A Hydrophilic Polyethylene Glycol-Blended Anion Exchange Membrane to Facilitate the Migration of Hydroxide Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures and Materials
2.2. Characterization and Measurements
3. Results and Discussion
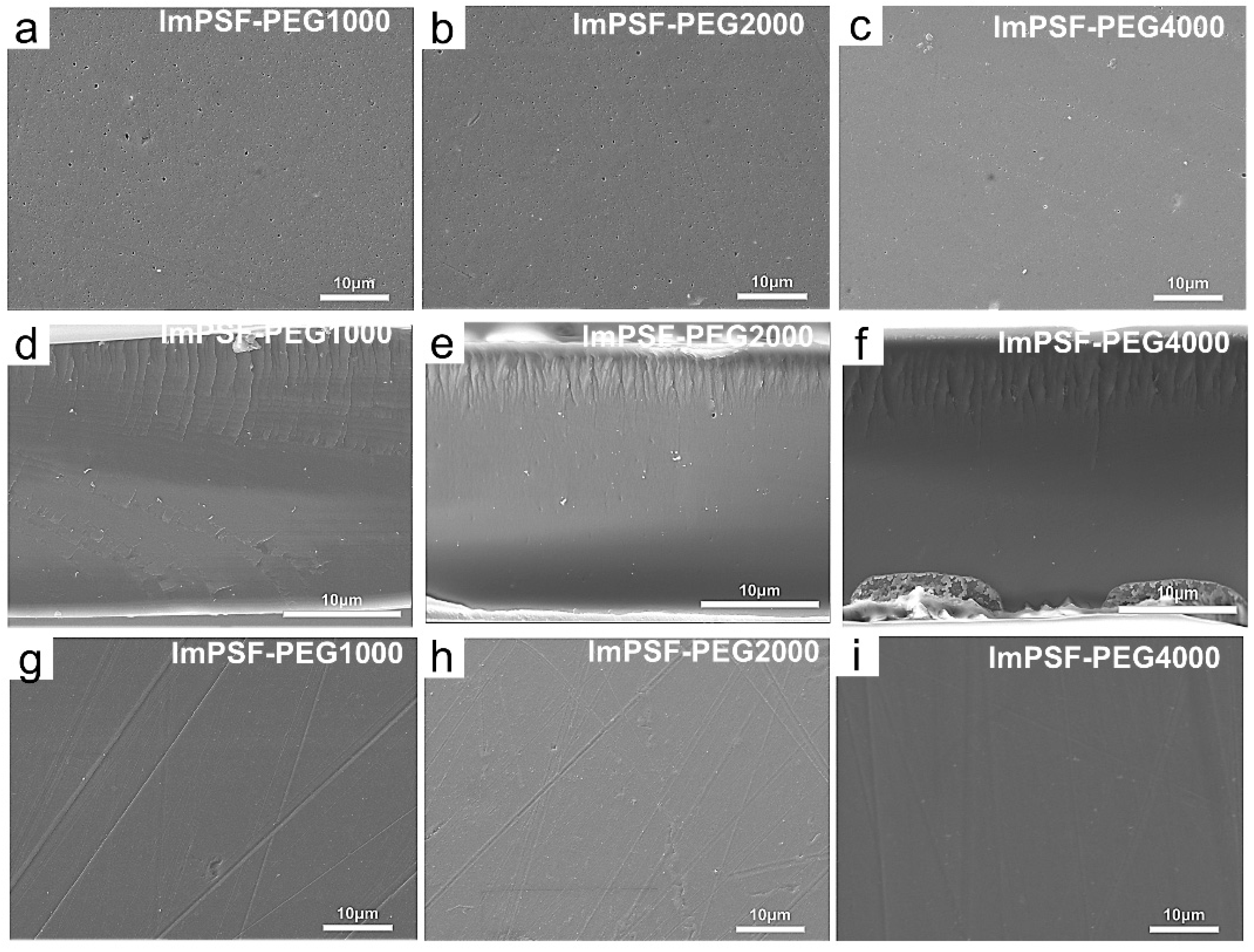
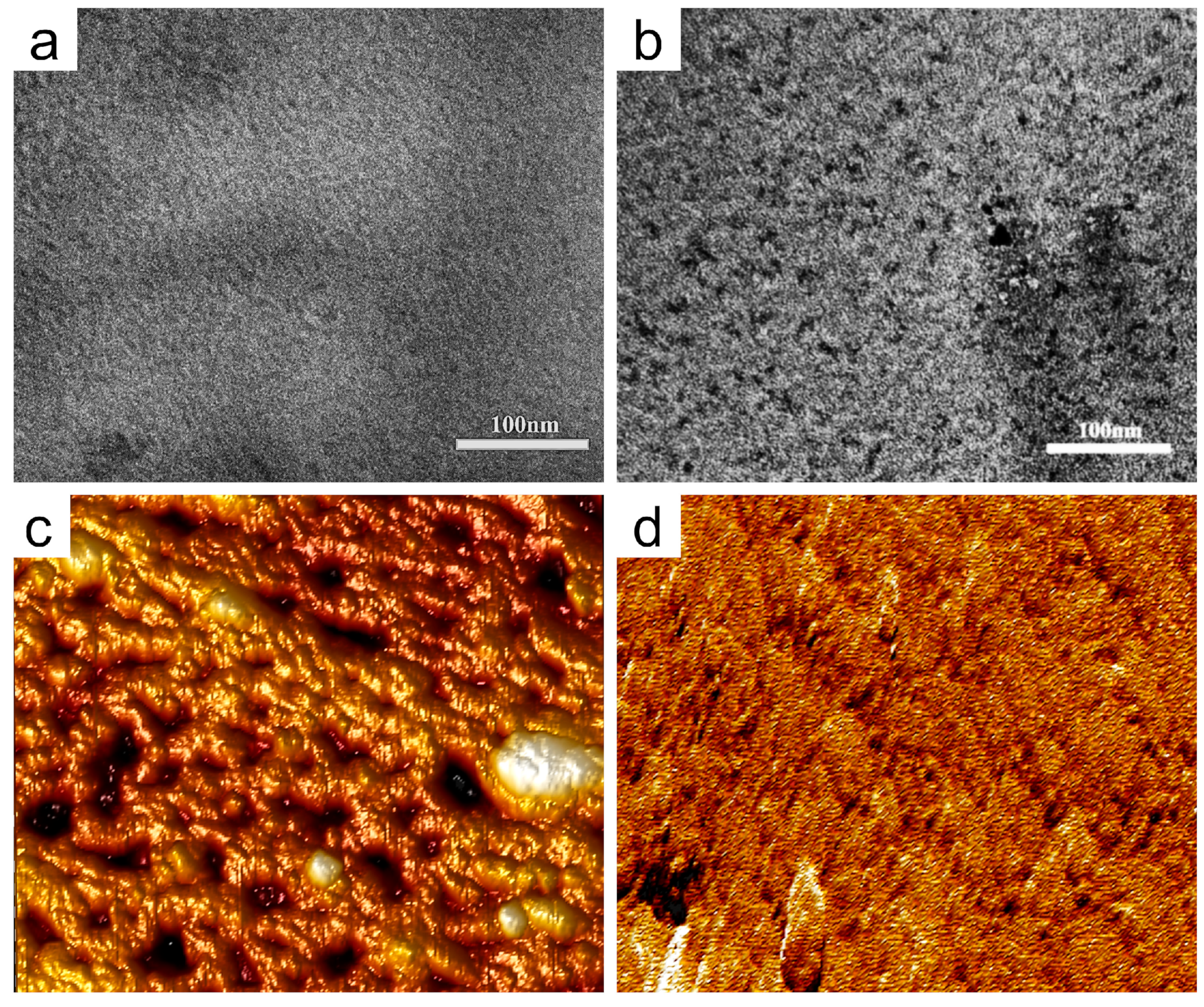
3.1. Synthesis and Characterization of ImPSF-PEGx Membrane
3.2. Ion Exchange Capacity, Water Uptake, and Swelling Ratio
3.3. Hydroxide Conductivity
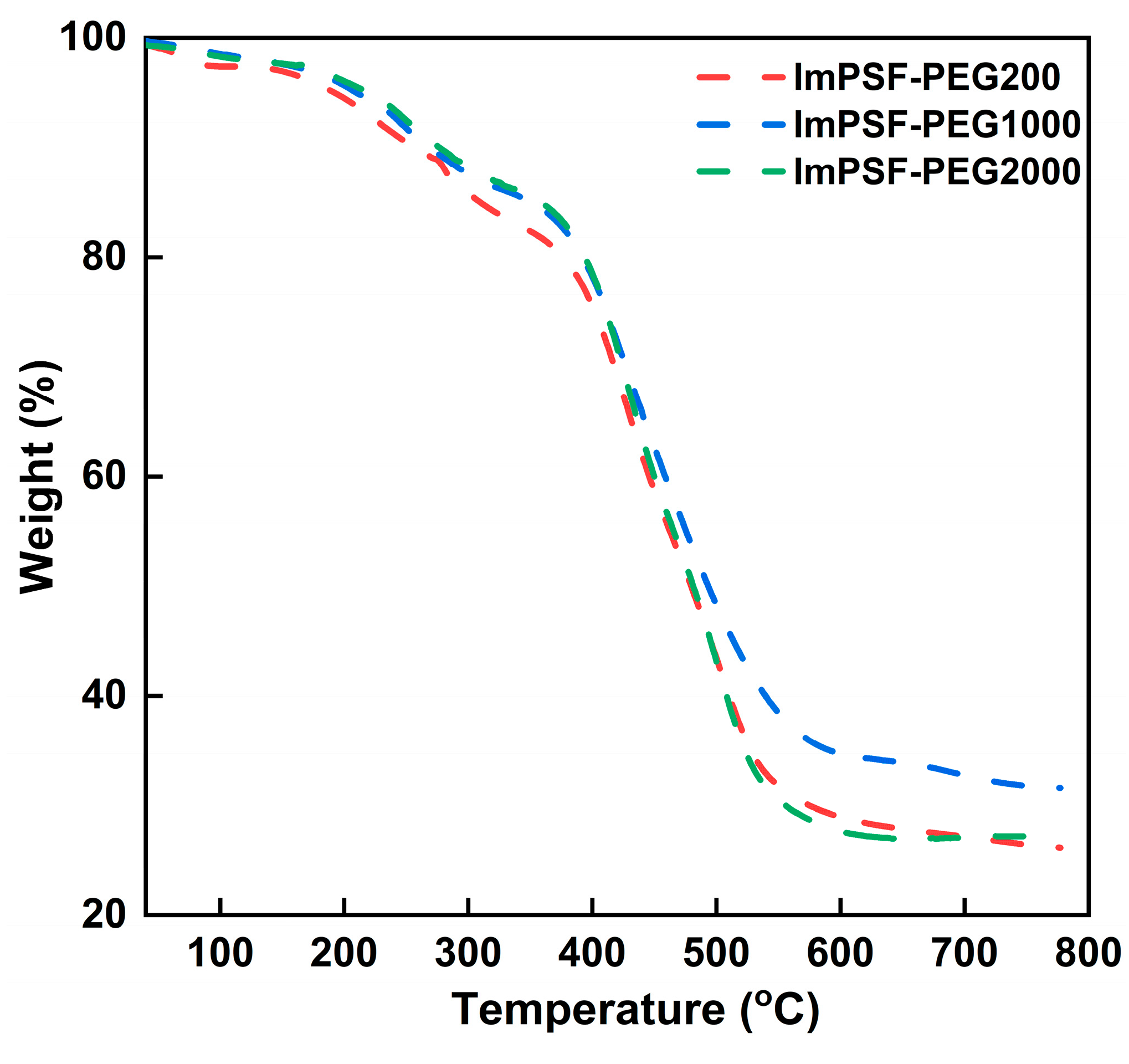
3.4. Mechanical and Thermal Stability
3.5. Alkaline Stability
3.6. The Performance of the Alkaline Water Electrolyzer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEMEWs | exchange membrane water electrolyzers |
| AEMWEs | anion exchange membrane water electrolyzers |
| AEMs | anion exchange membranes |
| PSF | polysulfone |
| CMPSF | chloromethylated polysulfone |
| ImPSF | imidazolium-functionalized polysulfone |
| OEG | oligo(ethylene glycol) |
| PEG | polyethylene glycol |
| CMOE | chloromethyl octyl ether |
| IEC | ionic exchange capacity |
| WU | water uptake |
| SR | swelling ratio |
| MEA | membrane electrode assembly |
| CCM | catalyst-coating membrane |
References
- Sriram, G.; Dhanabalan, K.; Ajeya, K.V.; Aruchamy, K.; Ching, Y.C.; Oh, T.H.; Jung, H.-Y.; Kurkuri, M. Recent progress in anion exchange membranes (AEMs) in water electrolysis: Synthesis, physio-chemical analysis, properties, and applications. J. Mater. Chem. A 2023, 11, 20886–21008. [Google Scholar] [CrossRef]
- Liu, C.; Geng, Z.; Wang, X.; Liu, W.; Wang, Y.; Xia, Q.; Li, W.; Jin, L.; Zhang, C. Development of advanced anion exchange membrane from the view of the performance of water electrolysis cell. J. Energy Chem. 2024, 90, 348–369. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef] [PubMed]
- Vinodh, R.; Kalanur, S.S.; Natarajan, S.K.; Pollet, B.G. Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review. Polymers 2023, 15, 2144. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, G.A.; Xu, Q.; Oener, S.Z.; Boettcher, S.W. Membrane Electrolyzers for Impure-Water Splitting. Joule 2020, 4, 2549–2561. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Abbasi, R.; Setzler, B.P.; Lin, S.; Wang, J.; Zhao, Y.; Xu, H.; Pivovar, B.; Tian, B.; Chen, X.; Wu, G.; et al. A Roadmap to Low-Cost Hydrogen with Hydroxide Exchange Membrane Electrolyzers. Adv. Mater. 2019, 31, 1805876. [Google Scholar] [CrossRef] [PubMed]
