Granulation of Lithium-Ion Sieves Using Biopolymers: A Review
Abstract
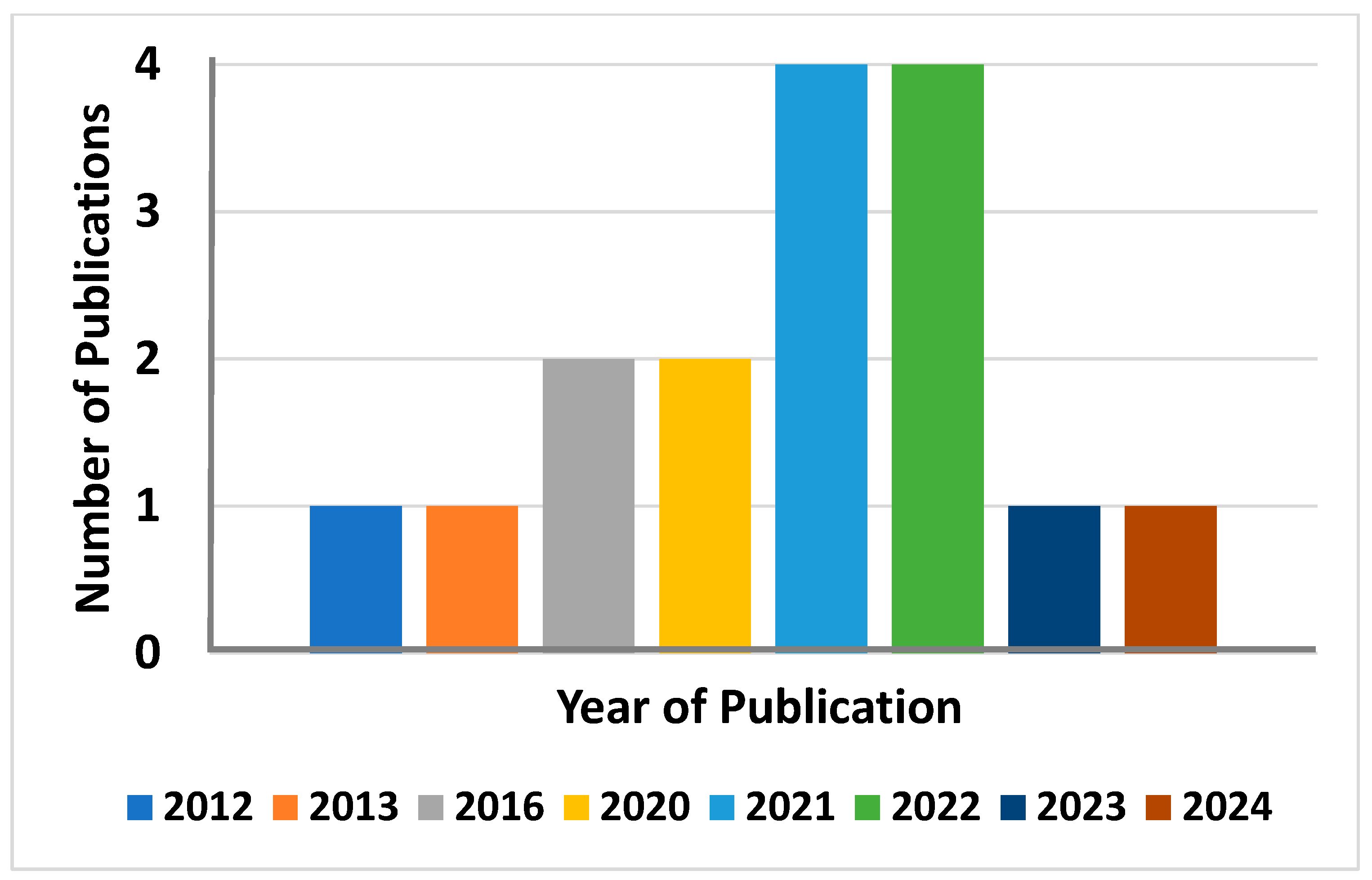
:1. Introduction
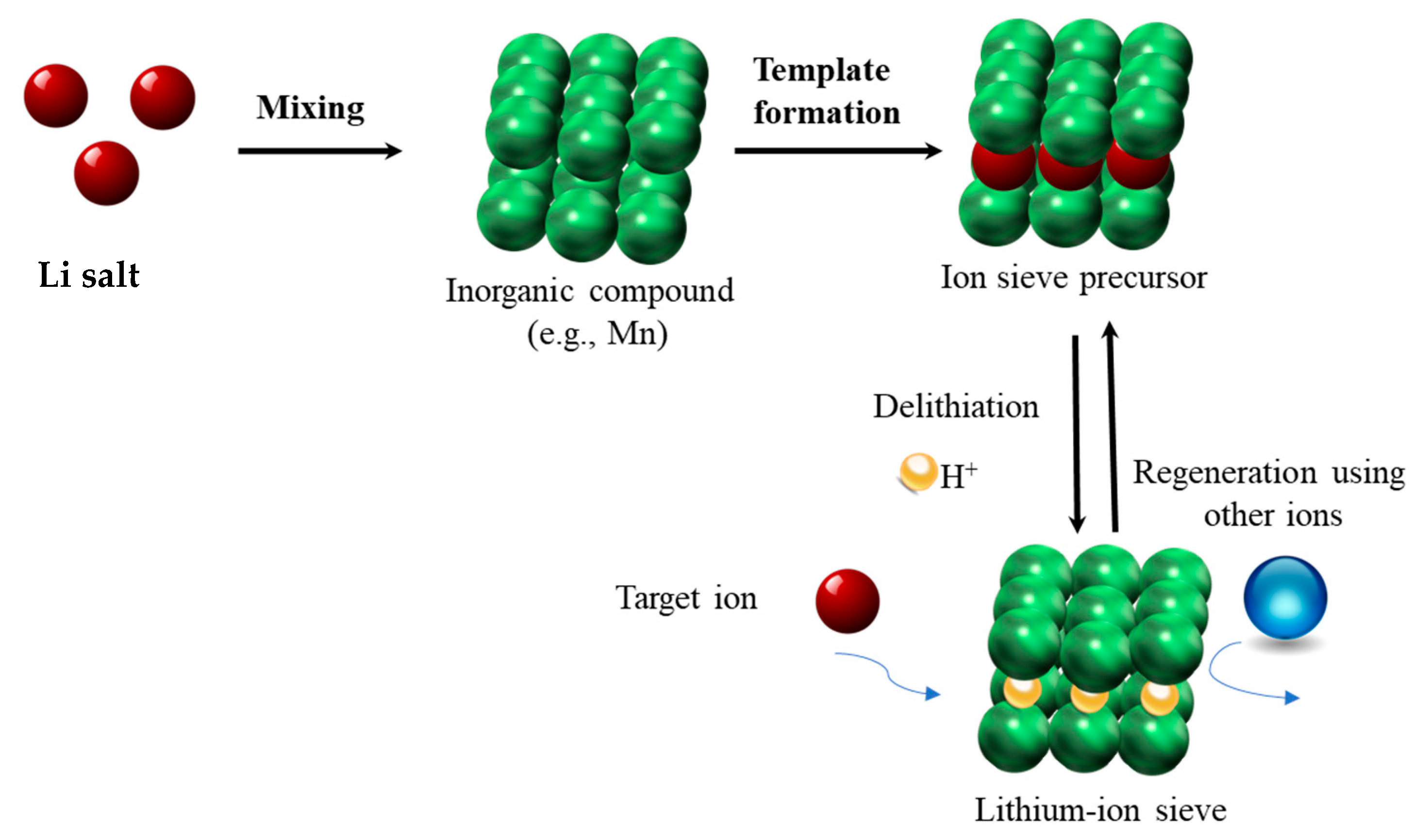
2. Preparation of Lithium-Ion Sieves: An Overview
3. Granulation of LISs
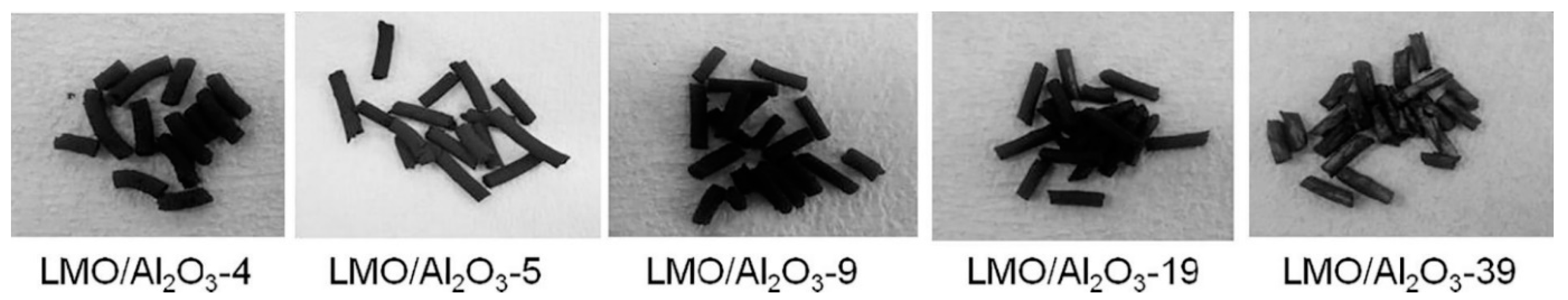
3.1. Granulation of LISs Using Inorganic and Synthetic Organic Materials
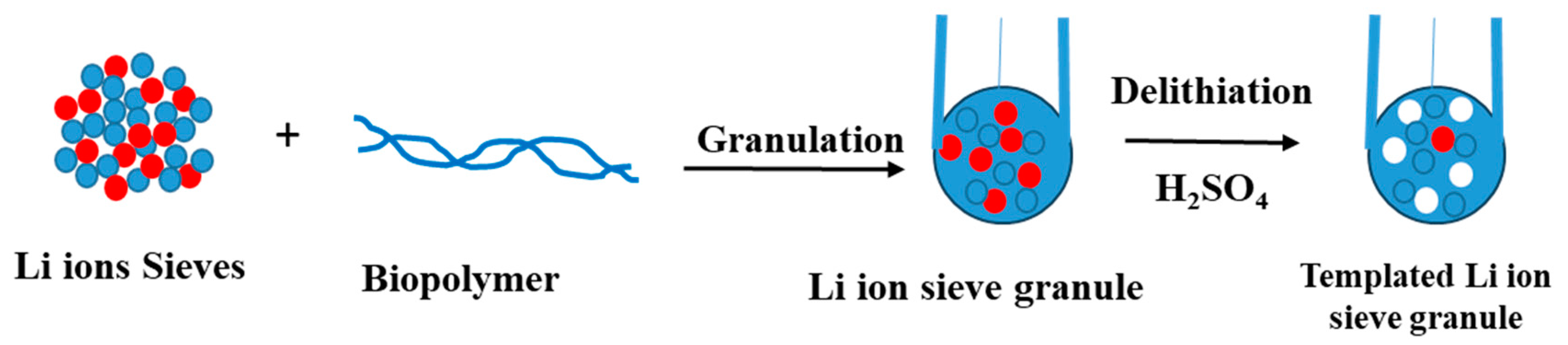
