A “2-in-1” Bioanalytical System Based on Nanocomposite Conductive Polymers for Early Detection of Surface Water Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Microorganisms
2.3. Cultivation of Microorganisms
2.4. Association Stability Assessment
2.5. Formation of Working Electrodes
2.6. CV Tests
2.7. Impedance Spectroscopy
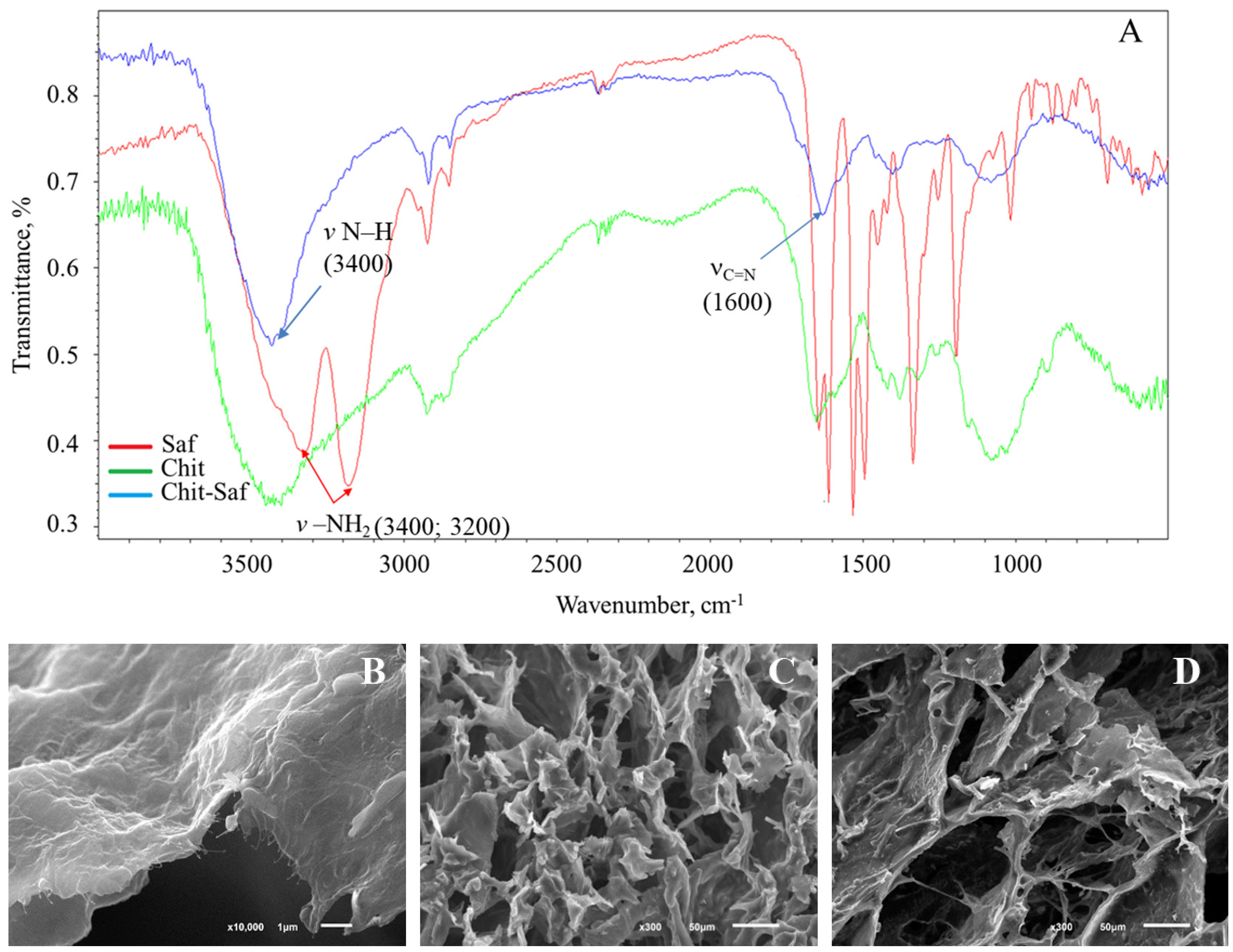
2.8. Electron Microscopy
2.9. IR Spectroscopy
2.10. Raman Spectroscopy
2.11. Biosensor Measurements
2.12. Reference Luminescence Method for Assessing the Toxicity of Water Samples
2.13. Reference Chlorella Method for Determining the Toxicity of Water Samples
2.14. Determination of BOD Using the Standard Method
3. Results and Discussion
3.1. Formation of Time-Stable Associations among Microorganisms for Rapid Assessment of Toxicity and BOD
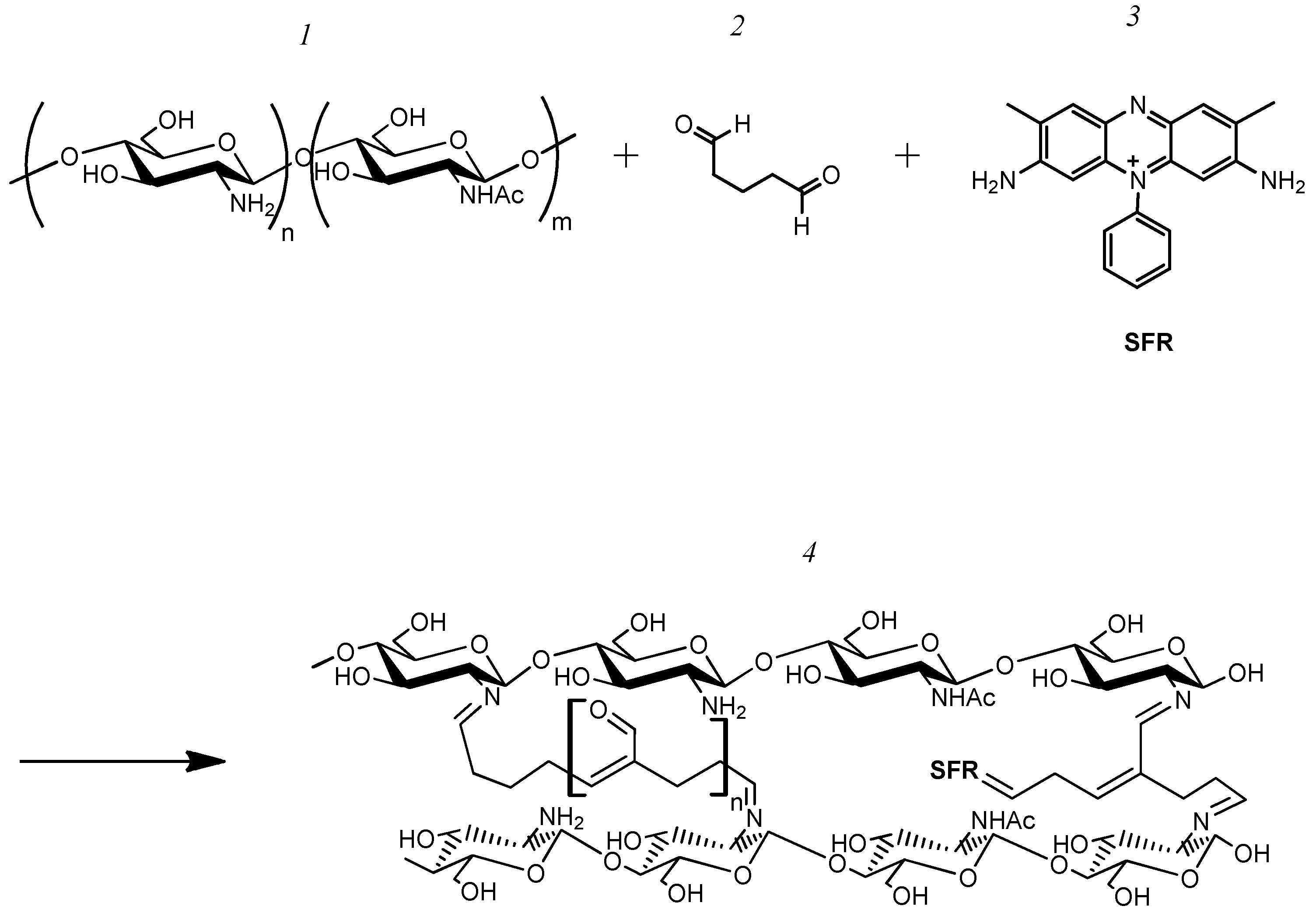
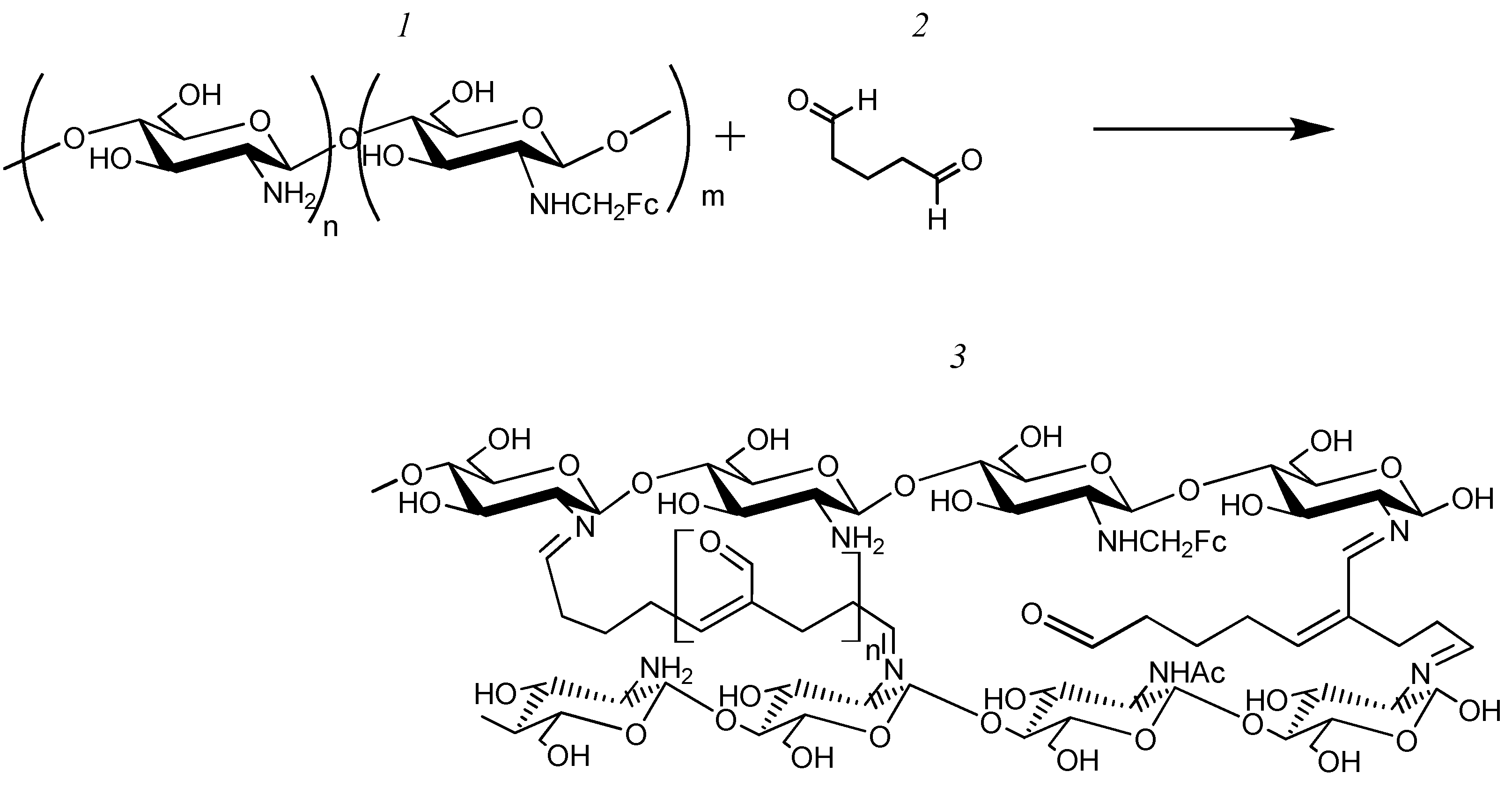
3.2. The Formation and Study of Chemical, Spatial Structures, and Electrochemical Properties of Redox-Active Polymers/Composites
3.3. Immobilization of Microorganisms into Redox-Active Polymers and Their Nanocomposites
3.4. Analytical and Metrological Parameters for the Development of Biosensors for the Rapid Assessment of BOD and Toxicity
3.5. Testing of the Developed Receptor Elements for Rapid Assessment of Toxicity and Biochemical Oxygen Consumption on Natural Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noor, R.; Maqsood, A.; Baig, A.; Pande, C.B.; Zahra, S.M.; Saad, A.; Anwar, M.; Singh, S.K. A comprehensive review on water pollution, South Asia Region: Pakistan. Urban Clim. 2023, 48, 101413. [Google Scholar] [CrossRef]
- Saberifar, R. Climate Change and Water Crisis (Case Study, Mashhad in Northeastern Iran). Pol. J. Environ. Stud. 2023, 32, 705–716. [Google Scholar] [CrossRef]
