Characterization of Cellulosic Pulps Isolated from Two Widespread Agricultural Wastes: Cotton and Sunflower Stalks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Conditioning of CS and SFS Wastes
2.2. Extraction of Cellulose from CS and SFS Fibers
2.3. Characterization of CS and SFS Fibers
2.3.1. Chemical Composition
2.3.2. Scanning Electron Microscopy (SEM), Fourier Transformed Infrared (FT-IR) Spectroscopy, X-ray Diffraction (XRD), and Thermogravimetric Analysis (TGA)
3. Results
3.1. Chemical Composition of CS and SFS Fibers and Cellulose Isolation Process
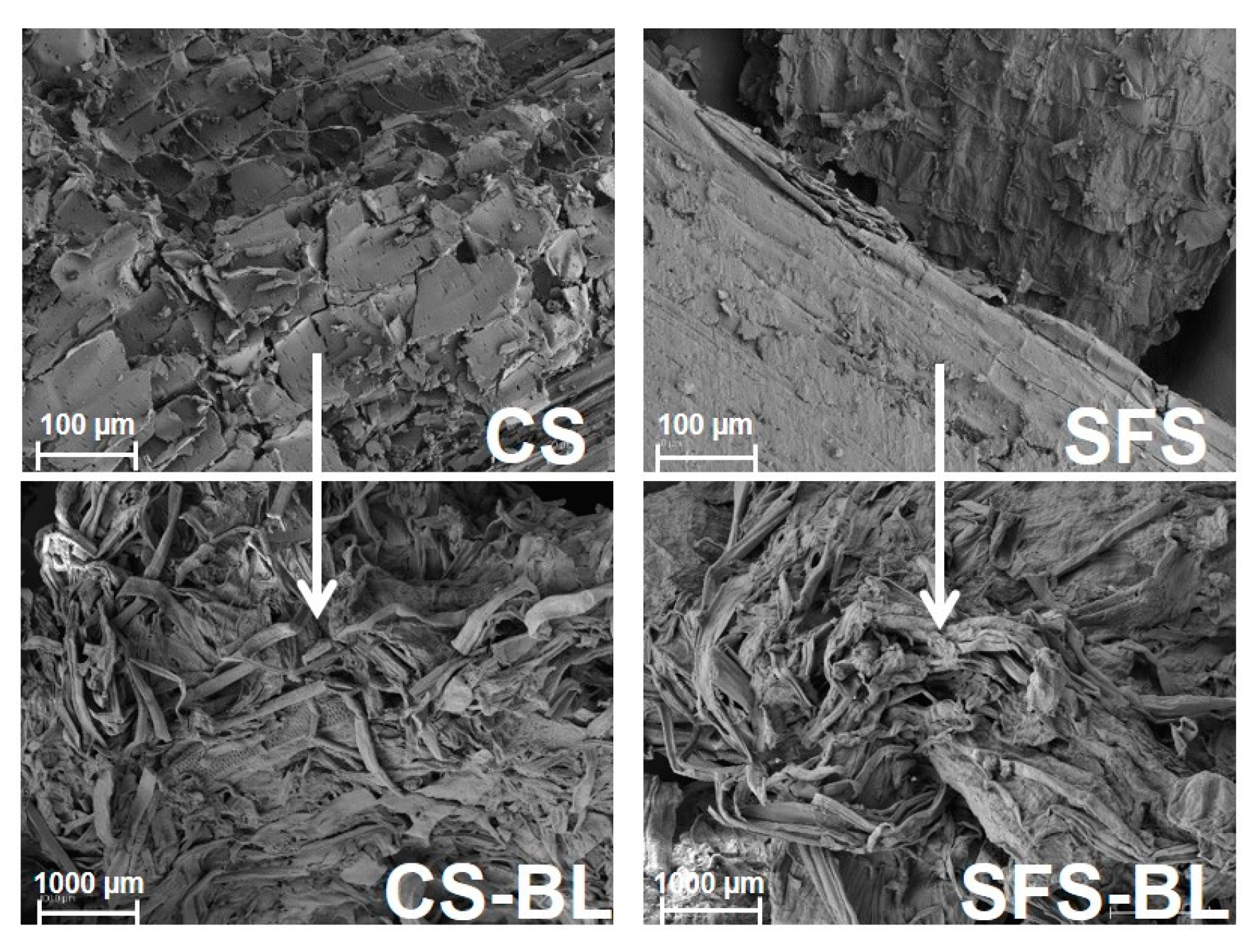
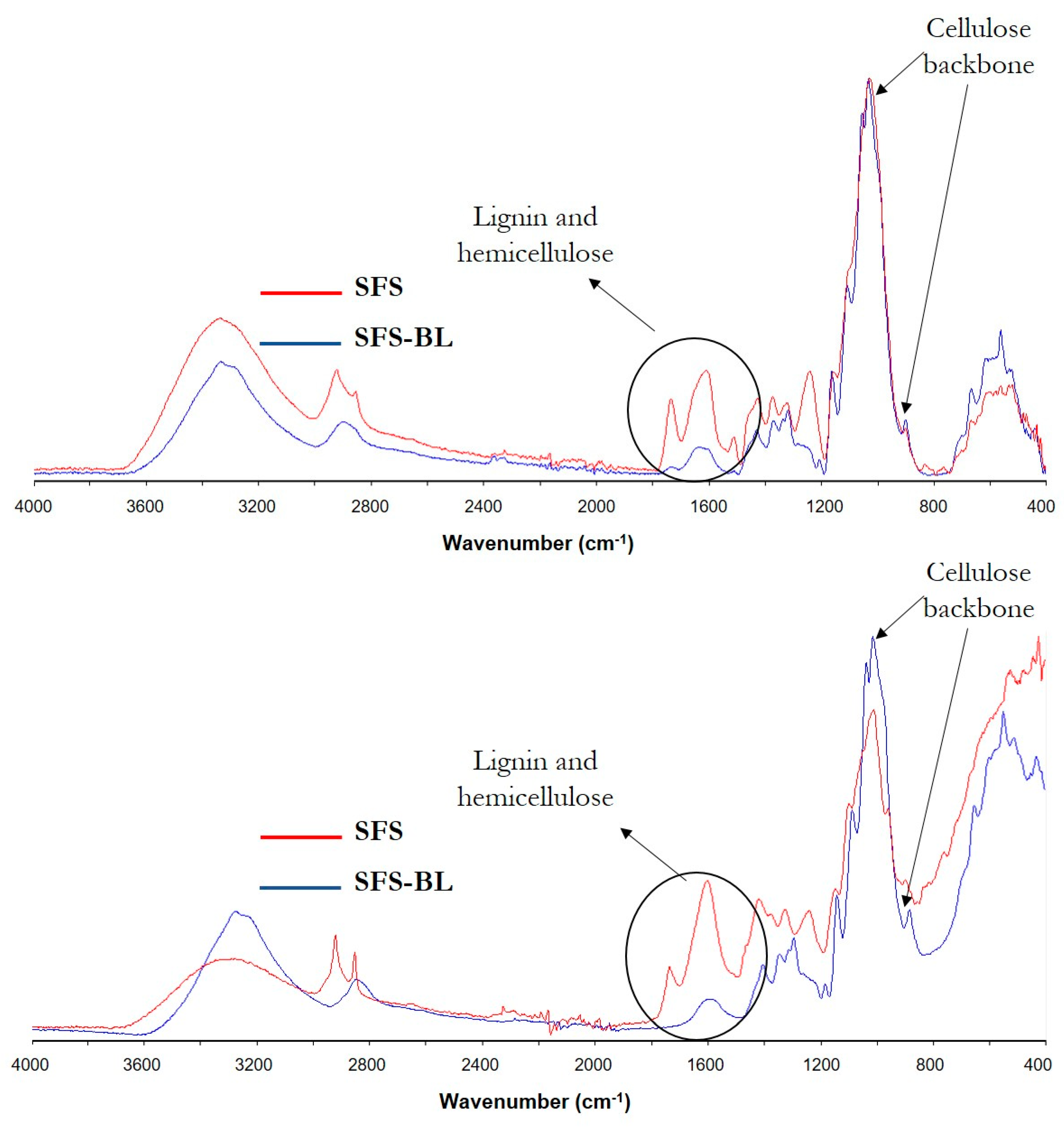
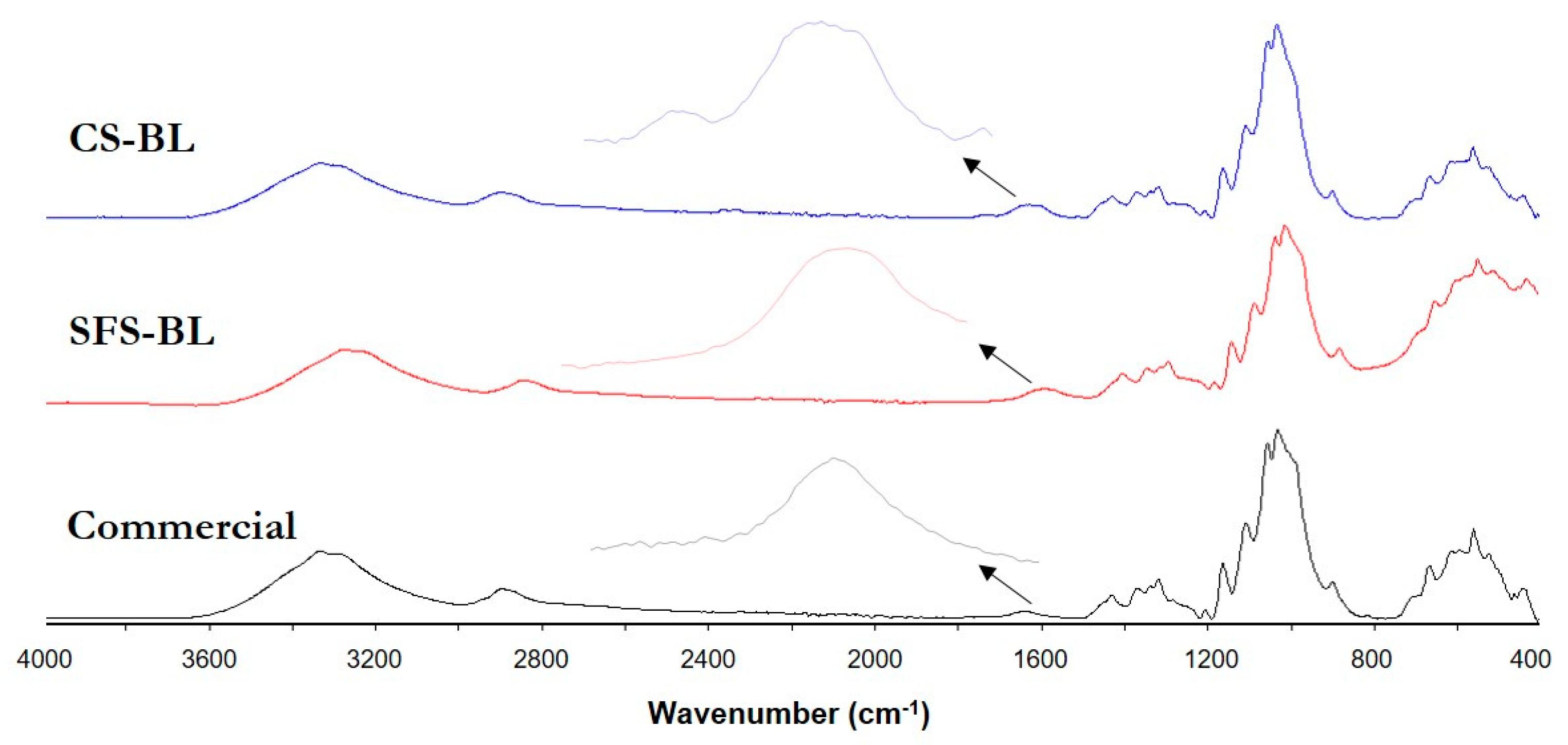
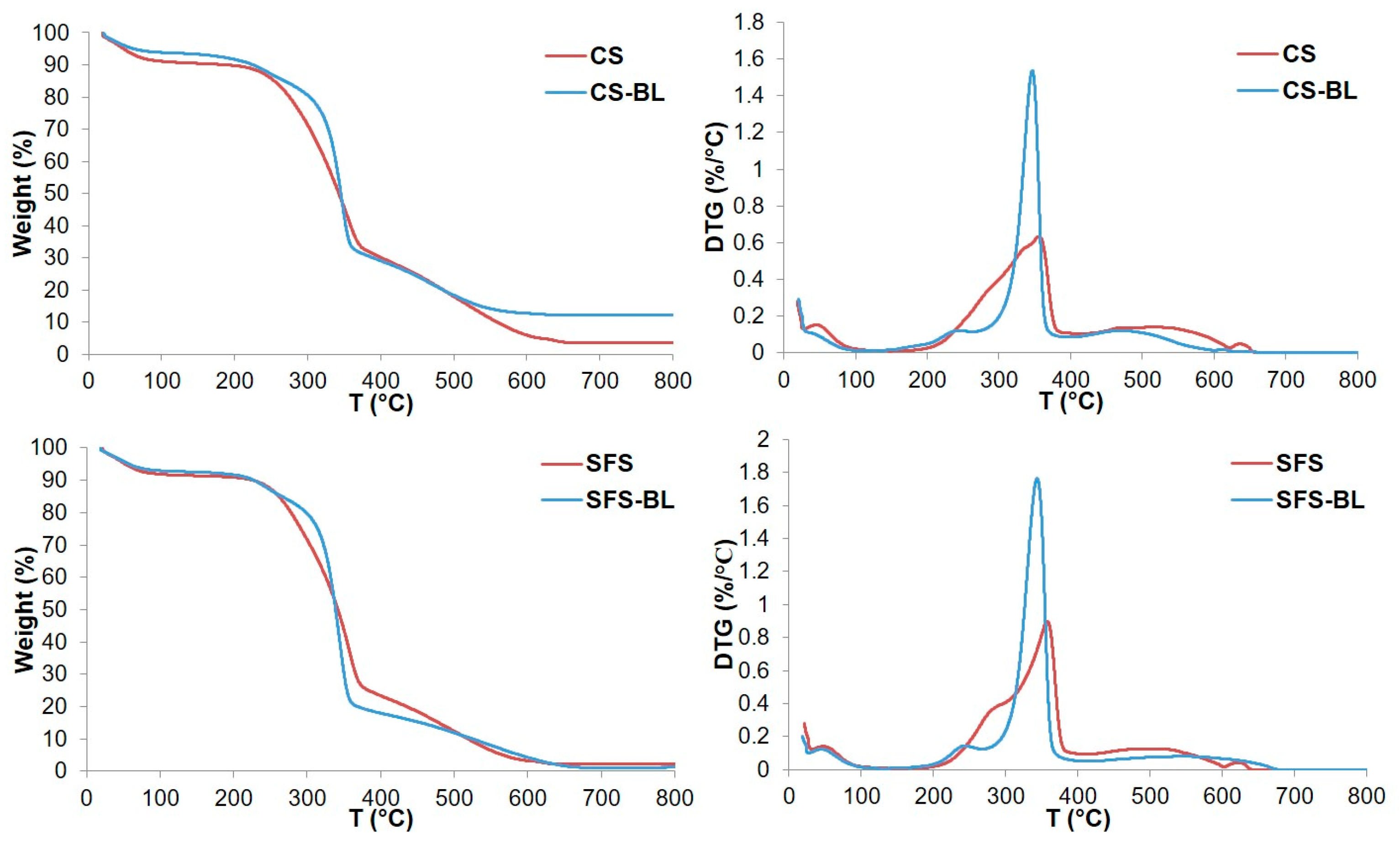
3.2. Monitoring of Cellulose Isolation. Characterization of the Fibers by SEM, FT-IR, XRD and TGA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Fernandes Antunes, F.A.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.D.; Patel, A.K.; Puri, M. Global status of lignocellulosic biorefinery: Challenges and perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef] [PubMed]
- Grace Karp, S.; Bittencourt Sydney, E.; Lorenci Woiciechowski, A.; Junior Letti, L.A.; de Carvalho, J.C.; Zevallos Torres, L.A.; Sprotte Kumlehn, G.; de Souza Candeo, E.; Soccol, C.R. Lignocellulosic biorefinery for value-added products: The emerging bioeconomy. In Biomass, Biofuels, Biochemicals. Circular Bioeconomy—Current Developments and Future Outlook; Pandey, A., Dayal Tyagi, R., Varjani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 221–391. [Google Scholar] [CrossRef]
- Ojo, A.O. An overview of lignocellulose and its biotechnological importance in high-value product production. Fermentation 2023, 9, 990. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Fermentation 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Dean Smith, M. An abbreviated historical and structural introduction to lignocellulose. In Understanding Lignocellulose: Synergistic Computational and Analytic Methods; Dean Smith, M., Ed.; ACS Symposium Series: Washington, DC, USA, 2019; Volume 1338, pp. 1–15. [Google Scholar] [CrossRef]
