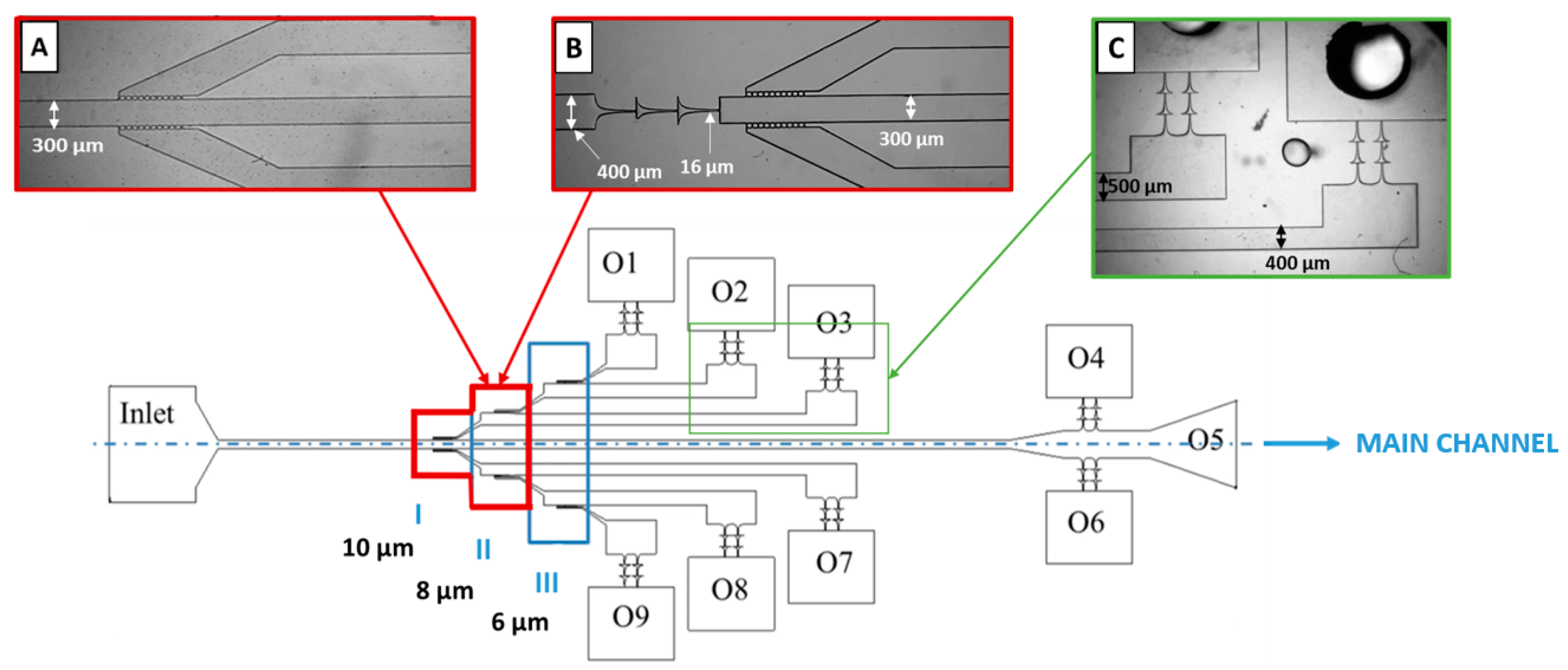
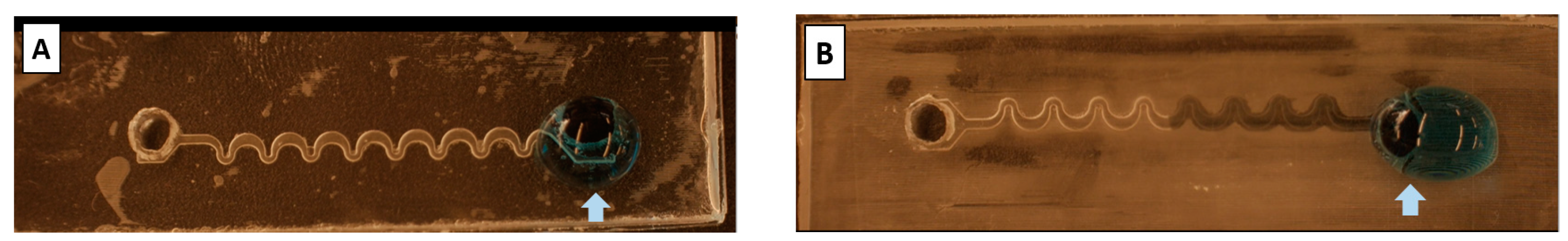
2.4.1. Microchannel Geometry and Fabrication
2.4.2. Blood Sample Processing Set-Up
3. Results
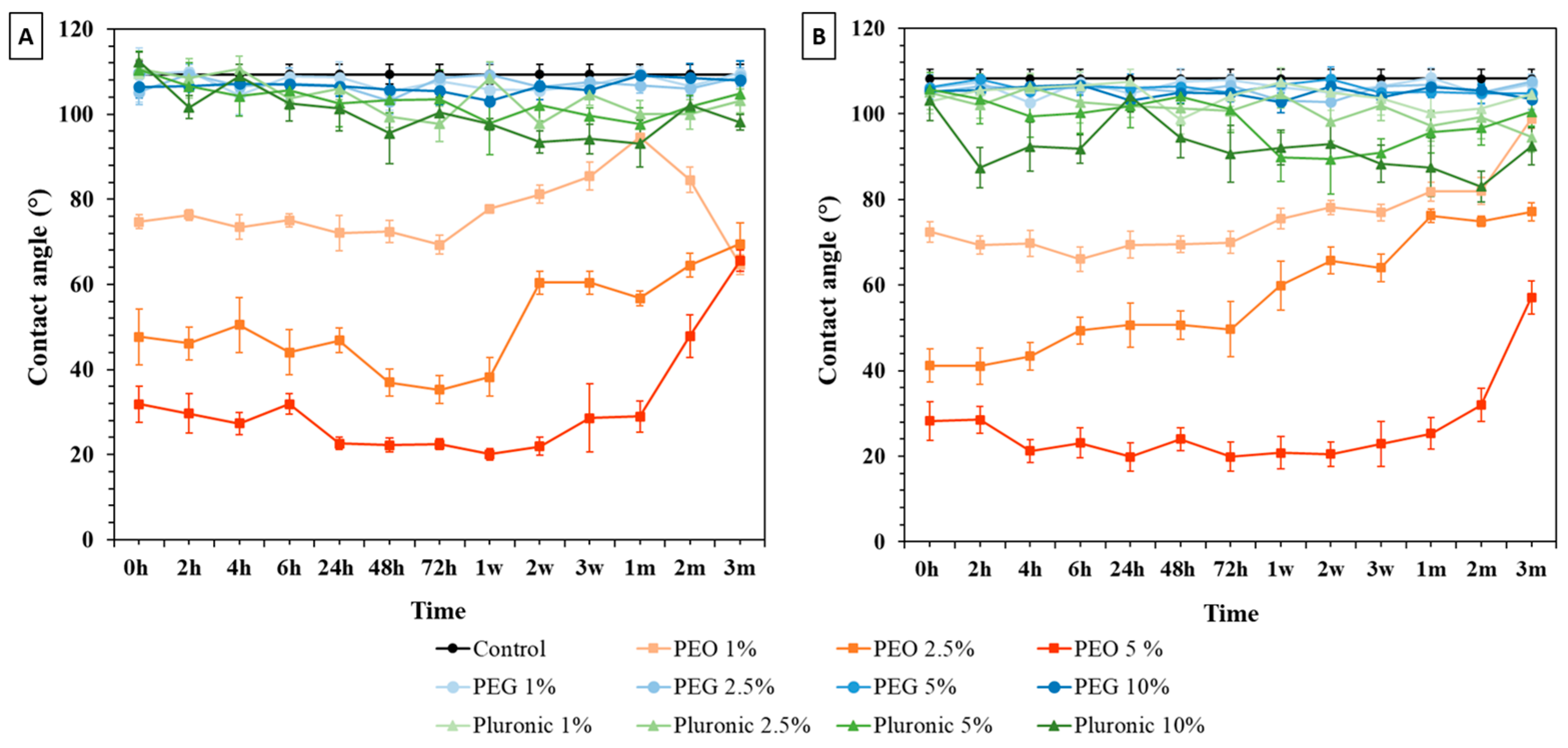
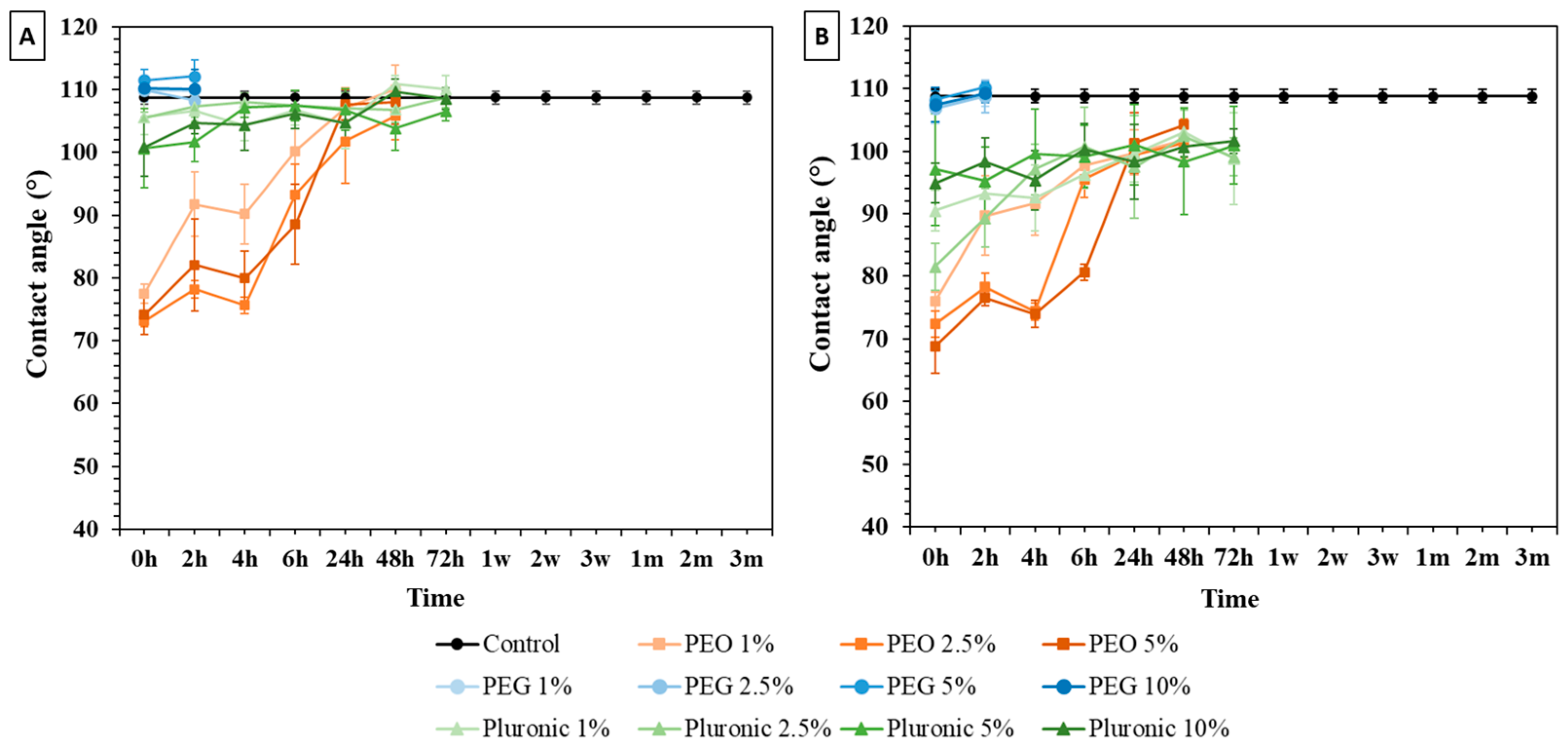
3.1. Water Contact Angle (WCA) Measurements
3.1.1. Bulk Modification
3.1.2. Surface Immersion Modification
3.2. Capillary Flow Studies
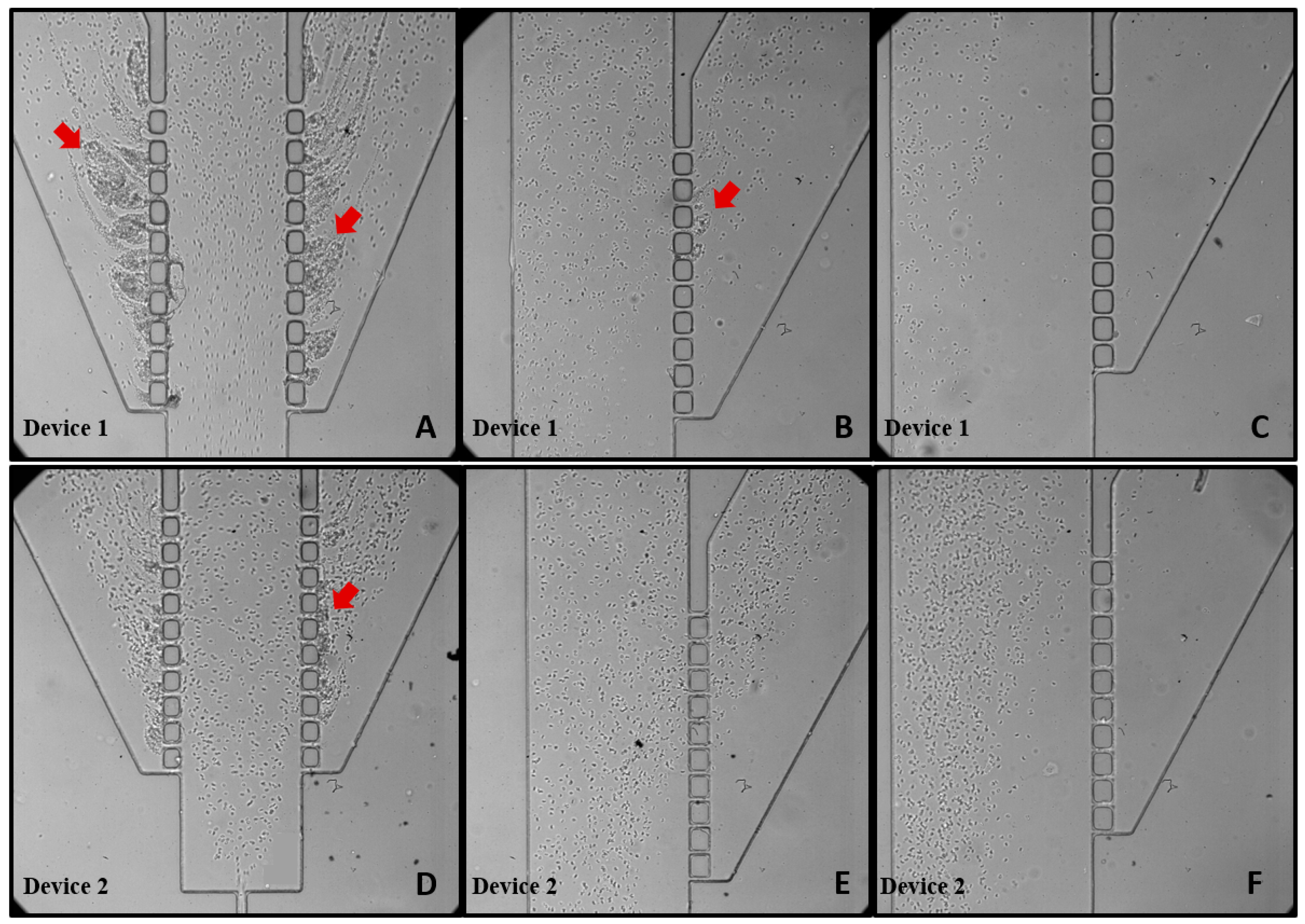
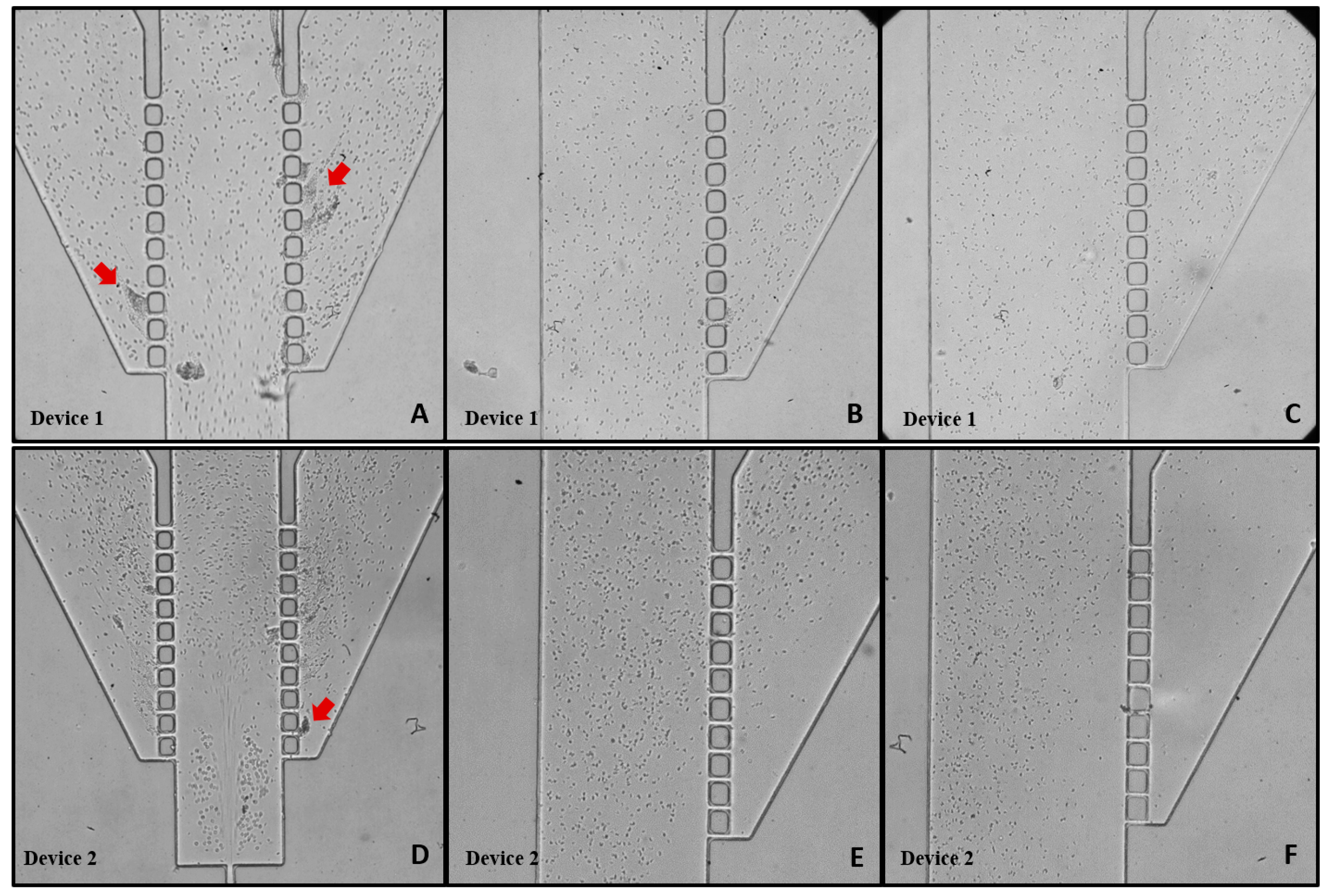
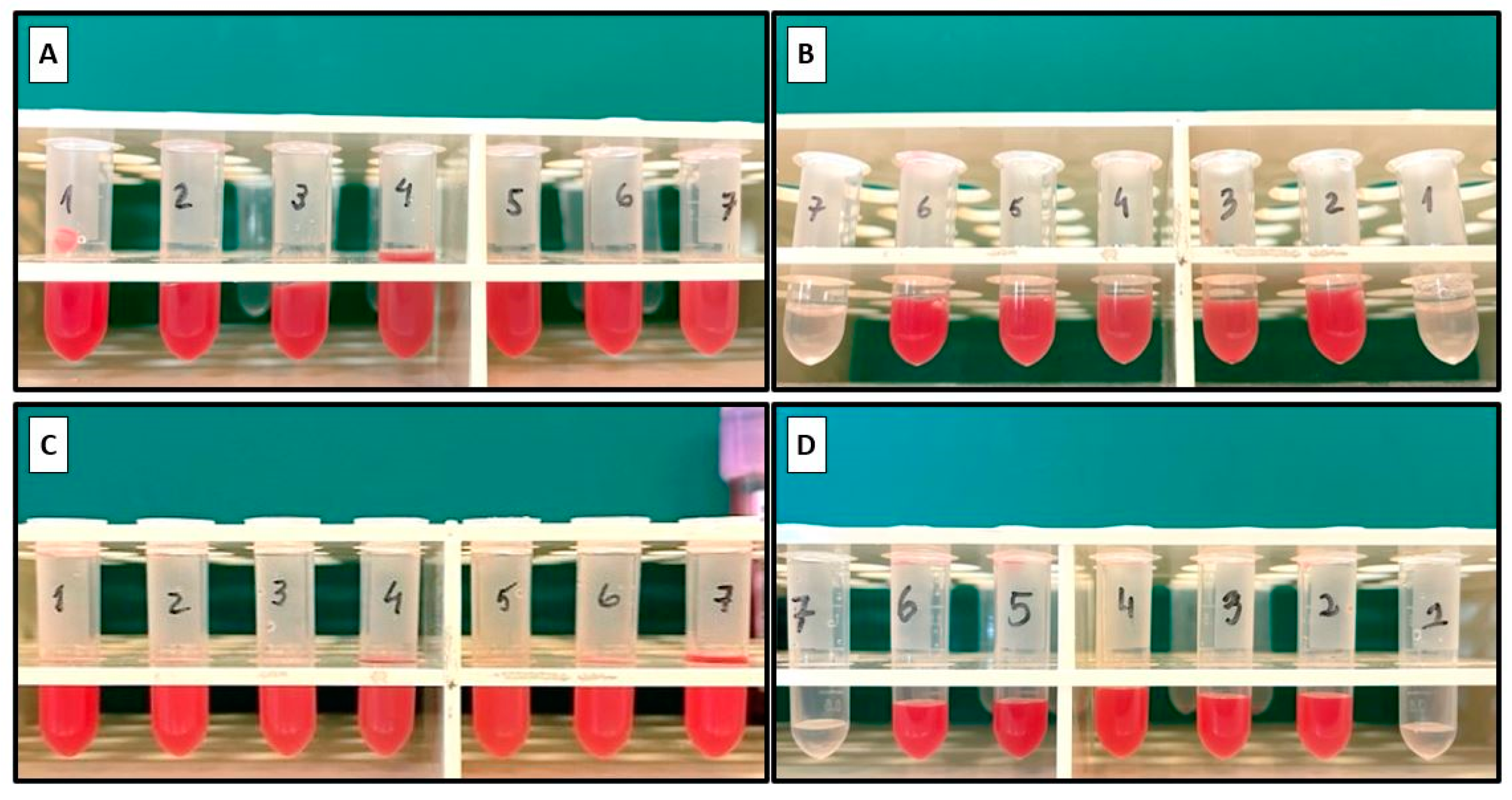
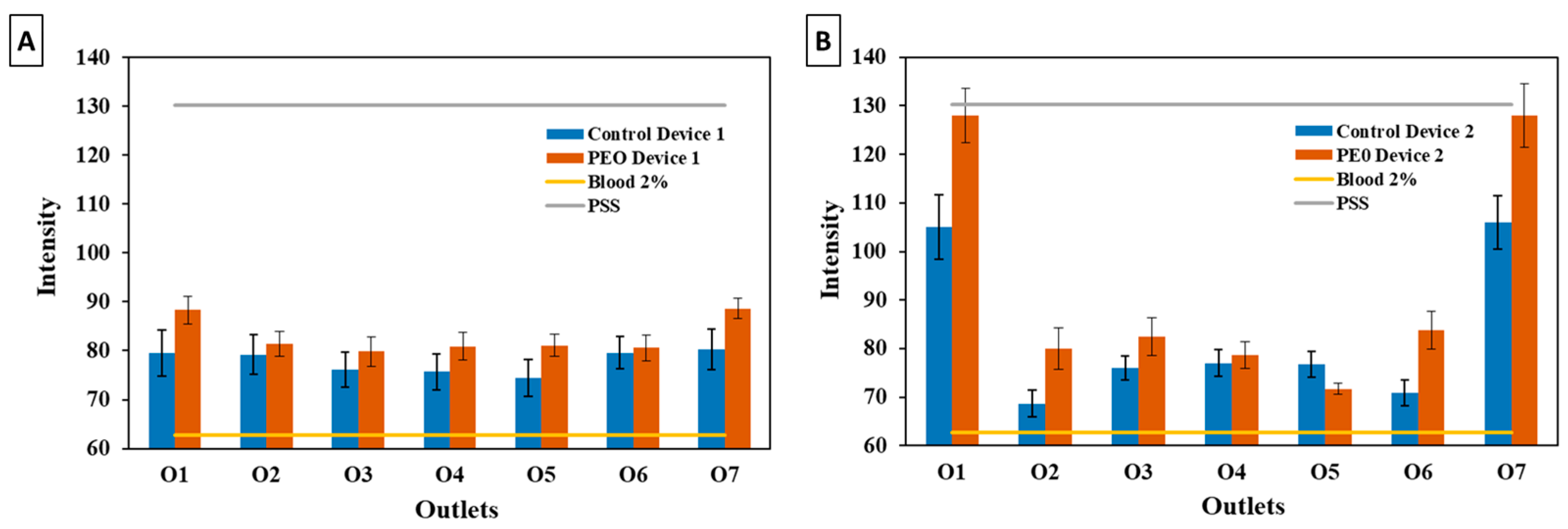
3.3. Blood Plasma Separation
4. Conclusions
- -
- Bulk surface modification emerged as the most efficient method for long-term PDMS surface wettability modification;
- -
- The surfactant PEO at a concentration of 2.5% was identified as the most suitable option, taking into account the surface properties of PDMS such as the water contact angle (WCA), capillarity, and optical features;
- -
- Device 2, featuring a double hyperbola-shaped contraction at the main channel branch, demonstrated superior efficiency for blood plasma separation compared to Device 1;
- -