- du Plessis, A. Water resources from a global perspective. In South Africa’s Water Predicament: Freshwater’s Unceasing Decline; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–25. [Google Scholar]
- Zhang, L.; Zhang, Y.; Zhu, M.; Chen, L.; Wu, B. A critical review on quantitative evaluation of aqueous toxicity in water quality assessment. Chemosphere 2023, 342, 140159. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.A.; Plekhanova, Y.V.; Kamanina, O.A.; Nakamura, H.; Reshetilov, A.N. Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects. Biosensors 2022, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Shvets, V.I.; Reshetilov, A.N. A mediator microbial biosensor for assaying general toxicity. Enzym. Microb. Technol. 2020, 132, 109435. [Google Scholar] [CrossRef] [PubMed]
- Yudina, N.Y.; Kozlova, T.N.; Bogachikhin, D.A.; Kosarenina, M.M.; Arlyapov, V.A.; Alferov, S.V. Electrochemical Biosensors for Express Analysis of the Integral Toxicity of Polymer Materials. Biosensors 2023, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Azizullah, A.; Häder, D.P. Azizullah, A.; Häder, D.P. A comparison of commonly used and commercially available bioassays for aquatic ecosystems. In Bioassays; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 347–368. [Google Scholar] [CrossRef]
- Moretti, A.I.; Heidi, L.I.; Skvaril, J. A review of the state-of-the-art wastewater quality characterization and measurement technologies. Is the shift to real-time monitoring nowadays feasible? J. Water Process Eng. 2024, 60, 105061. [Google Scholar] [CrossRef]