- Kumar, P.; Joshi, L. Pollution caused by agricultural waste burning and possible alternate uses of crop stubble: A case study of Punjab. In Knowledge Systems of Societies for Adaptation and Mitigation of Impacts of Climate Change; Nautiyal, S., Rao, K., Kaechele, H., Raju, K., Schaldach, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 367–385. [Google Scholar] [CrossRef]
- de Zárate, I.O.; Ezcurra, A.; Lacaux, J.P.; Van Dinh, P.; de Argandoña, J.D. Pollution by cereal waste burning in Spain. Atmos. Res. 2005, 73, 161–170. [Google Scholar] [CrossRef]
- Shi, T.; Liu, Y.; Zhang, L.; Hao, L.; Gao, Z. Burning in agricultural landscapes: An emerging natural and human issue in China. Landsc. Ecol. 2014, 29, 1785–1798. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Hao, L. Contributions of open crop straw burning emissions to PM2.5 concentrations in China. Environ. Res. Lett. 2016, 11, 014014. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023; pp. 235–246. [Google Scholar]
- Cotton. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/cotton_en (accessed on 15 February 2024).
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef] [PubMed]
- Production Volume of Sunflower Seed in Major Producer Countries in 2023/2024. Available online: https://www.statista.com/statistics/263928/production-of-sunflower-seed-since-2000-by-major-countries/ (accessed on 17 February 2024).
- OLEAGINOSAS EN ESPAÑA, EN LA UE-27 Y EN EL MUNDO (Girasol, Colza y Soja). Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/anexooleaginosas2021_tcm30-563740.pdf (accessed on 17 February 2024).
- Silanikove, N.; Levanon, D. Cotton straw: Composition, variability and effect of anaerobic preservation. Biomass 1986, 9, 101–112. [Google Scholar] [CrossRef]
- Marechal, V.; Rigal, L. Characterization of by-products of sunflower culture—Commercial applications for stalks and heads. Ind. Crop. Prod. 1999, 10, 185–200. [Google Scholar] [CrossRef]
- Rahbar Shamskar, K.; Heidari, H.; Rashidi, A. Preparation and evaluation of nanocrystalline cellulose aerogels from raw cotton and cotton stalk. Ind. Crop. Prod. 2016, 93, 203–211. [Google Scholar] [CrossRef]
