Improving Pore Characteristics, Mechanical Properties and Thermal Performances of Near-Net Shape Manufacturing Phenolic Resin Aerogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
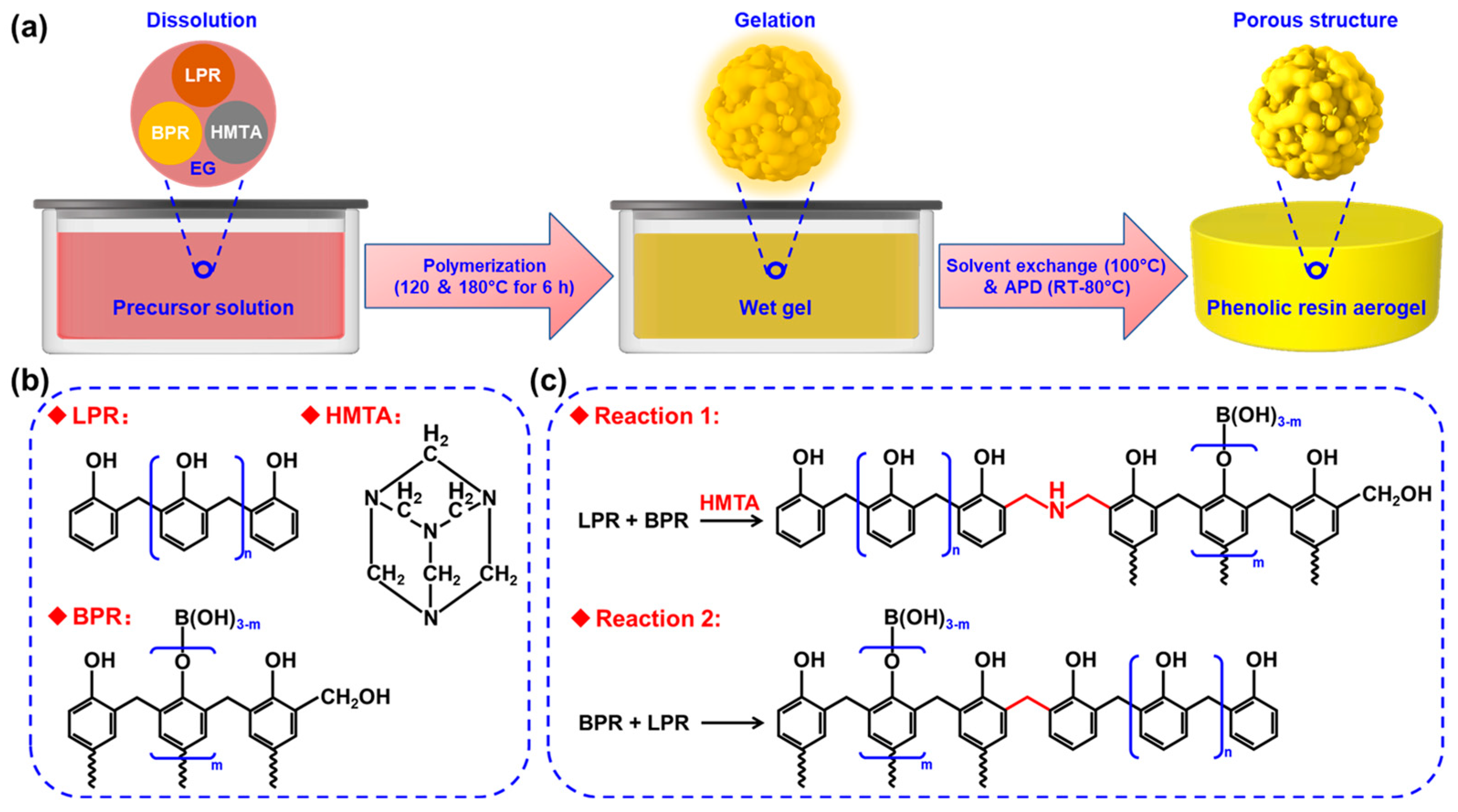
2.2. Synthesis of PRAs