- Cui, Y.; Lai, B.; Tang, X. Microbial fuel cell-based biosensors. Biosensors 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, J.M.; Ezugwu, C.I.; Ghosh, P.C. Microbial fuel cell-based biological oxygen demand sensors for monitoring wastewater: State-of-the-art and practical applications. ACS Sens. 2020, 5, 2297–2316. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, S.; Qin, W. Electrochemical biochemical oxygen demand biosensors and their applications in aquatic environmental monitoring. Sens. Bio-Sens. Res. 2024, 44, 100642. [Google Scholar] [CrossRef]
- Medvedeva, A.S.; Dyakova, E.I.; Kuznetsova, L.S.; Mironov, V.G.; Gurkin, G.K.; Rogova, T.V.; Kharkova, A.S.; Melnikov, P.V.; Naumova, A.O.; Butusov, D.N.; et al. A Two-Mediator System Based on a Nanocomposite of Redox-Active Polymer Poly(thionine) and SWCNT as an Effective Electron Carrier for Eukaryotic Microorganisms in Biosensor Analyzers. Polymers 2023, 15, 3335. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wan, N.; Fu, T.; Wang, L.; Li, C.; Qie, Z.; Zhu, A. A current sensing biosensor for BOD rapid measurement. Archaea 2020, 2020, 8894925. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Ilyukhina, A.S.; Ponamoreva, O.N.; Alferov, V.A.; Reshetilov, A.N. A kinetic approach to the formation of two-mediator systems for developing microbial biosensors as exemplified by a rapid biochemical oxygen demand assay. 3 Biotech 2021, 11, 222. [Google Scholar] [CrossRef]
- Meskher, H.; Ragdi, T.; Thakur, A.K.; Ha, S.; Khelfaoui, I.; Sathyamurthy, R.; Sharshir, S.W.; Pandey, A.K.; Saidur, R.; Singh, P.; et al. A review on CNTs-based electrochemical sensors and biosensors: Unique properties and potential applications. Crit. Rev. Anal. Chem. 2023, 1, 1–24. [Google Scholar] [CrossRef]
- Zare-Shehneh, N.; Mollarasouli, F.; Ghaedi, M. Recent advances in carbon nanostructure-based electrochemical biosensors for environmental monitoring. Crit. Rev. Anal. Chem. 2023, 53, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Sazawa, K.; Sugawara, K.; Kuramitz, H. Electrochemical Biosensor for Evaluation of Environmental Pollutants Toxicity. Environments 2023, 10, 63. [Google Scholar] [CrossRef]