3.2. Granulation of LISs Using Biopolymers
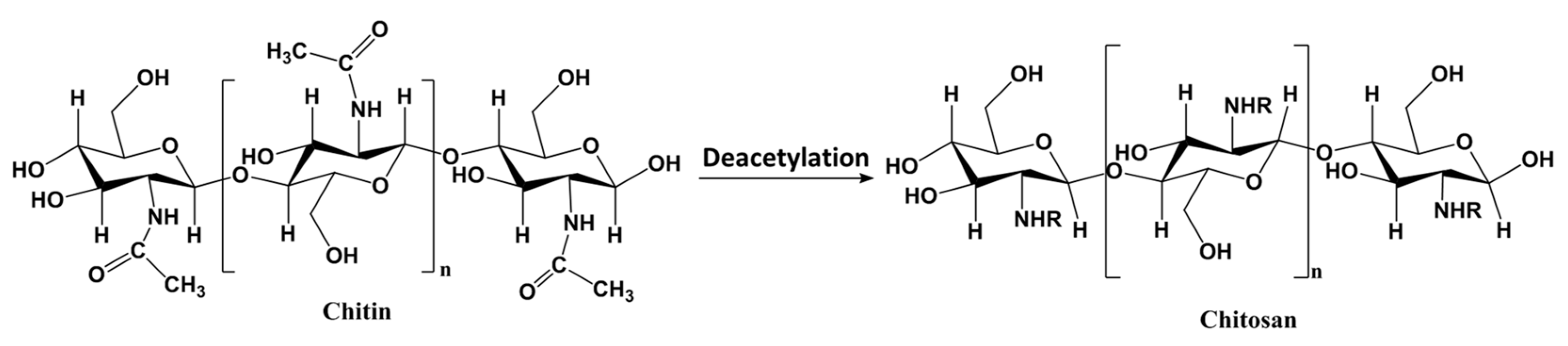
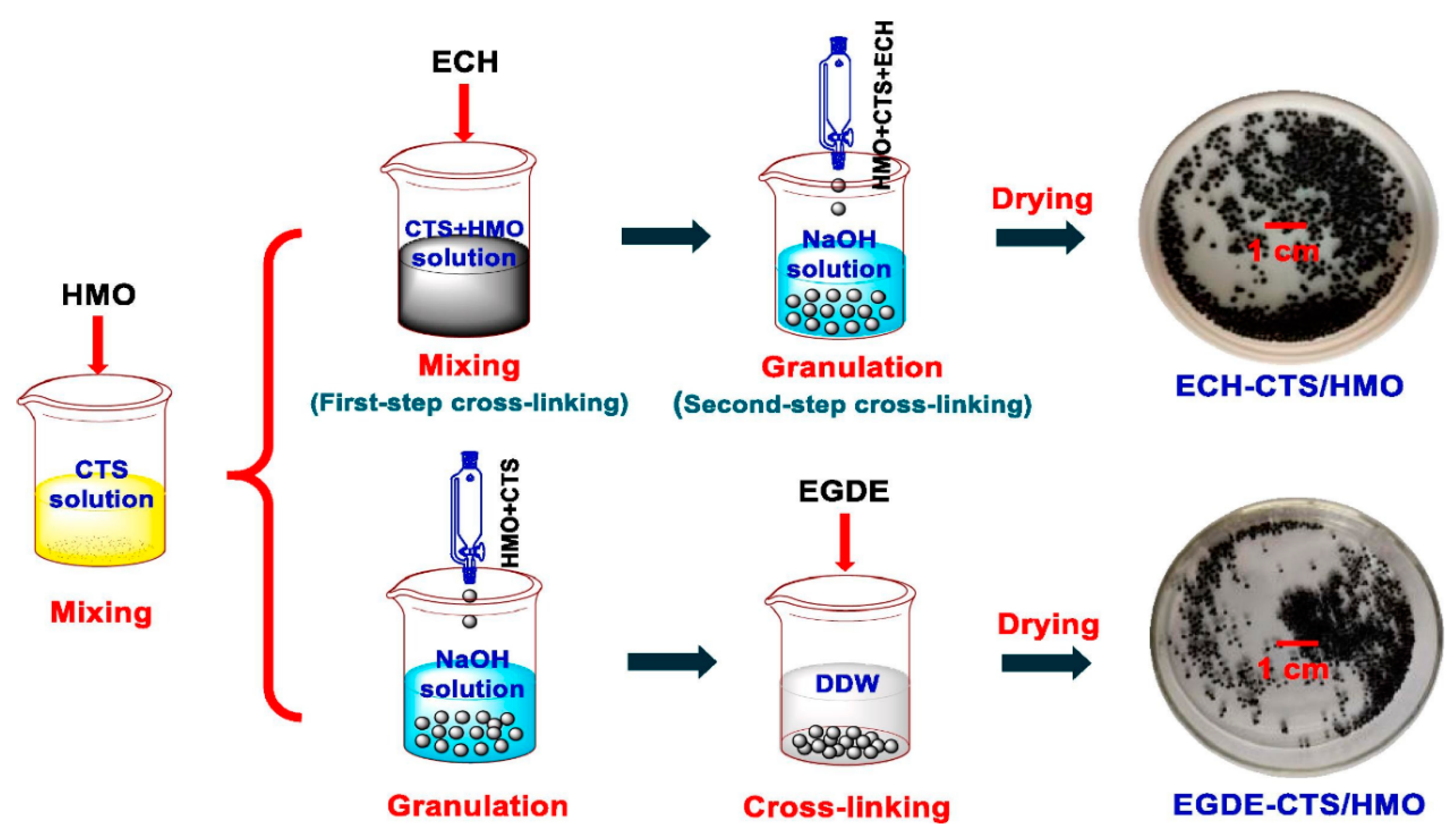
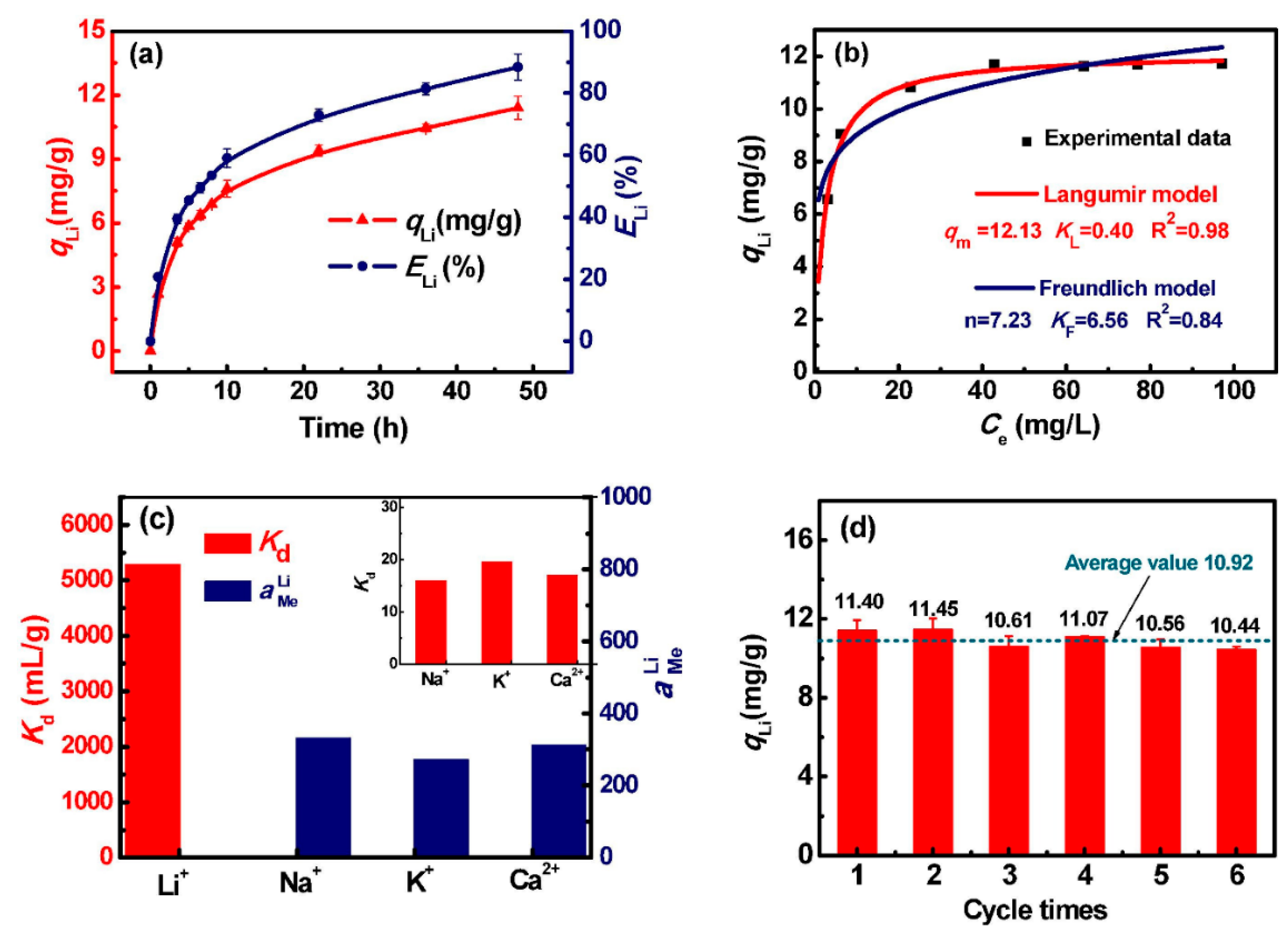
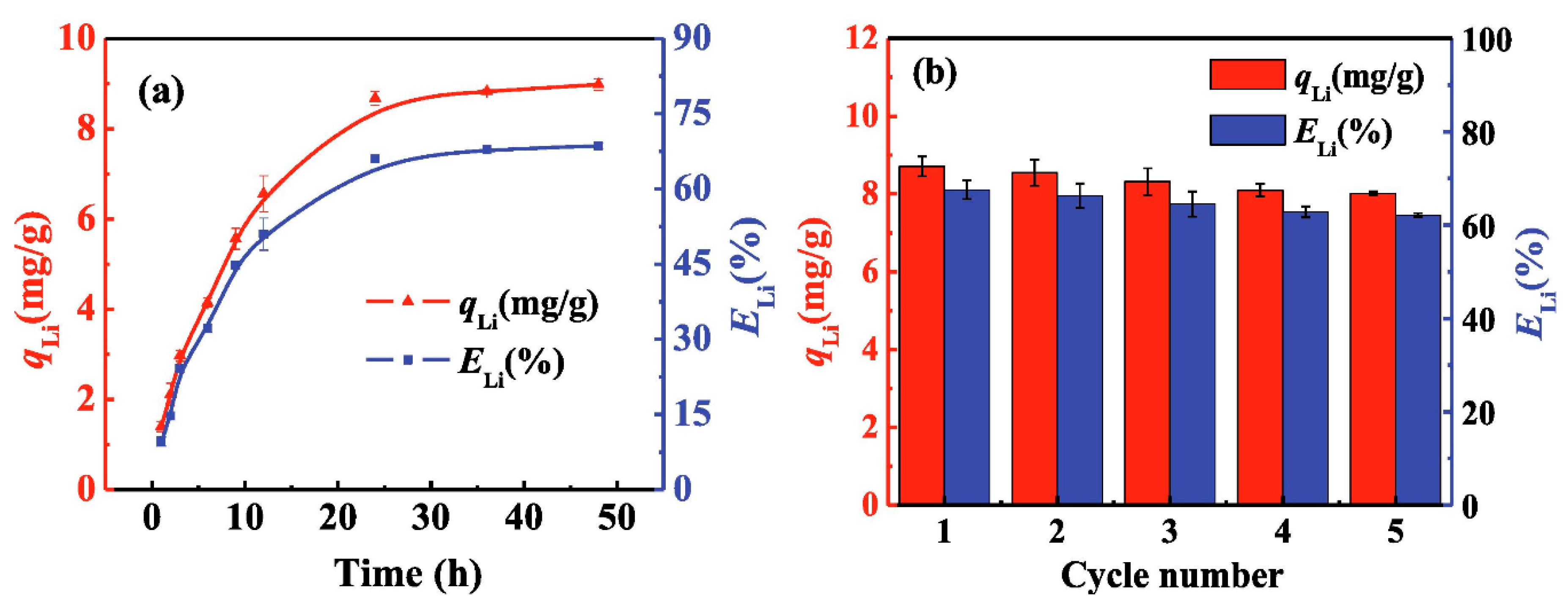
3.2.1. Metal-Based LISs Granulated with Chitosan
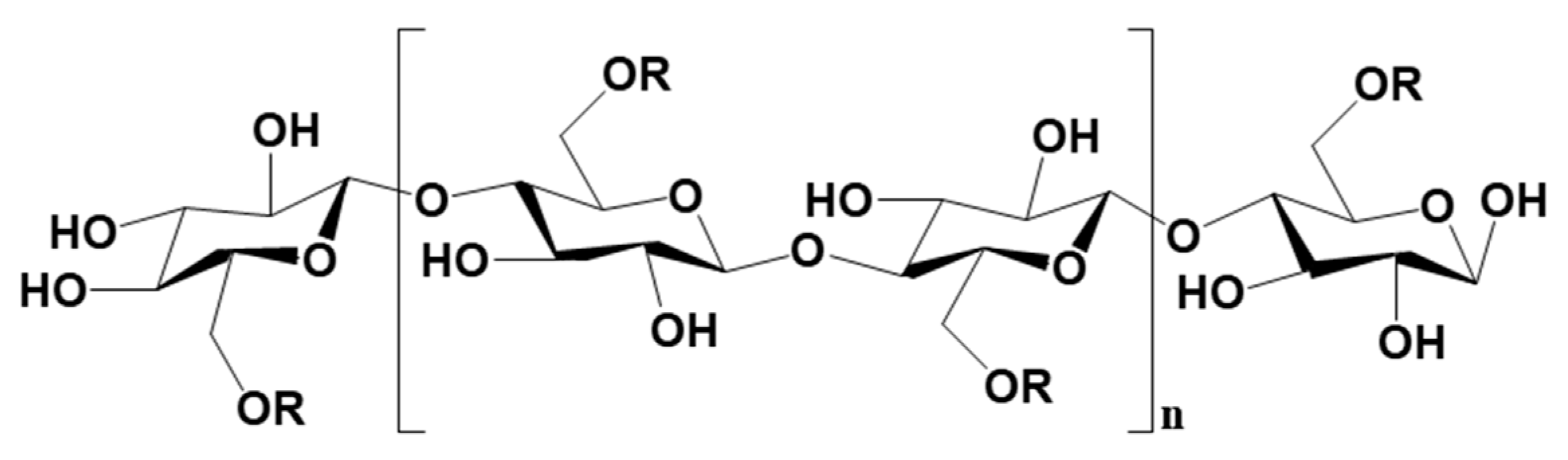
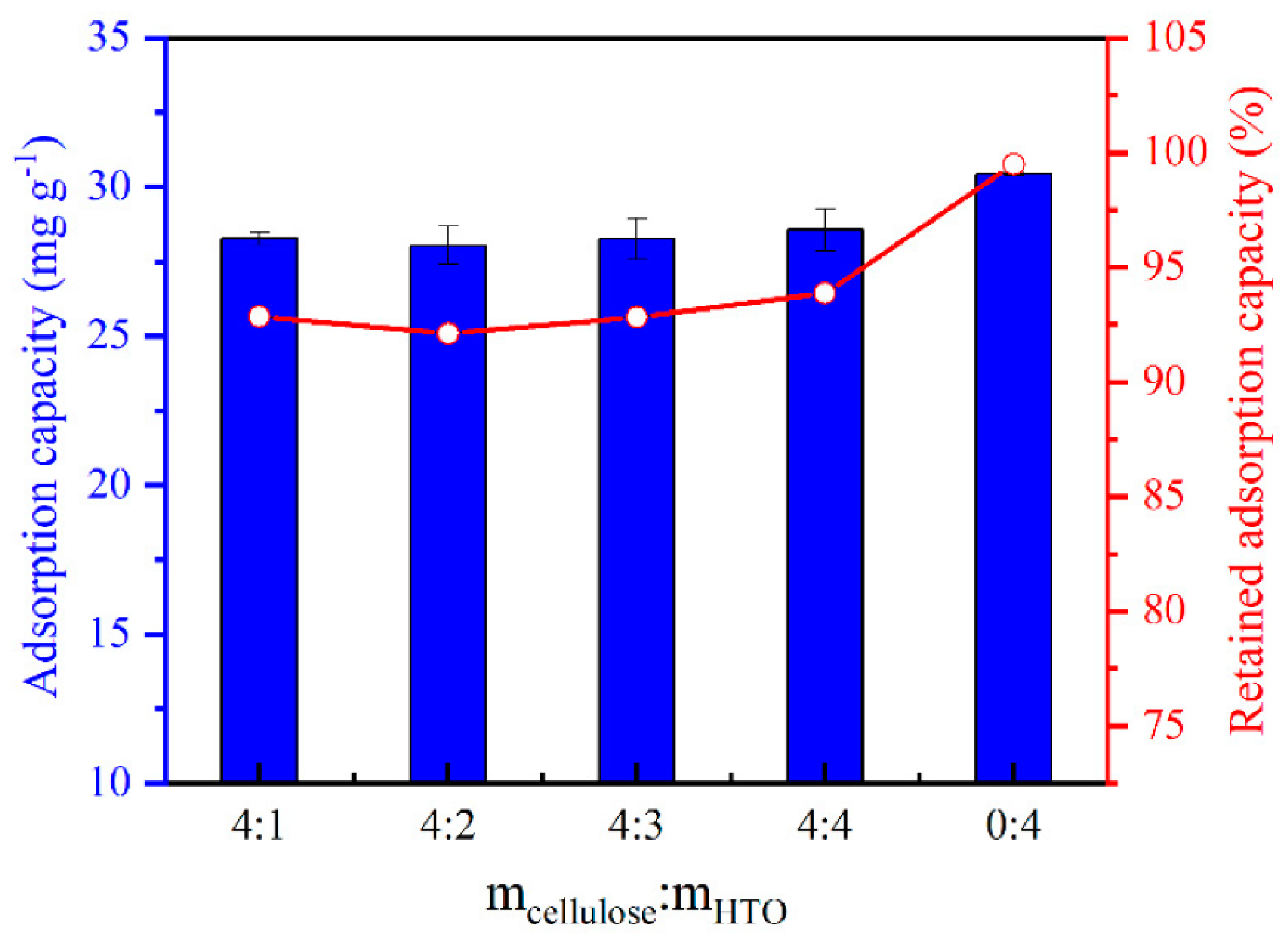
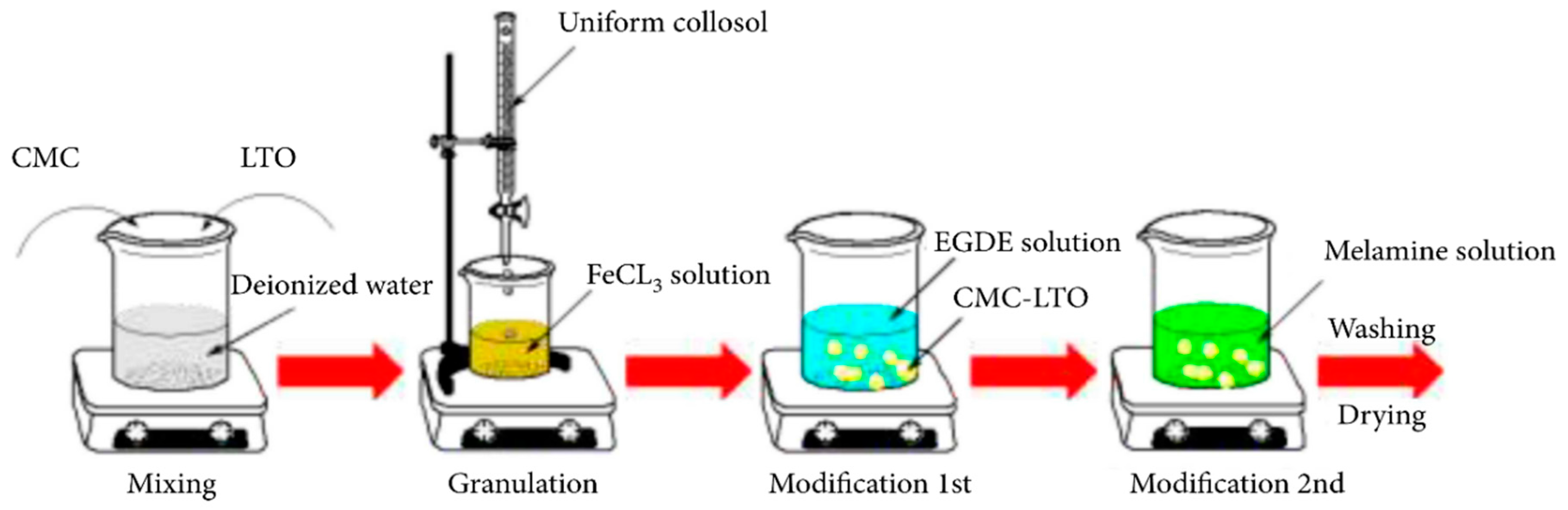
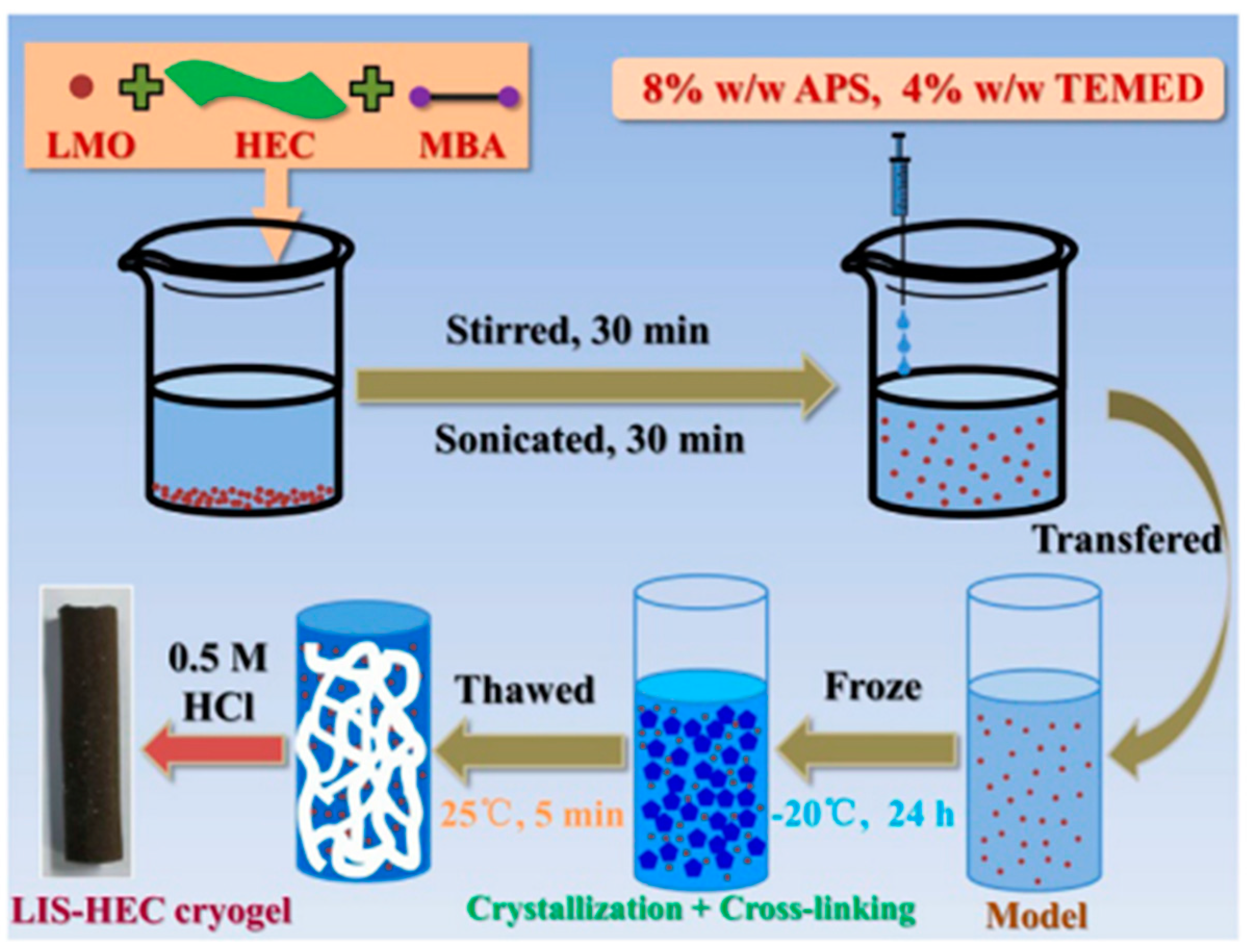