- Racchi, O.; Baldassari, R.; Araya-Hermosilla, E.; Mattoli, V.; Minei, P.; Pozio, A.; Pucci, A. Polyketone-Based Anion-Exchange Membranes for Alkaline Water Electrolysis. Polymers 2023, 15, 2027. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Jeong, J.; Noh, Y.; Jang, M.J.; Lee, J.; Lee, K.H.; Lim, D.C.; Seo, M.H.; Kim, W.B.; Yang, J.; et al. Commercial anion exchange membrane water electrolyzer stack through non-precious metal electrocatalysts. Appl. Catal. B-Environ. 2021, 292, 120170. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Zheng, X.; Sun, W.; Dou, S.X.; Ma, T.; Pan, H. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 2022, 51, 9620–9693. [Google Scholar] [CrossRef]
- Yin, L.; Ren, R.; He, L.; Zheng, W.; Guo, Y.; Wang, L.; Lee, H.; Du, J.; Li, Z.; Tang, T.; et al. Stable Anion Exchange Membrane Bearing Quinuclidinium for High-performance Water Electrolysis. Angew. Chem. Int. Ed. 2024, 63, e202400764. [Google Scholar] [CrossRef]
- Gjoshi, S.; Loukopoulou, P.; Plevova, M.; Hnat, J.; Bouzek, K.; Deimede, V. Cycloaliphatic Quaternary Ammonium Functionalized Poly(oxindole biphenyl) Based Anion-Exchange Membranes for Water Electrolysis: Stability and Performance. Polymers 2024, 16, 99. [Google Scholar] [CrossRef]
- Mardle, P.; Chen, B.; Holdcroft, S. Opportunities of Ionomer Development for Anion-Exchange Membrane Water Electrolysis. ACS Energy Lett. 2023, 8, 3330–3342. [Google Scholar] [CrossRef]
- Ren, G.; Wan, K.; Kong, H.; Guo, L.; Wang, Y.; Liu, X.; Wei, G. Recent advance in biomass membranes: Fabrication, functional regulation, and antimicrobial applications. Carbohydr. Polym. 2023, 305, 120537. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Kang, M.; Yun, H.; Park, H.; Hong, C.S. Emerging Porous Solid Electrolytes for Hydroxide Ion Transport. Adv. Funct. Mater. 2021, 31, 2100083. [Google Scholar] [CrossRef]
- Wan, K.; Fang, T.; Zhang, W.; Ren, G.; Tang, X.; Ding, Z.; Wang, Y.; Qi, P.; Liu, X. Enhanced antimony removal within lamellar nanoconfined interspaces through a self-cleaning MXene@CNF@FeOOH water purification membrane. Chem. Eng. J. 2023, 465, 143018. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, M.E.; Thomas, S.; Schechter, A.; Grynszpan, F. A perspective into recent progress on the tailored cationic group-based polymeric anion exchange membranes intended for electrochemical energy applications. Mater. Today Chem. 2024, 35, 101866. [Google Scholar] [CrossRef]
- Wang, W.; Cao, D.-F.; Sun, X.-W.; Pan, L.; Ma, Z.; Li, Y.-S. The Influence of Various Cationic Group on Polynorbornene Based Anion Exchange Membranes with Hydrophobic Large Steric Hindrance Arylene Substituent. Chin. J. Polym. Sci. 2023, 41, 278–287. [Google Scholar] [CrossRef]