- He, Q.; Wanga, B.; Liangc, J.; Liuab, J.; Liangd, B.; Liab, G.; Longa, Y.; Zhang, G.; Liu, H. Research on the construction of portable electrochemical sensors for environmental compounds quality monitoring. Mater. Today Adv. 2023, 17, 100340. [Google Scholar] [CrossRef]
- Dalkiran, B.; Brett, C.M. Polyphenazine and polytriphenylmethane redox polymer/nanomaterial–based electrochemical sensors and biosensors: A review. Microchim. Acta 2021, 188, 178. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Gao, G.; Yang, Y.; Wang, Y.; Gao, L.; Zhi, J. Redox Mediator-Based Microbial Biosensors for Acute Water Toxicity Assessment: A Critical Review. ChemElectroChem 2020, 9, 2513–2526. [Google Scholar] [CrossRef]
- Tan, G.; Wang, S.; Yu, J.; Chen, J.; Liao, D.; Liu, M.; Nezamzadeh-Ejhieh, A.; Pan, Y.; Liu, J. Detection mechanism and the outlook of metal-organic frameworks for the detection of hazardous substances in milk. Food Chem. 2024, 430, 136934. [Google Scholar] [CrossRef]
- Fang, D.; Gao, G.; Shen, J.; Yu, Y.; Zhi, J. A reagentless electrochemical biosensor based on thionine wrapped E. coli and chitosan-entrapped carbon nanodots film modified glassy carbon electrode for wastewater toxicity assessment. Electrochim. Acta 2016, 222, 303–311. [Google Scholar] [CrossRef]
- PND F T 14.1:2:3:4.11-04; PND F T Methodology for Determining the Integral Toxicity of Surface, Including Sea, Ground, Drinking, Waste Water, Aqueous Extracts of Soils, Waste, Sewage Sludge by Changes in the Intensity of Bacterial Bioluminescence Using the Ecolum Test System. Federal Environmental Regulatory Standard Toxicological Methods of Analysis: Moscow, Russia, 2004.