3.2.2. Metal-Based LISs Granulated with Cellulose
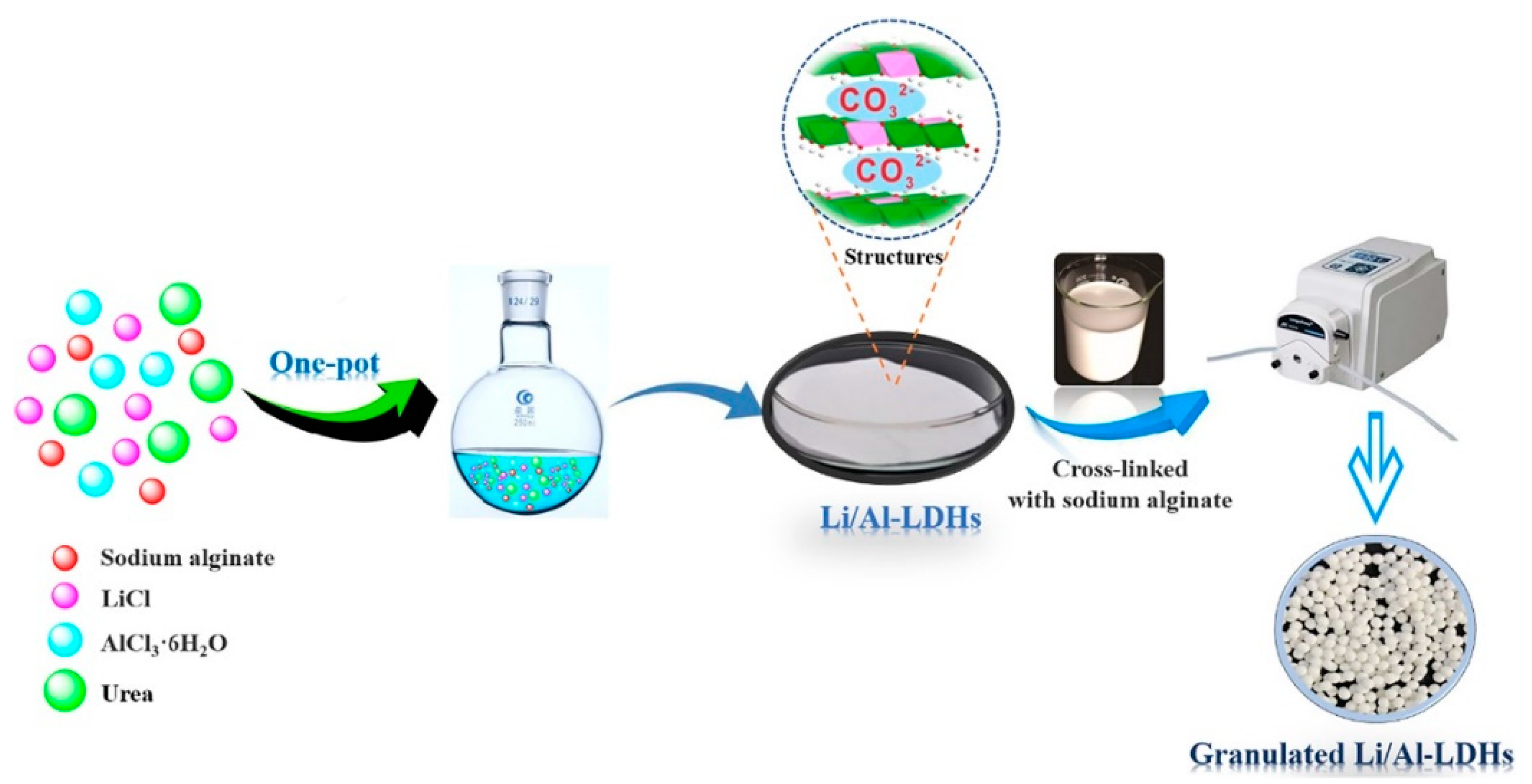
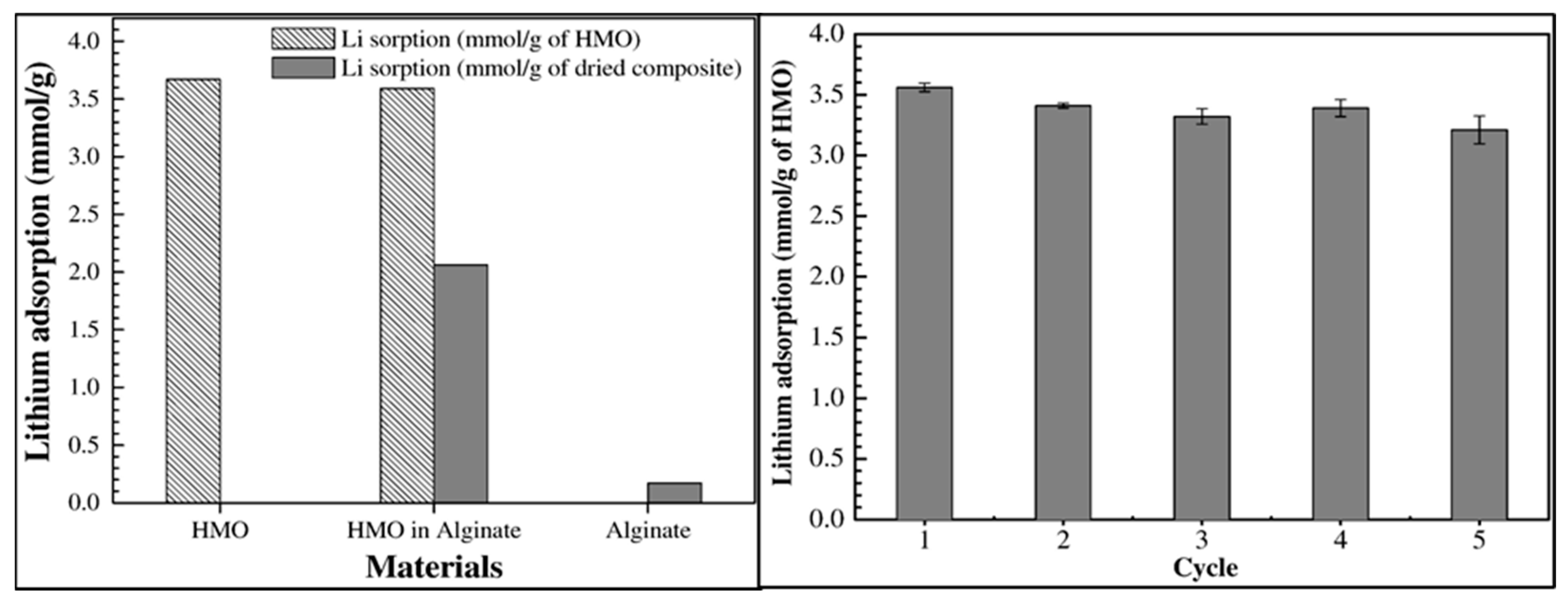
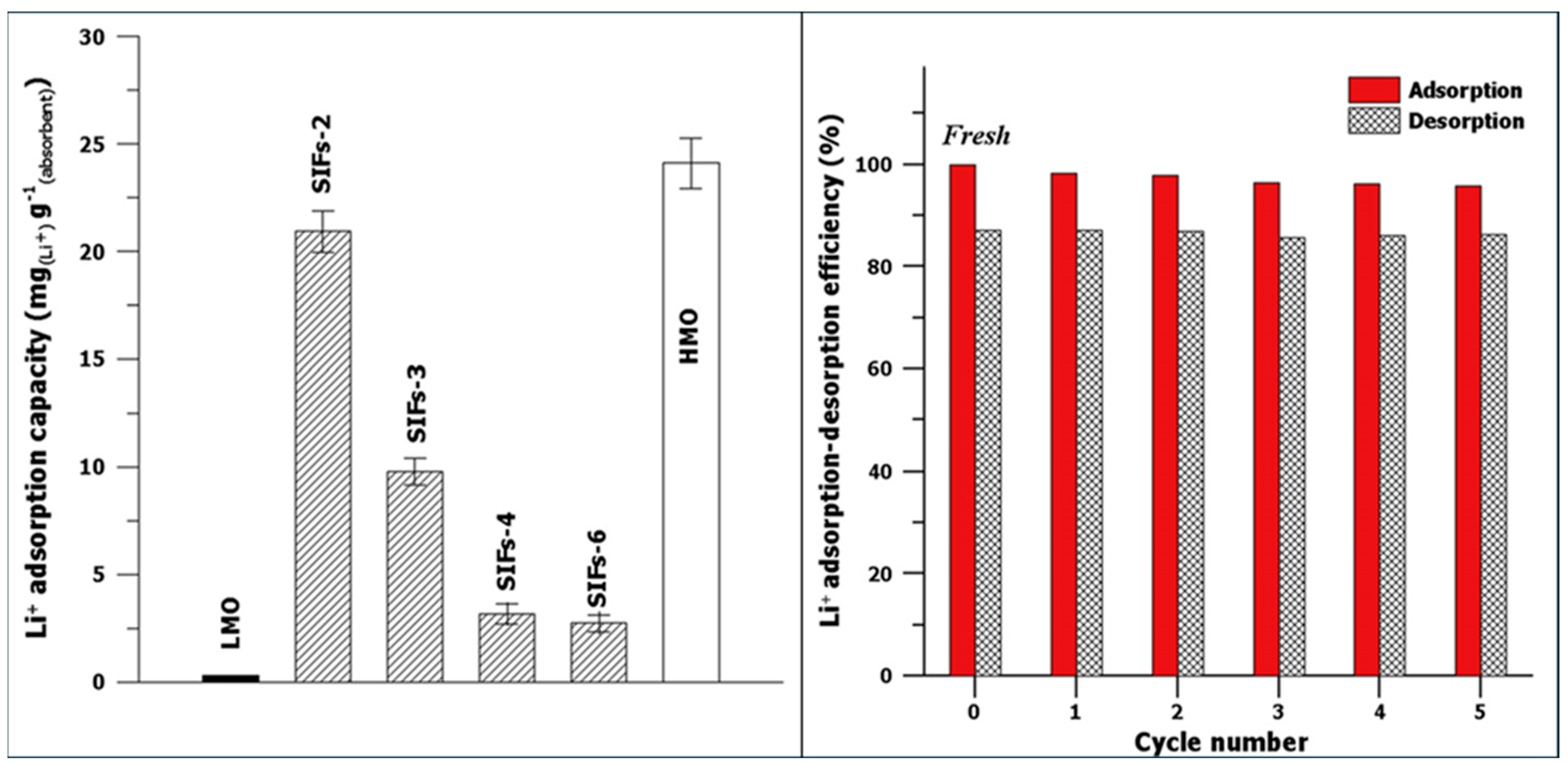
3.2.3. Metal-Based LISs Granulated with Alginate
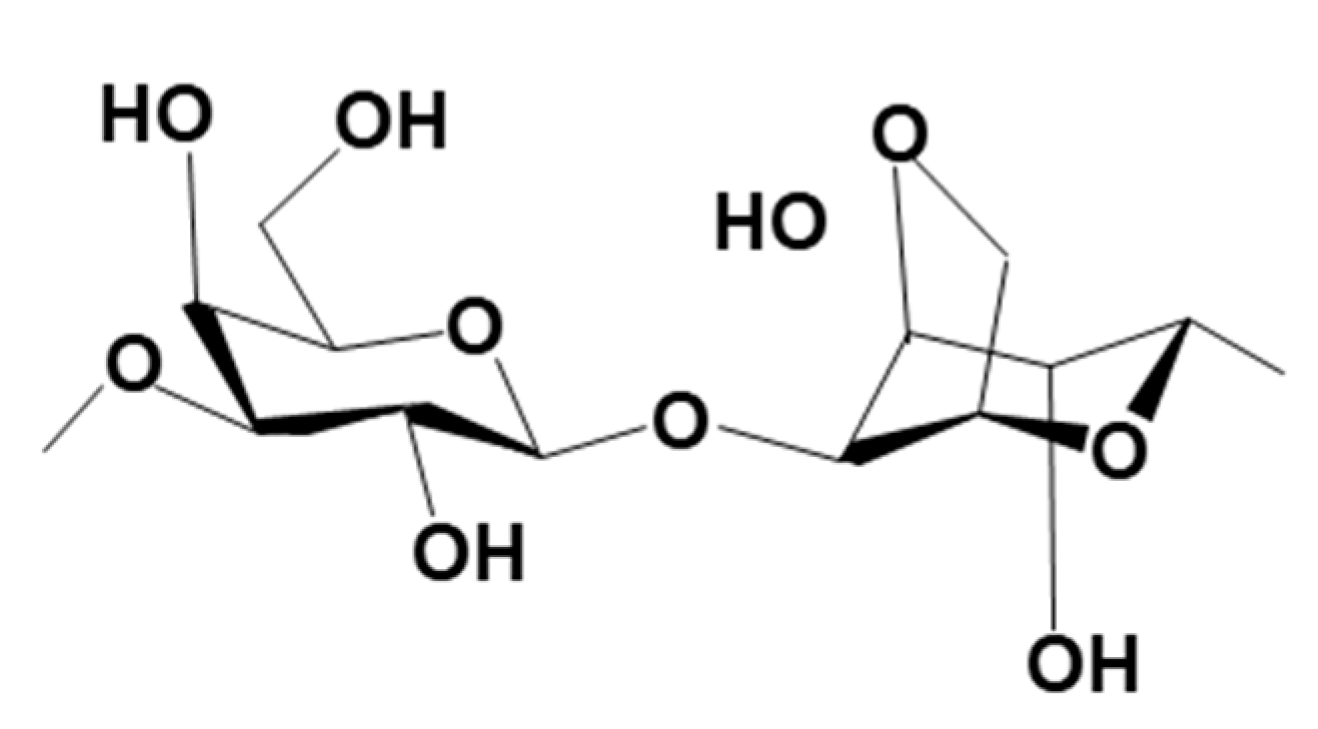
3.2.4. Metal-Based LISs Granulated with Agar
4. Comparison of the Sorption Properties of Granulated LISs
5. Prospects and Challenges
6. Conclusions
Funding
Conflicts of Interest
References
- Haddad, A.Z.; Hackl, L.; Akuzum, B.; Pohlman, G.; Magnan, J.-F.; Kostecki, R. How to Make Lithium Extraction Cleaner, Faster and Cheaper—In Six Steps. Nature 2023, 616, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Jaskula, B.W. Mineral Commodity Summaries. LITHIUM. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-lithium.pdf (accessed on 24 May 2023).
