Effect of Rapid High-Intensity Light-Curing on Increasing Transdentinal Temperature and Cell Viability: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
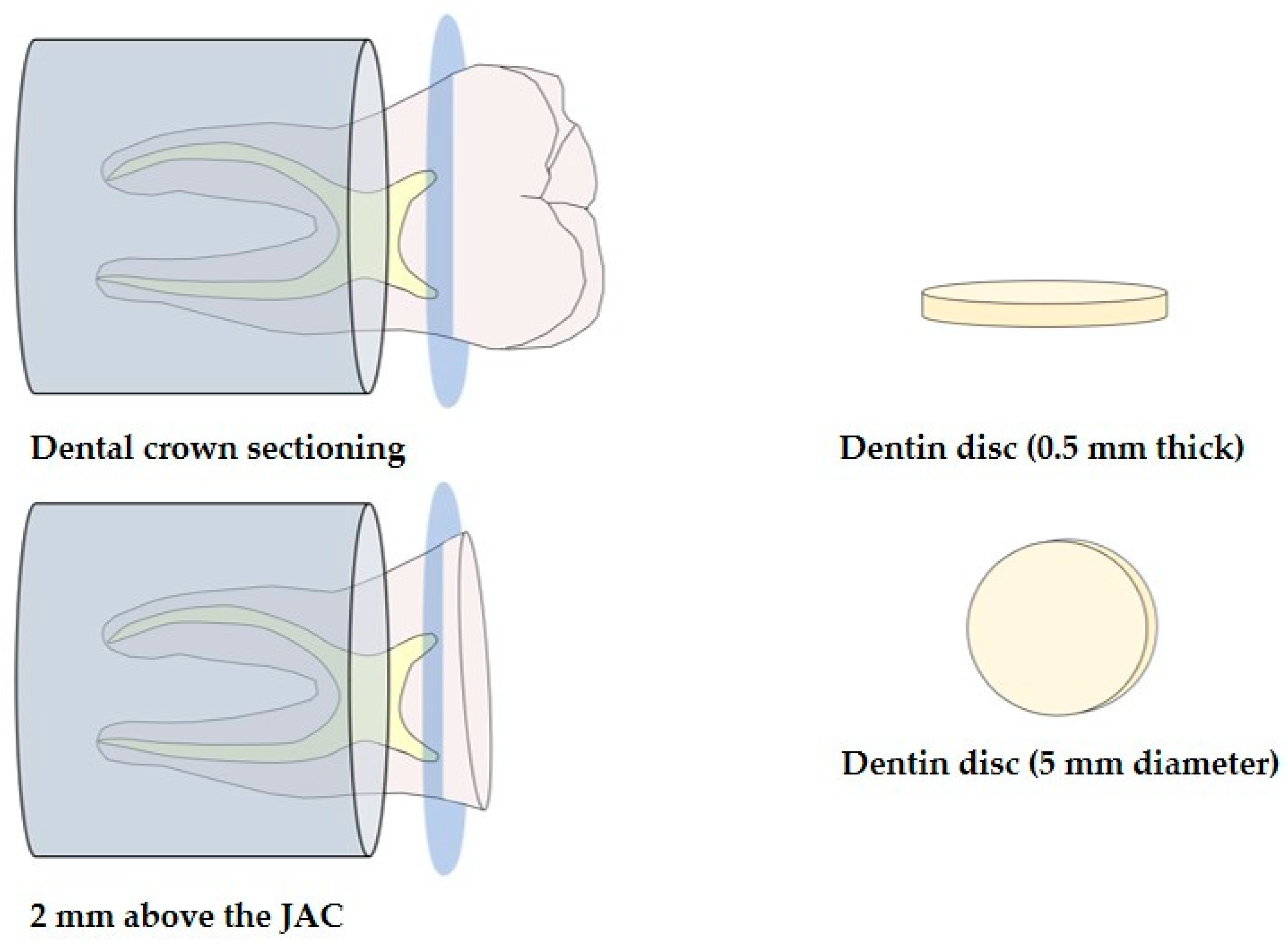
2.1. Obtaining Dentin Discs
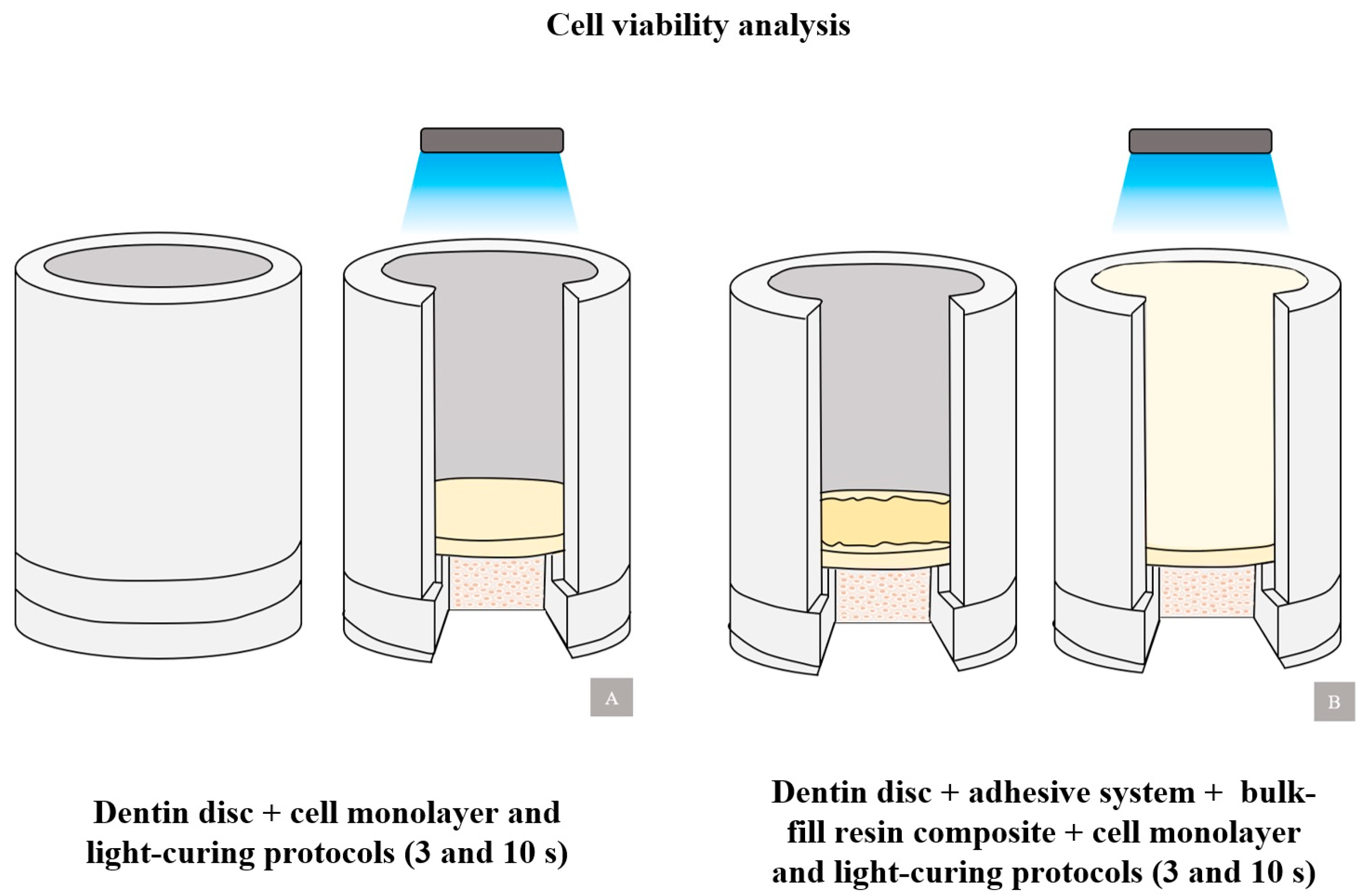
2.2. Artificial Pulp Chamber
2.3. Assessment of Transdentinal Temperature Changes
2.4. Cell Viability Analysis
2.4.1. Cell Cultivation
2.4.2. MTT Analysis
2.5. Statistical Analysis
3. Results
3.1. Increased Transdentinal Temperature
3.2. Cell Viability Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousavinasab, S.M.; Taromi, Z.; Zajkani, E. Thermal rise during photopolymerization and degree of conversion of bulk fill and conventional resin composites. Dent. Res. J. 2020, 17, 293–299. [Google Scholar]
- Chesterman, J.; Jowett, A.; Gallacher, A.; Nixon, P. Bulk-fill resin-based composite restorative materials: A review. Br. Dent. J. 2017, 222, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Tauböck, T.T.; Jäger, F.; Attin, T. Polymerization shrinkage and shrinkage force kinetics of high- and low-viscosity dimethacrylate- and ormocer-based bulk-fill resin composites. Odontology 2019, 107, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Paganini, A.; Attin, T.; Tauböck, T.T. Margin Integrity of Bulk-Fill Composite Restorations in Primary Teeth. Materials 2020, 13, 3802. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.F.; Vestphal, M.; Amaral, R.C.D.; Rodrigues, J.A.; Roulet, J.F.; Roscoe, M.G. Efficiency of polymerization of bulk-fill composite resins: A systematic review. Braz. Oral Res. 2017, 31 (Suppl. S1), e59. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Spanovic, N.; Marovic, D.; Attin, T.; Tarle, Z.; Tauböck, T.T. Rapid high-intensity light-curing of bulk-fill composites: A quantitative analysis of marginal integrity. J. Dent. 2021, 111, 103708. