Preparation of a Flame-Retardant Curing Agent Based on Phytic Acid–Melamine Ion Crosslinking and Its Application in Wood Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.2.1. Preparation of PM
2.2.2. Preparation of SUF Coatings and Their Application on Wood
2.3. Sample Characterization
2.3.1. Structural Characterization
2.3.2. Thermal Analysis
2.3.3. Flame Retardance Tests
2.3.4. Analysis of Physical Properties
3. Results and Discussion
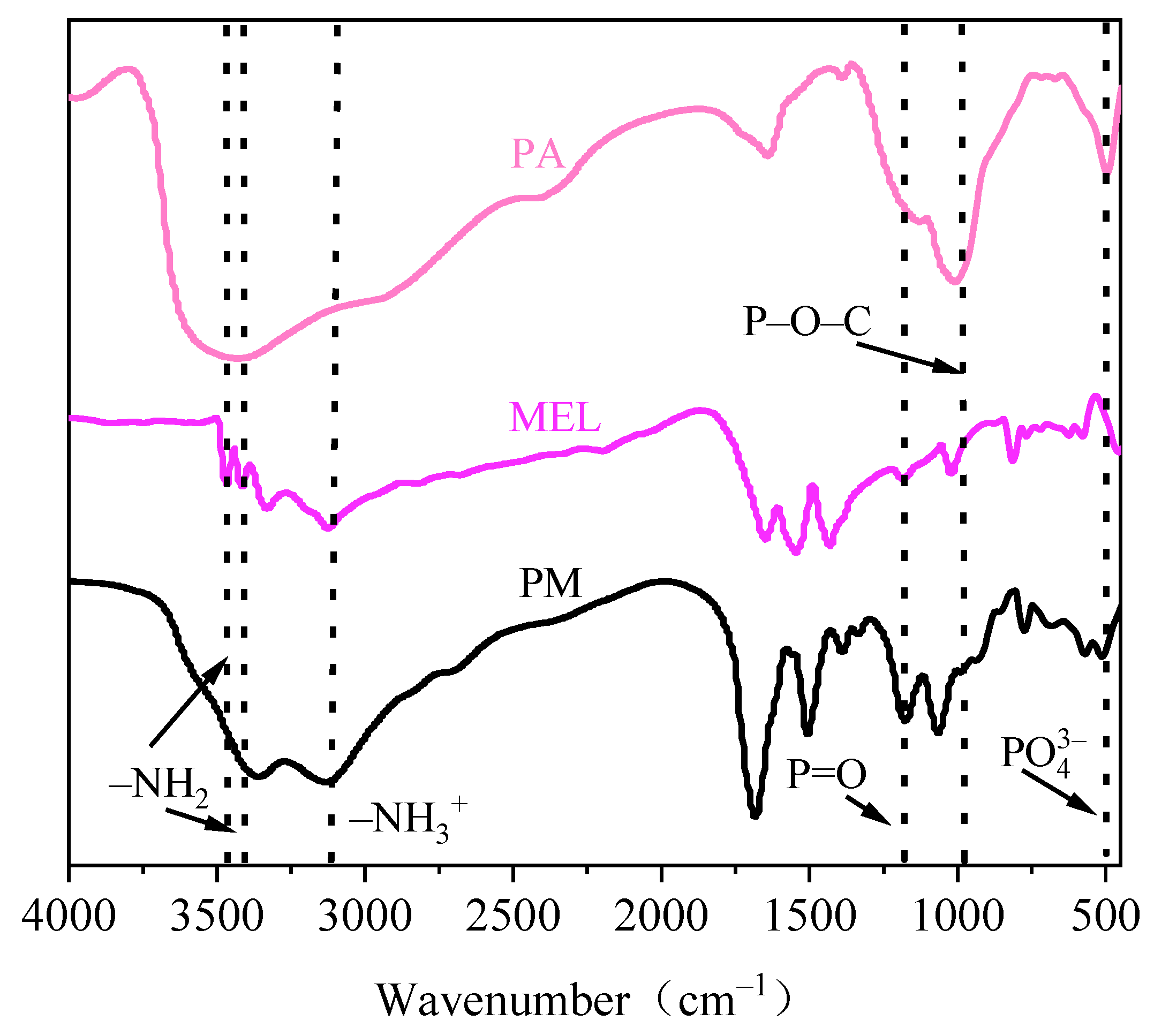
3.1. FTIR Analysis of the PM Structure
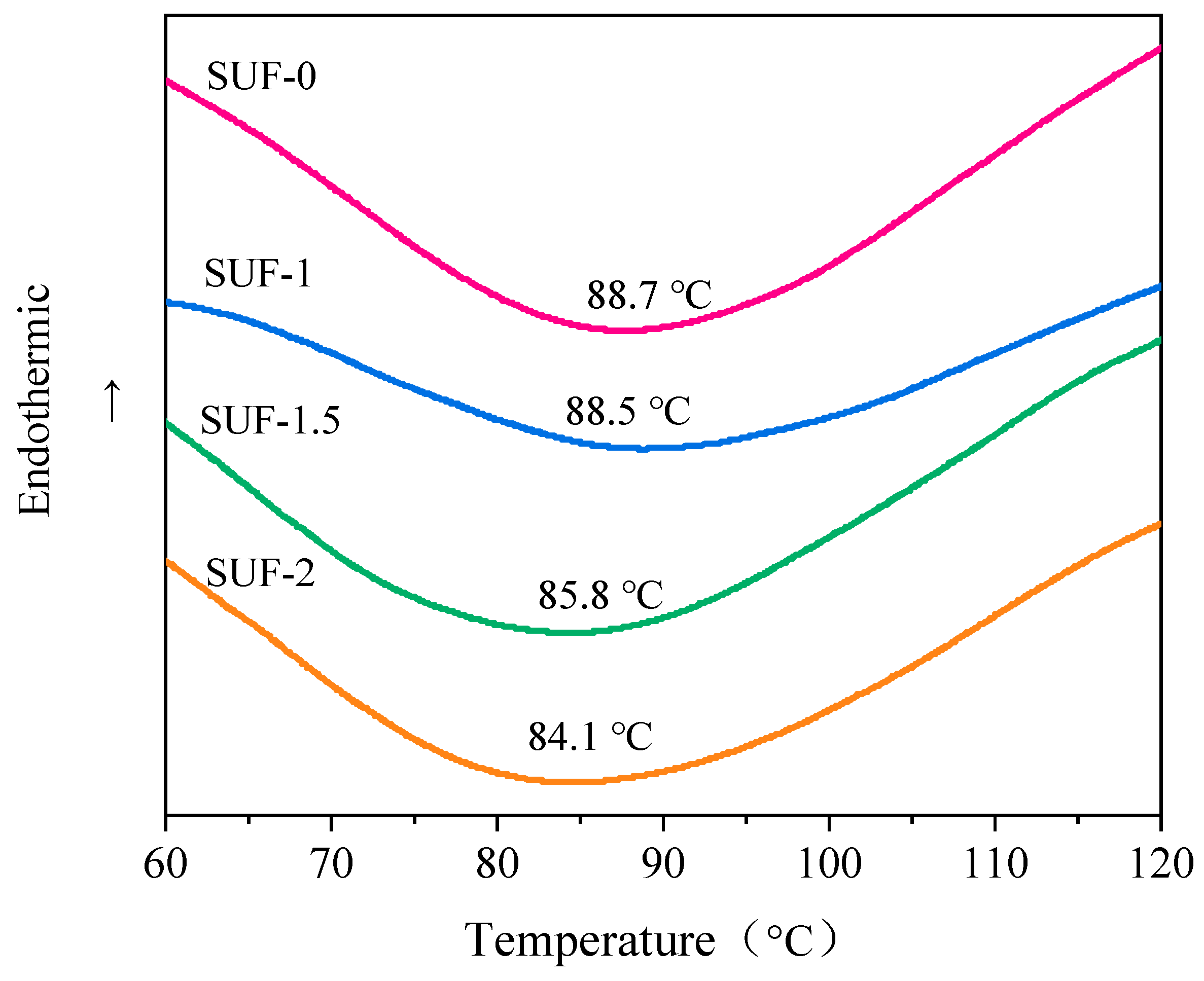
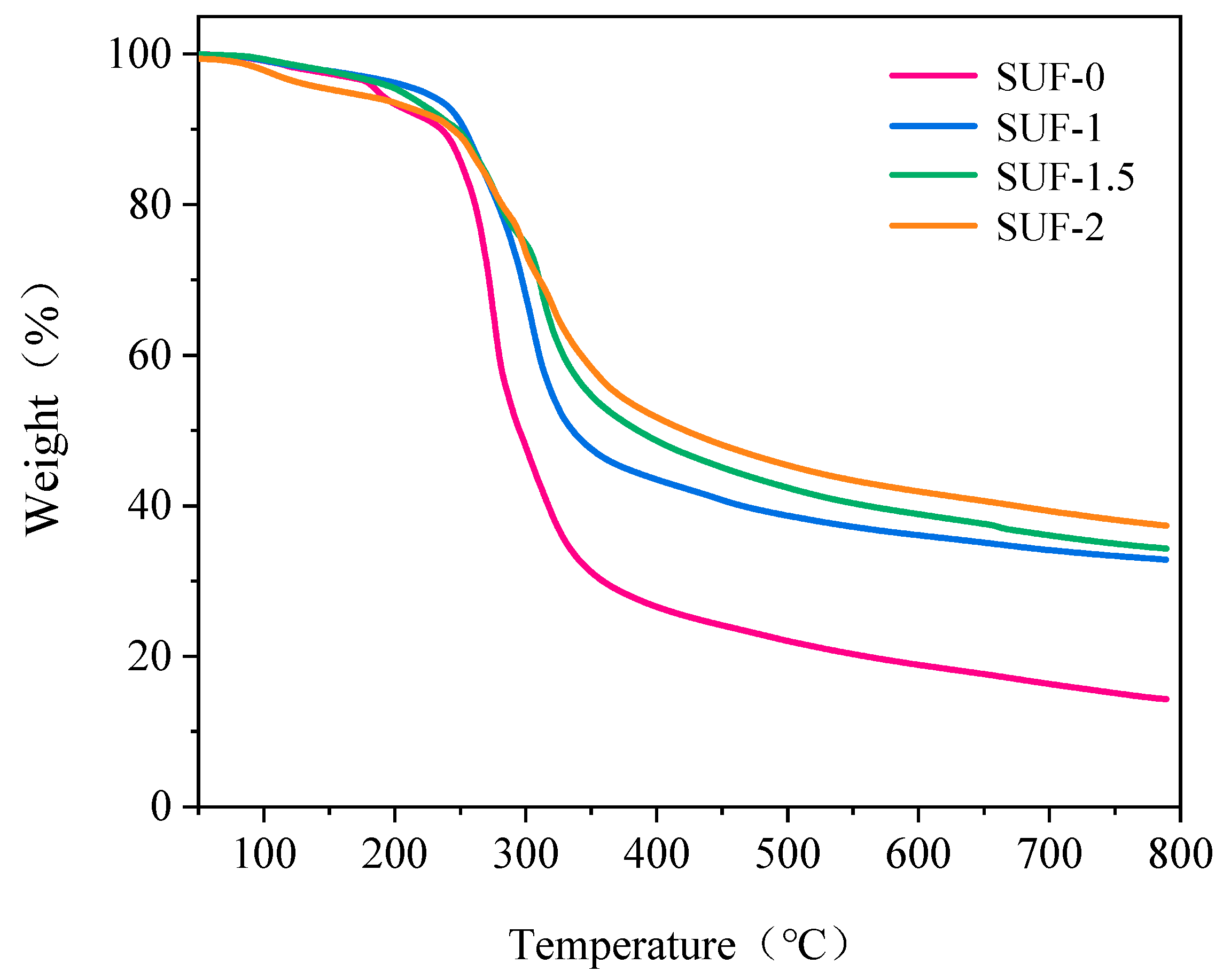
3.2. Thermal Performance of Flame-Retardant SUF Coating
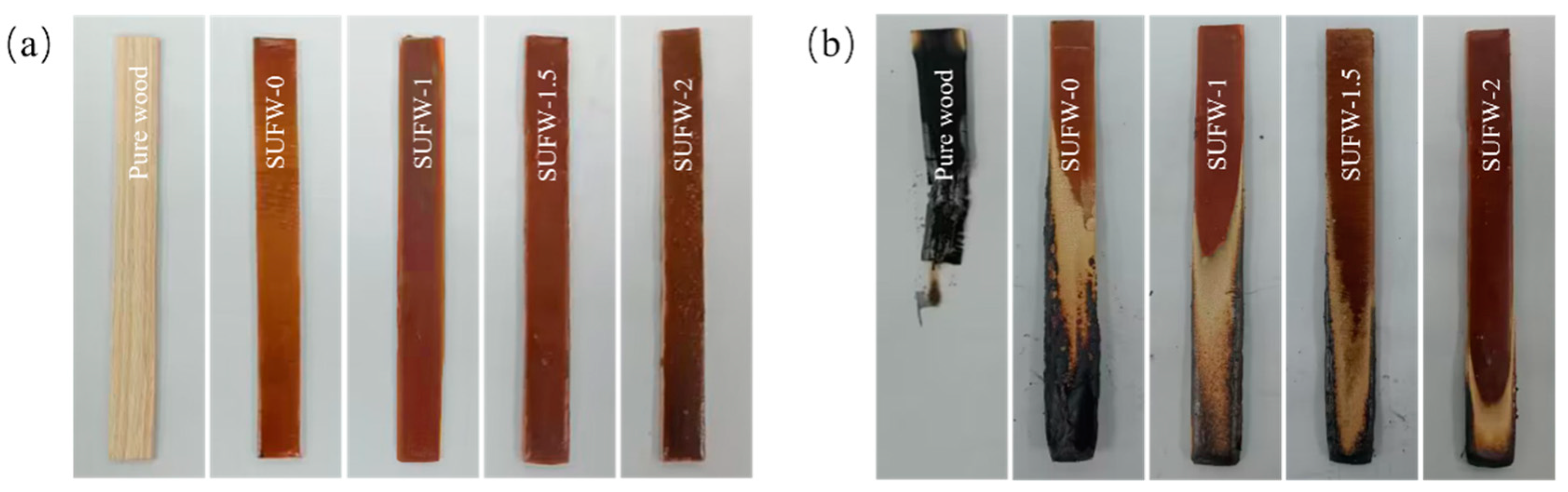
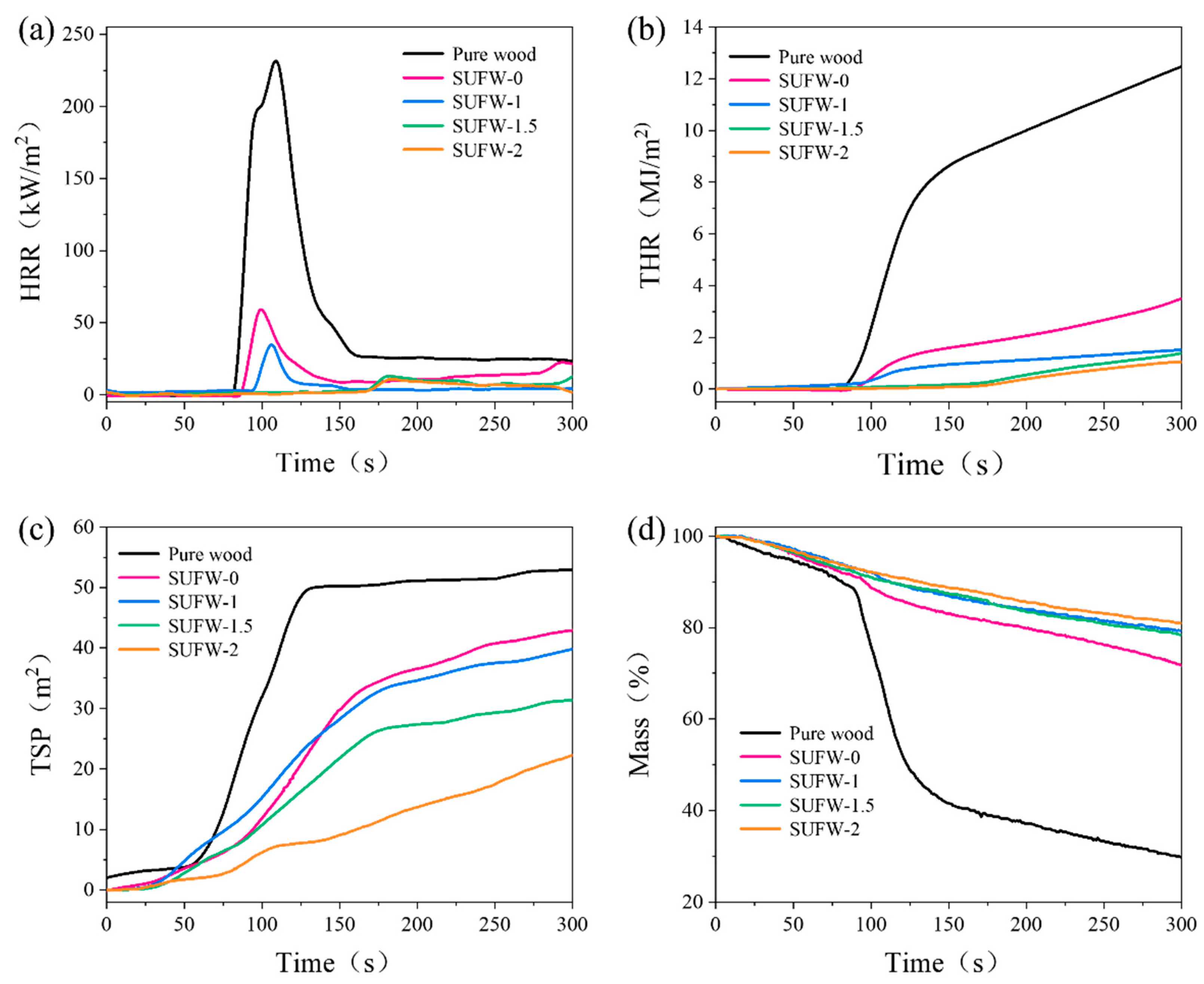
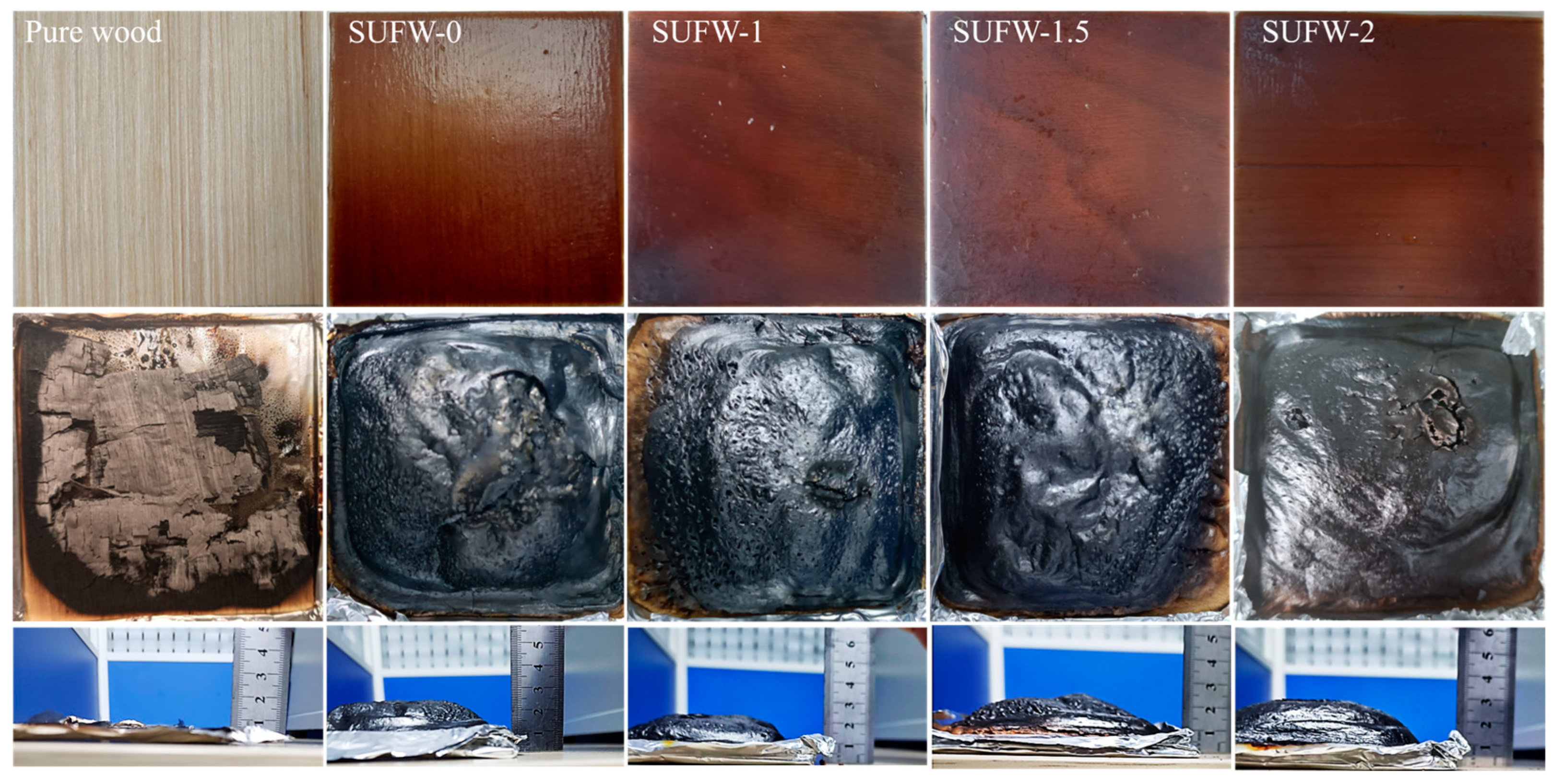
3.3. Flame-Retardant Performance of SUFW
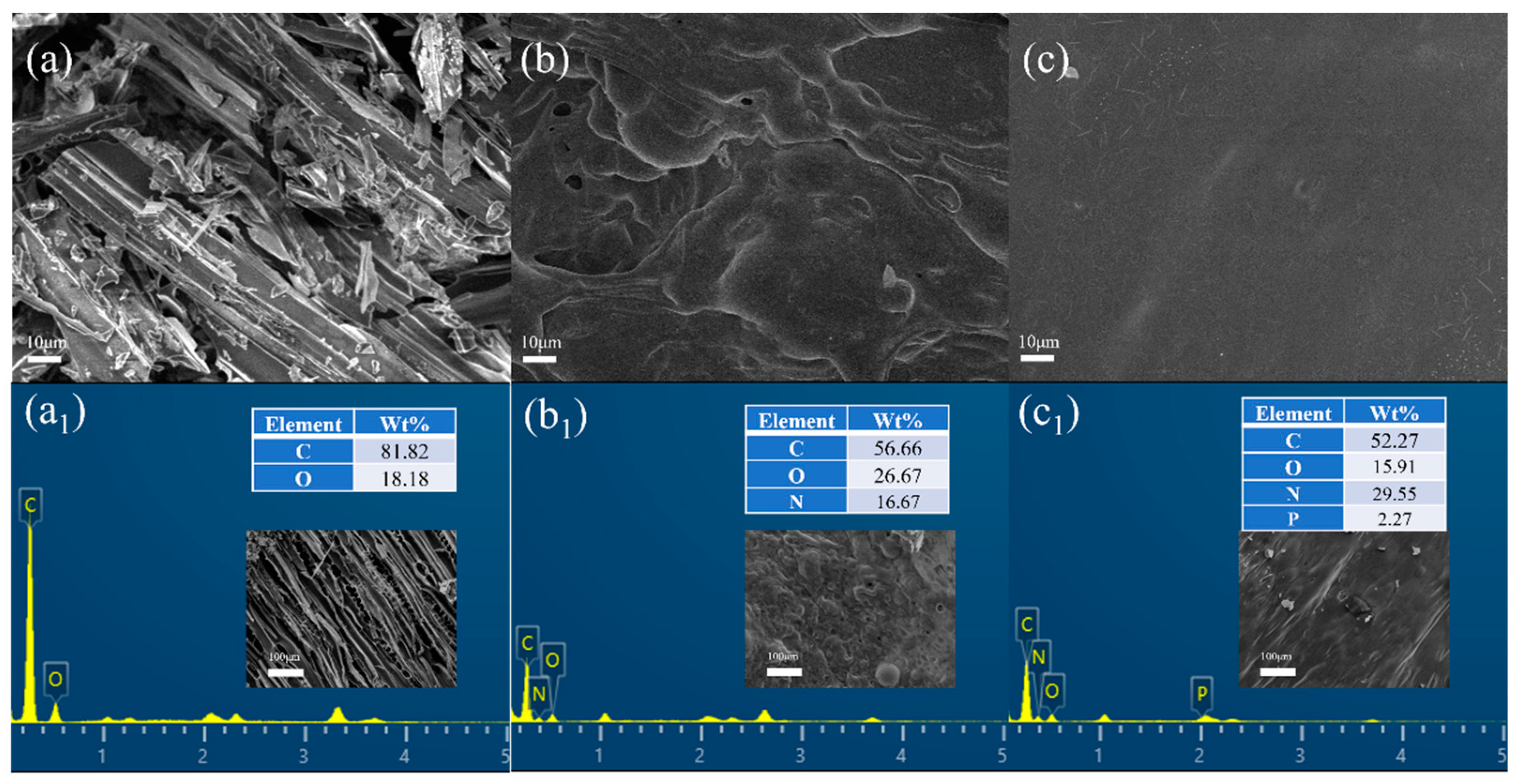
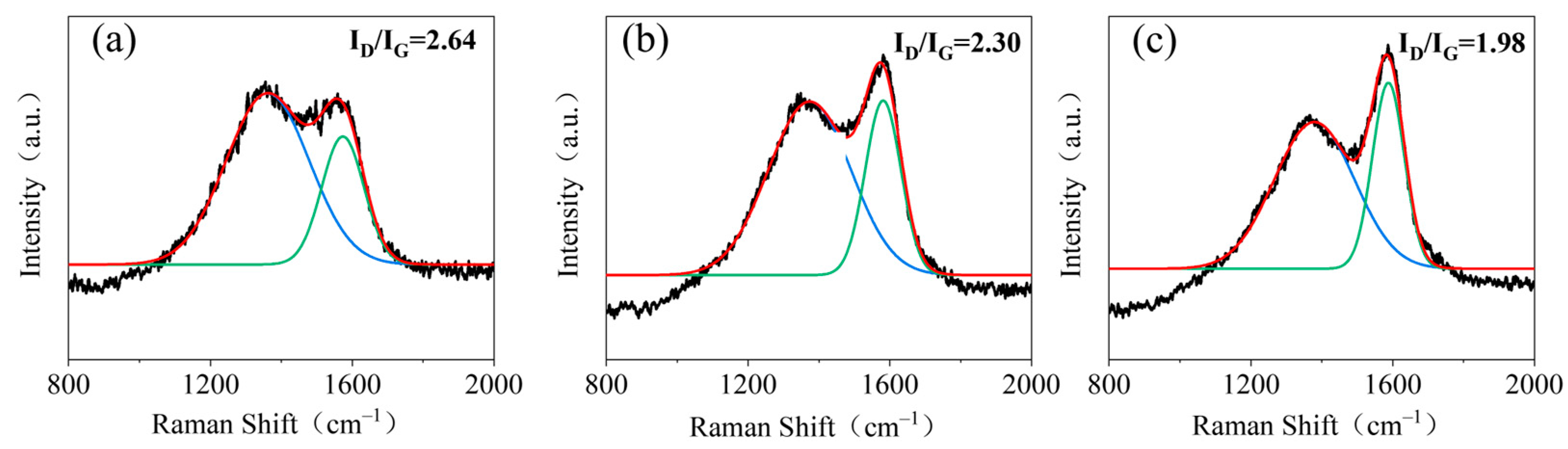
3.4. Investigating the Flame-Retardant Mechanism
3.5. Physical Properties of the Wood Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, C.; Chen, C.; Mi, R.; Li, T.; Dai, J.; Yang, Z.; Pei, Y.; He, S.; Bian, H.; Jang, S.H.; et al. Clear Wood toward High-Performance Building Materials. ACS Nano 2019, 13, 9993–10001. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, C.; Zhu, S.; Zhu, M.; Dai, J.; Ray, U.; Li, Y.; Kuang, Y.; Li, Y.; Quispe, N.; et al. Processing bulk natural wood into a high-performance structural material. Nature 2018, 554, 224–228. [Google Scholar] [CrossRef]