- Weng, D.; Duan, H.; Hou, Y.; Huo, J.; Chen, L.; Zhang, F.; Wang, J. Introduction of Manganese Based Lithium-Ion Sieve—A Review. Prog. Nat. Sci. Mater. Int. 2020, 30, 139–152. [Google Scholar] [CrossRef]
- Ambrose, H.; Kendall, A. Understanding the Future of Lithium: Part 1, Resource Model. J. Ind. Ecol. 2020, 24, 80–89. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M.S.; Brogioli, D.; La Mantia, F.; Trócoli, R. Electrochemical Methods for Lithium Recovery: A Comprehensive and Critical Review. Adv. Mater. 2020, 32, 1905440. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Naidu, G.; Zhang, C.; Wang, C.; Razmjou, A.; Han, D.S.; He, T.; Shon, H. Metal-Based Adsorbents for Lithium Recovery from Aqueous Resources. Desalination 2022, 539, 115951. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, L.; Sun, W. Systematic Review of Lithium Extraction from Salt-Lake Brines via Precipitation Approaches. Min. Eng. 2019, 139, 105868. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Y.; Du, B.; Zhao, Y.; Wang, M. Recovery of Both Magnesium and Lithium from High Mg/Li Ratio Brines Using a Novel Process. Hydrometallurgy 2018, 175, 102–108. [Google Scholar] [CrossRef]
- Shi, D.; Cui, B.; Li, L.; Xu, M.; Zhang, Y.; Peng, X.; Zhang, L.; Song, F.; Ji, L. Removal of Calcium and Magnesium from Lithium Concentrated Solution by Solvent Extraction Method Using D2EHPA. Desalination 2020, 479, 114306. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Yang, Y.; Yu, J. Synergic and Competitive Adsorption of Li–Na–MgCl2 onto Lithium–Aluminum Hydroxides. Adsorption 2020, 26, 1039–1049. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Wang, P.; Yu, T.; Du, X.; Hao, X.; Abudula, A.; Guan, G. Electrochemical Technologies for Lithium Recovery from Liquid Resources: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111813. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Ali, A.; Drioli, E.; Macedonio, F. Perspectives on Mining from Sea and Other Alternative Strategies for Minerals and Water Recovery—The Development of Novel Membrane Operations. J. Taiwan Inst. Chem. Eng. 2019, 94, 129–134. [Google Scholar] [CrossRef]
- Ryu, T.; Lee, D.-H.; Ryu, J.C.; Shin, J.; Chung, K.-S.; Kim, Y.H. Lithium Recovery System Using Electrostatic Field Assistance. Hydrometallurgy 2015, 151, 78–83. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-Based Technologies for Lithium Recovery from Water Lithium Resources: A Review. J. Memb. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Paredes, C.; Rodríguez de San Miguel, E. Selective Lithium Extraction and Concentration from Diluted Alkaline Aqueous Media by a Polymer Inclusion Membrane and Application to Seawater. Desalination 2020, 487, 114500. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent Advances in Magnesium/Lithium Separation and Lithium Extraction Technologies from Salt Lake Brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Z.; Qin, X.; Gao, X.; Wang, M.; Xiang, X. Recent Advances in Lithium Extraction from Salt Lake Brine Using Coupled and Tandem Technologies. Desalination 2023, 547, 116225. [Google Scholar] [CrossRef]
- Hu, S.; Sun, Y.; Pu, M.; Yun, R.; Xiang, X. Determination of Boundary Conditions for Highly Efficient Separation of Magnesium and Lithium from Salt Lake Brine by Reaction-Coupled Separation Technology. Sep. Purif. Technol. 2019, 229, 115813. [Google Scholar] [CrossRef]
- Swain, B. Recovery and Recycling of Lithium: A Review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Hamzaoui, A.H.; M’nif, A.; Hammi, H.; Rokbani, R. Contribution to the Lithium Recovery from Brine. Desalination 2003, 158, 221–224. [Google Scholar] [CrossRef]
- Masmoudi, A.; Zante, G.; Trébouet, D.; Barillon, R.; Boltoeva, M. Solvent Extraction of Lithium Ions Using Benzoyltrifluoroacetone in New Solvents. Sep. Purif. Technol. 2021, 255, 117653. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.; Ghahreman, A. Novel Approaches for Lithium Extraction from Salt-Lake Brines: A Review. Hydrometallurgy 2019, 187, 81–100. [Google Scholar] [CrossRef]
- Hong, H.-J.; Ryu, T.; Park, I.-S.; Kim, M.; Shin, J.; Kim, B.-G.; Chung, K.-S. Highly Porous and Surface-Expanded Spinel Hydrogen Manganese Oxide (HMO)/Al2O3 Composite for Effective Lithium (Li) Recovery from Seawater. Chem. Eng. J. 2018, 337, 455–461. [Google Scholar] [CrossRef]
- Luo, Q.; Dong, M.; Nie, G.; Liu, Z.; Wu, Z.; Li, J. Extraction of Lithium from Salt Lake Brines by Granulated Adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127256. [Google Scholar] [CrossRef]
- Safari, S.; Lottermoser, B.G.; Alessi, D.S. Metal Oxide Sorbents for the Sustainable Recovery of Lithium from Unconventional Resources. Appl. Mater. Today 2020, 19, 100638. [Google Scholar] [CrossRef]
- Orooji, Y.; Nezafat, Z.; Nasrollahzadeh, M.; Shafiei, N.; Afsari, M.; Pakzad, K.; Razmjou, A. Recent Advances in Nanomaterial Development for Lithium Ion-Sieving Technologies. Desalination 2022, 529, 115624. [Google Scholar] [CrossRef]
- Torrejos, R.E.C.; Nisola, G.M.; Song, H.S.; Limjuco, L.A.; Lawagon, C.P.; Parohinog, K.J.; Koo, S.; Han, J.W.; Chung, W.-J. Design of Lithium Selective Crown Ethers: Synthesis, Extraction and Theoretical Binding Studies. Chem. Eng. J. 2017, 326, 921–933. [Google Scholar] [CrossRef]
- Oral, I.; Tamm, S.; Herrmann, C.; Abetz, V. Lithium Selectivity of Crown Ethers: The Effect of Heteroatoms and Cavity Size. Sep. Purif. Technol. 2022, 294, 121142. [Google Scholar] [CrossRef]
- Dong, M.; Luo, Q.; Li, J.; Wu, Z.; Liu, Z. Lithium Adsorption Properties of Porous LiAl-Layered Double Hydroxides Synthesized Using Surfactants. J. Saudi Chem. Soc. 2022, 26, 101535. [Google Scholar] [CrossRef]
- Suresh, P.; Sreedhar, I.; Vaidhiswaran, R.; Venugopal, A. A Comprehensive Review on Process and Engineering Aspects of Pharmaceutical Wet Granulation. Chem. Eng. J. 2017, 328, 785–815. [Google Scholar] [CrossRef]
- Ma, L.-W.; Chen, B.-Z.; Shi, X.-C.; Zhang, W.; Zhang, K. Stability and Li+ extraction/adsorption properties of LiMxMn2−xO4 (M=Ni, Al, Ti; 0 ≤ x ≤ 1) in aqueous solution. Colloids Surf. A Physicochem. and Eng. Asp. 2010, 369, 88–94. [Google Scholar] [CrossRef]
