Theory-Driven Tailoring of the Microenvironment of Quaternary Ammonium Binding Sites on Electrospun Nanofibers for Efficient Bilirubin Removal in Hemoperfusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
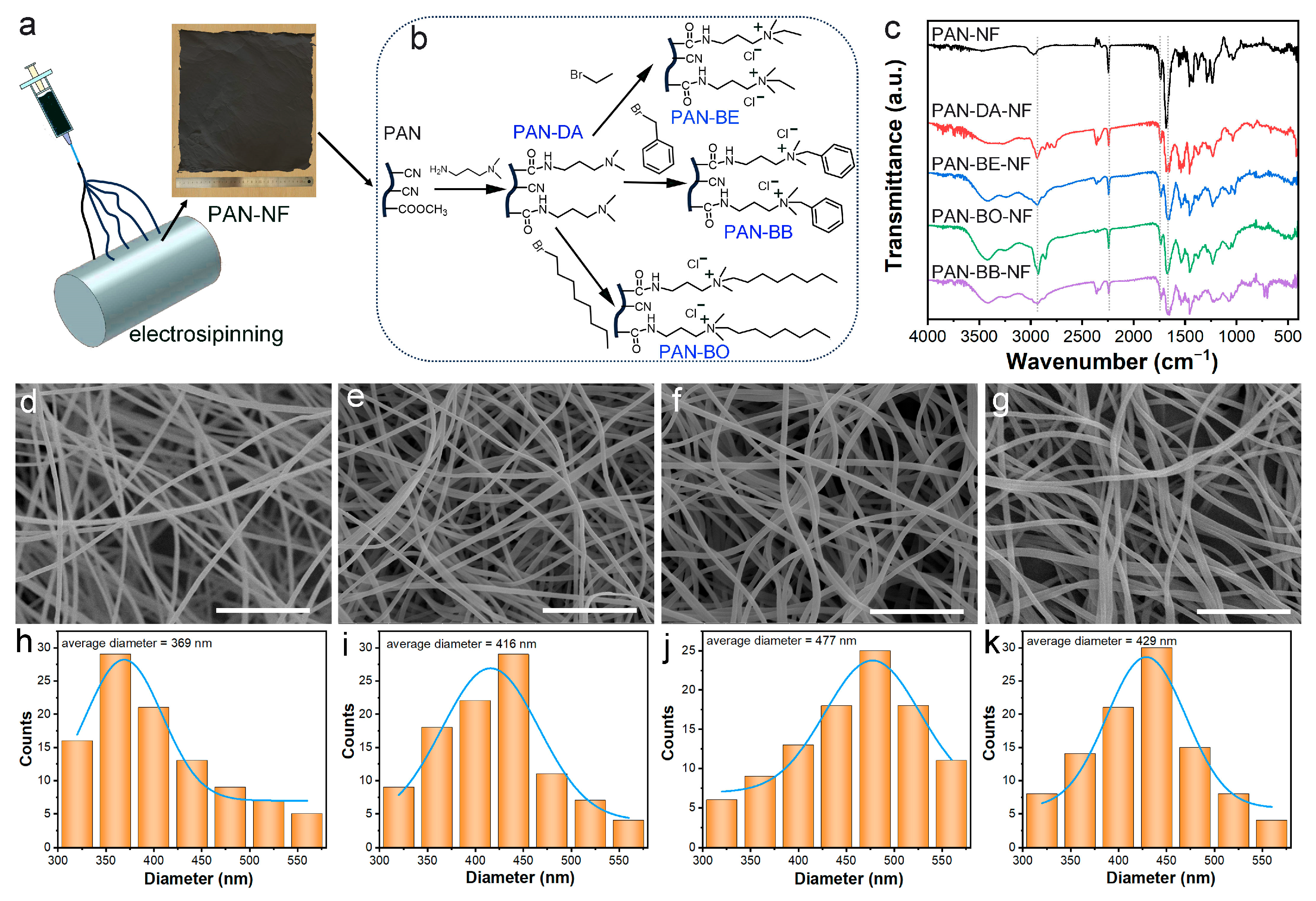
2.2. Synthesis of Grafted Electrospun Nanofibers
2.3. Bilirubin Adsorption Experiments
2.4. Blood Compatibility Experiments
2.5. Computational Method
3. Results
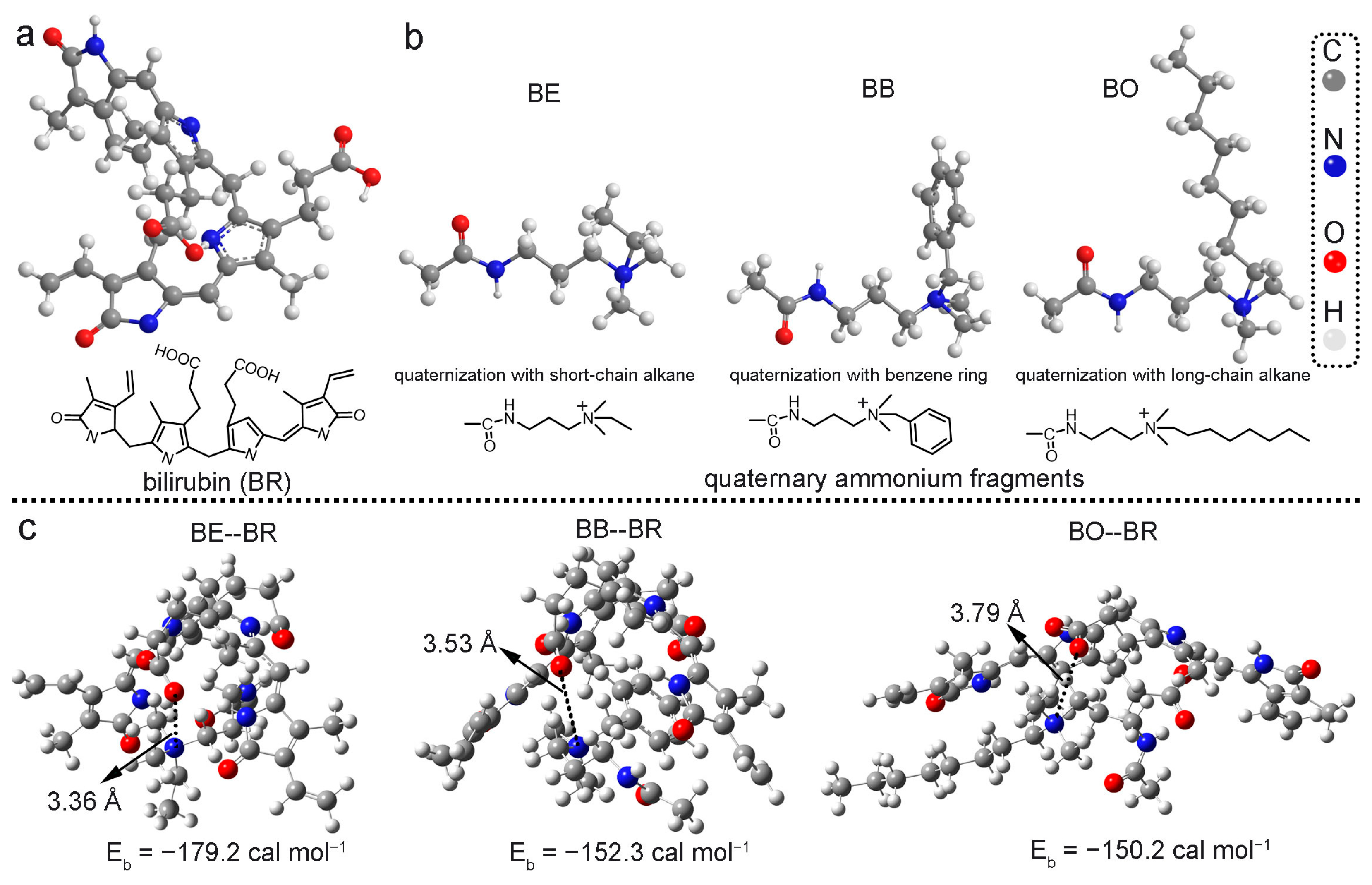
3.1. Design and Theoretical Calculations
3.2. Preparation and Characterization of Three Electrospun Nanofiber Adsorbents
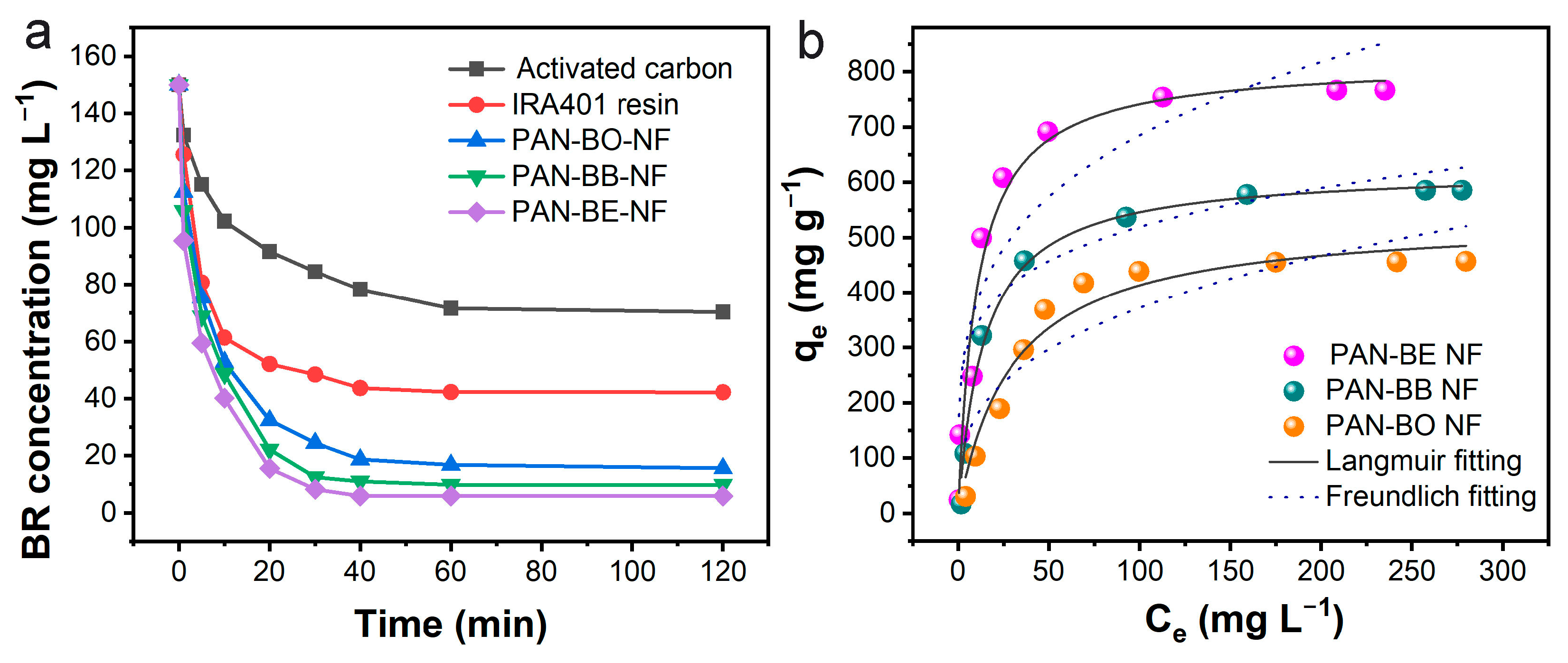
3.3. Bilirubin Adsorption Experiments
3.4. Blood Compatibility Test
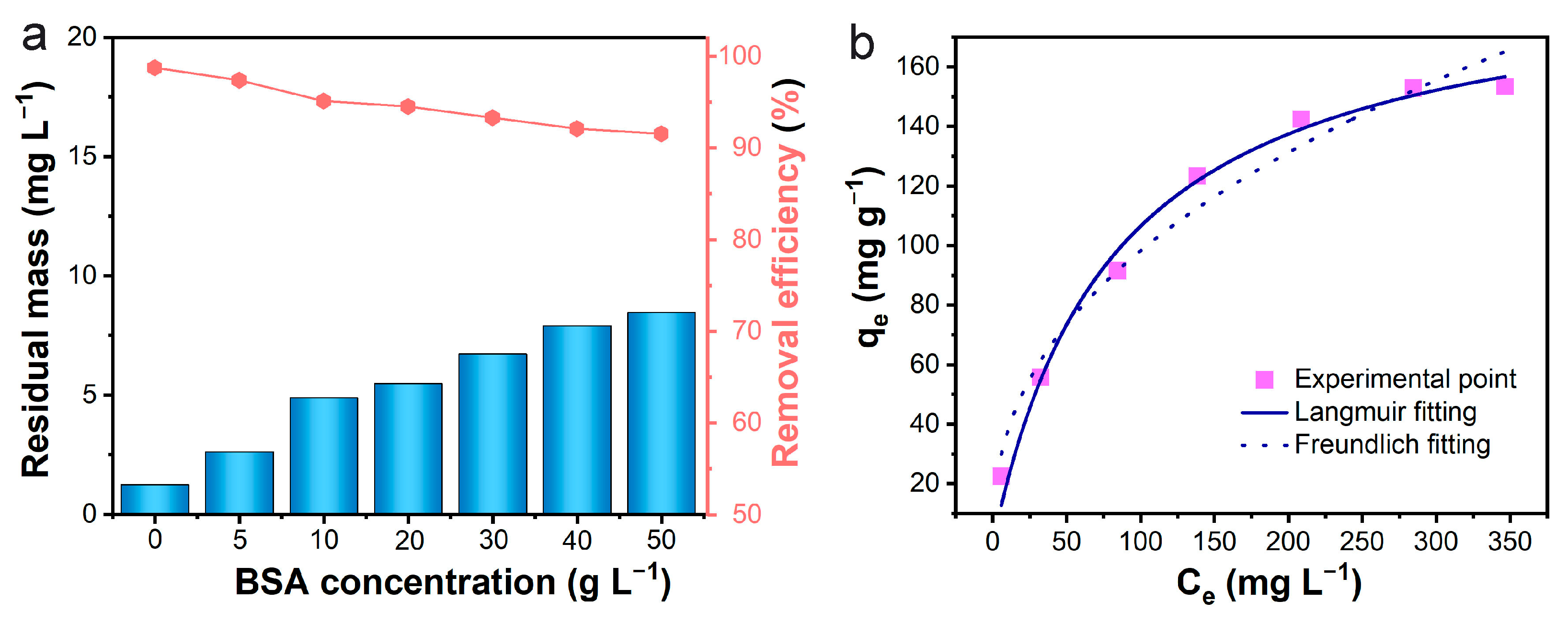
3.5. Dynamic Bilirubin Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Du, Y.; Chen, M.; Wang, B.; Chai, Y.; Wang, L.; Li, N.; Zhang, Y.; Zhuang, L.; Guo, C.; Jiang, X.; et al. TiO2/polystyrene nanocomposite antibacterial material as a hemoperfusion adsorbent for efficient bilirubin removal and prevention of bacterial infection. ACS Biomater. Sci. Eng. 2024, 10, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, M.; Liu, X.; Hussain, W.; Zhu, B.; Yu, H.; Wang, S.; Zhou, L. Collagen-modified chitosan microsphere as a novel hemoperfusion adsorbent for efficient removal of bilirubin from human plasma. Sep. Purif. Technol. 2024, 337, 126303. [Google Scholar] [CrossRef]