- Chu, T.; Gao, Y.; Yi, L.; Fan, C.; Yan, L.; Ding, C.; Liu, C.; Huang, Q.; Wang, Z. Highly fire-retardant optical wood enabled by transparent fireproof coatings. Adv. Compos. Hybrid Mater. 2022, 5, 1821–1829. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, Z.; Wang, J.; Lyu, C.; Lyu, Y.; Liang, Y.; Nie, B. Unveiling optimal wetting additives for extinguishing wood fires: Insights from molecular simulation and experimental investigations. Surf. Interfaces 2024, 44, 103805. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, T.; Wang, Q.; Guo, C. Synthesis of Biobased Flame-Retardant Carboxylic Acid Curing Agent and Application in Wood Surface Coating. ACS Sustain. Chem. Eng. 2019, 7, 14727–14738. [Google Scholar] [CrossRef]
- Soula, M.; Samyn, F.; Duquesne, S.; Landry, V. Innovative Polyelectrolyte Treatment to Flame-Retard Wood. Polymers 2021, 13, 2884. [Google Scholar] [CrossRef]
- Lainioti, G.C.; Koukoumtzis, V.; Andrikopoulos, K.S.; Tsantaridis, L.; Ostman, B.; Voyiatzis, G.A.; Kallitsis, J.K. Environmentally Friendly Hybrid Organic-Inorganic Halogen-Free Coatings for Wood Fire-Retardant Applications. Polymers 2022, 14, 4959. [Google Scholar] [CrossRef]
- Cho, W.; Shields, J.R.; Dubrulle, L.; Wakeman, K.; Bhattarai, A.; Zammarano, M.; Fox, D.M. Ion—Complexed chitosan formulations as effective fire-retardant coatings for wood substrates. Polym. Degrad. Stab. 2022, 197, 109870. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, Y.; Chen, Y.; Li, L.; Guo, C. Synthesis of eugenol-modified epoxy resin and application on wood flame retardant coating. Ind. Crops Prod. 2022, 183, 114979. [Google Scholar] [CrossRef]
- Bai, J.; Yu, Q. Preparation and characterization of a silicone RAFT-modified aqueous acrylic resin coating for wood antifouling. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132385. [Google Scholar] [CrossRef]