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.M.; Lien, W.; Mansell, M.R.; Risk, D.L.; Savett, D.A.; Vandewalle, K.S. Effect of high-intensity curing lights on the polymerization of bulk-fill composites. Dent. Mater. 2018, 34, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Watts, D.C. Outcomes of ultra-fast (3 s) photo-cure in a RAFT-modified resin-composite. Dent. Mater. 2020, 36, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Diegelmann, J. Impact of ultra-fast (3 s) light-cure on cell toxicity and viscoelastic behavior in a dental resin-based composite with RAFT-mediated polymerization. J. Mech. Behav. Biomed. Mater. 2021, 124, 104810. [Google Scholar] [CrossRef]
- Marovic, D.; Par, M.; Macan, M.; Klarić, N.; Plazonić, I.; Tarle, Z. Aging-Dependent Changes in Mechanical Properties of the New Generation of Bulk-Fill Composites. Materials 2022, 15, 902. [Google Scholar] [CrossRef]
- Marovic, D.; Par, M.; Crnadak, A.; Sekelja, A.; Negovetic Mandic, V.; Gamulin, O.; Rakic, M.; Tarle, Z. Rapid 3 s Curing: What Happens in Deep Layers of New Bulk-Fill Composites? Materials 2021, 14, 515. [Google Scholar] [CrossRef] [PubMed]
- Algamaiah, H.; Silikas, N.; Watts, D.C. Polymerization shrinkage and shrinkage stress development in ultra-rapid photo-polymerized bulk fill resin composites. Dent. Mater. 2021, 37, 559–567. [Google Scholar] [CrossRef]
- Maucoski, C.; Zarpellon, D.C.; Dos Santos, F.A.; Lipinski, L.C.; Campagnoli, E.B.; Rueggeberg, F.A.; Arrais, C.A.G. Analysis of temperature increase in swine gingiva after exposure to a Polywave® LED light curing unit. Dent. Mater. 2017, 33, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, S.; Aktuğ Karademir, S. Influence of Bulk-Fill Composites, Polimerization Modes, and Remaining Dentin Thickness on Intrapulpal Temperature Rise. BioMed Res. Int. 2019, 2019, 4250284. [Google Scholar] [CrossRef]