- Li, X.; Wei, F.; Li, Y.; Qi, F.; Wang, J.; Yao, Z.; Zhang, L.; Zhang, B. Fabricating polyacrylonitrile-polyethyleneimine nanofiber membranes with exceptional hemocompatibility and efficient bilirubin removal for hemoperfusion. React. Funct. Polym. 2024, 194, 105793. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, R.; Zhao, W.; Zhao, C. Bilirubin removal by polymeric adsorbents for hyperbilirubinemia therapy. Macromol. Biosci. 2023, 23, 2200567. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Zhou, W.; Zheng, X.; Wang, S.; Zhou, L. Ordered porous materials for blood purification. Sep. Purif. Technol. 2023, 327, 124844. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Bao, C.; Zhang, J.; Tian, Y.; Shen, J.; Feng, X. Freezing-induced chemical crosslinking to fabricate nanocellulose-based cryogels for efficient bilirubin removal. Sep. Purif. Technol. 2022, 300, 121865. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Y.; Zhou, Y.; Zhang, Q.; Wang, S. Influence of the acid groups on activated carbon on the adsorption of bilirubin. Mater. Chem. Phys. 2021, 260, 124133. [Google Scholar]
- Gao, X.; Liu, K.; Liu, P.; Bai, X.; Li, A.; Lyu, Z.; Li, Q. Preparation and properties of cellulose acetate graft copolymer-coated adsorbent resin for hemoperfusion device. J. Appl. Polym. Sci. 2023, 140, 53895. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, D.; Gao, N.; Yuan, L.; Hu, W.; Cui, F.; Tian, Y.; Shi, W.; Ma, S.; Zhu, G. Porous cationic electrospun fibers with sufficient adsorption sites for effective and continuous 99TcO4− uptake. Adv. Funct. Mater. 2022, 32, 2200618. [Google Scholar] [CrossRef]