- Mussana, H.; Yang, X.; Tessima, M.; Han, F.; Igbal, N.; Liu, L. Preparation of lignocellulose aerogels from cotton stalks in the ionic liquid-based co-solvent system. Ind. Crop. Prod. 2018, 113, 225–233. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.; Jiang, C.; Ma, L.; Yu, P. Cellulose nanocrystals from cotton stalk for reinforcement of poly(vinyl alcohol) composites. Cellul. Chem. Technol. 2017, 51, 109–119. [Google Scholar]
- Li, M.; He, B.; Chen, Y.; Zhao, L. Physicochemical properties of nanocellulose isolated from cotton stalk waste. ACS Omega 2021, 6, 25162–25169. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Cara, C.; Ballesteros, M.; Manzanares, P.; Ballesteros, I.; Castro, E. Ethanol production from pretreated olive tree wood and sunflower stalks by an SSF process. In Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals. ABAB Symposium; McMillan, J.D., Adney, W.S., Mielenz, J.R., Klasson, K.T., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 129–132, pp. 631–643. [Google Scholar] [CrossRef]
- Nargotra, P.; Sharma, V.; Gupta, M.; Kour, S.; Kumar Bajaj, B. Application of ionic liquid and alkali pretreatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour. Technol. 2018, 267, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Guo, X.M.; Latrille, E.; Trably, E.; Steyer, J.P.; Carrere, H. Predictive models of biohydrogen and biomethane production based on the compositional and structural features of lignocellulosic materials. Environ. Sci. Technol. 2012, 46, 12217–12225. [Google Scholar] [CrossRef] [PubMed]
- Passos Santos, B.L.; Santos Jesus, M.; Mata, F.; Oliveira Santos Prado, A.A.; Monteiro Vieira, I.M.; Castor Ramos, L.; López, J.A.; Vaz-Velho, M.; Santos Ruzene, D.; Pereira Silva, D. Use of agro-industrial waste for biosurfactant production: A comparative study of hemicellulosic liquors from corncobs and sunflower stalks. Sustainability 2023, 15, 6341. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, L.; Ma, X.; Zhou, X.; Xu, Y. Revalorization of sunflower stalk pith as feedstock for the coproduction of pectin and glucose using a two-step dilute acid pretreatment process. Biotechnol. Biofuels 2021, 14, 194. [Google Scholar] [CrossRef]