2.3. Characterization
3. Results and Discussion
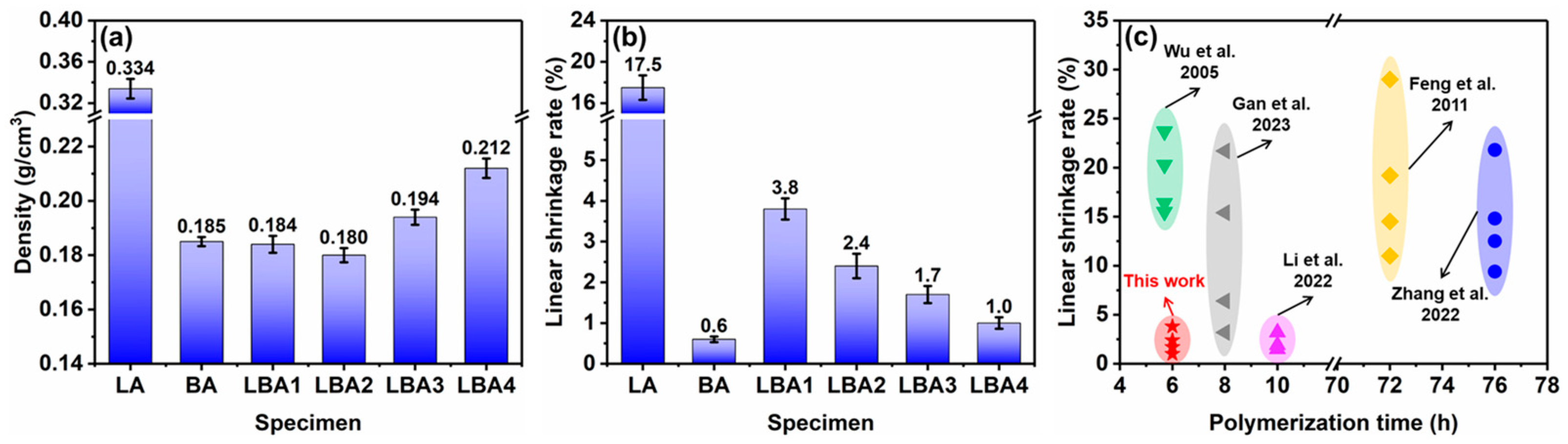
3.1. Shrinkage of Wet Gels
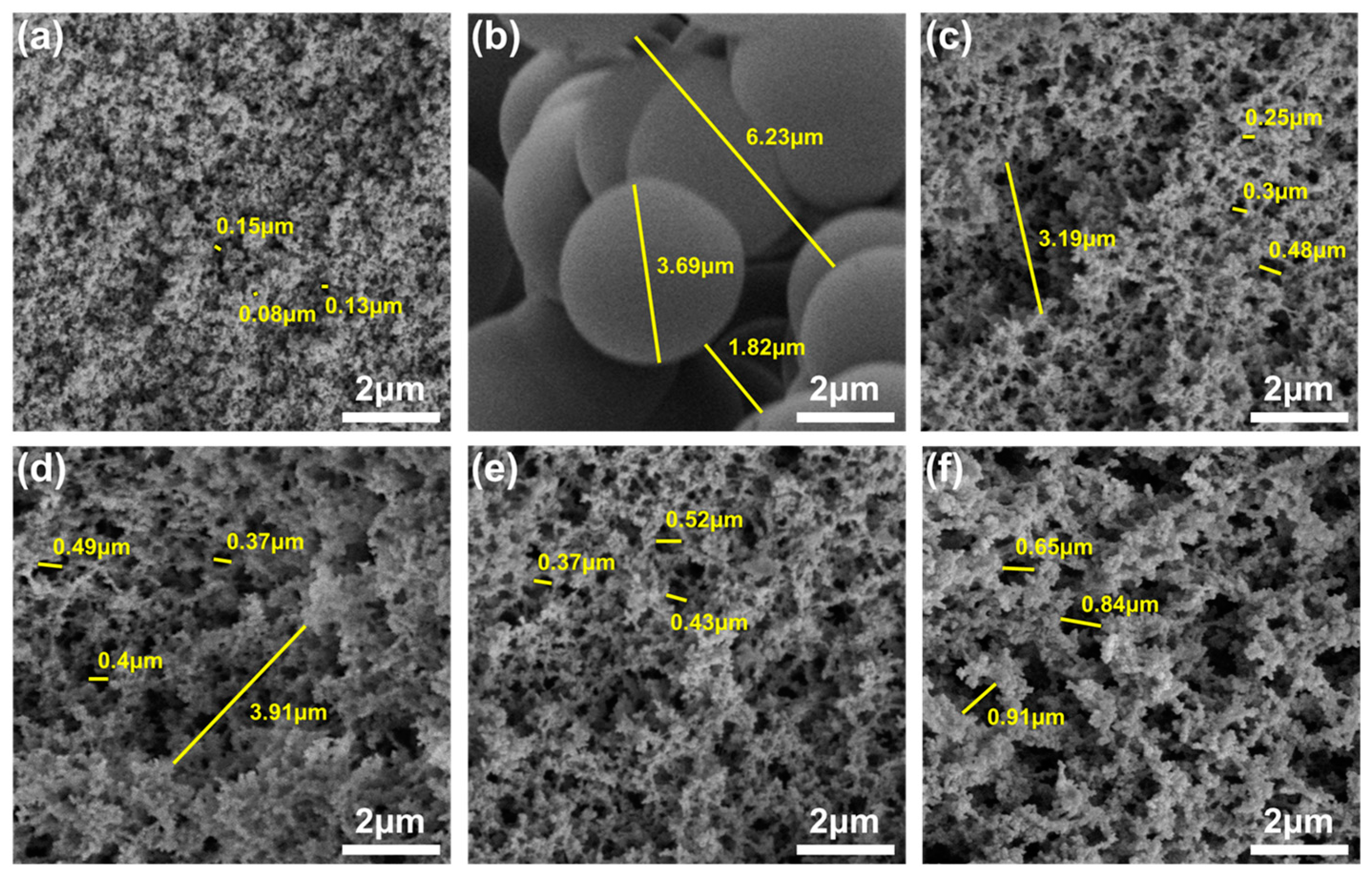
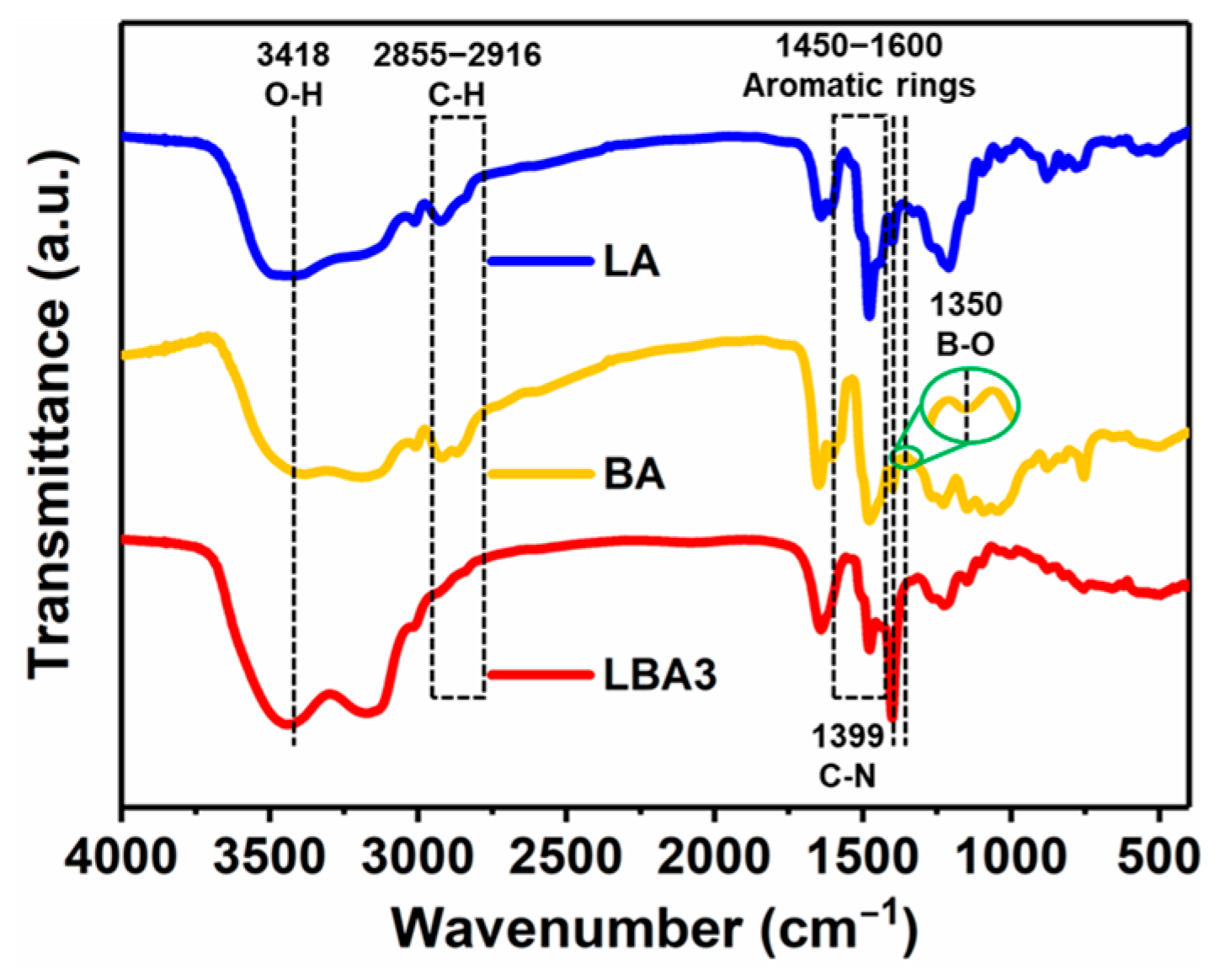
3.2. Microstructure
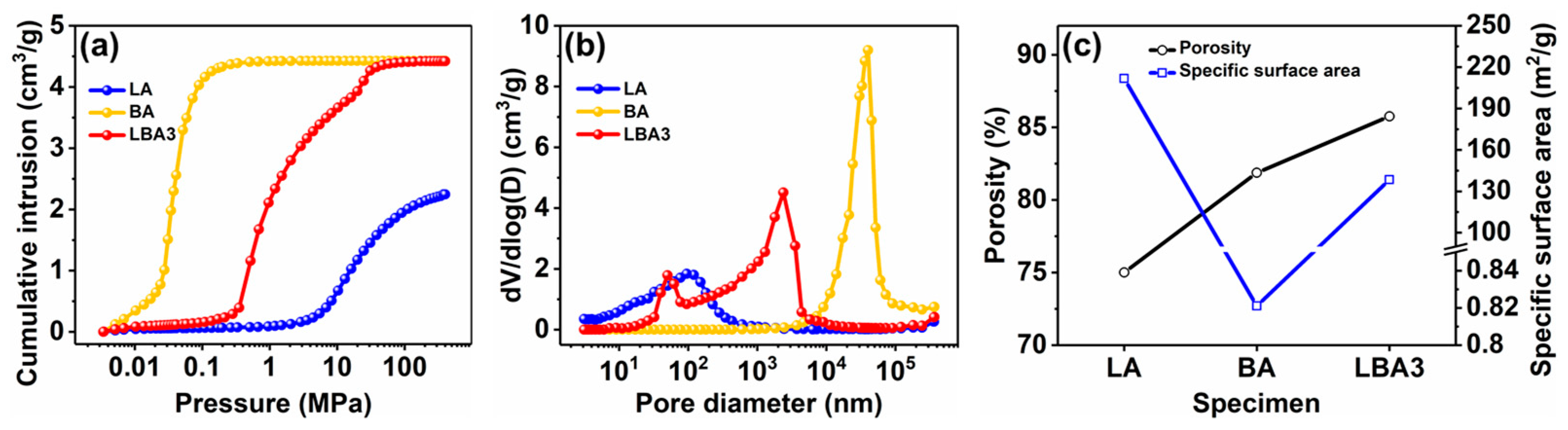