- The PEO bulk-modified Device 2 proved to be the most efficient microfluidic system for achieving pure plasma separation.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, S.; Joshi, R. Microfluidic Devices: Applications and Role of Surface Wettability in Its Fabrication. In 21st Century Surface Science—A Handbook; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Lu, N.; Tay, H.M.; Petchakup, C.; He, L.; Gong, L.; Maw, K.K.; Leong, S.Y.; Lok, W.W.; Ong, H.B.; Guo, R.; et al. Label-free microfluidic cell sorting and detection for rapid blood analysis. Lab Chip 2023, 23, 1226–1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nunna, B.B.; Talukder, N.; Etienne, E.E.; Lee, E.S. Blood plasma self-separation technologies during the self-driven flow in microfluidic platforms. Bioengineering 2021, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Preetam, S.; Nahak, B.K.; Patra, S.; Toncu, D.C.; Park, S.; Syväjärvi, M.; Orive, G.; Tiwari, A. Emergence of microfluidics for next generation biomedical devices. Biosens. Bioelectron. X 2022, 10, 100106. [Google Scholar] [CrossRef]
- Kirby, B.J. Micro- and Nanoscale Fluid Mechanics; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Team, E. PDMS: A Review on Polydimethylsiloxane in Microfluidics—Elveflow; Elveflow: Paris, France, 2021. [Google Scholar]