- Liu, H.; Lai, W.; Shi, Y.; Tian, L.; Li, K.; Bian, L.; Xi, Z.; Lin, B. One-step fast fabrication of electrospun fiber membranes for efficient particulate matter removal. Polymers 2024, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, M.; Ma, L.; Mao, L.; Yao, Y.; Xu, X.; Xu, L.; Han, J.; Xue, X.; Xu, G.; et al. Ultrafast strategy to self-assemble polyamidoxime PVA nanofibers with solar-induced synergy for enhancing uranium extraction from seawater. Chem. Eng. J. 2023, 474, 145718. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Ramakrishna, S. Electrospun cellulose nanofiber as affinity membrane. J. Membr. Sci. 2005, 265, 115–123. [Google Scholar] [CrossRef]
- Wu, K.; Yang, W.; Liu, X.; Jiao, Y.; Zhou, C. Electrospun porous polyethersulfone (PES) fiber mats with high bilirubin adsorption capacity. Mater. Lett. 2016, 185, 252–255. [Google Scholar] [CrossRef]
- Wu, K.; Song, X.; Cui, S.; Li, Z.; Jiao, Y.; Zhou, C. Immobilization of bovine serum albumin via mussel-inspired polydopamine coating on electrospun polyethersulfone (PES) fiber mat for effective bilirubin adsorption. Appl. Surf. Sci. 2018, 451, 45–55. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Li, X.; Li, Y.; Sun, B.; Chao, S.; Wang, C. Facile hydrothermal synthesis of branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane as a highly efficient and reusable bilirubin adsorbent in hemoperfusion. J. Colloid Interface Sci. 2018, 514, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Zhang, P.; Yuan, L.; Li, H.; Zhan, J. Enhanced hemocompatibility of lysine grafted polyacrylonitrile electrospun nanofiber membranes as a potential bilirubin adsorption in hemoperfusion. J. Polym. Environ. 2023, 31, 1553–1567. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.; Cui, F.; Tian, Y.; Zhu, G. Porous aromatic framework with tailored binding sites and pore sizes as a High-Performance hemoperfusion adsorbent for bilirubin removal. Adv. Sci. 2020, 7, 2001899. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; He, L.; Zhao, F.; Tao, Y.; Sun, W.; Tai, B.; Guo, Q.; Zhang, W.; Peng, F.; Chen, J.; et al. Theory-driven design of phosphazene-based porous polymer beads for enhanced iodine adsorption. Sep. Purif. Technol. 2024, 345, 127321. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Dong, J.; Ma, S.; Ou, J. One-pot synthesis of glucose-derived carbonaceous material with high hydrophilicity and adsorption capacity as bilirubin adsorbent. J. Mater. Sci. 2021, 56, 18006–18018. [Google Scholar] [CrossRef]
- Lei, H.; Huang, S.; Pan, N.; Zou, H.; Wang, X.; Tuo, X. Novel Phytic Acid-Based Supramolecular Material for Uranium Removal from Highly Acidic Media: Synthesis, Performance, and Mechanistic Insights. ACS EST Water 2023, 3, 2522–2534. [Google Scholar] [CrossRef]
- Li, L.; Ren, H.; Yuan, Y.; Yu, G.; Zhu, G. Construction and adsorption properties of porous aromatic frameworks via AlCl3-triggered coupling polymerization. J. Mater. Chem. A 2014, 2, 11091. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, J.; Zhang, S.; Niu, P.; Li, H.; Liu, Z.; Zhang, N.; Li, C.; Wang, L.; Wang, Y. A comparative study of removal of acid red 27 by adsorption on four different chitosan morphologies. Polymers 2024, 16, 1019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, W.; Zhan, Q.; Zhang, M.; Liu, X.; Hussain, W.; Yu, H.; Wang, S.; Zhou, L. Novel hemoperfusion adsorbents based on collagen for efficient bilirubin removal–A thought from yellow skin of patients with hyperbilirubinemia. Int. J. Biol. Macromol. 