- Niu, Y.; Li, J.; Yu, W.; Chen, W.; Wu, X.; He, G.; Yu, M.; Li, T. Comb-shaped anion exchange membrane with segmented hydrophilic/hydrophobic side chain. Mater. Today Commun. 2024, 38, 108241. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.; Yin, T.; Chen, S.; Zhang, X.; Li, N.; Liu, L. Oligo (ethylene glycol)-grafted poly(terphenyl indole piperidinium) with high water diffusivity for anion exchange membrane fuel cells. J. Membr. Sci. 2024, 694, 122424. [Google Scholar] [CrossRef]
- Cao, D.; Sun, X.; Gao, H.; Pan, L.; Li, N.; Li, Y. Crosslinked Polynorbornene-Based Anion Exchange Membranes with Perfluorinated Branch Chains. Polymers 2023, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ge, X.; Liang, X.; Zhang, J.; Shehzad, M.A.; Zhu, Y.; Yang, Z.; Wu, L.; Xu, T. Anion exchange membranes with branched ionic clusters for fuel cells. J. Mater. Chem. A 2018, 6, 5993–5998. [Google Scholar] [CrossRef]
- Hu, J.; Chen, K.; Zhang, X.; Qian, J.; Li, J.; Ren, Q.; Wang, C. Synthesis and performance of poly(aryl ether nitrile) anion exchange membranes containing highly dense quaternary ammonium cationic side chains for water electrolysis. Int. J. Hydrogen Energy 2024, 62, 498–509. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Chen, W.; Pang, B.; Liu, Y.; Guo, Y.; Wu, X.; Cui, F.; He, G. Polybenzimidazole Ultrathin Anion Exchange Membrane with Comb-Shape Amphiphilic Microphase Networks for a High-Performance Fuel Cell. ACS Appl. Mater. Interfaces 2021, 13, 49840–49849. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, C.; Yi, G.; Jiang, Z.; Su, X.; Liu, Q.; Lee, J.Y.; Lee, S.Y.; Lee, Y.M.; Zhang, Q. Durable Multiblock Poly(biphenyl alkylene) Anion Exchange Membranes with Microphase Separation for Hydrogen Energy Conversion. Angew. Chem. Int. Ed. 2023, 62, e202311509. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, X.; Liu, S.; Liu, L.; Li, N. Poly(terphenyl piperidinium) containing hydrophilic crown ether units in main chains as anion exchange membranes for alkaline fuel cells and water electrolysers. J. Membr. Sci. 2022, 653, 120558. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, C. Facilitated belt for ion transport by comb-shape Poly(terphenyl piperidone) carried with macrocyclic crown ether as anchor in anion exchange membrane. J. Power Sources 2024, 602, 234324. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Wang, S.; Wang, Y.; Lei, Y.; Wu, J.; Zhang, Y.; Yu, J.; Wang, F.; Sui, Z.; et al. Highly conductive anion-exchange membrane based on poly(acenaphthylenyl aryl piperidinium) π-π self-assembly. Next Energy 2023, 1, 100075. [Google Scholar] [CrossRef]