- Vlachopoulou, M.E.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Beltsios, K.; Gogolides, E. Effect of surface nanostructuring of PDMS on wetting properties, hydrophobic recovery and protein adsorption. Microelectron. Eng. 2009, 86, 1321–1324. [Google Scholar] [CrossRef]
- Tayyaba, S.; Ashraf, M.W.; Ahmad, Z.; Wang, N.; Afzal, M.J.; Afzulpurkar, N. Article fabrication and analysis of polydimethylsiloxane (PDMS) microchannels for biomedical application. Processes 2021, 9, 57. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Farè, S. Characterization of Polymeric Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Fatona, A.; Chen, Y.; Reid, M.; Brook, M.A.; Moran-Mirabal, J.M. One-step in-mould modification of PDMS surfaces and its application in the fabrication of self-driven microfluidic channels. Lab Chip 2015, 15, 4322–4330. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, W.S.; Obaje, E.A.; Bachmann, T.T.; Kersaudy-Kerhoas, M. Microfluidic blood plasma separation for medical diagnostics: Is it worth it? Lab Chip 2016, 16, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Hatami, A.; Saadatmand, M. Extremely Precise Blood–Plasma Separation from Whole Blood on a Centrifugal Microfluidic Disk (Lab-on-a-Disk) Using Separator Gel. Diagnostics 2022, 12, 2873. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Chung, D.R.; Kim, T.; Park, E.; Kang, M. A self-pressure-driven blood plasma-separation device for point-of-care diagnostics. Talanta 2022, 247, 123562. [Google Scholar] [CrossRef]
- Liu, S.C.; Yoo, P.B.; Garg, N.; Lee, A.P.; Rasheed, S. A microfluidic device for blood plasma separation and fluorescence detection of biomarkers using acoustic microstreaming. Sens. Actuators A Phys. 2021, 317, 112482. [Google Scholar] [CrossRef]
- Bajestani, M.I.; Ahmadzadeh, H. Modified polysulfone membrane facilitates rapid separation of plasma from whole blood for an effective anti-SARS-CoV-2-IgM diagnosis. Sci. Rep. 2023, 13, 13712. [Google Scholar] [CrossRef]
- Leong, S.Y.; Lok, W.W.; Goh, K.Y.; Ong, H.B.; Tay, H.M.; Su, C.; Kong, F.; Upadya, M.; Wang, W.; Radnaa, E.; et al. High-Throughput Microfluidic Extraction of Platelet-free Plasma for MicroRNA and Extracellular Vesicle Analysis. ACS Nano 2024, 18, 6623–6637. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, Y.V.B.V.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 083001. [Google Scholar] [CrossRef]