- Zach, L.; Cohen, G. Pulp response to externally applied heat. Oral Surg. Oral Med. Oral Pathol. 1965, 19, 515–530. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, R.J.; Ferracane, J.; Lee, I.B. Thermographic analysis of the effect of composite type, layering method, and curing light on the temperature rise of photo-cured composites in tooth cavities. Dent. Mater. 2017, 33, e373–e383. [Google Scholar] [CrossRef]
- Carrillo-Cotto, R.; Etges, A.; Jardim, P.S.; Torre, E.; Kaizer, M.R.; Ferrúa, C.P.; Nedel, F.; Cuevas-Suárez, C.E.; Moraes, R.R. Cytotoxicity of contemporary resin-based dental materials in contact with dentin. Eur. J. Oral Sci. 2020, 128, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Lempel, E.; Ori, Z.; Kincses, D.; Lovasz, B.V.; Kunsagi-Mate, S.; Szalma, J. Degree of conversion and in vitro temperature rise of pulp chamber during polymerization of flowable and sculptable conventional, bulk-fill and short-fibre reinforced resin composites. Dent. Mater. 2021, 37, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, V.B.; Moe, G.; Strom, M.B.; Jensen, E.; Lygre, H. Quantitative analysis of TEGDMA and HEMA eluted into saliva from two dental composites by use of GC/MS and tailor-made internal standards. Dent. Mater. 2008, 24, 724–731. [Google Scholar] [CrossRef]
- Şişman, R.; Aksoy, A.; Yalçın, M.; Karaöz, E. Cytotoxic effects of bulk fill composite resins on human dental pulp stem cells. J. Oral Sci. 2016, 58, 299–305. [Google Scholar] [CrossRef]
- Geurtsen, W. Biocompatibility of resin-modified filling materials. Crit. Rev. Oral Biol. Med. 2000, 11, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Algamaiah, H.; Watts, D.C. Spatio-temporal temperature fields generated coronally with bulk-fill resin composites: A thermography study. Dent. Mater. 2021, 37, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.A.; Lee, C.H.; Kim, M.J.; Ferracane, J.; Lee, I.B. Effect of pulse-width-modulated LED light on the temperature change of composite in tooth cavities. Dent. Mater. 