- Yuan, Y.; Du, X.; Sui, Z.; Wang, S.; Dong, T.; Chi, X.; Wang, Z. Host-guest coordination self-assembly gives anion exchange membranes better stability. Chem. Eng. J. 2023, 464, 142563. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Q.; Yan, X.; Liu, J.; Xu, X.; Wang, T.; El Hamouti, I.; Ruan, X.; Hao, C.; He, G. Hydrophilic side chain assisting continuous ion-conducting channels for anion exchange membranes. J. Membr. Sci. 2018, 552, 286–294. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, K.; Sun, X.; Ma, S.; So, Y.-M.; Ma, H.; Yan, X.; Zhang, N.; He, G. Facilitating ionic conduction for anion exchange membrane via employing star-shaped block copolymer. J. Membr. Sci. 2021, 630, 119290. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, T.-Y.; Yan, X.-M.; Xu, X.-W.; Zhang, Q.-D.; Zhao, B.-L.; El Hamouti, I.; Hao, C.; He, G.-H. Benzimidazolium functionalized polysulfone-based anion exchange membranes with improved alkaline stability. Chin. J. Polym. Sci. 2018, 36, 129–138. [Google Scholar] [CrossRef]
- Arbring Sjöström, T.; Jonsson, A.; Gabrielsson, E.; Kergoat, L.; Tybrandt, K.; Berggren, M.; Simon, D.T. Cross-Linked Polyelectrolyte for Improved Selectivity and Processability of Iontronic Systems. ACS Appl. Mater. Interfaces 2017, 9, 30247–30252. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, J.; Wang, L.; Wang, J. Preparation and Characterization of A Functional Polysulfone Anion Exchange Membrane of Polyethylene Glycol Modified Crown Ether. J. Petrochem. Univ. 2024, 37, 59–65. [Google Scholar]
- Warshawsky, A.; Deshe, A. Halomethyl octyl ethers: Convenient halomethylation reagents. J. Polym. Sci. Polym. Chem. 1985, 23, 1839–1841. [Google Scholar] [CrossRef]
- Yang, R.; Wu, X.; Chen, W.; Wang, X.; Li, T.; Yan, X.; Wang, L.; Cui, F.; He, G. Engineering of Interconnected Ionic Channels in Polybenzimidazole Anion-Exchange Membranes. Ind. Eng. Chem. Res. 2023, 62, 16746–16756. [Google Scholar] [CrossRef]
- Xu, Z.; Li, J.; Zhou, H.; Jiang, X.; Yang, C.; Wang, F.; Pan, Y.; Li, N.; Li, X.; Shi, L.; et al. Morphological and swelling behavior of cellulose nanofiber (CNF)/poly(vinyl alcohol) (PVA) hydrogels: Poly(ethylene glycol) (PEG) as porogen. RSC Adv. 2016, 6, 43626–43633. [Google Scholar] [CrossRef]
- Singh, T.R.R.; Garland, M.J.; Migalska, K.; Salvador, E.C.; Shaikh, R.; Mccarthy, H.O.; David Woolfson, A.; Donnelly, R.F. Influence of a pore-forming agent on swelling, network parameters, and permeability of poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels: Application in transdermal delivery systems. J. Appl. Polym. Sci. 2012, 125, 2680–2694. [Google Scholar] [CrossRef]