3.3. Pore Characteristic
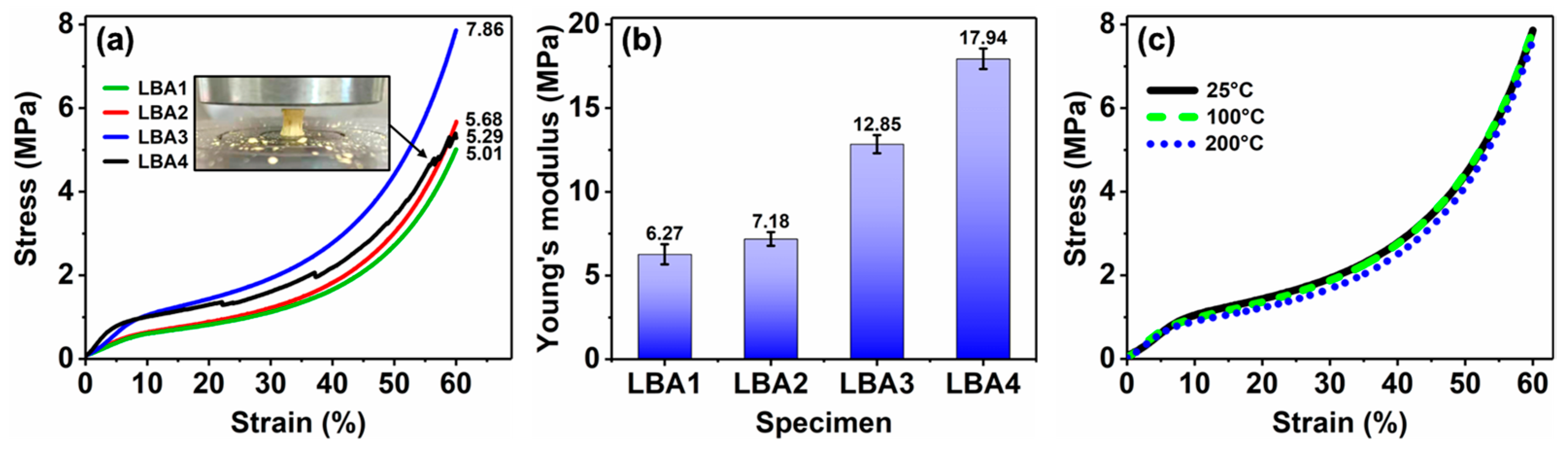
3.4. Mechanical Property
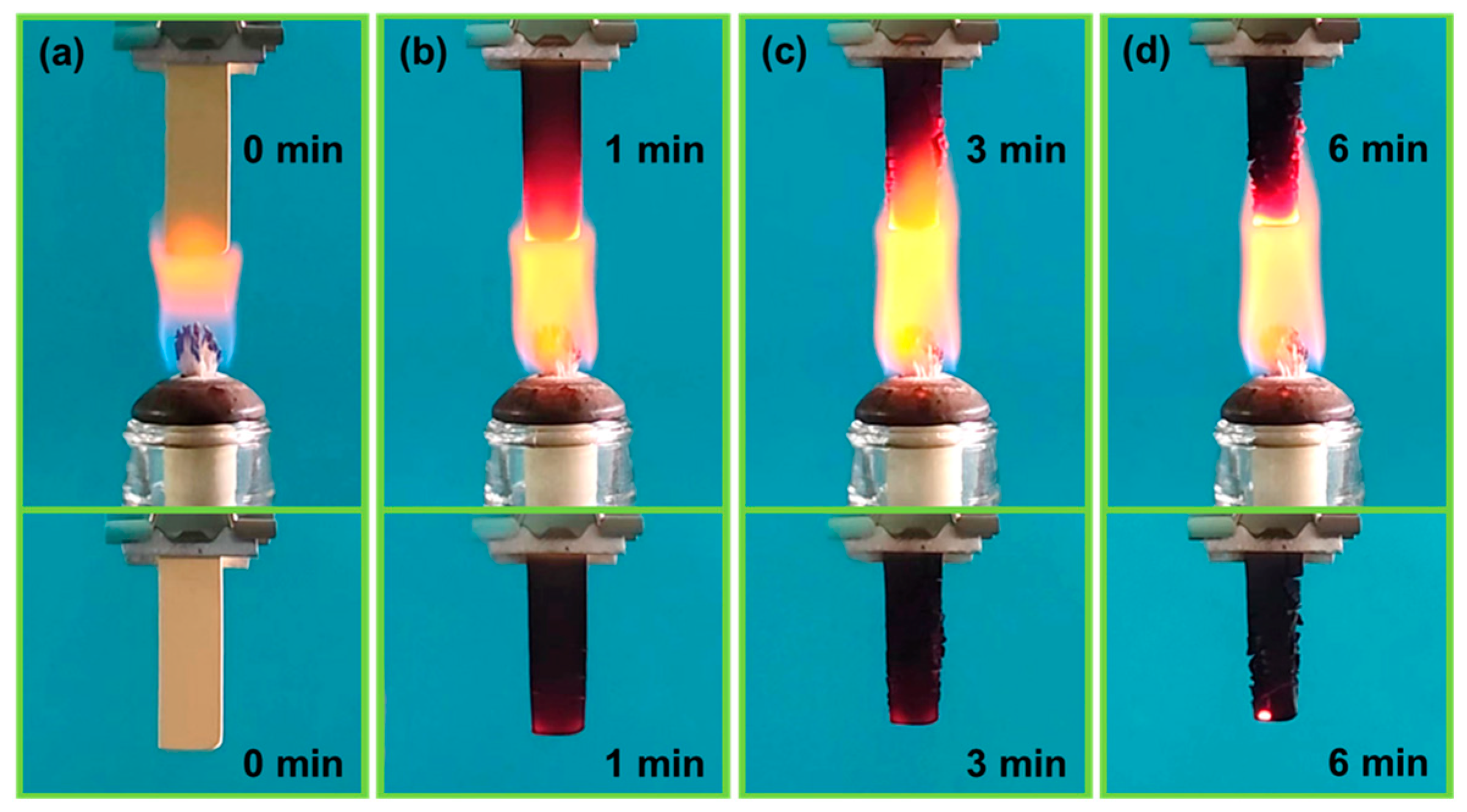
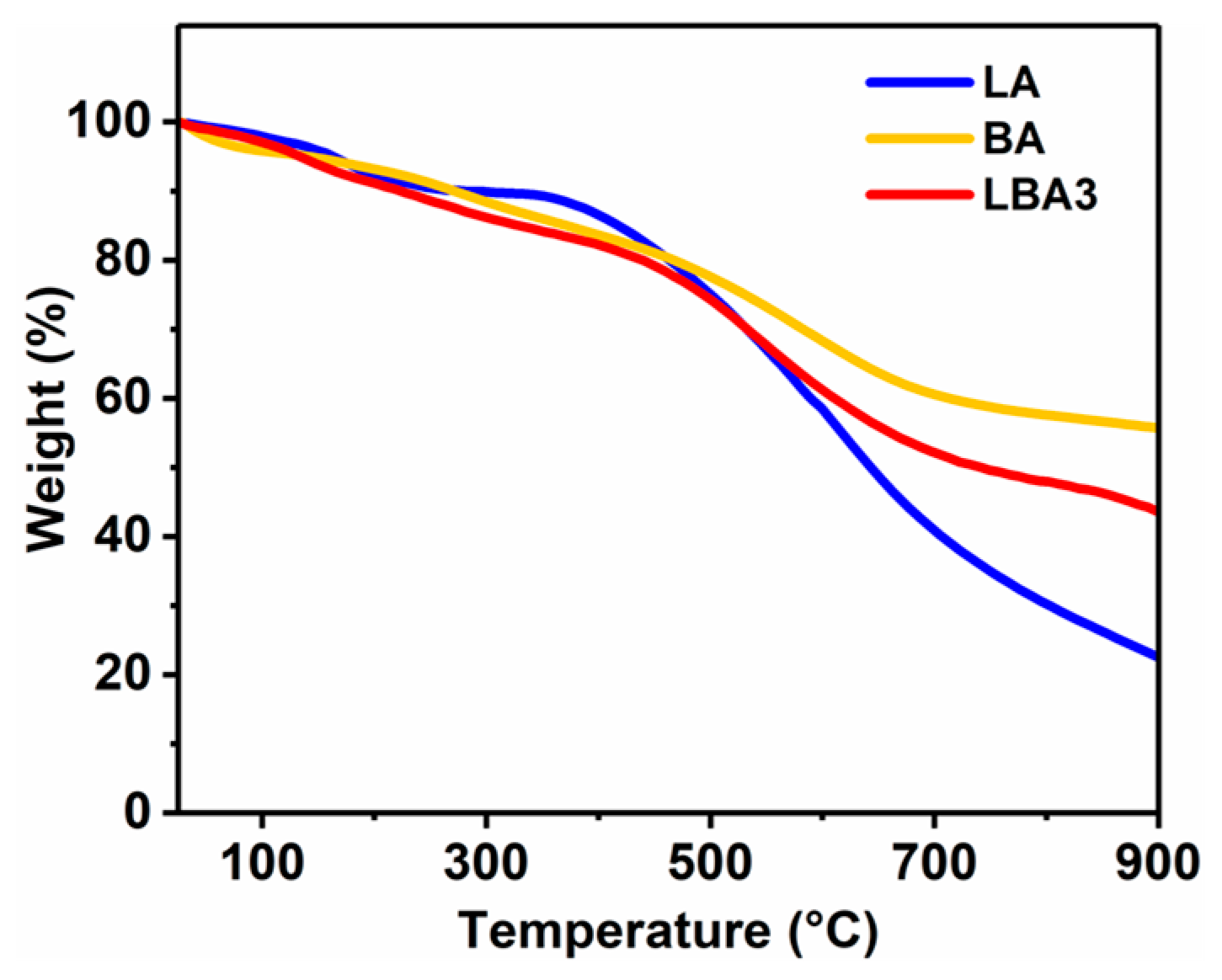
3.5. Thermal Performance
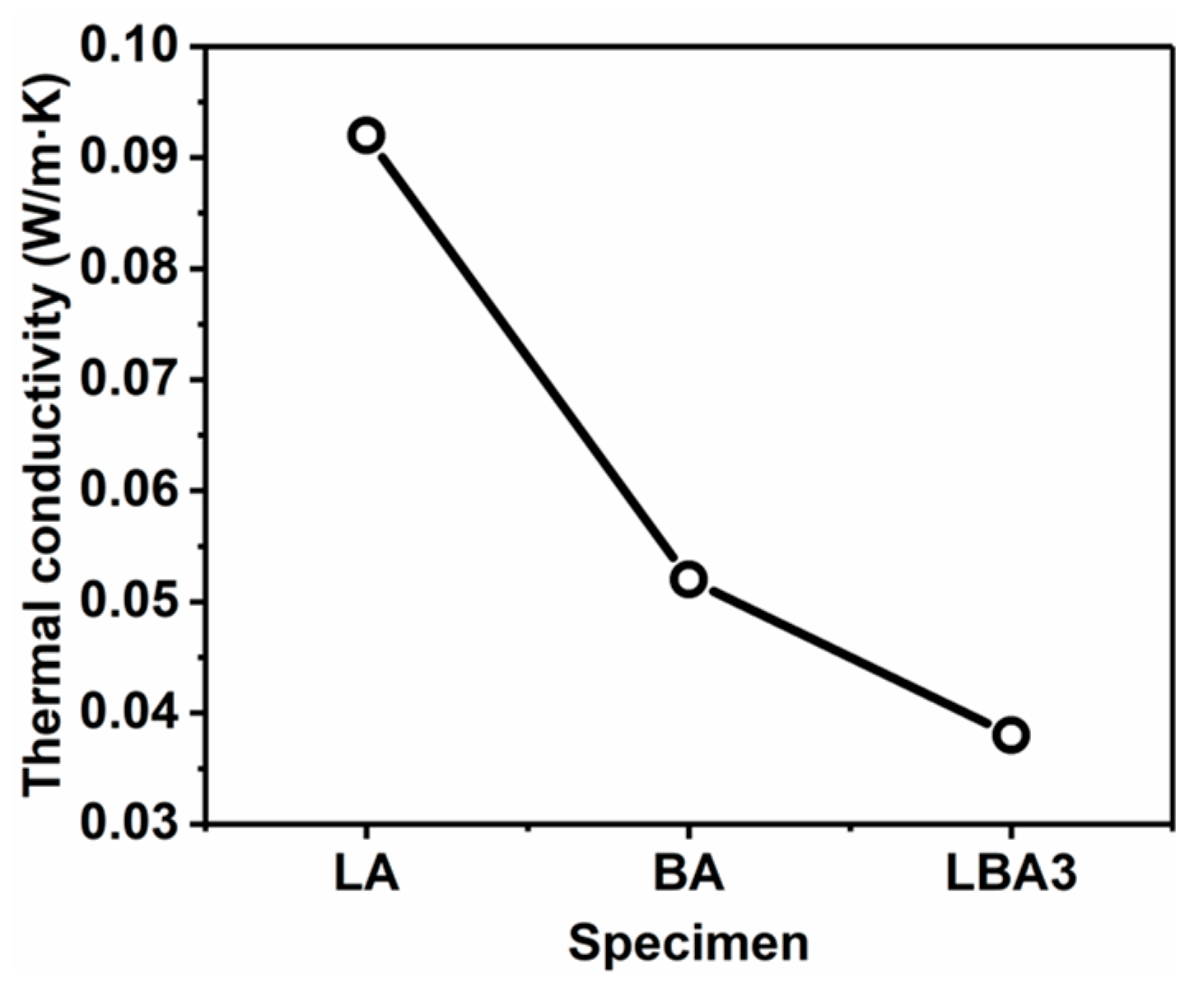
3.6. Thermal Conductivity
4. Conclusions
- (1)
- Through the synergistic effect of LPR and BPR, the shrinkage rate of PRAs can be significantly reduced. The shrinkage rate is dependent on the ratio of raw materials, which varied from 17.5 to 1.7%. The low shrinkage rate is attributed to the enhanced rigidity and the unique bimodal pore size distribution. The bimodal pore size distribution (50.4 nm and 2.4 μm) can effectively decrease the capillary force generated by the solvent evaporation during drying.