- Rosu, L.; Mustata, F.; Rosu, D.; Varganici, C.-D.; Rosca, I.; Rusu, T. Bio-based coatings from epoxy resins crosslinked with a rosin acid derivative for wood thermal and anti–fungal protection. Prog. Org. Coat. 2021, 151, 106008. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Liu, D. Synthesis and application of novel magnesium phosphate ester flame retardants for transparent intumescent fire-retardant coatings applied on wood substrates. Prog. Org. Coat. 2019, 129, 327–337. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, H.; Gao, Z.; Huang, Y.; Liang, J.; Zhou, H.; Sun, J. Preparation of waterborne intumescent flame-retardant coatings using adenosine-based phosphonates for wood surfaces. Prog. Org. Coat. 2024, 187, 108061. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Deng, N. Effects of polyethylene glycol borate on the flame retardancy and smoke suppression properties of transparent fire-retardant coatings applied on wood substrates. Prog. Org. Coat. 2019, 135, 123–134. [Google Scholar] [CrossRef]
- Song, F.; Liu, T.; Fan, Q.; Li, D.; Ou, R.; Liu, Z.; Wang, Q. Sustainable, high-performance, flame-retardant waterborne wood coatings via phytic acid based green curing agent for melamine-urea-formaldehyde resin. Prog. Org. Coat. 2022, 162, 106597. [Google Scholar] [CrossRef]
- Yu, X.; Su, X.; Liu, Y.; Yu, D.; Ren, Y.; Liu, X. Biomass intumescent flame retardant polyacrylonitrile composite: Flame retardancy, smoke suppression and recycling. Compos. Part A Appl. Sci. Manuf. 2023, 173, 107647. [Google Scholar] [CrossRef]
- Yan, B.-R.; Hu, X.-M.; Cheng, W.-M.; Zhao, Y.-Y.; Wang, W.; Liang, Y.-T.; Liu, T.-Y.; Feng, Y.; Xue, D. A novel intumescent flame-retardant to inhibit the spontaneous combustion of coal. Fuel 2021, 297, 120768. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Wu, C.; Dong, S.; Shen, J.