- PND F T 14.1:2:3:4.10-04; PND F T Methodology for Measuring the Optical Density of the Chlorella Algae Culture (Chlorella vulgaris beijer) for Determining the Toxicity of Drinking, Fresh Natural and Waste Water, Water Extracts From Soils, Soils, Sewage Sludge, Waste Production and Consumption. Federal Environmental Regulatory Standard Toxicological Methods of Analysis: Moscow, Russia, 2004.
- PND F 14. 1:2:3:4. 123-97; PND F Quantitative Chemical Analysis of Water. Methodology for Performing Measurements of Biochemical Oxygen Demand after n-Days of Incubation (BODtotal) in Surface Fresh, Underground (Ground), Drinking, Sewage and Treated Wastewater. Federal Environmental Regulatory Standard: Moscow, Russia, 1997.
- PND F T 14.1:2:3:4.2-98; PND F T Methods for Determining the Toxicity of Samples of Natural, Drinking, Household Drinking, Household Waste, Treated Waste, Sewage, Melt, and Process Waters Using an Express Method Using a Biotester Series Device. Federal Environmental Regulatory Standard Toxicological Methods of Analysis: Moscow, Russia, 1998.
- Elias, S.; Banin, E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- GOST 32426-2013; GOST Test Methods for Chemical Products That Are Hazardous to the Environment. Testing Duckweed for Growth Inhibition. Interstate Standard: Moscow, Russia, 2013.
- Kuznetsova, L.S.; Arlyapov, V.A.; Plekhanova, Y.V.; Tarasov, S.E.; Kharkova, A.S.; Saverina, E.A.; Reshetilov, A.N. Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells. Polymers 2023, 15, 3783. [Google Scholar] [CrossRef] [PubMed]
- Haipeng, Y.; Daming, K.; Hojong, Z.; Zhijia, M.; Shenghua, H. A Ferricyanide-mediated Activated Sludge Bioassay for Determination of the Toxicity of Water. Electroanalysis 2016, 28, 580–587. [Google Scholar] [CrossRef]
- Gao, G.; Fang, D.; Yu, Y.; Wu, L.; Wang, Y.; Zhi, J. A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta 2017, 167, 208–216. [Google Scholar] [CrossRef]
- Gao, G.; Qian, J.; Fang, D.; Yu, Y.; Zhi, J. Development of a mediated whole cell-based electrochemical biosensor for joint toxicity assessment of multi-pollutants using a mixed microbial consortium. Anal. Chim. Acta 2016, 924, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, G.; Sun, M.; Cai, Z.; Yin, Z.; Cai, Y. Bacterial cellulose immobilized S. cerevisiae as microbial sensor for rapid BOD detection. Fibers Polym. 2021, 22, 1208–1217. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Alferov, V.A.; Reshetilov, A.N. Registration of BOD using Paracoccus yeei bac-teria isolated from activated sludge. 3 Biotech 2020, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.H.; Abd, K.I.; Rahmalan, A.; Shafinaz, S. A redox mediated UME biosensor using immobilized Chromobacterium violaceum strain R1 for rapid biochemical oxygen demand measurement. Electrochim. Acta 2015, 176, 777–783. [Google Scholar] [CrossRef]
- Lin, V.J.C.; Koenig, J.L. Raman studies of bovine serum albumin. Biopolym. Orig. Res. Biomol. 1976, 15, 203–218. [Google Scholar] [CrossRef]
- Mažeikienė, R.; Balskus, K.; Eicher-Lorka, O.; Niaura, G.; Meškys, R. Raman spectroelectrochemical study of electrode processes at Neutral red-and poly (Neutral red) modified electrodes. Vib. Spectrosc. 2009, 51, 238–247. [Google Scholar] [CrossRef]
- Casado, N.; Hernándeza, G.; Sardon, H.; Mecerreyes, D. Current trends in redox polymers for energy and medicine. Prog. Polym. Sci. 2016, 52, 107–135. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, I. Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal. Chem 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Niyomdecha, S.; Limbut, W.; Numnuam, A.; Asawatreratanakul, P.; Kanatharana, P.; Thavarungkul, P. A novel BOD biosensor based on entrapped activated sludge in a porous chitosan-albumin cryogel incorporated with graphene and methylene blue. Sens. Actuators B Chem. 2017, 241, 473–481. [Google Scholar] [CrossRef]