- Gao, X.; Du, S.; Si, J.; Liu, X.; Cui, Z.; Wang, Q. Fabrication and characterization of cellulose acetate ultrafiltration membrane and its application for efficient bovine serum albumin separation. Polym. Eng. Sci. 2023, 63, 1423–1438. [Google Scholar] [CrossRef]
- Li, T.; Yan, X.; Liu, J.; Wu, X.; Gong, X.; Zhen, D.; Sun, S.; Chen, W.; He, G. Friedel-Crafts alkylation route for preparation of pendent side chain imidazolium-functionalized polysulfone anion exchange membranes for fuel cells. J. Membr. Sci. 2019, 573, 157–166. [Google Scholar] [CrossRef]
- Guo, M.; Ban, T.; Wang, Y.; Wang, Y.; Zhang, Y.; Zhang, J.; Zhu, X. Exploring highly soluble ether-free polybenzimidazole as anion exchange membranes with long term durability. J. Membr. Sci. 2022, 647, 120299. [Google Scholar] [CrossRef]
- Su, X.; Nan, S.; Wei, W.; Xu, S.; He, R. Highly conductive and robustly stable anion exchange membranes with a star-branched crosslinking structure. J. Membr. Sci. 2023, 683, 121843. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Y.; Guo, W. Preparation and drug release performance of amphiphilic medical hot-melt pressure sensitive adhesives based on polystyrene-isoprene-styrene. J. Appl. Polym. Sci. 2023, 140, e53600. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Chen, Y.; Sun, L.; Wei, C.; Zhang, H.; Zhang, H.; Ye, B.; Wu, L.; Ge, X.; et al. Helical configuration channels boost performance in anion exchange membranes. Chem. Eng. J. 2023, 455, 140938. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Liu, C.; Li, Y.; Xing, W.; Sun, J. Self-Healing Proton-Exchange Membranes Composed of Nafion–Poly(vinyl alcohol) Complexes for Durable Direct Methanol Fuel Cells. Adv. Mater. 2018, 30, 1707146. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.N.; Lin, C.X.; Liu, F.H.; Wang, X.Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Poly(arylene ether nitrile) anion exchange membranes with dense flexible ionic side chain for fuel cells. J. Membr. Sci. 2018, 550, 254–265. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Z.; Zhang, M.; Xie, S.; Xie, D.; Liu, P.; Wang, S.; Cheng, F.; Xu, T. Macromolecular crosslinked poly(aryl piperidinium)-based anion exchange membranes with enhanced ion conduction for water electrolysis. J. Membr. Sci. 2024, 700, 122717. [Google Scholar] [CrossRef]