- (2)
- The HMTA can affect the porous structure of PRAs. When the HMTA concentration is 0.03 g/mL, the PRAs present a homogeneous and nanoparticles-assembled fibrous 3D network structure. Such a porous structure endows the PRAs with excellent thermal insulation performance. As a result, the PRAs with a density of 0.194 g/cm3 possess a low thermal conductivity of 0.038 W/(m·K).
- (3)
- The PRAs possess outstanding thermo-mechanical stability and almost have no mechanical degradation from 25 to 200 °C. The PRAs can bear a stress of 7.86 MPa without failure at a compressive strain of 60% at 25 °C. Furthermore, the PRAs also show excellent fire retardancy and thermal stability. They are incombustible when exposed to a flame (~600 °C for 6 min). And the obvious pyrolysis starts at temperatures beyond 300 °C based on the TGA test.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Coufal, R.; Fijalkowski, M.; Adach, K.; Bu, H.; Karl, C.W.; Mikyskova, E.; Petrik, S. Preparation and Investigation of High Surface Area Aerogels from Crosslinked Polypropylenes. Polymers 2024, 16, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Leventis, N. Polyurea Aerogels: Synthesis, Material Properties, and Applications. Polymers 2022, 14, 969–1015. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yuan, C.F.; Ding, J.; Zhuang, Y.L.; Li, Y.; Wang, C.K.; Huang, Z.X. A novel method for low-cost and rapid preparation of nanoporous phenolic aerogels and its performance regulation mechanism. Rev. Adv. Mater. Sci. 2022, 61, 817–828. [Google Scholar] [CrossRef]
- Yuan, C.F.; Wang, D.G.; Zhang, Y.J.; Li, K.; Ding, J. Research progress on preparation, modification, and application of phenolic aerogel. Nanotechnol. Rev. 2023, 12, 20230109–20230128. [Google Scholar] [CrossRef]
- Cheng, H.M.; Fan, Z.H.; Hong, C.Q.; Zhang, X.H. Lightweight multiscale hybrid carbon-quartz fiber fabric reinforced phenolic-silica aerogel nanocomposite for high temperature thermal protection. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106313–106321. [Google Scholar] [CrossRef]
- Abdeali, G.; Samani, F.; Hadizadeh-Raiesi, H.; Bahramian, A.R. Enhancement of Novolac aerogel nanostructure and cellulose cork on thermal performance and ablation properties of lightweight heat shields: With regard to omission of thermal convection. Exp. Heat Transf. 2020, 33, 633–649. [Google Scholar] [CrossRef]
- Bian, L.P.; Xiao, J.Y.; Zeng, J.C.; Xing, S.L.; Yin, C.P. Microstructural interpretation of the ablative properties of phenolic-quartz hybrid fabric reinforced phenolic resin composites. Mater. Des. 2014, 62, 424–429. [Google Scholar] [CrossRef]
- De Almeida, L.E.N.; Cunha, F.A.L.; Batista, N.L.; Rocco, J.; Iha, K.; Botelho, E.C. Processing and characterization of ablative composites used in rocket motors. J. Reinf. Plast. Compos. 2014, 33, 1474–1484. [Google Scholar] [CrossRef]
- Harpale, A.; Sawant, S.; Kumar, R.; Levin, D.; Chew, H.B. Ablative thermal protection systems: Pyrolysis modeling by scale-bridging molecular dynamics. Carbon 2018, 130, 315–324. [Google Scholar] [CrossRef]
- Liu, Y.M.; Liu, T.T.; Ye, S.D.; Liu, Y.S. Cost-benefit analysis for Energy Efficiency Retrofit of existing buildings: A case study in China. J. Clean. Prod. 2018, 177, 493–506. [Google Scholar] [CrossRef]
- Lee, J.; Shepley, M.M.; Choi, J. Exploring the effects of a building retrofit to improve energy performance and sustainability: A case study of Korean public buildings. J. Build. Eng. 2019, 25, 100822–100831. [Google Scholar] [CrossRef]
- Yu, Z.L.; Yang, N.; Apostolopoulou-Kalkavoura, V.; Qin, B.; Ma, Z.Y.; Xing, W.Y.; Qiao, C.; Bergstrom, L.; Antonietti, M.; Yu, S.H. Fire-Retardant and Thermally Insulating Phenolic-Silica Aerogels. Angew. Chem. Int. Ed. Engl. 2018, 57, 4538–4542. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.L.; Li, J.; Pang, S.Y.; Hu, C.L.; Tang, S.F.; Cheng, H.M. Ultralight carbon fiber felt reinforced monolithic carbon aerogel composites with excellent thermal insulation performance. Carbon 2021, 183, 525–529. [Google Scholar] [CrossRef]
- Liu, H.; Wang, P.; Zhang, B.; Li, H.; Li, J.; Li, Y.; Chen, Z. Morphology controlled carbon aerogel with enhanced thermal insulation and mechanical properties: A simple route for the regulated synthesis. J. Non-Cryst. Solids 2021, 564, 120828–120833. [Google Scholar] [CrossRef]
- Albert, D.F.; Andrews, G.R.; Mendenhall, R.S.; Bruno, J.W. Supercritical methanol drying as a convenient route to phenolic-furfural aerogels. J. Non-Cryst. Solids 2001, 296, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Gong, L.L.; Xu, X.J.; Zhao, L.; Li, K.; Liang, G.J.; Li, L.; Xie, Q. Synthesis of carbon aerogels with controlled morphology and pore structure to modulate their bulk density and thermal conductivity via a quick one-pot preparation strategy. Carbon 2024, 216, 118487–118495. [Google Scholar] [CrossRef]