- Yang, Y.; Liua, Y.; Chenab, Y.; Wangc, Y.; Shaoc, P.; Liua, R.; Gaoa, G.; Zhi, J. A portable instrument for monitoring acute water toxicity based on mediated electrochemical biosensor: Design, testing and evaluation. Chemosphere 2020, 255, 126964. [Google Scholar] [CrossRef]
- Nam, I.H.; Chang, Y.S.; Hong, H.B.; Lee, Y.E. A novel catabolic activity of Pseudomonas veronii in biotransformation of pentachlorophenol. Appl. Microbiol. Biotechnol. 2003, 62, 284–290. [Google Scholar] [CrossRef]
- Strandberg, G.W.; Shumate, S.E.; Parrott, J.R. Microbial cells as biosorbents for heavy metals: Accumulation of uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1981, 41, 237–245. [Google Scholar] [CrossRef]
- Lehmann, M.; Riedel, K.; Adler, K.; Kunze, G. Amperometric measurement of copper ions with a deputy substrate using a novel Saccharomyces cerevisiae sensor. Biosens. Bioelectron. 2000, 15, 211–219. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Lavrova, D.G.; Arlyapov, V.A.; Alferov, V.A.; Ponamoreva, O.N. Silica sol-gel encapsulated methylotrophic yeast as filling of biofilters for the removal of methanol from industrial wastewater. Enzyme Microb. Technol. 2016, 92, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Ponamoreva, O.N.; Kamanina, T.V.; Alferov, V.A.; Machulin, A.V.; Rogova, T.V.; Arlyapov, S.V.; Suzina, V.A.; Alferov, S.V.; Ivanova, E.P. Yeast-based self-organized hybrid bio-silica sol–gels for the design of biosensors. Biosens. Bioelectron. 2015, 67, 321–326. [Google Scholar] [CrossRef]
- Yudina, N.Y.; Arlyapov, V.A.; Chepurnova, M.A.; Alferov, S.V.; Reshetilov, A.N. A yeast co-culture-based biosensor for determination of waste water contamination levels. Enzyme Microb. Technol. 2015, 78, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Lehmann, M.; Tag, K.; Lung, M.; Kunze, G.; Riedeld, K.; Gruendige, B.; Renneberg, R. Measurement of biodegradable substances using the salt-tolerant yeast Arxula adeninivorans for a microbial sensor immobilized with poly(carbamoyl)sulfonate (PCS). Part II: Application of the novel biosensor to real samples from coastal and island regions. Biosens. Bioelectron. 1999, 14, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Arlyapov, V.A.; Kamanina, O.A.; Popova, N.M.; Zaitsev, N.K. Optical Oxygen Sensing and Clark Electrode: Face-to-Face in a Biosensor Case Study. Sensors 2022, 19, 7626. [Google Scholar] [CrossRef] [PubMed]
- Galler, H.; Feierl, G.; Petternel, C.; Reinthaler, F.F.; Haas, D.; Habib, J.; Kittinger, C.; Luxner, J.; Zarfel, G. Multiresistant Bacteria Isolated from Activated Sludge in Austria. Int. J. Environ. Res. Public Health 2018, 15, 479. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, A.; Arlyapov, V.; Medvedeva, A.; Lepikash, R.; Melnikov, P.; Reshetilov, A. Mediator Microbial Biosensor Analyzers for Rapid Determination of Surface Water Toxicity. Sensors 2022, 22, 8522. [Google Scholar] [CrossRef]
- Eom, H.; Park, M.; Jang, A.; Kim, S.; Oh, S.-E. A simple and rapid algal assay kit to assess toxicity of heavy metal-contaminated water. Environ. Pollut. 2020, 269, 116135. [Google Scholar] [CrossRef]
- Order of the Ministry of Agriculture of the Russian Federation N. 552. In On Approval of Water Quality Standards for Water Bodies of Fishery Importance, Including Standards for Maximum Permissible Concentrations of Harmful Substances in the Waters of Water Bodies of Fishery Importance; Ministry of Agriculture of the Russian Federation: Moscow, Russia, 2009.