- Lin, B.; Xu, F.; Su, Y.; Han, J.; Zhu, Z.; Chu, F.; Ren, Y.; Zhu, L.; Ding, J. Ether-Free Polybenzimidazole Bearing Pendant Imidazolium Groups for Alkaline Anion Exchange Membrane Fuel Cells Application. ACS Appl. Energy Mater. 2020, 3, 1089–1098. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; He, M.; Shen, Y.; Liu, S.; Liu, L.; Li, N. Poly(biphenyl piperidinium) Anion Exchange Membranes for Alkaline Water Electrolyzers: The Effect of Nonionic Pendant Groups. ACS Appl. Energy Mater. 2023, 6, 11396–11407. [Google Scholar] [CrossRef]
- Lin, B.; Dong, H.; Li, Y.; Si, Z.; Gu, F.; Yan, F. Alkaline Stable C2-Substituted Imidazolium-Based Anion-Exchange Membranes. Chem. Mater. 2013, 25, 1858–1867. [Google Scholar] [CrossRef]
- Xue, B.; Wang, Q.; Zheng, J.; Li, S.; Zhang, S. Bi-guanidinium-based crosslinked anion exchange membranes: Synthesis, characterization, and properties. J. Membr. Sci. 2020, 601, 117923. [Google Scholar] [CrossRef]
- Yuan, C.; Li, P.; Zeng, L.; Duan, H.; Wang, J.; Wei, Z. Poly(vinyl alcohol)-Based Hydrogel Anion Exchange Membranes for Alkaline Fuel Cell. Macromolecules 2021, 54, 7900–7909. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, W.; Ge, X.; Wu, L.; Xu, T. Molecular dynamics insight into phase separation and transport in anion-exchange membranes: Effect of hydrophobicity of backbones. J. Membr. Sci. 2022, 661, 120922. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Li, N.; Hu, Z.; Chen, S. Homologous flexible multi-cationic cross-linkers modified poly(aryl ether sulfone) anion exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2022, 47, 17329–17340. [Google Scholar] [CrossRef]
- Lai, A.N.; Li, S.C.; Zheng, P.Z.; Zhou, S.F.; Wu, S.J. Comb-shaped block poly(arylene ether sulfone ketone)s for anion exchange membranes. J. Appl. Polym. Sci. 2024, 141, e55047. [Google Scholar] [CrossRef]
- Li, S.; Du, S.; Xie, N.; Zhang, T.; Xu, Y.; Ning, X.; Chen, P.; Chen, X.; An, Z. Imidazole-Functionalized Multiquaternary Side-Chain Polyethersulfone Anion-Exchange Membrane for Fuel Cell Applications. ACS Appl. Energy Mater. 2022, 5, 10023–10033. [Google Scholar] [CrossRef]
- Caielli, T.; Ferrari, A.R.; Bonizzoni, S.; Sediva, E.; Caprì, A.; Santoro, M.; Gatto, I.; Baglio, V.; Mustarelli, P. Synthesis, characterization and water electrolyzer cell tests of poly(biphenyl piperidinium) Anion exchange membranes. J. Power Sources 2023, 557, 232532. [Google Scholar] [CrossRef]
- Liu, L.; Bai, L.; Liu, Z.; Miao, S.; Pan, J.; Shen, L.; Shi, Y.; Li, N. Side-chain structural engineering on poly(terphenyl piperidinium) anion exchange membrane for water electrolysers. J. Membr. Sci. 2023, 665, 121135. [Google Scholar] [CrossRef]