-C.; Guan, J.-P. A sustainable and reactive intumescent flame-retardant containing phytate and triazine-trione for durable functional coating of silk fabric. Prog. Org. Coat. 2023, 182, 107630. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Wu, J. Synergistic catalytic flame retardant effect of zirconium phosphate on the poplar plywood. Constr. Build. Mater. 2021, 290, 123208. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, S.; Zhang, Z.; Mai, C.; Xie, Y.; Wang, Q. Fire retardancy of an aqueous, intumescent, and translucent wood varnish based on guanylurea phosphate and melamine-urea-formaldehyde resin. Prog. Org. Coat. 2018, 121, 64–72. [Google Scholar] [CrossRef]
- Taib, M.N.A.M.; Antov, P.; Savov, V.; Fatriasari, W.; Madyaratri, E.W.; Wirawan, R.; Osvaldová, L.M.; Hua, L.S.; Ghani, M.A.A.; Edrus, S.S.A.O.A.; et al. Current progress of biopolymer-based flame retardant. Polym. Degrad. Stab. 2022, 205, 110153. [Google Scholar] [CrossRef]
- Chen, X.-L.; Zeng, F.-R.; Li, W.-X.; Zhang, L.; Deng, C.; Tan, Y.; Chen, M.-J.; Huang, S.-C.; Liu, B.-W.; Wang, Y.-Z.; et al. Durable flame-retardant, smoke-suppressant, and thermal-insulating biomass polyurethane foam enabled by a green bio-based system. J. Mater. Sci. Techol. 2023, 162, 179–188. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, Y.; Tu, F.; Sun, J.; Zhi, H.; Yang, J. The synergistic flame-retardant behaviors of soybean oil phosphate-based polyols and modified ammonium polyphosphate in polyurethane foam. J. Polym. Res. 2023, 30, 84. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, P.; Liu, Y.; Zhu, P. Green flame-retardant flexible polyurethane foam based on polyphenol-iron-phytic acid network to improve the fire safety. Compos. Part B Eng. 2022, 239, 109958. [Google Scholar] [CrossRef]