- Malankowska, M.; Pellejero, I.; Julian, I.; Rho, H.S.; Pinczowski, P.; Tiggelaar, R.M.; Gardeniers, H.; Mallada, R.; Pina, M.P. On the Improvement of Alveolar-Like Microfluidic Devices for Efficient Blood Oxygenation. Adv. Mater. Technol. 2021, 6, 2001027. [Google Scholar] [CrossRef]
- Potkay, J.A. The promise of microfluidic artificial lungs. Lab Chip 2014, 14, 4122–4138. [Google Scholar] [CrossRef]
- Mortensen, K. PEO-related block copolymer surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183–185, 277–292. [Google Scholar] [CrossRef]
- Yao, M.; Fang, J. Hydrophilic PEO-PDMS for microfluidic applications. J. Micromech. Microeng. 2012, 22, 025012. [Google Scholar] [CrossRef]
- Gelardi, G.; Mantellato, S.; Marchon, D.; Palacios, M.; Eberhardt, A.B.; Flatt, R.J. Chemistry of chemical admixtures. In Science and Technology of Concrete Admixtures; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar] [CrossRef]
- Plegue, T.J.; Kovach, K.M.; Thompson, A.J.; Potkay, J.A. Stability of Polyethylene Glycol and Zwitterionic Surface Modifications in PDMS Microfluidic Flow Chambers. Langmuir 2018, 34, 492–502. [Google Scholar] [CrossRef]