- Lin, X.; Varcoe, J.R.; Poynton, S.D.; Liang, X.; Ong, A.L.; Ran, J.; Li, Y.; Xu, T. Alkaline polymer electrolytes containing pendant dimethylimidazolium groups for alkaline membrane fuel cells. J. Mater. Chem. A 2013, 1, 7262–7269. [Google Scholar] [CrossRef]
- Zeng, M.; He, X.; Wen, J.; Zhang, G.; Zhang, H.; Feng, H.; Qian, Y.; Li, M. N-Methylquinuclidinium-Based Anion Exchange Membrane with Ultrahigh Alkaline Stability. Adv. Mater. 2023, 35, 2306675. [Google Scholar] [CrossRef]
- Lee, J.; Min, K.; Jeon, S.; Park, S.; Kim, H.; Kim, T.-H. Development of crosslinked SEBS-based anion exchange membranes for water electrolysis: Investigation of the crosslinker effect. Int. J. Hydrogen Energy 2023, 48, 24180–24195. [Google Scholar] [CrossRef]
- López-Fernández, E.; Gómez-Sacedón, C.; Gil-Rostra, J.; Espinós, J.P.; Brey, J.J.; González-Elipe, A.R.; De Lucas-Consuegra, A.; Yubero, F. Optimization of anion exchange membrane water electrolyzers using ionomer-free electrodes. Renew. Energy 2022, 197, 1183–1191. [Google Scholar] [CrossRef]
- Carbone, A.; Zignani, S.C.; Gatto, I.; Trocino, S.; Aricò, A.S. Assessment of the FAA3-50 polymer electrolyte in combination with a NiMn2O4 anode catalyst for anion exchange membrane water electrolysis. Int. J. Hydrogen Energy 2020, 45, 9285–9292. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.Y.; Lee, T.K.; Kim, H.-J.; Lee, Y.M. N3-butyl imidazolium-based anion exchange membranes blended with Poly(vinyl alcohol) for alkaline water electrolysis. J. Membr. Sci. 2020, 611, 118355. [Google Scholar] [CrossRef]
- Wang, X.; Lammertink, R.G.H. Dimensionally stable multication-crosslinked poly(arylene piperidinium) membranes for water electrolysis. J. Mater. Chem. A 2022, 10, 8401–8412. [Google Scholar] [CrossRef]
- Qian, J.; Wang, C.; Zhang, X.; Hu, J.; Zhao, X.; Li, J.; Ren, Q. Quaternary ammonium-functionalized crosslinked poly(aryl ether sulfone)s anion exchange membranes with enhanced alkaline stability for water electrolysis. J. Membr. Sci. 2023, 685, 121946. [Google Scholar] [CrossRef]
- Khataee, A.; Shirole, A.; Jannasch, P.; Krüger, A.; Cornell, A. Anion exchange membrane water electrolysis using Aemion™ membranes and nickel electrodes. J. Mater. Chem. A 2022, 10, 16061–16070. [Google Scholar] [CrossRef]
- Motealleh, B.; Liu, Z.; Masel, R.I.; Sculley, J.P.; Richard Ni, Z.; Meroueh, L. Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes. Int. J. Hydrogen Energy 2021, 46, 3379–3386. [Google Scholar] [CrossRef]