- Zheng, X.-T.; Dong, Y.-Q.; Liu, X.-D.; Xu, Y.-L.; Jian, R.-K. Fully bio-based flame-retardant cotton fabrics via layer-by-layer self assembly of laccase and phytic acid. J. Clean. Prod. 2022, 350, 131525. [Google Scholar] [CrossRef]
- Cao, X.; Huang, J.; Tang, Z.; Tong, Y.; Yuen, A.C.Y.; Zhao, W.; Huang, Q.; Li, R.K.Y.; Wu, W. Self-assembled biobased chitosan hybrid carrying N/P/B elements for polylactide with enhanced fire safety and mechanical properties. Int. J. Biol. Macromol. 2023, 236, 123947. [Google Scholar] [CrossRef] [PubMed]
- Patankar, K.C.; Biranje, S.; Pawar, A.; Maiti, S.; Shahid, M.; More, S.; Adivarekar, R.V. Fabrication of chitosan-based finishing agent for flame-retardant, UV-protective, and antibacterial cotton fabrics. Mater. Today Commun. 2022, 33, 104637. [Google Scholar] [CrossRef]
- Li, C.; Wang, B.; Zhou, L.; Hou, X.; Su, S. Effects of lignin-based flame retardants on flame-retardancy and insulation performances of epoxy resin composites. Iran. Polym. J. 2022, 31, 949–962. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Qi, Y.; Wang, H.; Liu, B.; Zhao, Q.; Zhang, J.; Duan, J.; Zhang, L.; Sun, Z.; et al. Improved Mechanical Properties and Flame Retardancy of Wood/PLA All-Degradable Biocomposites with Novel Lignin-Based Flame Retardant and TGIC. Macromo. Master. Eng. 2020, 305, 1900840–1900850. [Google Scholar] [CrossRef]
- Jiang, X.; Chu, F.; Luo, X.; Hu, Y.; Hu, W. Exploring the effects of cardanol-based co-curing agents with different phosphorus structures on the mechanical and flame-retardant properties of bismaleimide resin. Compos. Part B Eng. 2022, 241, 110047. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Gangireddy, C.S.R.; Wang, J.; Pan, Y.; Xing, W.; Song, L.; Hu, Y. Cardanol derived benzoxazine in combination with boron-doped graphene toward simultaneously improved toughening and flame retardant epoxy composites. Compos. Part A Appl. Sci. Manuf. 2019, 116, 13–23. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, T.; Li, J.; Tan, J.; Zhang, M.; Zhou, Y.; Zhu, X. Facile Synthesis of Eugenol-Based Phosphorus/Silicon-Containing Flame Retardant and Its Performance on Fire Retardancy of Epoxy Resin. ACS Appl. Polym. Mater. 2022, 4, 1794–1804. [Google Scholar] [CrossRef]
- Bo, C.; Shi, Z.; Hu, L.; Pan, Z.; Hu, Y.; Yang, X.; Jia, P.; Ren, X.; Zhang, M.; Zhou, Y. Cardanol derived P, Si and N based precursors to develop flame retardant phenolic foam. Sci. Rep. 2020, 10, 12082. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Li, Z.; Bo, C.; Song, F.; Xu, Y.; Hu, L.; Zhou, Y.; Jia, P. Design lignin doped with nitrogen and phosphorus for flame retardant phenolic foam materials. React. Funct. Polym. 2023, 185, 105535. [Google Scholar] [CrossRef]
- He, S.; Deng, C.; Zhao, Z.-Y.; Chen, Z.-X.; Wang, Y.-Z. Hyperbranched polyamide-amine based phosphorous-containing flame retardant for simultaneous flame retardancy and high performance of polypropylene. Compos. Part B Eng. 2023, 250, 110431. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Liu, Y. Green synthesis of chitosan–phytic acid polymers and nanoparticles. Ind. Crops Prod. 2023, 199, 116747. [Google Scholar] [CrossRef]
- Cao, L.; Pizzi, A.; Zhang, Q.; Tian, H.; Lei, H.; Xi, X.; Du, G. Preparation and characterization of a novel environment-friendly urea-glyoxal resin of improved bonding performance. Eur. Polym. J. 2022, 162, 110915. [Google Scholar] [CrossRef]