| Mediator Biosensors for Toxicity Assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomaterial/ Mediator | Concentrations of Toxic Substances (IC50), Causing a 50% Decrease in the Activity of a Receptor Element, mg/L | Long-Term Stability, Days | Operational Stability, % | Reproducibility, % | Ref. | |||||
| Cu2+ | Cd2+ | Phenol | Cu2+ | Phenol | Cu2+ | Phenol | Cu2+ | Phenol | ||
| Association of S. cerevisiae and P. yeei/ferrocene | 15.9 | 15.6 | 1.6 | 5 | 5 | 7.5 | 7.0 | 5.6 | 6.7 | This work |
| Association of E. coli and P. yeei/ferrocene | 23.8 | 7.5 | 8.1 | 4 | 4 | 7.3 | 10.3 | 5.2 | 9.6 | [7] |
| S. cerevisiae/ferrocene | 2.7 | 17.5 | 1.8 | 5 | 5 | 6.9 | 11.5 | 4.9 | 5.1 | [7] |
| E. coli/ferrocene | 47.6 | 8.9 | 17.6 | 3 | 3 | 6.92 | 10.81 | 7 | 9 | [7] |
| P. yeei/ferrocene | 21.1 | 18.2 | 9.9 | 9 | 10 | 4.9 | 5.3 | 4.5 | 4.8 | [6] |
| Activated sludge/K3[Fe(CN)6] | 19.8 | 13.4 | -* | - | - | - | - | - | - | [31] |
| S. cerevisiae/menadione and K3[Fe(CN)6] | 10.1 | 13.9 | 44.5 | - | - | - | - | - | - | [32] |
| E. coli/thionine | 20.2 | 36.2 | - | - | - | - | - | - | - | [23] |
| E. coli, B. subtilis, S. cerevisiae/p-benzoquinone | 16.5 | 20.5 | - | - | - | - | - | - | - | [33] |
| Mediator Biosensors for BOD5 Assessment | ||||||||||
| Biomaterial/ Mediator | Number of Oxidized Substrates, Pcs. | Long-Term Stability, Days | Linear Range BOD5, mg/L | Ref. | ||||||
| P. yeei, E. coli/ferrocene | 16 | 20 | 61–164 | This work | ||||||
| O. polymorpha, B. adeninivorans/ferrocene | 16 | 19 | 2–140 | This work | ||||||
| P. yeei, B. adeninivorans/ferrocene | 12 | 5 | 49–290 | This work | ||||||
| S. cerevisiae/menadione and K3[Fe(CN)6] | -* | -* | 10–220 | [34] | ||||||
| E. coli/ferrocene | 7 | 17 | 0.7–1.59 | [15] | ||||||
| O. polymorpha/ferrocene | 14 | 9 | -* | [15] | ||||||
| B. adeninivorans/ferrocene | 16 | 5 | 2.5–21 | [15] | ||||||
| P. yeei/ferrocene | 22 | 22 | 1.3–200 | [35] | ||||||
| Chromobacterium violaceum/K3[Fe(CN)6] | -* | -* | 20–225 | [35,36] | ||||||
| Redox-Active Polymers/Nanocomposite | Limiting Stages of the Electronic Process | Rate Constant for Heterogeneous Electron Transfer to the Electrode, s−1 | Charge-Transfer Resistance, 105 Ohm |
|---|---|---|---|
| BSA-FEN | Surface reaction | 0.32 ± 0.02 | 17.4 ± 0.4 |
| BSA-FEN-CNT/COOH | Surface reaction | 0.42 ± 0.02 | 75 ± 2 |
| BSA-FEN-CNT/CONH2 | Surface reaction | 0.36 ± 0.02 | 93 ± 4 |
| CHIT-FEN | Surface reaction | 0.34 ± 0.03 | 4.83 ± 0.06 |
| CHIT-FEN-CNT/COOH | Surface reaction | 0.54 ± 0.03 | 24 ± 4 |
| CHIT-FEN-CNT/CONH2 | Surface reaction | 0.41 ± 0.02 | 89 ± 4 |
| BSA-SFR | Surface reaction | 0.26 ± 0.02 | 57 ± 3 |
| BSA-SFR-CNT/COOH | Hopping mechanism | - | 121 ± 3 |
| BSA-SFR-CNT/CONH2 | Hopping mechanism | - | 243 ± 8 |
| CHIT-SFR | Surface reaction | 0.23 ± 0.02 | 6.7 ± 0.1 |
| CHIT-SFR-CNT/COOH | Surface reaction | 0.57 ± 0.02 | 19 ± 1 |
| CHIT-SFR-CNT/CONH2 | Surface reaction | 0.54 ± 0.05 | 41 ± 4 |