- Gao, W.; Gao, X.; Choo, Y.; Jun, W.J.; Cai, Z.H.; Gen, Q.; Mei, Z.A.; Liu, Q. Durable dual-methylpiperidinium crosslinked poly(binaphthyl-co-terphenyl piperidinium) anion exchange membranes with high ion transport and electrochemical performance. Chem. Eng. J. 2023, 466, 143107. [Google Scholar] [CrossRef]






| Run | Membrane | IEC a (mmol g−1) | WU b (%) | SR b (%) | λc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Theo. | Expt. | 40 °C | 80 °C | 40 °C | 80 °C | 40 °C | 80 °C | ||
| 1 | ImPSF | 1.56 | 1.55 | 26 | 56 | 21 | 31 | 9.3 | 19.9 |
| 2 | ImPSF-PEG200 | 1.48 | 1.48 | 45 | 93 | 16 | 32 | 16.9 | 34.9 |
| 3 | ImPSF-PEG600 | 1.48 | 1.46 | 43 | 86 | 15 | 31 | 16.4 | 32.7 |
| 4 | ImPSF-PEG1000 | 1.48 | 1.45 | 42 | 82 | 17 | 30 | 16.1 | 31.4 |
| 5 | ImPSF-PEG2000 | 1.48 | 1.43 | 40 | 71 | 20 | 35 | 15.6 | 27.6 |
| 6 | ImPSF-PEG4000 | 1.48 | 1.44 | 36 | 68 | 23 | 36 | 13.9 | 26.2 |
| Run | Membrane | IEC (mmol g−1) | σ (mS cm−1) | |
|---|---|---|---|---|
| 40 °C | 80 °C | |||
| 1 | ImPSF | 1.56 | 19.0 | 43.3 |
| 2 | ImPSF-PEG200 | 1.48 | 25.1 | 87.0 |
| 3 | ImPSF-PEG600 | 1.46 | 24.6 | 85.8 |
| 4 | ImPSF-PEG1000 | 1.45 | 25.3 | 82.6 |
| 5 | ImPSF-PEG2000 | 1.43 | 22.6 | 61.3 |
| 6 | ImPSF-PEG4000 | 1.44 | 22.7 | 60.8 |
| Membrane | Tensile Strength at Break (Mpa) | Elongation at Break (%) | ||
|---|---|---|---|---|
| Dry | Wet | Dry | Wet | |
| ImPSF | 20.7 | 15.1 | 5.0 | 14.5 |
| ImPSF-PEG200 | 23.7 | 17.3 | 9.2 | 16.5 |
| ImPSF-PEG600 | 24.8 | 18.5 | 10.5 | 19.7 |
| ImPSF-PEG1000 | 26.1 | 20.3 | 13.6 | 23.4 |
| ImPSF-PEG2000 | 27.0 | 21.8 | 14.5 | 28.3 |
| ImPSF-PEG4000 | 28.0 | 23.3 | 19.1 | 29.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Jin, C.; Li, X.; So, Y.-M.; Pan, Y. A Hydrophilic Polyethylene Glycol-Blended Anion Exchange Membrane to Facilitate the Migration of Hydroxide Ions. Polymers 2024, 16, 1464. https://doi.org/10.3390/polym16111464
Gao H, Jin C, Li X, So Y-M, Pan Y. A Hydrophilic Polyethylene Glycol-Blended Anion Exchange Membrane to Facilitate the Migration of Hydroxide Ions. Polymers. 2024; 16(11):1464. https://doi.org/10.3390/polym16111464
Chicago/Turabian StyleGao, Huaiming, Chenglou Jin, Xia Li, Yat-Ming So, and Yu Pan. 2024. "A Hydrophilic Polyethylene Glycol-Blended Anion Exchange Membrane to Facilitate the Migration of Hydroxide Ions" Polymers 16, no. 11: 1464. https://doi.org/10.3390/polym16111464
APA StyleGao, H., Jin, C., Li, X., So, Y.-M., & Pan, Y. (2024). A Hydrophilic Polyethylene Glycol-Blended Anion Exchange Membrane to Facilitate the Migration of Hydroxide Ions. Polymers, 16(11), 1464. https://doi.org/10.3390/polym16111464