- Bacigalupe, A.; Molinari, F.; Eisenberg, P.; Escobar, M.M. Adhesive properties of urea-formaldehyde resins blended with soy protein concentrate. Adv. Compos. Hybrid Mater. 2020, 3, 213–221. [Google Scholar] [CrossRef]
- Gao, S.; Cheng, Z.; Zhou, X.; Liu, Y.; Chen, R.; Wang, J.; Wang, C.; Chu, F.; Xu, F.; Zhang, D. Unexpected role of amphiphilic lignosulfonate to improve the storage stability of urea formaldehyde resin and its application as adhesives. Int. J. Biol. Macromol. 2020, 161, 755–762. [Google Scholar] [CrossRef]
- ASTM D3801; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. American Society of Testing Materials: West Conshohocken, PA, USA, 2020.
- ASTM D2863; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-like Combustion of Plastics (Oxygen Index). American Society of Testing Materials: West Conshohocken, PA, USA, 2019.
- ASTM E1354; Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter. American Society of Testing Materials: West Conshohocken, PA, USA, 2023.
- ISO 5660; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate. ISO: Geneva, Switzerland, 2015.
- ASTM D3363-2005; Standard Test Method for Film Hardness by Pencil Test. American Society of Testing Materials: West Conshohocken, PA, USA, 2005.
- ASTM D3359-09; Standard Test Methods for Measuring Adhesion by Tape Test. American Society of Testing Materials: West Conshohocken, PA, USA, 2009.
- ASTM D523; Standard Test Methods for Specular Gloss. American Society of Testing Materials: West Conshohocken, PA, USA, 1999.
- ASTM D870-02; Standard Practice for Testing Water Resistance of Coatings Using Water Immersion. American Society of Testing Materials: West Conshohocken, PA, USA, 2002.