- Wu, Z.; Hjort, K. Surface modification of PDMS by gradient-induced migration of embedded Pluronic. Lab Chip 2009, 9, 1500–1503. [Google Scholar] [CrossRef]
- Ziółkowska, K.; Zukowski, K.; Chudy, M.; Dybko, A.; Brzózka, Z. Enhancing efficiency of double casting prototyping by thermal aging of poly(dimethylsiloxane). In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences 2011, MicroTAS 2011, Seattle, WA, USA, 2–6 October 2011. [Google Scholar]
- Ansari, A.; Trehan, R.; Watson, C.; Senyo, S. Increasing silicone mold longevity: A review of surface modification techniques for PDMS-PDMS double casting. Soft Mater. 2021, 19, 388–399. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Faustino, V.; Pinho, D.; Catarino, S.O.; Minas, G.; Lima, R.A. Geometry effect in multi-step crossflow microfluidic devices for red blood cells separation and deformability assessment. Biomed. Microdevices 2022, 24, 20. [Google Scholar] [CrossRef]
- Pinto, V.C.; Sousa, P.J.; Cardoso, V.F.; Minas, G. Optimized SU-8 processing for low-cost microstructures fabrication without cleanroom facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, H.; Chen, D.; Zhou, M. Microscale investigation on the wettability and bonding mechanism of oxygen plasma-treated PDMS microfluidic chip. Appl. Surf. Sci. 2022, 574, 151704. [Google Scholar] [CrossRef]