- de Souza, J.B.; Michelin, M.; Amâncio, F.L.R.; Vital Brazil, O.A.; Polizeli, M.d.L.T.M.; Ruzene, D.S.; Silva, D.P.; Mendonça, M.d.C.; López, J.A. Sunflower stalk as a carbon source inductive for fungal xylanase production. Ind. Crop. Prod. 2020, 153, 112368. [Google Scholar] [CrossRef]
- Mati-Bouche, N.; De Baynast, H.; Lebert, A.; Sun, S.; Sacristan Lopez-Mingo, C.J.; Leclaire, P.; Michaud, P. Mechanical, thermal and acoustical characterizations of an insulating bio-based composite made from sunflower stalks particles and chitosan. Ind. Crop. Prod. 2014, 58, 244–250. [Google Scholar] [CrossRef]
- Rudi, H.; Resalati, H.; Behrooz Eshkiki, R.; Kermanian, H. Sunflower stalk neutral sulfite semi-chemical pulp: An alternative fiber source for production of fluting paper. J. Clean. Prod. 2016, 127, 562–566. [Google Scholar] [CrossRef]
- Ewulonu, C.M.; Liu, X.; Wu, M.; Huang, Y. Ultrasound-assisted mild sulphuric acid ball milling preparation of lignocellulose nanofibers (LCNFs) from sunflower stalks (SFS). Cellulose 2019, 26, 4371–4389. [Google Scholar] [CrossRef]
- Rodríguez-Liébana, J.A.; Navas-Martos, F.J.; Jurado-Contreras, S.; Morillas-Gutiérrez, F.; Mateo, S.; Moya, A.J.; La Rubia, M.D. Manufacture and characterisation of polylactic acid biocomposites with high-purity cellulose isolated from olive pruning waste. J. Reinf. Plast. Compos. 2024, 43, 318–338. [Google Scholar] [CrossRef]
- Camacho-Núñez, L.; Jurado-Contreras, S.; La Rubia, M.D.; Navas-Martos, F.J.; Rodríguez-Liébana, J.A. Cellulose-Based Upcycling of Brewer’s Spent Grains: Extraction and Acetylation. J. Polym. Environ. 2023; in press. [Google Scholar] [CrossRef]