- Kozawa, A.; Powers, R.A. The Manganese Dioxide Electrode in Alkaline Electrolyte; The Electron-Proton Mechanism for the Discharge Process from MnO2 to MnO1.5. J. Electrochem. Soc. 1966, 113, 870. [Google Scholar] [CrossRef]
- Hunter, J.C. Preparation of a New Crystal Form of Manganese Dioxide: λ-MnO2. J. Solid State Chem. 1981, 39, 142–147. [Google Scholar] [CrossRef]
- Yang, X.; Kanoh, H.; Tang, W.; Ooi, K. Synthesis of Li1.33Mn1.67O4 Spinels with Different Morphologies and Their Ion Adsorptivities after Delithiation. J. Mater. Chem. 2000, 10, 1903–1909. [Google Scholar] [CrossRef]
- Chitrakar, R.; Sakane, K.; Umeno, A.; Kasaishi, S.; Takagi, N.; Ooi, K. Synthesis of Orthorhombic LiMnO2 by Solid-Phase Reaction under Steam Atmosphere and a Study of Its Heat and Acid-Treated Phases. J. Solid State Chem. 2002, 169, 66–74. [Google Scholar] [CrossRef]
- Takada, T.; Hayakawa, H.; Akiba, E. Preparation and Crystal Structure Refinement of Li4Mn5O12 by the Rietveld Method. J. Solid State Chem. 1995, 115, 420–426. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Z.; Zhou, D.; Zhang, L.; Chen, B.; Yu, L. Synthesis of Li+ Adsorbent (H2TiO3) and Its Adsorption Properties. Trans. Nonferrous Met. Soc. China 2013, 23, 253–259. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Lithium Recovery from Salt Lake Brine by H2TiO3. Dalton Trans. 2014, 43, 8933–8939. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, D.; Yao, Q.; Zhou, J. Preparation of H2TiO3-Lithium Adsorbent by the Sol–Gel Process and Its Adsorption Performance. Appl. Surf. Sci. 2016, 368, 82–87. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Cui, W.; Zhang, H.; Wang, H.; Zheng, S.; Zhang, Y. Hydrothermal Synthesis of Lithium-Enriched β-Li2TiO3 with an Ion-Sieve Application: Excellent Lithium Adsorption. RSC Adv. 2016, 6, 102608–102616. [Google Scholar] [CrossRef]
- Moazeni, M.; Hajipour, H.; Askari, M.; Nusheh, M. Hydrothermal Synthesis and Characterization of Titanium Dioxide Nanotubes as Novel Lithium Adsorbents. Mater. Res. Bull. 2015, 61, 70–75. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Katoh, S.; Maeda, H.; Abe, M. Topotactic Lithium (1+) Insertion to .Lambda.-Manganese Dioxide in the Aqueous Phase. Langmuir 1989, 5, 150–157. [Google Scholar] [CrossRef]
- Shen, X.-M.; Clearfield, A. Phase Transitions and Ion Exchange Behavior of Electrolytically Prepared Manganese Dioxide. J. Solid State Chem. 1986, 64, 270–282. [Google Scholar] [CrossRef]
- Koyanaka, H.; Matsubaya, O.; Koyanaka, Y.; Hatta, N. Quantitative Correlation between Li Absorption and H Content in Manganese Oxide Spinel λ-MnO2. J. Electroanal. Chem. 2003, 559, 77–81. [Google Scholar] [CrossRef]
- Feng, Q.; Miyai, Y.; Kanoh, H.; Ooi, K. Lithium (1+) Extraction/Insertion with Spinel-Type Lithium Manganese Oxides. Characterization of Redox-Type and Ion-Exchange-Type Sites. Langmuir 1992, 8, 1861–1867. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Sakakihara, J. Mechanism of Lithium (1+) Insertion in Spinel-Type Manganese Oxide. Redox and Ion-Exchange Reactions. Langmuir 1991, 7, 1167–1171. [Google Scholar] [CrossRef]
- Hong, H.-J.; Park, I.-S.; Ryu, T.; Ryu, J.; Kim, B.-G.; Chung, K.-S. Granulation of Li1.33Mn1.67O4 (LMO) through the Use of Cross-Linked Chitosan for the Effective Recovery of Li+ from Seawater. Chem. Eng. J. 2013, 234, 16–22. [Google Scholar] [CrossRef]
- Ryu, T.; Haldorai, Y.; Rengaraj, A.; Shin, J.; Hong, H.-J.; Lee, G.-W.; Han, Y.-K.; Huh, Y.S.; Chung, K.-S. Recovery of Lithium Ions from Seawater Using a Continuous Flow Adsorption Column Packed with Granulated Chitosan–Lithium Manganese Oxide. Ind. Eng. Chem. Res. 2016, 55, 7218–7225. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, J.; Liu, Y.; Guo, Y.; Deng, T.; Yu, X. Synthesis of Granulated H4Mn5O12/Chitosan with Improved Stability by a Novel Cross-Linking Strategy for Lithium Adsorption from Aqueous Solutions. Chem. Eng. J. 2021, 426, 131689. [Google Scholar] [CrossRef]
- Yüksel Özşen, A.; Kahvecioğlu, A. Adsorbent Synthesis for the Recovery of Lithium Water Resources. Master’s Thesis, Izmir Institute of Technology, Izmir, Turkey, 2022. [Google Scholar]
- Wang, H.; Cui, J.; Li, M.; Guo, Y.; Deng, T.; Yu, X. Selective Recovery of Lithium from Geothermal Water by EGDE Cross-Linked Spherical CTS/LMO. Chem. Eng. J. 2020, 389, 124410. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Qin, J.; Samadiy, M.; Deng, T. Synthesis of Spherical Composite CMC-LTO-EGDE-ME for Lithium Recovery from Geothermal Water. J. Chem. 2022, 2022, 6884947. [Google Scholar] [CrossRef]
- Li, X.; Qi, Y.; Li, Y.; Zhang, Y.; He, X.; Wang, Y. Novel Magnetic Beads Based on Sodium Alginate Gel Crosslinked by Zirconium (IV) and Their Effective Removal for Pb2+ in Aqueous Solutions by Using a Batch and Continuous Systems. Bioresour. Technol. 2013, 142, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K. Lithium Ion Rechargeable Batteries: Materials, Technology, and New Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 3527644652. [Google Scholar]
- Darul, J.; Nowicki, W.; Piszora, P. Unusual Compressional Behavior of Lithium–Manganese Oxides: A Case Study of Li4Mn5O12. J. Phys. Chem. C 2012, 116, 17872–17879. [Google Scholar] [CrossRef]
- Robinson, D.M.; Go, Y.B.; Greenblatt, M.; Dismukes, G.C. Water Oxidation by λ-MnO2: Catalysis by the Cubical Mn4O4 Subcluster Obtained by Delithiation of Spinel LiMn2O4. J. Am. Chem. Soc. 2010, 132, 11467–11469. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Tong, K.; Zhou, L.; Xiao, J.; Sun, S.; Li, P.; Yu, J. Adsorption and Desorption Behavior of Lithium Ion in Spherical PVC–MnO2 Ion Sieve. Ind. Eng. Chem. Res. 2012, 51, 10921–10929. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, P.; Qi, P.; Gao, C. Adsorption and Desorption Properties of Li+ on PVC-H1.6Mn1.6O4 Lithium Ion-Sieve Membrane. Chem. Eng. J. 2014, 235, 340–348. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Ma, P. Synthesis of H2TiO3-PVC Lithium-Ion Sieves via an Antisolvent Method and Its Adsorption Performance. Ceram. Int. 2022, 48, 30127–30134. [Google Scholar] [CrossRef]