2019, 35, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Gamulin, O.; Marovic, D.; Klaric, E.; Tarle, Z. Effect of temperature on post-cure polymerization of bulk-fill composites. J. Dent. 2014, 42, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Yap, A.; Loo, C.; Ng, J.; Goh, C.Y.; Hong, C.; Toh, W.S. Comparison of cytotoxicity test models for evaluating resin-based composites. Hum. Exp. Toxicol. 2017, 36, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, C.M.E.; Bahari, M.; Safyari, L.; Safarvand, H.; Shafaei, H.; Jafari Navimipour, E.; Alizadeh, O.P.; Ajami, A.A.; Abed, K.M. Effect of preheating on the cytotoxicity of bulk-fill composite resins. J. Dent. Res. Dent. Clin. Dent. Prospects 2020, 14, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Caldas, I.P.; da Silva, E.M.; Lourenço, E.S.; Martins do Nascimento, J.C.; Leite, P.E.C.; Leão, M.P.; Alves, G.; Scelza, M.Z. The influence of methodology on the comparison of cytotoxicity of total-etch and self-etch adhesive systems. J. Dent. 2022, 122, 104158. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.H.; Sauro, S.; Lima, A.F.; Loguercio, A.D.; Della Bona, A.; Mazzoni, A.; Collares, F.M.; Staxrud, F.; Ferracane, J.; Tsoi, J.; et al. RoBDEMAT: A risk of bias tool and guideline to support reporting of pre-clinical dental materials research and assessment of systematic reviews. J. Dent. 2022, 127, 104350. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.; Penelas, A.G.; Simão, M.S.; Filho, J.D.; Poskus, L.T.; Guimarães, J.G. Influence of the degree of dentine mineralization on pulp chamber temperature increase during resin-based composite (RBC) light-activation. J. Dent. 2010, 38, 336–342. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO—International Organization for Standardization: Geneva, Switzerland, 2009.