- Rodríguez-Liébana, J.A.; Robles-Solano, E.; Jurado-Contreras, S.; Morillas-Gutiérrez, F.; Moya, A.J.; Mateo, S.; Navas-Martos, F.J.; La Rubia, M.D. Production and characterization of cellulose acetate using olive tree pruning biomass as feedstock. Biofuels Bioprod. Bioref. 2024; in press. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using X-Ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Kassab, Z.; Abdellaoui, Y.; Hamid Salim, M.; Bouhfid, R.; El Kacem Qaiss, A.; El Achaby, M. Micro- and nano-celluloses derived from hemp stalks and their effects as polymer reinforcing materials. Carbohydr. Polym. 2020, 245, 116506. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Abu-Jdayil, B.; Banat, F.; Al-Marzouqi, A.H. Isolation and characterization of cellulose nanocrystals from date palm waste. ACS Omega 2022, 7, 25366–25379. [Google Scholar] [CrossRef] [PubMed]
- Rubiyah, M.H.; Melethil, K.; Varghese, S.; Kurian, M.; Babu, S.; Jojo, L.; Thomas, B. Isolation and characterization of cellulose nanofibrils from agro-biomass of Jackfruit (Artocarpus heterophyllus) rind, using a soft and benign acid hydrolysis. Carbohydr. Polym. Technol. Appl. 2023, 6, 100374. [Google Scholar] [CrossRef]
- Jiménez, L.; Pérez, I.; de la Torre, M.J.; García, J.C. The effect of processing variables on the soda pulping of olive tree wood. Bioresour. Technol. 1999, 69, 95–102. [Google Scholar] [CrossRef]
- Candido, R.G.; Conçalves, A.R. Synthesis of cellulose acetate and carboxymethylcellulose from sugarcane straw. Carbohydr. Polym. 2016, 152, 679–686. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Rocha, G.J.M.; Roberto, I.C. Hydrogen peroxide bleaching of cellulose pulps obtained from brewer’s spent grain. Cellulose 2008, 15, 641–649. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Andrade Alves, J.A.; Lisboa dos Santos, M.D.; Cintra Morais, C.; Ramirez Ascheri, J.L.; Signini, R.; dos Santos, D.M.; Cavalcante Bastos, S.M.; Ramirez Ascheri, D.P. Sorghum straw: Pulping and bleaching process optimization and synthesis of cellulose acetate. Int. J. Biol. Macromol. 2019, 135, 877–886. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Sun, X.F.; Jing, Z.; Fowler, P.; Wu, Y.; Rajaratnam, M. Structural characterization and isolation of lignin and hemicelluloses from barley straw. Ind. Crop. Prod. 2011, 33, 588–598. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; De Lacerda Bukzem, A.; Ascheri, D.P.R.; Signini, R.; De Aquino, G.L.B. Microwave-assisted carboxymethylation of cellulose extracted from brewer´s spent grain. Carbohydr. Polym. 2015, 131, 125–133. [Google Scholar] [CrossRef]