- Nisola, G.M.; Limjuco, L.A.; Vivas, E.L.; Lawagon, C.P.; Park, M.J.; Shon, H.K.; Mittal, N.; Nah, I.W.; Kim, H.; Chung, W.J. Macroporous Flexible Polyvinyl Alcohol Lithium Adsorbent Foam Composite Prepared via Surfactant Blending and Cryo-Desiccation. Chem. Eng. J. 2015, 280, 536–548. [Google Scholar] [CrossRef]
- Limjuco, L.A.; Nisola, G.M.; Lawagon, C.P.; Lee, S.-P.; Seo, J.G.; Kim, H.; Chung, W.-J. H2TiO3 Composite Adsorbent Foam for Efficient and Continuous Recovery of Li+ from Liquid Resources. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 267–279. [Google Scholar] [CrossRef]
- Xiao, J.-L.; Sun, S.-Y.; Song, X.; Li, P.; Yu, J.-G. Lithium Ion Recovery from Brine Using Granulated Polyacrylamide–MnO2 Ion-Sieve. Chem. Eng. J. 2015, 279, 659–666. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, J.; Guo, R. Preparation and Characterization of Porous HMO/PAN Composite Adsorbent and Its Adsorption–Desorption Properties in Brine. J. Porous Mater. 2019, 26, 705–716. [Google Scholar] [CrossRef]
- Lai, X.; Yijia, Y.; Chen, Z.; Peng, J.; Sun, H.; Zhong, H. Adsorption-Desorption Properties of Granular EP/HMO Composite and Its Application in Lithium Recovery from Brine. Ind. Eng. Chem. Res. 2020, 59, 7913–7925. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, X.; Yan, X.; Chen, Y.; Lang, W. Zr-Doped Titanium Lithium Ion Sieve and EP-Granulated Composite: Superb Adsorption and Recycling Performance. Particuology 2022, 69, 100–110. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Miao, Y.; Yang, Y.; Li, P. Alkaline Resins Enhancing Li+/H+ Ion Exchange for Lithium Recovery from Brines Using Granular Titanium-Type Lithium Ion-Sieves. Ind. Eng. Chem. Res. 2021, 60, 16457–16468. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yang, Y.; Lin, S.; Li, P. Preparation of Granular Titanium-Type Lithium-Ion Sieves and Recyclability Assessment for Lithium Recovery from Brines with Different PH Value. Sep. Purif. Technol. 2021, 267, 118613. [Google Scholar] [CrossRef]
- Hong, H.-J.; Park, I.-S.; Ryu, J.; Ryu, T.; Kim, B.-G.; Chung, K.-S. Immobilization of Hydrogen Manganese Oxide (HMO) on Alpha-Alumina Bead (AAB) to Effective Recovery of Li+ from Seawater. Chem. Eng. J. 2015, 271, 71–78. [Google Scholar] [CrossRef]
- Qian, H.; Huang, S.; Ba, Z.; Wang, W.; Yu, F.; Liang, D.; Xie, Y.; Wang, Y.; Wang, Y. Hto/Cellulose Aerogel for Rapid and Highly Selective Li+ Recovery from Seawater. Molecules 2021, 26, 4054. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, X.; Xu, T.; Zhang, Y.; Li, G.; Li, Z. Synthesis of High Specific Surface Lithium-Ion Sieve Templated by Bacterial Cellulose for Selective Adsorption of Li. Molecules 2023, 28, 3191. [Google Scholar] [CrossRef]
- Liu, C.; Tao, B.; Wang, Z.; Wang, D.; Guo, R.; Chen, L. Preparation and Characterization of Lithium Ion Sieves Embedded in a Hydroxyethyl Cellulose Cryogel for the Continuous Recovery of Lithium from Brine and Seawater. Chem. Eng. Sci. 2021, 229, 115984. [Google Scholar] [CrossRef]
- Li, J.; Luo, Q.; Dong, M.; Nie, G.; Liu, Z.; Wu, Z. Synthesis of Granulated Li/Al-LDHs Adsorbent and Application for Recovery of Li from Synthetic and Real Salt Lake Brines. Hydrometallurgy 2022, 209, 105828. [Google Scholar] [CrossRef]
- Koilraj, P.; Smith, S.M.; Yu, Q.; Ulrich, S.; Sasaki, K. Encapsulation of a Powdery Spinel-Type Li+ Ion Sieve Derived from Biogenic Manganese Oxide in Alginate Beads. Powder Technol. 2016, 301, 1201–1207. [Google Scholar] [CrossRef]
- Park, S.H.; Yan, Y.-Z.; Kim, J.; Ha, C.-S.; Lee, S.J. Rapid and Selective Adsorption of Li+ from Concentrated Seawater Using Repulsive Force of Al3+–Crosslinked Alginate Composite Incorporated with Hydrogen Manganese Oxide. Hydrometallurgy 2022, 208, 105812. [Google Scholar] [CrossRef]
- Cheng, M.; Yao, C.; Su, Y.; Liu, J.; Xu, L.; Hou, S. Synthesis of Membrane-Type Graphene Oxide Immobilized Manganese Dioxide Adsorbent and Its Adsorption Behavior for Lithium Ion. Chemosphere 2021, 279, 130487. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, H.; Park, J. Millimeter-Sized Spherical Ion-Sieve Foams with Hierarchical Pore Structure for Recovery of Lithium from Seawater. Chem. Eng. J. 2012, 210, 482–489. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Wei, Z.; Hu, J.; Guo, Y.; Deng, T. Titanium-Based Ion Sieve with Enhanced Post-Separation Ability for High Performance Lithium Recovery from Geothermal Water. Chem. Eng. J. 2021, 410, 128320. [Google Scholar] [CrossRef]
- Recepoğlu, Y.K.; Arabacı, B.; Kahvecioğlu, A.; Yüksel, A. Granulation of Hydrometallurgically Synthesized Spinel Lithium Manganese Oxide Using Cross-Linked Chitosan for Lithium Adsorption from Water. J. Chromatogr. A 2024, 1719, 464712. [Google Scholar] [CrossRef] [PubMed]
- Udoetok, I. Modified Biopolymer Sorbents for the Uptake of Naphthenic Acid Fraction Components (NAFCs) from Aqueous Solutions. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2018. [Google Scholar]
- Ibrahim, S.; Riahi, O.; Said, S.M.; Sabri, M.F.M.; Rozali, S. Biopolymers from Crop Plants. In Reference Module in Materials Science and Materials Engineering; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Kaith, B.S.; Mittal, H.; Bhatia, J.K.; Kalia, S. Polysaccharide Graft Copolymers—Synthesis, Properties and Applications. In Biopolymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 35–57. ISBN 9781118164792. [Google Scholar]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A Review of Chitin and Chitosan Applications; Elsevier Science B.V.: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Zhang, Q.; Wu, Q.; Lin, D.; Yao, S. Effect and Mechanism of Sodium Chloride on the Formation of Chitosan–Cellulose Sulfate–Tripolyphosphate Crosslinked Beads. Soft Matter 2013, 9, 10354–10363. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J. V Self-Assembled and Cross-Linked Animal and Plant-Based Polysaccharides: Chitosan–Cellulose Composites and Their Anion Uptake Properties. ACS Appl. Mater. Interfaces 2016, 8, 33197–33209. [Google Scholar] [CrossRef] [PubMed]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. “Pillaring Effects” in Cross-Linked Cellulose Biopolymers: A Study of Structure and Properties. Int. J. Polym. Sci. 2018, 2018, 6358254. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef]