- Sha, R.Y.; Cheng, X.P.; Dai, J.X.; Zu, Y.F.; Zeng, Y.Y.; Sha, J.J. Lightweight phenolic resin aerogel with excellent thermal insulation and mechanical properties via an ultralow shrinkage process. Mater. Lett. 2022, 324, 132626–132629. [Google Scholar] [CrossRef]
- Li, J.; Sun, B.H.; Zuo, L.X.; Qu, L.F.; Luo, R.; Pang, S.Y.; Hu, C.L.; Zhao, R.D.; Liang, B.; Cheng, H.M.; et al. Facile preparation of lightweight and robust carbon aerogel monoliths for high-temperature thermal insulation and oil-water separation. Carbon 2024, 218, 118734–118743. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Guo, P.L.; Pang, S.Y.; Hu, C.L.; Zhao, R.D.; Tang, S.F.; Cheng, H.M. Tailoring microstructures of carbon fiber reinforced carbon aerogel-like matrix composites by carbonization to modulate their mechanical properties and thermal conductivities. Carbon 2022, 196, 807–818. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.L.; Wang, W.; Jin, X.Y.; Wang, H.B.; Wu, C.; Pan, Y.W.; Han, W.B.; Hong, C.Q.; Zhang, X.H. Lightweight, Flexible, and Covalent-Bonding Phenolic Fabric Reinforced Phenolic Ablator for Thermal Insulation and Conformal Infrared Stealth. ACS Appl. Mater. Interfaces 2023, 15, 59866–59875. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Hong, C.Q.; Zhang, X.H.; Xue, H.F.; Meng, S.H.; Han, J.C. Super flame-retardant lightweight rime-like carbon-phenolic nanofoam. Sci. Rep. 2016, 6, 33480–33488. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Xue, H.F.; Hong, C.Q.; Zhang, X.H. Preparation, mechanical, thermal and ablative properties of lightweight needled carbon fibre felt/phenolic resin aerogel composite with a bird’s nest structure. Compos. Sci. Technol. 2017, 140, 63–72. [Google Scholar] [CrossRef]
- Wang, H.B.; Pan, Y.W.; Jin, X.Y.; Wu, C.; Huang, H.; Yan, X.J.; Hong, C.Q.; Zhang, X.H. Gradient fiber-reinforced aerogel composites using surface ceramicizable-resin densification with outstanding ablation resistance for high-temperature thermal protection. Compos. Sci. Technol. 2022, 230, 109798–109806. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Su, P.G.; Xiao, L.X.; Zhao, H.B.; Fu, T.; Wang, X.L.; Wang, Y.Z. 4-Hydroxybenzenesulfonic acid triggers rapid preparation of phenolic aerogel composites by ambient pressure drying. Chem. Eng. J. 2024, 479, 147856–147865. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Abidli, A.; Tafreshi, O.A.; Ghaffari-Mosanenzadeh, S.; Buahom, P.; Naguib, H.E.; Park, C.B. Nanocomposite Aerogel Network Featuring High Surface Area and Superinsulation Properties. Chem. Mater. 2024, 36, 642–656. [Google Scholar] [CrossRef]
- Xu, J.G.; Hong, C.Q.; Geng, J.; Jin, X.Y.; Pan, Y.W.; Wang, H.B.; Luo, X.G.; Zhang, X.H. Facile synthesis, mechanical toughening, low thermal conductivity and fire-retardant of lightweight quartz fiber reinforced polymer nanocomposites. Compos. Sci. Technol. 2021, 211, 108836–108844. [Google Scholar] [CrossRef]
- Wu, C.; Huang, H.; Jin, X.Y.; Yan, X.J.; Wang, H.B.; Pan, Y.W.; Zhang, X.H.; Hong, C.Q. Water-assisted synthesis of phenolic aerogel with superior compression and thermal insulation performance enabled by thick-united nano-structure. Chem. Eng. J. 2023, 464, 142805–142813. [Google Scholar] [CrossRef]
- Li, J.; Guo, P.; Hu, C.; Pang, S.; Ma, J.; Zhao, R.; Tang, S.; Cheng, H.-M. Fabrication of Large Aerogel-Like Carbon/Carbon Composites with Excellent Load-Bearing Capacity and Thermal-Insulating Performance at 1800 °C. ACS Nano 2022, 16, 6565–6577. [Google Scholar] [CrossRef]
- Jia, X.F.; Dai, B.W.; Zhu, Z.X.; Wang, J.T.; Qiao, W.M.; Long, D.H.; Ling, L.C. Strong and machinable carbon aerogel monoliths with low thermal conductivity prepared via ambient pressure drying. Carbon 2016, 108, 551–560. [Google Scholar] [CrossRef]
- Wu, D.C.; Fu, R.W.; Sun, Z.Q.; Yu, Z.Q. Low-density organic and carbon aerogels from the sol-gel polymerization of phenol with formaldehyde. J. Non-Cryst. Solids 2005, 351, 915–921. [Google Scholar] [CrossRef]
- Feng, J.Z.; Zhang, C.R.; Feng, J.; Jiang, Y.G.; Zhao, N. Carbon Aerogel Composites Prepared by Ambient Drying and Using Oxidized Polyacrylonitrile Fibers as Reinforcements. ACS Appl. Mater. Interfaces 2011, 3, 4796–4803. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.R.; Guo, L.F.; Zhang, H.N.; Zhai, H.M.; Ren, H. Research status, industrial application demand and prospects of phenolic resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef] [PubMed]
- ASTM D5930; Standard Test Method for Thermal Conductivity of Plastics by Means of a Transient Line-Source Technique. ASTM International: West Conshohocken, PA, USA, 2017.