| BSA-NR | Surface reaction | 0.0119 ± 0.0006 | 72 ± 2 |
| BSA-NR-CNT/COOH | Surface reaction | 0.89 ± 0.03 | 4.5 ± 0.3 |
| BSA-NR-CNT/CONH2 | Surface reaction | 0.64 ± 0.05 | 14 ± 1 |
| CHIT-NR | Hopping mechanism | - | 30.5 ± 0.5 |
| CHIT-NR-CNT/COOH | Surface reaction | 0.77 ± 0.06 | 10.6 ± 0.3 |
| CHIT-NR-CNT/CONH2 | Surface reaction | 0.44 ± 0.03 | 57 ± 3 |
| BSA-FC | Surface reaction | 0.45 ± 0.01 | 14 ± 1 |
| BSA-FC-CNT/COOH | Surface reaction | 0.87 ± 0.04 | 9.3 ± 0.1 |
| BSA-FC-CNT/CONH2 | Surface reaction | 0.63 ± 0.09 | 19 ± 2 |
| CHIT-FC | Surface reaction | 0.44 ± 0.02 | 4.10 ±0.05 |
| CHIT-FC-CNT/COOH | Surface reaction | 0.86 ± 0.04 | 7.1 ± 0.2 |
| CHIT-FC-CNT/CONH2 | Surface reaction | 0.56 ± 0.08 | 22 ± 5 |
| Biomaterial/Immobilization 1 | Redox Compound | Parameters 2 | Ref. |
|---|---|---|---|
| Association of O. polymorpha and B. adeninivorans/CHIT-NR-CNT/COOH | Neutral red, covalently bonded to chitosan | R: 0.6—20 mg/L S: 22 compound L: 34 days T: 4–5 min | This work |
| Association of O. polymorpha and B. adeninivorans/D | Ferrocene in graphite paste | R: 61–164 mg/L O: 6% S: 16 compound L: 19 days T: 4–5 min | This work |
| B. adeninivorans/D | Ferrocene in graphite paste | R: 2.5–21 mg/L S: 16 compound L: 5 days T: 4–5 min | [15] |
| Saccharomyces cerevisiae/BC-CB | Solution of potassium hexacyanoferrate(III) and menadione | R: 10–220 mg/L T: 5 min | [35] |
| Activated sludge/AC-G | Methylene blue | R: 1–100 mg/L L: 65 min | [41] |
| Biox-1010 analyzer | - | R: 5–100,000 mg/L T: 3–15 min | [5] |
| BioMonitor analyzer | - | R: 1–200,000 mg/L T: 3–4 min | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharkova, A.S.; Medvedeva, A.S.; Kuznetsova, L.S.; Gertsen, M.M.; Kolesov, V.V.; Arlyapov, V.A.; Reshetilov, A.N. A “2-in-1” Bioanalytical System Based on Nanocomposite Conductive Polymers for Early Detection of Surface Water Pollution. Polymers 2024, 16, 1431. https://doi.org/10.3390/polym16101431
Kharkova AS, Medvedeva AS, Kuznetsova LS, Gertsen MM, Kolesov VV, Arlyapov VA, Reshetilov AN. A “2-in-1” Bioanalytical System Based on Nanocomposite Conductive Polymers for Early Detection of Surface Water Pollution. Polymers. 2024; 16(10):1431. https://doi.org/10.3390/polym16101431
Chicago/Turabian StyleKharkova, Anna S., Anastasia S. Medvedeva, Lyubov S. Kuznetsova, Maria M. Gertsen, Vladimir V. Kolesov, Vyacheslav A. Arlyapov, and Anatoly N. Reshetilov. 2024. "A “2-in-1” Bioanalytical System Based on Nanocomposite Conductive Polymers for Early Detection of Surface Water Pollution" Polymers 16, no. 10: 1431. https://doi.org/10.3390/polym16101431
APA StyleKharkova, A. S., Medvedeva, A. S., Kuznetsova, L. S., Gertsen, M. M., Kolesov, V. V., Arlyapov, V. A., & Reshetilov, A. N. (2024). A “2-in-1” Bioanalytical System Based on Nanocomposite Conductive Polymers for Early Detection of Surface Water Pollution. Polymers, 16(10), 1431. https://doi.org/10.3390/polym16101431