- Odum, N.C.; Ross, J.T.; Citrin, N.S.; Tantbirojn, D.; Versluis, A. Fast Curing with High-power Curing Lights Affects Depth of Cure and Post-gel Shrinkage and Increases Temperature in Bulk-fill Composites. Oper. Dent. 2023, 48, 98–107. [Google Scholar] [CrossRef]
- Ridha, A.M.; Aidinis, K.; Suliman, A.H. Temperature Rise at the Pulp-Dentin Junction for a Multi-Layered Composite Restoration using the Finite Element Method. Open Dent. J. 2021, 15, 487–494. [Google Scholar] [CrossRef]
- Millen, C.; Ormond, M.; Richardson, G.; Santini, A.; Miletic, V.; Kew, P. A study of temperature rise in the pulp chamber during composite polymerization with different light-curing units. J. Contemp. Dent. Pract. 2007, 8, 29–37. [Google Scholar] [CrossRef]
- Novta, E.; Lainovic, T.; Grujic, D.; Savic-Sevi, S.S.; Toth, E.; Cvejic, Z.; Blazic, L. Internal photo-activation of a dental composite using optical fibers: A holographic, thermographic and Raman study. Opt. Quantum Electron. 2022, 54, 1–18. [Google Scholar] [CrossRef]
- Al-Qudah, A.A.; Mitchell, C.A.; Biagioni, P.A.; Hussey, D.L. Effect of composite shade, increment thickness and curing light on temperature rise during photocuring. J. Dent. 2007, 35, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Lee, I.B.; Yoo, J.Y.; Park, S.J.; Kim, S.Y.; Yi, Y.A.; Hwang, J.Y.; Seo, D.G. Real-Time Analysis of Temperature Changes in Composite Increments and Pulp Chamber during Photopolymerization. BioMed Res. Int. 2015, 2015, 923808. [Google Scholar] [CrossRef] [PubMed]
- Rueggeberg, F.A.; Giannini, M.; Arrais, C.A.G.; Price, R.B.T. Light curing in dentistry and clinical implications: A literature review. Braz. Oral Res. 2017, 31, e61. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.; Choi, J.J.E.; Uy, C.E.; Ramani, R.S.; Ganjigatti, R.; Waddell, J.N. Real-time pulp temperature change at different tooth sites during fabrication of temporary resin crowns. Heliyon 2019, 5, e02971. [Google Scholar] [CrossRef]
- Baldissara, P.; Catapano, S.; Scotti, R. Clinical and histological evaluation of thermal injury thresholds in human teeth: A preliminary study. J. Oral Rehabil. 1997, 24, 791–801. [Google Scholar] [CrossRef]
- Wang, W.J.; Grymak, A.; Waddell, J.N.; Choi, J.J.E. The effect of light curing intensity on bulk-fill composite resins: Heat generation and chemomechanical properties. Biomater. Investig. Dent. 2021, 8, 137–151. [Google Scholar] [CrossRef]
- Runnacles, P.; Arrais, C.A.G.; Maucoski, C.; Coelho, U.; De Goes, M.S.; Rueggeberg, F.A. Comparison of in vivo and in vitro models to evaluate pulp temperature rise during exposure to a Polywave® LED light curing unit. J. Appl. Oral Sci. 2019, 27, e20180480. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, A.; Veríssimo, C.; Tantbirojn, D.; Garcia-Godoy, F.; Soares, C.J.; Versluis, A. Heat generated during light-curing of restorative composites: Effect of curing light, exotherm, and experiment substrate. Am. J. Dent. 2016, 29, 234–2240. [Google Scholar] [PubMed]