- Gan, Z.C.; Yang, Z.C.; Zhang, Z.; Li, K.F.; Fei, Z.F.; Li, X.H.; Zhang, P.; Song, Y.L.; Zhao, S. Low-density, high-strength and large-scaled monolithic carbon aerogels fabricated via modified ambient pressure drying. J. Mater. Sci. 2023, 58, 3038–3052. [Google Scholar] [CrossRef]
- Wang, F.Y.; Huang, Z.X.; Guo, Z.G. Bionic boron/silicon-modified phenolic resin system with multifunctional groups: Synthesis, thermal properties and ablation mechanism. Biosurf. Biotribol. 2018, 4, 85–93. [Google Scholar] [CrossRef]
- He, H.; Geng, L.Y.; Liu, F.; Ma, B.; Huang, W.X.; Qu, L.J.; Xu, B.S. Facile preparation of a phenolic aerogel with excellent flexibility for thermal insulation. Eur. Polym. J. 2021, 163, 110905–110913. [Google Scholar] [CrossRef]
- He, Y.L.; Xie, T. Advances of thermal conductivity models of nanoscale silica aerogel insulation material. Appl. Therm. Eng. 2015, 81, 28–50. [Google Scholar] [CrossRef]
- Noisser, T.; Reichenauer, G.; Hüsing, N. In Situ Modification of the Silica Backbone leading to Highly Porous Monolithic Hybrid Organic-Inorganic Materials via Ambient Pressure Drying. ACS Appl. Mater. Interfaces 2014, 6, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.X.; Wang, P.; Cai, H.X.; Niu, B.; Zhang, Y.Y.; Long, D.H. Lightweight Nitrogen-Doped Phenolic Aerogels with Flame-Retardant and Thermal-Insulation Properties. ACS Appl. Polym. Mater. 2023, 5, 10276–10288. [Google Scholar] [CrossRef]
- Su, R.Y.; Wang, X.B.; Wang, D.G.; Li, L.; Liang, G.J.; Zheng, Z.X.; Li, K. Preparation of carbon foam-reinforced carbon aerogels and their copyrolysis mechanism. Microporous Mesoporous Mater. 2021, 319, 111059. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180–1194. [Google Scholar] [CrossRef]
- Li, B.; Feng, S.H.; Niasar, H.S.; Zhang, Y.S.; Yuan, Z.S.; Schmidt, J.; Xu, C. Preparation and characterization of bark-derived phenol formaldehyde foams. RSC Adv. 2016, 6, 40975–40981. [Google Scholar] [CrossRef]
- Chen, X.K.; Yu, W.C.; Ma, L.; Zhou, S.S.; Liu, X.X. Mechanical properties and thermal characteristics of different-density phenolic foams. J. Therm. Anal. Calorim. 2021, 144, 393–401. [Google Scholar] [CrossRef]
- Wu, K.D.; Dong, W.; Pan, Y.K.; Cao, J.X.; Zhang, Y.Y.; Long, D.H. Lightweight and Flexible Phenolic Aerogels with Three-Dimensional Foam Reinforcement for Acoustic and Thermal Insulation. Ind. Eng. Chem. Res. 2021, 60, 1241–1249. [Google Scholar] [CrossRef]
- Deng, P.; Shi, Y.; Liu, Y.S.; Liu, Y.; Wang, Q. Solidifying process and flame retardancy of epoxy resin cured with boron-containing phenolic resin. Appl. Surf. Sci. 2018, 427, 894–904. [Google Scholar] [CrossRef]
- Feng, J.J.; Chen, L.X.; Gu, J.W.; He, Z.Y.; Yun, J.; Wang, X.B. Synthesis and characterization of aryl boron-containing thermoplastic phenolic resin with high thermal decomposition temperature and char yield. J. Polym. Res. 2016, 23, 97–103. [Google Scholar] [CrossRef]
- Zhang, W.F.; Liu, C.L.; Ying, Y.G.; Dong, W.S. The preparation and characterization of boron-containing phenolic fibers. Mater. Chem. Phys. 2010, 121, 89–94. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]


| Specimen | LPR (g/mL) | BPR (g/mL) | HMTA (g/mL) |
|---|---|---|---|
| LA | 0.193 | 0 | 0.03 |
| BA | 0 | 0.193 | 0.03 |
| LBA1 | 0.15 | 0.043 | 0.017 |
| LBA2 | 0.15 | 0.043 | 0.021 |
| LBA3 | 0.15 | 0.043 | 0.03 |
| LBA4 | 0.15 | 0.043 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, R.; Dai, J.; Wang, B.; Sha, J. Improving Pore Characteristics, Mechanical Properties and Thermal Performances of Near-Net Shape Manufacturing Phenolic Resin Aerogels. Polymers 2024, 16, 1593. https://doi.org/10.3390/polym16111593
Sha R, Dai J, Wang B, Sha J. Improving Pore Characteristics, Mechanical Properties and Thermal Performances of Near-Net Shape Manufacturing Phenolic Resin Aerogels. Polymers. 2024; 16(11):1593. https://doi.org/10.3390/polym16111593
Chicago/Turabian StyleSha, Ruyi, Jixiang Dai, Bingzhu Wang, and Jianjun Sha. 2024. "Improving Pore Characteristics, Mechanical Properties and Thermal Performances of Near-Net Shape Manufacturing Phenolic Resin Aerogels" Polymers 16, no. 11: 1593. https://doi.org/10.3390/polym16111593
APA StyleSha, R., Dai, J., Wang, B., & Sha, J. (2024). Improving Pore Characteristics, Mechanical Properties and Thermal Performances of Near-Net Shape Manufacturing Phenolic Resin Aerogels. Polymers, 16(11), 1593. https://doi.org/10.3390/polym16111593