- Li, W.X.; Zhang, H.J.; Hu, X.P.; Yang, W.X.; Cheng, Z.; Xie, C.Q. Highly efficient replacement of traditional intumescent flame retardants in polypropylene by manganese ions doped melamine phytate nanosheets. J. Hazard. Mater. 2020, 398, 123001. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; He, X.; Chen, Y.; Zhang, L.; Zhang, X.; Sheng, X. Green construction of melamine phytate nanosheets for enhancing anti-corrosive performance of water-borne epoxy coatings. J. Mater. Sci. 2022, 57, 4820–4833. [Google Scholar] [CrossRef]
- Li, D.-F.; Zhao, X.; Jia, Y.-W.; Wang, X.-L.; Wang, Y.-Z. Tough and flame-retardant poly(lactic acid) composites prepared via reactive blending with biobased ammonium phytate and in situ formed crosslinked polyurethane. Compos. Commun. 2018, 8, 52–57. [Google Scholar] [CrossRef]
- Qin, Y.; Li, M.; Huang, T.; Shen, C.; Gao, S. A study on the modification of polypropylene by a star-shaped intumescent flame retardant containing phosphorus and nitrogen. Polym. Degrad. Stab. 2022, 195, 109801. [Google Scholar] [CrossRef]
- Sodeifian, G.; Nikooamal, H.R.; Yousefi, A.A. Molecular dynamics study of epoxy/clay nanocomposites: Rheology and molecular confinement. J. Polym. Res. 2012, 19, 9897. [Google Scholar] [CrossRef]

| Coating | MUF (g) | DOP (g) | NH4Cl (g) | PM (g) |
|---|---|---|---|---|
| SUF-0 | 100 | 1 | 1 | 0 |
| SUF-1 | 100 | 1 | 0 | 1 |
| SUF-1.5 | 100 | 1 | 0 | 1.5 |
| SUF-2 | 100 | 1 | 0 | 2 |
| Sample | Td5% (°C) | Tpeak (°C) | R800 (wt.%) |
|---|---|---|---|
| SUF-0 | 235.6 | 275.1 | 14.3 |
| SUF-1 | 253.1 | 305.2 | 32.8 |
| SUF-1.5 | 248.3 | 312.2 | 34.5 |
| SUF-2 | 244.9 | 297.7 | 37.4 |
| Sample | LOI (%) | t1 (s) | t2 (s) | Dropping | UL-94 Rating |
|---|---|---|---|---|---|
| Pure wood | 20.5 | >60 | - | Yes | NR |
| SUFW-0 | 27.2 | 10.7 | 14.5 | No | V-1 |
| SUFW-1 | 29.1 | 5.4 | 11.8 | No | V-0 |
| SUFW-1.5 | 30.4 | 3.8 | 5.6 | No | V-0 |
| SUFW-2 | 32.1 | 1.2 | 4.3 | No | V-0 |
| Samples | UL-94 Rating | LOI (%) | Reference |
|---|---|---|---|
| SUFW-2 | V-0 | 32.1 | This work |
| P-E/DDM+W | V-0 | 32.1 | [9] |
| HCPVC-EP | V-0 | 30.7 | [5] |
| TPLA/PA-HDA10 | V-0 | 30 | [50] |
| EP/14IFR/2Ba | V-0 | 30.5 | [22] |
| Sample | Pure Wood | SUFW-0 | SUFW-1 | SUFW-1.5 | SUFW-2 |
|---|---|---|---|---|---|
| TTI (s) | 95 | 102 | 107 | 173 | 174 |
| PHRR (kW/m2) | 246.48 | 65.56 | 43.26 | 13.87 | 11.25 |
| THR (MJ/m2) | 12.48 | 3.50 | 1.52 | 1.38 | 1.06 |
| TSP (m2) | 52.89 | 42.88 | 39.89 | 31.30 | 22.32 |
| AV-EHC (MJ/kg) | 12.57 | 7.93 | 3.15 | 2.95 | 2.38 |
| FGI (kW/m2/s) | 2.24 | 0.68 | 0.42 | 0.08 | 0.06 |
| Mass (%) | 29.72 | 71.72 | 79.30 | 78.43 | 80.93 |
| Sample | Pencil Hardness | Adhesion | 60° Gloss (Gloss Units) | Water Resistance |
|---|---|---|---|---|
| SUFW-0 | 9H | 5B | 42.3 | No effect on surface |
| SUFW-1 | 8H | 5B | 40.4 | No effect on surface |
| SUFW-1.5 | 8H | 5B | 35.2 | Slight peeling of coating |
| SUFW-2 | 8H | 5B | 31.5 | Slight peeling of coating |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, A.; Wang, S.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Preparation of a Flame-Retardant Curing Agent Based on Phytic Acid–Melamine Ion Crosslinking and Its Application in Wood Coatings. Polymers 2024, 16, 1557. https://doi.org/10.3390/polym16111557
Wei A, Wang S, Zou Y, Xiang C, Xu F, Sun L. Preparation of a Flame-Retardant Curing Agent Based on Phytic Acid–Melamine Ion Crosslinking and Its Application in Wood Coatings. Polymers. 2024; 16(11):1557. https://doi.org/10.3390/polym16111557
Chicago/Turabian StyleWei, An, Shunxiang Wang, Yongjin Zou, Cuili Xiang, Fen Xu, and Lixian Sun. 2024. "Preparation of a Flame-Retardant Curing Agent Based on Phytic Acid–Melamine Ion Crosslinking and Its Application in Wood Coatings" Polymers 16, no. 11: 1557. https://doi.org/10.3390/polym16111557
APA StyleWei, A., Wang, S., Zou, Y., Xiang, C., Xu, F., & Sun, L. (2024). Preparation of a Flame-Retardant Curing Agent Based on Phytic Acid–Melamine Ion Crosslinking and Its Application in Wood Coatings. Polymers, 16(11), 1557. https://doi.org/10.3390/polym16111557