- Algamaiah, H.; Yang, J.; Alayed, A.; Alshabib, A.; Alshehri, A.; Watts, D.C. Temperature rise in photopolymerized adhesively-bonded resin composite: A thermography study. Dent. Mater. 2023, 40, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, S.; Demirci, M.; Schweikl, H.; Erguven, M.; Bilir, A.; Kara, T.A. Inhibition of cell survival, viability and proliferation by dentin adhesives after direct and indirect exposure in vitro. Clin. Oral Investig. 2012, 16, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Kierklo, A.; Pawińska, M.; Tokajuk, G.; Popławska, B.; Bielawska, A. Cytotoxicity evaluation of three light-cured dentin adhesive materials on human gingival fibroblasts, ex vivo. Adv. Med. Sci. 2012, 57, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, C.; Mascarenhas, P.; Avelar, M.; Ribeiro, A.C.; Barahona, I. Biocompatibility of bulk-fill resins in vitro. Clin. Oral Investig. 2023, 27, 7851–7858. [Google Scholar] [CrossRef] [PubMed]
- Kamalak, H.; Kamalak, A.; Taghizadehghalehjoughi, A.; Hacımüftüoğlu, A.; Nalcı, K.A. Cytotoxic and biological effects of bulk fill composites on rat cortical neuron cells. Odontology 2018, 106, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, R.; Eskandarizadeh, A.; Lashkarizadeh, L.; Derakhshani, A.; Arjmand, F. Cytotoxicity effects of nanohybrid, bulk-fill, and ormocer composites on dental pulp stem cells and human gingival fibroblast cells. Dent. Res. J. 2022, 19, 101. [Google Scholar]
- Lee, S.M.; Kim, S.Y.; Kim, J.H.; Jun, S.K.; Kim, H.W.; Lee, J.H.; Lee, H.H. Depth-Dependent Cellular Response from Dental Bulk-Fill Resins in Human Dental Pulp Stem Cells. Stem Cells Int. 2019, 2019, 1251536. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Rezende, M.; Gutierrez, M.F.; Costa, T.F.; Armas-Vega, A.; Reis, A. Randomized 36-month follow-up of posterior bulk-filled resin composite restorations. J. Dent. 2019, 85, 93–102. [Google Scholar] [CrossRef]
- Elias, S.T.; Santos, A.F.; Garcia, F.C.; Pereira, P.N.; Hilgert, L.A.; Fonseca-Bazzo, Y.M.; Guerra, E.N.; Ribeiro, A.P. Cytotoxicity of universal, self-etching and etch-and-rinse adhesive systems according to the polymerization time. Braz. Dent. J. 2015, 26, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Pupo, Y.M.; Bernardo, C.F.F.; de Souza, F.F.F.A.; Michél, M.D.; Ribeiro, C.N.M.; Germano, S.; Maluf, D.F. Cytotoxicity of Etch-and-Rinse, Self-Etch, and Universal Dental Adhesive Systems in Fibroblast Cell Line 3T3. Scanning 2017, 2017, 9650420. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Ribeiro, A.P.; Carrilho, M.R.; Pashley, D.H.; de Souza Costa, C.A.; Hebling, J. Cytotoxicity of adhesive systems of different hydrophilicities on cultured odontoblast-like cells. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.C.A.; Hebling, J.; Scheffel, D.L.; Soares, D.G.; Basso, F.G.; Ribeiro, A.P. Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent. Mater. 2014, 30, 769–784. [Google Scholar] [CrossRef]
- Volk, J.; Leyhausen, G.; Wessels, M.; Geurtsen, W. Reduced glutathione prevents camphorquinone-induced apoptosis in human oral keratinocytes. Dent. Mater. 2014, 30, 215–226. [Google Scholar] [CrossRef]