- Elyamine, A.M.; Moussa, M.G.; Afzal, J.; Rana, M.S.; Imran, M.; Zhao, X.; Hu, C.X. Modified rice straw enhanced cadmium (II) immobilization in soil and promoted the degradation of phenantrene in co-contaminated soil. Int. J. Mol. Sci. 2019, 20, 2189. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Harris, D.; DeBolt, S. Relative crystallinity of plant biomass: Studies on assembly, adaptation and acclimation. PLoS ONE 2008, 3, e2897. [Google Scholar] [CrossRef]
- Puris, V.P. Effect of crystallinity and degree of polymerization of cellulose on enzymatic saccharification. Biotechnol. Bioeng. 1984, 26, 1219–1222. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]


| Coded Values | Real Values | |||||
|---|---|---|---|---|---|---|
| RUN | t | T | c | t (min) | T (°C) | c (% w/v) |
| AH1 | −1 | −1 | −1 | 120 | 60 | 2 |
| AH2 | 1 | −1 | −1 | 240 | 60 | 2 |
| AH3 | −1 | 1 | −1 | 120 | 90 | 2 |
| AH4 | 1 | 1 | −1 | 240 | 90 | 2 |
| AH5 | −1 | −1 | 1 | 120 | 60 | 8 |
| AH6 | 1 | −1 | 1 | 240 | 60 | 8 |
| AH7 | −1 | 1 | 1 | 120 | 90 | 8 |
| AH8 | 1 | 1 | 1 | 240 | 90 | 8 |
| AH9 | −1.41 | 0 | 0 | 95.4 | 75 | 5 |
| AH10 | 1.41 | 0 | 0 | 264.6 | 75 | 5 |
| AH11 | 0 | −1.41 | 0 | 180 | 53.85 | 5 |
| AH12 | 0 | 1.41 | 0 | 180 | 96.15 | 5 |
| AH13 | 0 | 0 | −1.41 | 180 | 75 | 0.77 |
| AH14 | 0 | 0 | 1.41 | 180 | 75 | 9.23 |
| AH15 | 0 | 0 | 0 | 180 | 75 | 5 |
| AH16 | 0 | 0 | 0 | 180 | 75 | 5 |
| AH17 | 0 | 0 | 0 | 180 | 75 | 5 |
| Waste Biomass | Cellulose (%) | Hemicellulose (%) | Lignin (AIL; %) | Yield (%) |
|---|---|---|---|---|
| CS | 23.1 ± 0.2 | 14.2 ± 0.4 | 21.9 ± 0.1 | — |
| CS-AH 1 | 52.6 ± 1.9 | 7.5 ± 1.7 | 11.1 ± 2.5 | 29.1 |
| CS-BH | 75.7 ± 3.8 | 4.3 ± 0.2 | 7.8 ± 0.8 | 75.5 |
| CS-BL | 90.6 ± 0.6 | 0.36 ± 0.06 | 2.4 ± 0.1 | 91.7 |
| YiedWHOLE 20.1 | ||||
| SFS | 28.0 ± 0.5 | 14.7 ± 0.5 | 20.2 ± 0.4 | — |