- Yamazawa, A.; Iikura, T.; Shino, A.; Date, Y.; Kikuchi, J. Solid-, Solution-, and Gas-State NMR Monitoring of 13C-Cellulose Degradation in an Anaerobic Microbial Ecosystem. Molecules 2013, 18, 9021–9033. [Google Scholar] [CrossRef]
- Boufi, S.; Alila, S. Modified Cellulose Fibres as a Biosorbent for the Organic Pollutants. In Biopolymers: Biomedical and Environmental Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 483–524. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.-X.; Yu, J.-Y. Kinetics of Cellulose Regeneration from Cellulose-NaOH/Thiourea/Urea/H2O System. Cellul. Chem. Technol. 2011, 45, 593. [Google Scholar]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Qin, X.; Lu, A.; Zhang, L. Effect of Stirring Conditions on Cellulose Dissolution in NaOH/Urea Aqueous Solution at Low Temperature. J. Appl. Polym. Sci. 2012, 126, E470–E477. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic Liquid Processing of Cellulose. Chem. Soc. Rev. 2012, 41, 1519. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Moulthrop, J.S.; Swatloski, R.P.; Moyna, G.; Rogers, R.D. High-Resolution 13C NMR Studies of Cellulose and Cellulose Oligomers in Ionic Liquid Solutions. Chem. Commun. 2005, 1557. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/Alginate Crosslinked Hydrogels: Preparation, Characterization and Application for Cell Growth Purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Faye, O.; Wilson, L.D. Adsorption of Phosphate Dianions by Hybrid Inorganic–Biopolymer Polyelectrolyte Complexes: Experimental and Computational Studies. ACS Appl. Polym. Mater. 2020, 2, 899–910. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Jameel, E.; Mohanta, B.; Dhara, A.K.; Alkahtani, S.; Nayak, A.K. Chapter 1—Alginates: Sources, Structure, and Properties. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–17. ISBN 978-0-12-817640-5. [Google Scholar]
- Hasnain, M.S.; Nayak, A.K. Alginates: Versatile Polymers in Biomedical Applications and Therapeutics; CRC Press: Boca Raton, FL, USA, 2019; ISBN 042965748X. [Google Scholar]
- Yu, Q.; Morioka, E.; Sasaki, K. Characterization of Lithium Ion Sieve Derived from Biogenic Mn Oxide. Microporous Mesoporous Mater. 2013, 179, 122–127. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, M.; Qian, F.; Qian, Z.; Xu, N.; Wu, Z.; Liu, Z. Hydrothermal Synthesis and Adsorption Behavior of H4Ti5O12 Nanorods along [100] as Lithium Ion-Sieves. RSC Adv. 2020, 10, 35153–35163. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, F.S.; Zaeim, D. Agar-Based Edible Films for Food Packaging Applications—A Review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of Unpurified Agar-Based Extracts from Red Seaweed Gelidium Sesquipedale by Means of Simplified Extraction Protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Armisén, R.; Gaiatas, F. 4—Agar. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 82–107. ISBN 978-1-84569-414-2. [Google Scholar]
- Schmidt, É.C.; Dos Santos, R.; Horta, P.A.; Maraschin, M.; Bouzon, Z.L. Effects of UVB Radiation on the Agarophyte Gracilaria Domingensis (Rhodophyta, Gracilariales): Changes in Cell Organization, Growth and Photosynthetic Performance. Micron 2010, 41, 919–930. [Google Scholar] [CrossRef]
- Nieto, M.B.; Akins, M. Hydrocolloids in Bakery Fillings. In Hydrocolloids in Food Processing; Blackwell Publishing, Ltd.: Hoboken, NJ, USA, 2010; pp. 67–107. [Google Scholar]
- Nieto, M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Huber, K., Embuscado, M., Eds.; Springer: New York, NY, USA, 2009; pp. 57–112. [Google Scholar]
- Han, Y.; Kim, S.; Kim, H.; Park, J. Preparation of Sizable and Uniform-sized Spherical Ceramic Foams: Drop-in-oil and Agar Gelation. J. Am. Ceram. Soc. 2011, 94, 2742–2745. [Google Scholar] [CrossRef]





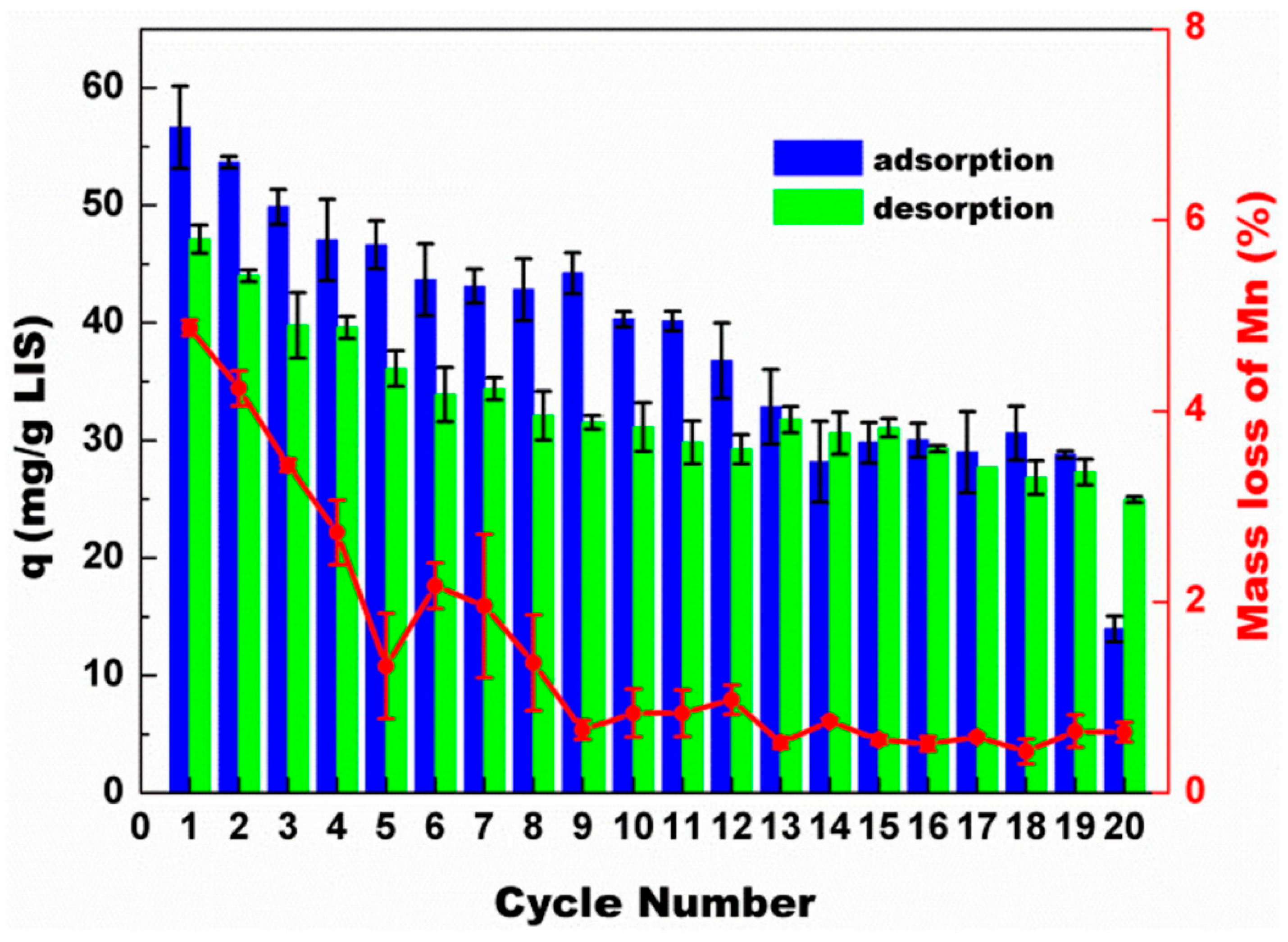
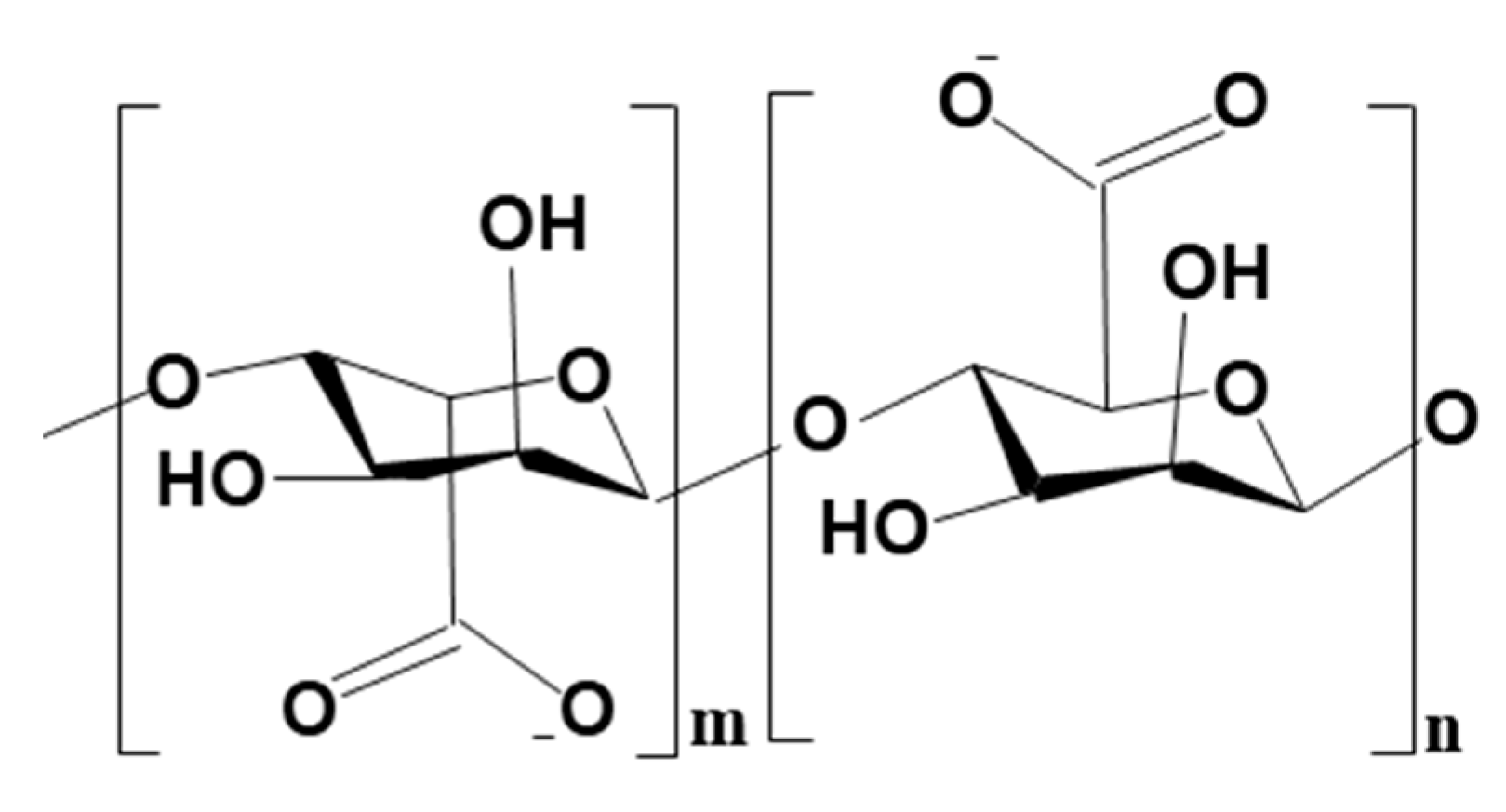
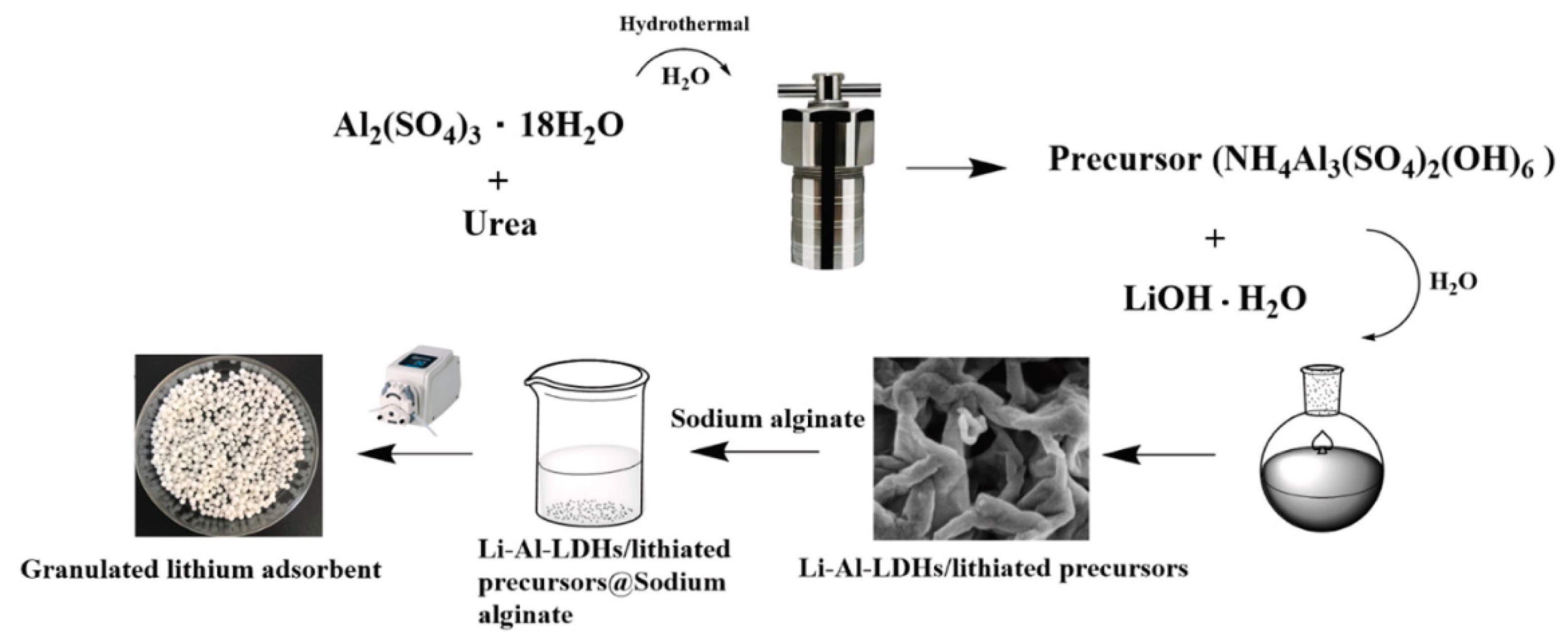
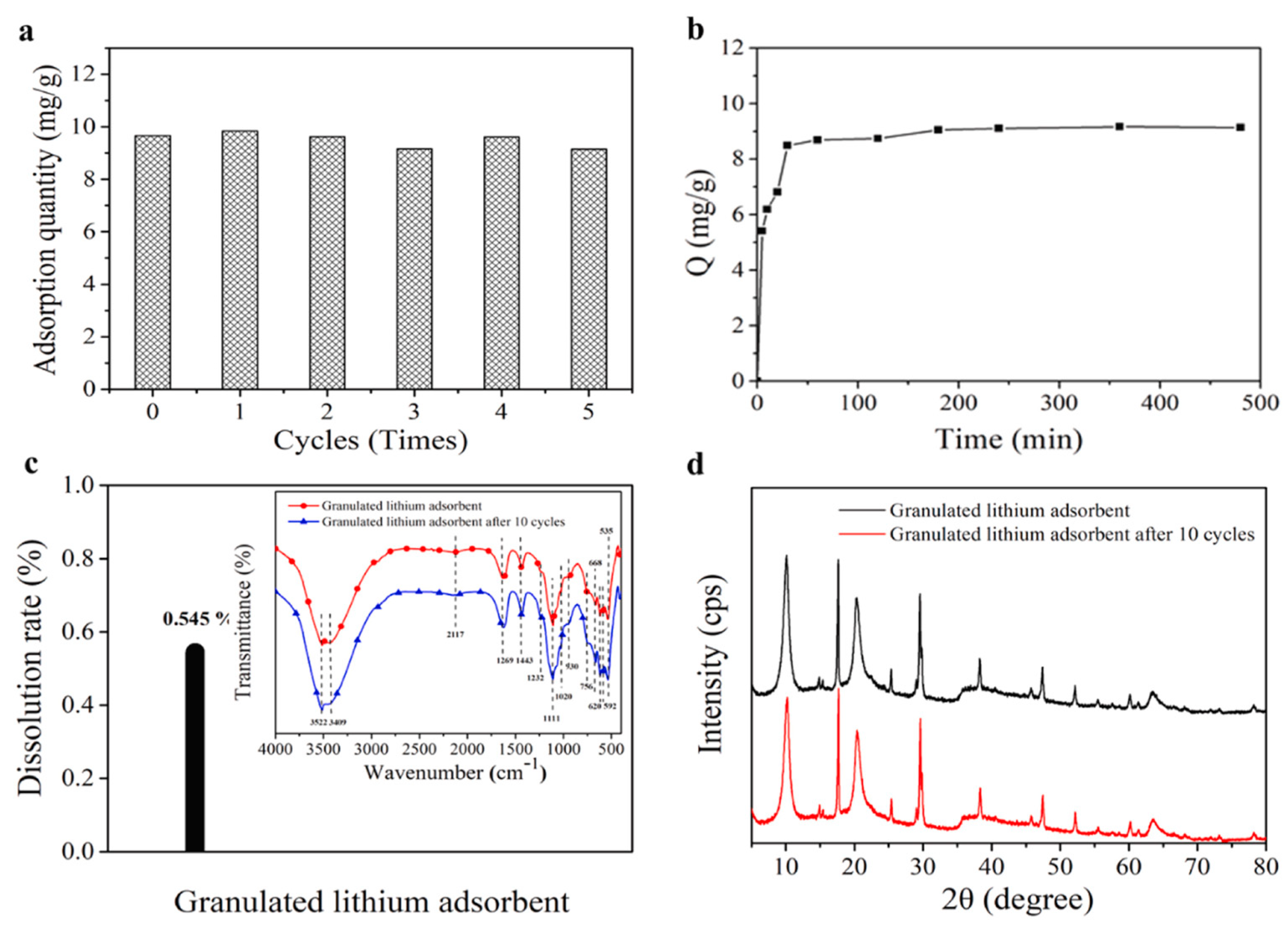

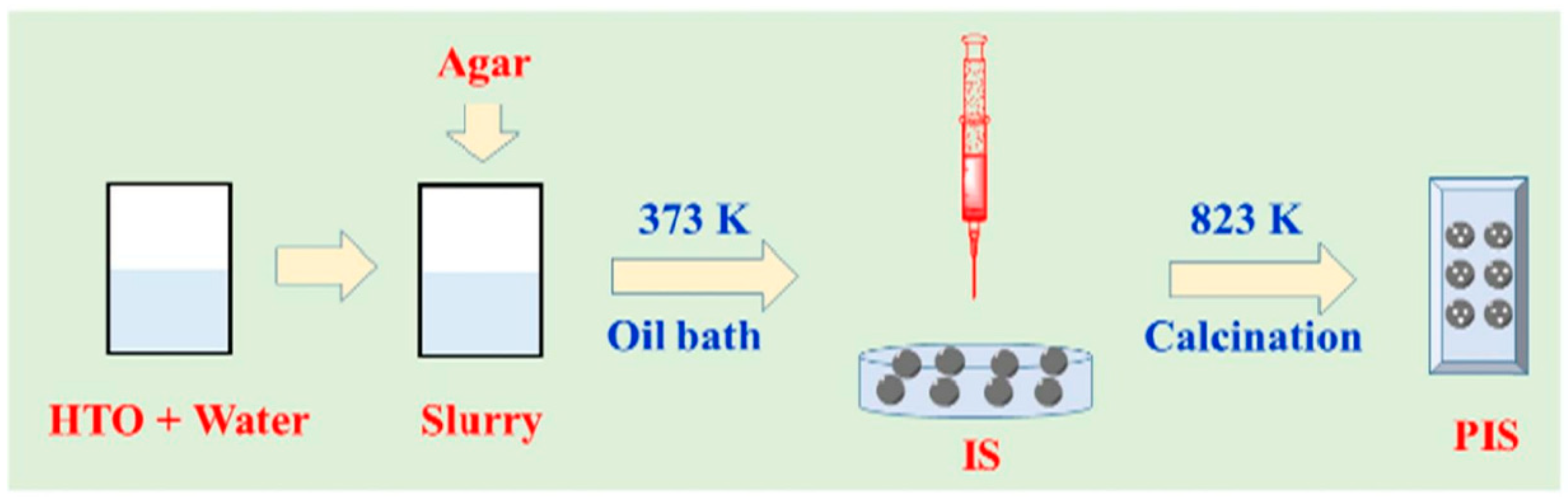
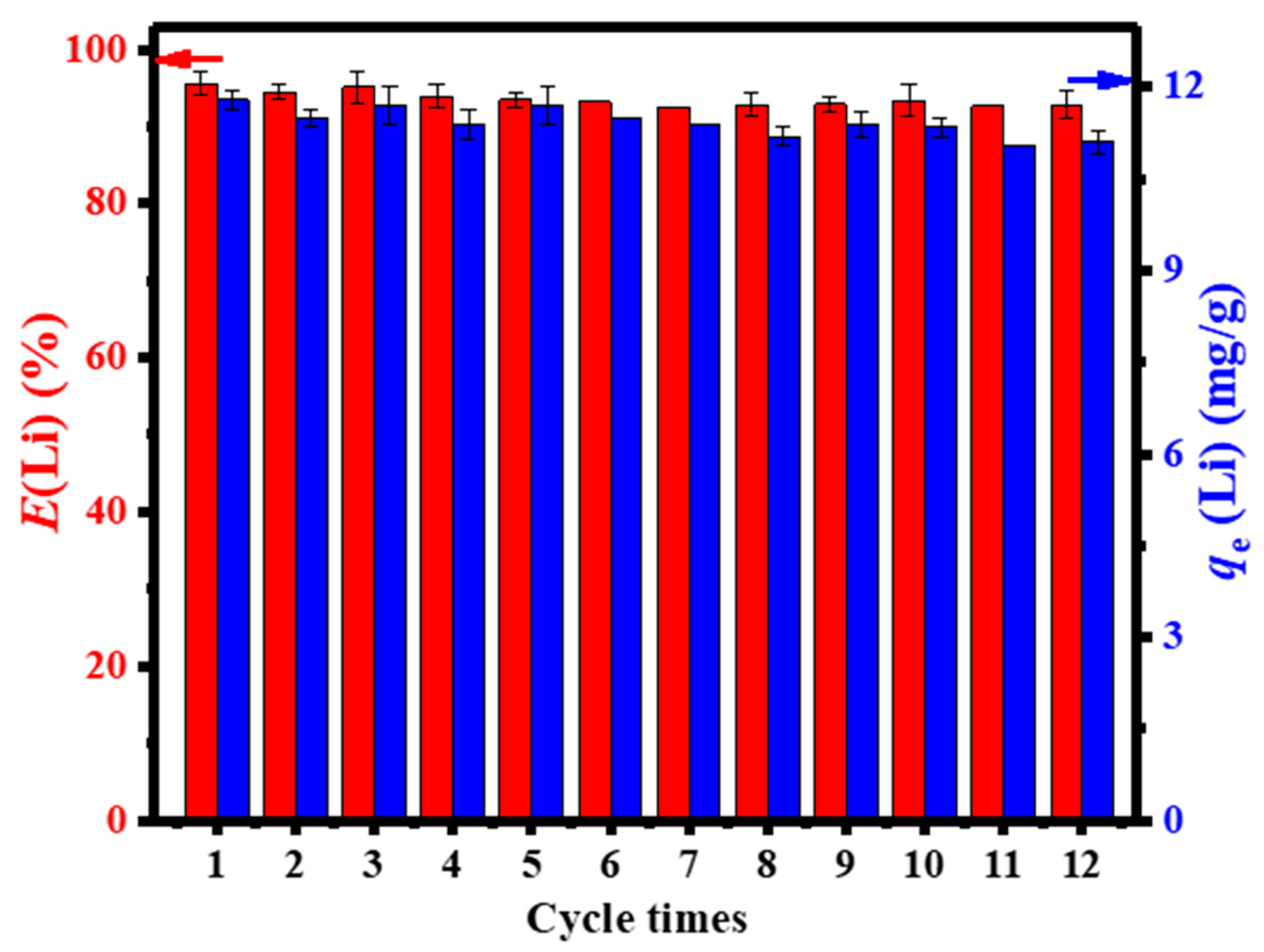
| S/N | Binder | Crosslinker | Sorbate | Uptake Capacity (mg/g) | Reference |
|---|---|---|---|---|---|
| 1 | Chitosan | Sulfuric acid | Spiked seawater | 10.0 | Hong et al. [47] |
| 2 | Chitosan | Sulfuric acid | Spiked seawater | 54.7 | Ryu et al. [48] |
| 3 | Chitosan | ECH and EDGE | Geothermal water | 11.4 | Ding et al. [49] |
| 4 | Chitosan | ECH | LiCl | 5.0 | Yüksel Özşen and Kahvecioğlu [50] |
| 5 | Chitosan | EDGE, ECH and glutaraldehyde | Geothermal water | 8.98 | Wang et al. [51] |
| 6 | Chitosan | ECH | LiCl | 4.94 | Recepoğlu et al. [78] |
| 7 | Cellulose | NA | LiCl | 28.6 | Qian et al. [69] |
| 8 | Carboxymethyl cellulose | FeCl3, EGDE, and ME | Geothermal water | 12.0 | Xie et al. [52] |
| 9 | Bacterial cellulose | NA | LiCl | 35.5 | Zhang et al. [70] |
| 10 | Hydroxyethyl cellulose | MBA | LiCl | 54.2 | Liu et al. [71] |
| 11 | Sodium alginate | CaCl2 | Salt Lake brine | 9.16 | Luo et al. [24] |
| 12 | Sodium alginate | CaCl2 | Salt Lake brine | 14.5 | Li et al. [72] |
| 13 | Sodium alginate | CaCl2 | LiCl | 24.9 | Koilraj et al. [73] |
| 14 | Sodium alginate | AlCl3/ZrCl4 | Concentrated seawater | 2.52 | Park et al. [74] |
| 15 | Agar | NA | Seawater | 3.40 | Han et al. [76] |
| 16 | Agar | NA | Geothermal water | 12.3 | Chen et al. [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udoetok, I.A.; Karoyo, A.H.; Ubuo, E.E.; Asuquo, E.D. Granulation of Lithium-Ion Sieves Using Biopolymers: A Review. Polymers 2024, 16, 1520. https://doi.org/10.3390/polym16111520
Udoetok IA, Karoyo AH, Ubuo EE, Asuquo ED. Granulation of Lithium-Ion Sieves Using Biopolymers: A Review. Polymers. 2024; 16(11):1520. https://doi.org/10.3390/polym16111520
Chicago/Turabian StyleUdoetok, Inimfon A., Abdalla H. Karoyo, Emmanuel E. Ubuo, and Edidiong D. Asuquo. 2024. "Granulation of Lithium-Ion Sieves Using Biopolymers: A Review" Polymers 16, no. 11: 1520. https://doi.org/10.3390/polym16111520