2023, 253, 127321. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, H.; Zhang, Z.; Luo, M.; Wang, Y.; Liu, Q.; Chen, Y.; Li, M.; Wang, D. Amine-functionalized PVA-co-PE nanofibrous membrane as affinity membrane with high adsorption capacity for bilirubin. Colloids. Surf. B. 2017, 150, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhao, D.; Zhang, K.; Wang, F.; Wang, C.; Wen, Y. High efficiency 3D nanofiber sponge for bilirubin removal used in hemoperfusion. Colloids. Surf. B. 2018, 172, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gao, Q.; Zhou, J.; Chen, Q.; Han, B.; Xia, K.; Zhou, C. Facile one-pot synthesis of magnetic nitrogen-doped porous carbon for high-performance bilirubin removal from BSA-rich solution. RSC Adv. 2017, 7, 2081. [Google Scholar] [CrossRef]
- Ma, C.F.; Gao, Q.; Xia, K.S.; Huang, Z.Y.; Han, B.; Zhou, C.G. Three-dimensionally porous graphene: A high-performance adsorbent for removal of albumin-bonded bilirubin. Colloids. Surf. B. 2017, 149, 146–153. [Google Scholar] [CrossRef] [PubMed]


| Sample | Weight Gain (%) | Functionality (mmol g–1) |
|---|---|---|
| PAN-NF | -- | -- |
| PAN-DA-NF | 25.30% | 1.96 |
| PAN-BE-NF | 5.50% | 1.79 |
| PAN-BB-NF | 19.80% | 1.82 |
| PAN-BO-NF | 25.70% | 1.81 |
| Isotherm Model | PAN-BE-NF | PAN-BB-NF | PAN-BO-NF |
|---|---|---|---|
| Langmuir isotherm | |||
| qmax (mg g−1) | 818.9 | 623.8 | 536.8 |
| b (L mg−1) | 0.096 | 0.07 | 0.033 |