- Yaginuma, T.; Oliveira, M.S.N.; Lima, R.; Ishikawa, T.; Yamaguchi, T. Human red blood cell behavior under homogeneous extensional flow in a hyperbolic-shaped microchannel. Biomicrofluidics 2013, 7, 54110. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Lopes, R.; Pinho, D.; Pereira, A.I.; Garcia, V.; Gassmann, S.; Sousa, P.C.; Lima, R. In vitro blood flow and cell-free layer in hyperbolic microchannels: Visualizations and measurements. Biochip J. 2016, 10, 9–15. [Google Scholar] [CrossRef]
- Sollier, E.; Go, D.E.; Che, J.; Gossett, D.R.; O’Byrne, S.; Weaver, W.M.; Kummer, N.; Rettig, M.; Goldman, J.; Nickols, N.; et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip 2014, 14, 63–77. [Google Scholar] [CrossRef]

| Microchannels | Time (s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control PDMS | O2 Plasma Treatment | PEO 2.5% | |||||||
| 0 h | 2 h | 0 h | 2 h | 48 h | 0 h | 2 h | 48 h | ||
| A |  | − * | − * | 1.74 | 7.46 | −* | 7.80 | 8.10 | 9.20 |
| B |  | − * | − * | 2.90 | 17.79 | −* | 27.27 | 25.15 | 26.30 |
| C |  | − * | − * | 22.87 | 135.71 | −* | 6.02 | 6.30 | 6.10 |