- Schmalz, G. Resin-based composites. In Biocompatibility of Dental Materials; Schmalz, G., Arenholt-Bindslev, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 99–137. [Google Scholar]


| Material | Type, Color | Manufacturer | Composition |
|---|---|---|---|
| Single Bond Universal | One-step universal adhesive system | 3M ESPE, St. Paul, EUA | MDP, phosphate monomer, dimethacrylate, HEMA, Bis-GMA, copolymerizer, dimethylaminobenzoate, vitrabond, ethanol, water, silane, and primer. |
| Filtek Bulk-Fill Flow | Bulk-Fill Flow Resin, A2 | 3M ESPE, St. Paul, EUA | Bis-EMA, Bis-GMA, UDMA, treated silanized ceramic, Benzotriazole, substituted dimethacrylate, TEGDMA, and ytterbium fluoride. |
| Tetric PowerFill | Bulk-Fill Flow Resin, IVA | Ivoclar Vivadent AG, Schaan, Liechtenstein | Dimethacrylates, barium glass, ytterbium trifluoride, and copolymers. |
| Light-Curing Unit | Emission Spectrum | Mode | Time | Irradiance (mW/cm2) | Manufacturer |
|---|---|---|---|---|---|
| Valo Grand | Polywave | Xtra | 10 s | 1000 | Ultradent, South Jordan, EUA |
| Standard | 3 s | 3200 |
| Group | n | Procedure | Light-Curing |
|---|---|---|---|
| Valo-10 s | 5 | Artificial pulp chamber + dentin disc | 10 s, 1000 mW/cm2 |
| Valo-3 s | 5 | Artificial pulp chamber + dentin disc | 3 s, 3200 mW/cm2 |
| FBF-10 s | 5 | Artificial pulp chamber + dentin disc + adhesive system + Filtek Bulk-Fill Flow | 10 s, 1000 mW/cm2 |
| TPF-3 s | 5 | Artificial pulp chamber + dentin disc + adhesive system + Tetric PowerFlow | 3 s, 3200 mW/cm2 |
| Group | Transdentinal Temperature (ΔT-°C) |
|---|---|
| Valo-10 s | 7.04 (1.4) C |
| Valo-3 s | 11.70 (0.5) A |
| FBF-10 s | 5.52 (1.3) C |
| TPF-3 s | 9.16 (0.7) B |
| Group | Cell Viability (%) |
|---|---|
| Valo-10 s | 73.79 (6.8) AB |
| Valo-3 s | 88.09 (5.5) A |
| FBF-10 s | 69.27 (10.0) B |
| TPF-3 s | 69.50 (12.5) B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, S.B.; Lins, R.B.E.; Santi, M.R.; Denucci, G.C.; Silva, C.C.S.; da Silva, S.d.F.F.; Marques, D.d.A.V.; Montes, M.A.J.R. Effect of Rapid High-Intensity Light-Curing on Increasing Transdentinal Temperature and Cell Viability: An In Vitro Study. Polymers 2024, 16, 1466. https://doi.org/10.3390/polym16111466
Miranda SB, Lins RBE, Santi MR, Denucci GC, Silva CCS, da Silva SdFF, Marques DdAV, Montes MAJR. Effect of Rapid High-Intensity Light-Curing on Increasing Transdentinal Temperature and Cell Viability: An In Vitro Study. Polymers. 2024; 16(11):1466. https://doi.org/10.3390/polym16111466
Chicago/Turabian StyleMiranda, Samille Biasi, Rodrigo Barros Esteves Lins, Marina Rodrigues Santi, Giovanna Corrêa Denucci, Cleyton Cézar Souto Silva, Silvana de Fátima Ferreira da Silva, Daniela de Araújo Viana Marques, and Marcos Antônio Japiassú Resende Montes. 2024. "Effect of Rapid High-Intensity Light-Curing on Increasing Transdentinal Temperature and Cell Viability: An In Vitro Study" Polymers 16, no. 11: 1466. https://doi.org/10.3390/polym16111466