| SFS-AH 1 | 50.3 ± 1.7 | 12.3 ± 1.3 | 4.7 ± 1.1 | 51.5 |
| SFS-BH | 74.0 ± 1.8 | 7.5 ± 0.4 | 5.7 ± 0.2 | 70.2 |
| SFS-BL | 90.8 ± 1.2 | 0.59 ± 0.09 | 0.27 ± 0.01 | 88.6 |
| YiedWHOLE 32.0 |
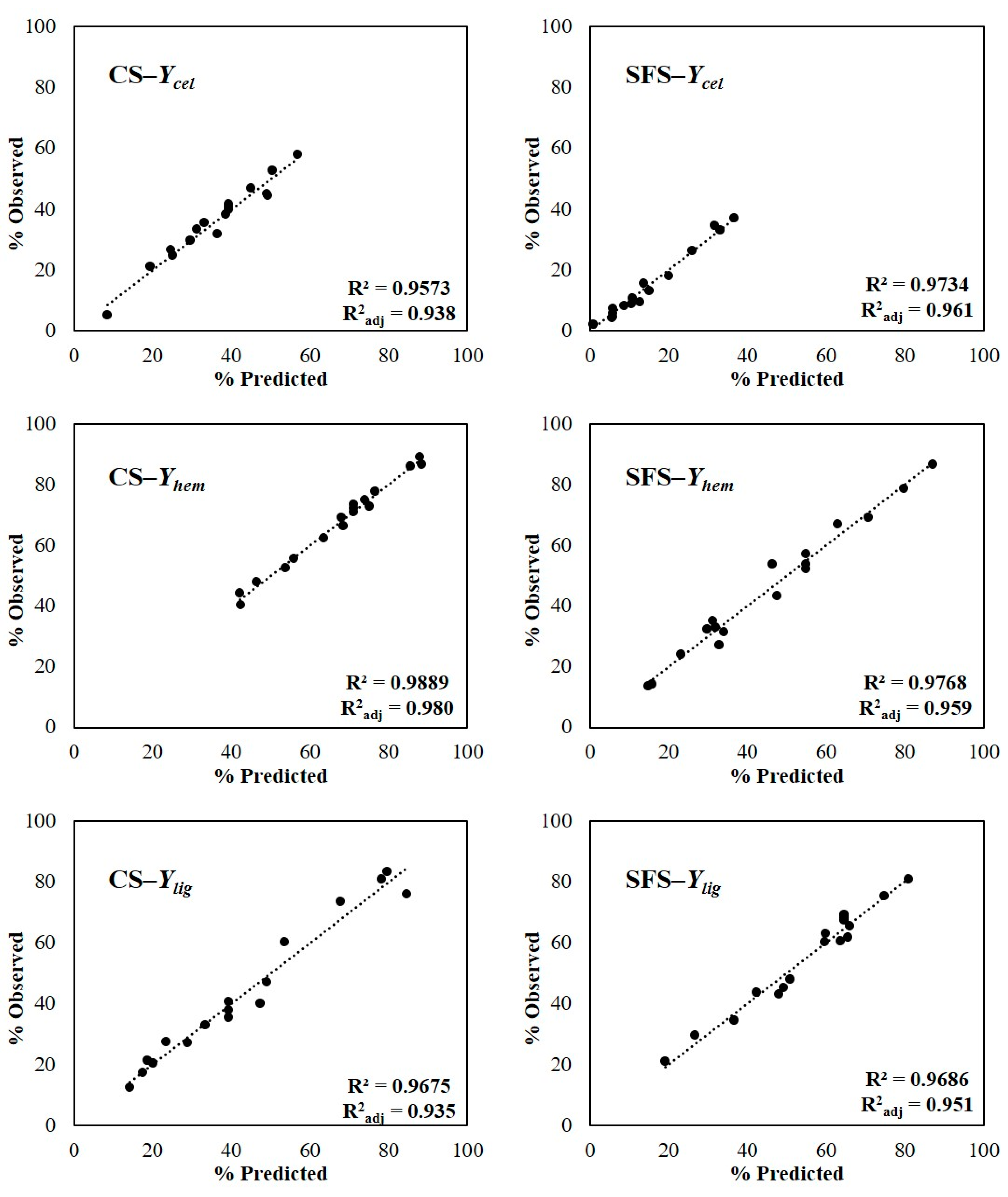
| RUN | t (min) | T (°C) | c (% w/v) | Ycel (%) | Yhem (%) | Ylig (%) | YieldAH |
|---|---|---|---|---|---|---|---|
| AH1 | 120 | 60 | 2 | 26.7 | 44.1 | 21.3 | 69.9 |
| AH2 | 240 | 60 | 2 | 21.1 | 47.9 | 32.9 | 53.0 |
| AH3 | 120 | 90 | 2 | 31.7 | 55.6 | 12.4 | 61.4 |
| AH4 | 240 | 90 | 2 | 33.3 | 66.3 | 27.2 | 54.1 |
| AH5 | 120 | 60 | 8 | 38.2 | 75.0 | 60.4 | 50.4 |
| AH6 | 240 | 60 | 8 | 35.5 | 62.4 | 40.0 | 54.9 |
| AH7 | 120 | 90 | 8 | 52.8 | 88.9 | 75.9 | 32.8 |
| AH8 | 240 | 90 | 8 | 46.9 | 86.1 | 81.0 | 31.7 |
| AH9 | 95.4 | 75 | 5 | 58.0 | 72.7 | 17.3 | 57.9 |
| AH10 | 264.6 | 75 | 5 | 44.4 | 77.7 | 27.6 | 50.2 |
| AH11 | 180 | 53.85 | 5 | 5.2 | 40.3 | 47.1 | 67.6 |
| AH12 | 180 | 96.15 | 5 | 24.9 | 69.0 | 73.7 | 31.5 |
| AH13 | 180 | 75 | 0.77 | 29.6 | 52.5 | 20.3 | 69.4 |
| AH14 | 180 | 75 | 9.23 | 45.1 | 86.6 | 83.2 | 33.0 |
| AH15 | 180 | 75 | 5 | 39.9 | 71.1 | 40.6 | 54.1 |
| AH16 | 180 | 75 | 5 | 41.8 | 73.3 | 35.3 | 54.9 |
| AH17 | 180 | 75 | 5 | 40.8 | 72.2 | 38.0 | 56.6 |
| RUN | t (min) | T (°C) | c (% w/v) | Ycel (%) | Yhem (%) | Ylig (%) |
|---|---|---|---|---|---|---|
| AH1 | 120 | 60 | 2 | 26.7 | 44.1 | 21.3 |
| AH2 | 240 | 60 | 2 | 21.1 | 47.9 | 32.9 |
| AH3 | 120 | 90 | 2 | 31.7 | 55.6 | 12.4 |
| AH4 | 240 | 90 | 2 | 33.3 | 66.3 | 27.2 |
| AH5 | 120 | 60 | 8 | 38.2 | 75.0 | 60.4 |
| AH6 | 240 | 60 | 8 | 35.5 | 62.4 | 40.0 |
| AH7 | 120 | 90 | 8 | 52.8 | 88.9 | 75.9 |
| AH8 | 240 | 90 | 8 | 46.9 | 86.1 | 81.0 |
| AH9 | 95.4 | 75 | 5 | 58.0 | 72.7 | 17.3 |
| AH10 | 264.6 | 75 | 5 | 44.4 | 77.7 | 27.6 |
| AH11 | 180 | 53.85 | 5 | 5.2 | 40.3 | 47.1 |
| AH12 | 180 | 96.15 | 5 | 24.9 | 69.0 | 73.7 |
| AH13 | 180 | 75 | 0.77 | 29.6 | 52.5 | 20.3 |
| AH14 | 180 | 75 | 9.23 | 45.1 | 86.6 | 83.2 |
| AH15 | 180 | 75 | 5 | 39.9 | 71.1 | 40.6 |
| AH16 | 180 | 75 | 5 | 41.8 | 73.3 | 35.3 |
| AH17 | 180 | 75 | 5 | 40.8 | 72.2 | 38.0 |