| D |  | − * | − * | 13.18 | 185.12 | −* | 5.48 | 5.88 | 5.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.; Gonçalves, I.M.; Borges, J.; Faustino, V.; Soares, D.; Vaz, F.; Minas, G.; Lima, R.; Pinho, D. Polydimethylsiloxane Surface Modification of Microfluidic Devices for Blood Plasma Separation. Polymers 2024, 16, 1416. https://doi.org/10.3390/polym16101416
Gonçalves M, Gonçalves IM, Borges J, Faustino V, Soares D, Vaz F, Minas G, Lima R, Pinho D. Polydimethylsiloxane Surface Modification of Microfluidic Devices for Blood Plasma Separation. Polymers. 2024; 16(10):1416. https://doi.org/10.3390/polym16101416
Chicago/Turabian StyleGonçalves, Margarida, Inês Maia Gonçalves, Joel Borges, Vera Faustino, Delfim Soares, Filipe Vaz, Graça Minas, Rui Lima, and Diana Pinho. 2024. "Polydimethylsiloxane Surface Modification of Microfluidic Devices for Blood Plasma Separation" Polymers 16, no. 10: 1416. https://doi.org/10.3390/polym16101416
APA StyleGonçalves, M., Gonçalves, I. M., Borges, J., Faustino, V., Soares, D., Vaz, F., Minas, G., Lima, R., & Pinho, D. (2024). Polydimethylsiloxane Surface Modification of Microfluidic Devices for Blood Plasma Separation. Polymers, 16(10), 1416. https://doi.org/10.3390/polym16101416