| R2 | 0.9662 | 0.9886 | 0.9561 |
| Freundlich isotherm | |||
| KF | 210.9 | 222.9 | 83.2 |
| n | 3.91 | 5.44 | 3.07 |
| R2 | 0.8452 | 0.8782 | 0.7982 |
| Isotherm Model | PAN-BE-NF |
|---|---|
| Langmuir isotherm | |
| qmax (mg g−1) | 163.7 |
| b (L mg−1) | 0.012 |
| R2 | 0.9868 |
| Freundlich isotherm | |
| KF | 14.4 |
| n | 2.4 |
| R2 | 0.9281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Shi, M.; Chen, D.; Zhao, X.; Jiang, T.; Zhao, R. Theory-Driven Tailoring of the Microenvironment of Quaternary Ammonium Binding Sites on Electrospun Nanofibers for Efficient Bilirubin Removal in Hemoperfusion. Polymers 2024, 16, 1599. https://doi.org/10.3390/polym16111599
Fu X, Shi M, Chen D, Zhao X, Jiang T, Zhao R. Theory-Driven Tailoring of the Microenvironment of Quaternary Ammonium Binding Sites on Electrospun Nanofibers for Efficient Bilirubin Removal in Hemoperfusion. Polymers. 2024; 16(11):1599. https://doi.org/10.3390/polym16111599
Chicago/Turabian StyleFu, Xingyu, Minsi Shi, Dingyang Chen, Xinyue Zhao, Tingting Jiang, and Rui Zhao. 2024. "Theory-Driven Tailoring of the Microenvironment of Quaternary Ammonium Binding Sites on Electrospun Nanofibers for Efficient Bilirubin Removal in Hemoperfusion" Polymers 16, no. 11: 1599. https://doi.org/10.3390/polym16111599
APA StyleFu, X., Shi, M., Chen, D., Zhao, X., Jiang, T., & Zhao, R. (2024). Theory-Driven Tailoring of the Microenvironment of Quaternary Ammonium Binding Sites on Electrospun Nanofibers for Efficient Bilirubin Removal in Hemoperfusion. Polymers, 16(11), 1599. https://doi.org/10.3390/polym16111599