| Experimental Conditions | Experimental Values | Predicted Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| t (min) | T (°C) | c (%) | Ycel (%) | Yhem (%) | Ylig (%) | Ycel (%) | Yhem (%) | Ylig (%) | |
| CS | 190 | 96.2 | 6.3 | 32.72 | 72.90 | 88.78 | 27.95 | 73.69 | 83.35 |
| SFS | 130 | 73.8 | 8.7 | 0.78 | 54.25 | 87.04 | 0.62 | 52.88 | 86.25 |
| Type of Fiber | Amorphous Fraction | Crystalline Fraction | CrI (%) | ||
|---|---|---|---|---|---|
| 2θ (°) | Iam | 2θ (°) | I200 | ||
| CS | 17.8 | 26,435 | 22.0 | 43,776 | 39.6 |
| CS-BL | 18.1 | 6990 | 22.3 | 22,597 | 69.1 |
| SFS | 17.5 | 27,079 | 21.9 | 51,527 | 47.4 |
| SFS-BL | 18.6 | 9136 | 22.4 | 30,019 | 69.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rubia, M.D.; Jurado-Contreras, S.; Navas-Martos, F.J.; García-Ruiz, Á.; Morillas-Gutiérrez, F.; Moya, A.J.; Mateo, S.; Rodríguez-Liébana, J.A. Characterization of Cellulosic Pulps Isolated from Two Widespread Agricultural Wastes: Cotton and Sunflower Stalks. Polymers 2024, 16, 1594. https://doi.org/10.3390/polym16111594
La Rubia MD, Jurado-Contreras S, Navas-Martos FJ, García-Ruiz Á, Morillas-Gutiérrez F, Moya AJ, Mateo S, Rodríguez-Liébana JA. Characterization of Cellulosic Pulps Isolated from Two Widespread Agricultural Wastes: Cotton and Sunflower Stalks. Polymers. 2024; 16(11):1594. https://doi.org/10.3390/polym16111594
Chicago/Turabian StyleLa Rubia, M. Dolores, Sofía Jurado-Contreras, Francisco Javier Navas-Martos, Ángeles García-Ruiz, Francisca Morillas-Gutiérrez, Alberto J. Moya, Soledad Mateo, and José Antonio Rodríguez-Liébana. 2024. "Characterization of Cellulosic Pulps Isolated from Two Widespread Agricultural Wastes: Cotton and Sunflower Stalks" Polymers 16, no. 11: 1594. https://doi.org/10.3390/polym16111594
APA StyleLa Rubia, M. D., Jurado-Contreras, S., Navas-Martos, F. J., García-Ruiz, Á., Morillas-Gutiérrez, F., Moya, A. J., Mateo, S., & Rodríguez-Liébana, J. A. (2024). Characterization of Cellulosic Pulps Isolated from Two Widespread Agricultural Wastes: Cotton and Sunflower Stalks. Polymers, 16(11), 1594. https://doi.org/10.3390/polym16111594








